Abstract
Background
Ticks and tick-borne diseases are a major impediment to livestock production worldwide. Cattle trade and transnational transhumance create risks for the spread of ticks and tick-borne diseases and threaten cattle production in the absence of an effective tick control program. Few studies have been undertaken on cattle ticks in the Central African region; therefore, the need to assess the occurrence and the spatial distribution of tick vectors with the aim of establishing a baseline for monitoring future spread of tick borne-diseases in the region is urgent.
Results
A total of 7091 ixodid ticks were collected during a countrywide cross-sectional field survey and identified using morphological criteria. Of these, 4210 (59.4%) ticks were Amblyomma variegatum, 1112 (15.6%) Rhipicephalus (Boophilus) microplus, 708 (10.0%) Rhipicephalus (Boophilus) decoloratus, 28 (0.4%) Rhipicephalus (Boophilus) annulatus, 210 (3.0%) Hyalomma rufipes, 768 (10.8%) Hyalomma truncatum, and 19 (0.3%) Rhipicephalus sanguineus. Three ticks of the genus Hyalomma spp. and 33 of the genus Rhipicephalus spp. were not identified to the species level. Cytochrome c oxidase subunit 1 (cox1) gene sequencing supported the data from morphological examination and led to identification of three additional species, namely Hyalomma dromedarii, Rhipicephalus sulcatus and Rhipicephalus pusillus. The finding of the invasive tick species R. microplus in such large numbers and the apparent displacement of the indigenous R. decoloratus is highly significant since R. microplus is a highly efficient vector of Babesia bovis.
Conclusions
This study reports the occurrence and current geographical distribution of important tick vectors associated with cattle in Cameroon. It appears that R. microplus is now well established and may be displacing native Rhipicephalus (Boophilus) species, such as R. decoloratus. This calls for an urgent response to safeguard the livestock sector in western central Africa.
Keywords: Tick-borne diseases, Ticks, Identification, Cattle, cox1, Agro-ecological zones
Background
Ticks rank first among vectors of diseases affecting livestock globally [1]. Their direct effects on the hosts include anemia and excessive grooming, stress, toxicosis and immunosuppression, which often lead to diminished productivity [2]. Ticks also transmit a great variety of pathogenic microorganisms that cause disease in both humans and livestock [3]. Data on the economic impact of ticks and tick-borne diseases (TBDs) are scarce but it has been estimated that, globally, about US$ 20–30 billion are lost annually due TBDs [4].
In a study conducted in 1982 in Cameroon, approximately 63% of animal mortality in the Wakwa research station situated in the principal cattle rearing region was attributed to TBDs [5]. This situation has seriously constrained attempts to rear high performing exotic dairy cattle breeds which are highly susceptible to tick-borne diseases including babesiosis, ehrlichiosis and dermatophilosis [6].
Increased demand for animal food products in West and Central Africa due to rapid population growth has accelerated transboundary livestock movements for trade across the region. Consequently, there is an increased risk of animal disease transmission [7, 8]. In addition, animal movements in sub-Saharan Africa are also linked to transnational transhumance [9]. This regular movement of herders and their livestock across national boundaries to exploit the seasonal availability of pastures is a socio-cultural phenomenon [10]. It represents the transhumant communities’ key resilience strategy to combat fluctuations and long term change in climate [9]. Unfortunately, disease surveillance at the borders of most sub-Saharan African countries is limited or altogether lacking [11]. This has created a situation that allows the importation of exotic tick species and their pathogens into many countries. The recent finding of the cattle tick Rhipicephalus (Boophilus) microplus in West African countries such as Ivory coast, Burkina Faso, Mali, Togo, Benin and Nigeria is worrisome because this tick species is an efficient vector of Babesia bovis which causes a virulent form of babesiosis, the most important tick-transmitted disease of cattle globally [12–14]. It is widely believed that R. microplus was introduced into West Africa, seemingly through cattle imported from Brazil and has rapidly spread across the sub-region through transboundary cattle movements [14, 15].
Small-scale livestock keepers play an important role in the livestock industry in developing countries, contributing greatly to food security and rural development [16]. Most small scale livestock farmers cannot afford regular tick control with acaricides, relying on labor intensive manual control of ticks, combined with limited chemical treatment, particularly during the rainy seasons [17].
The few studies undertaken on ticks infesting livestock in Cameroon have been limited to the principal cattle rearing areas, namely the Far North, North, Adamawa and North West regions [5, 17–27]. Areas with low animal densities, including the eastern region bordering Central African Republic (C.A.R.) are understudied, yet this area has been the focus of very extensive livestock movements [28]. Such animal movement can contribute to a shift in the tick ‘population landscape’ [29]. Additionally, it has been demonstrated that the distribution of many species will expand or contract as a consequence to global warming and climate change [30]. Despite the high impact of TBDs on the global economy, there is a lack of reliable data since data on the incidence of TBDs and the distribution map of many tick vectors is either not available for many African countries or are outdated. It is therefore urgent to accurately identify and update the distribution of ticks in order to predict the risk of emergence or re-emergence of TBDs in the sub-region.
Most studies on the identification of ticks are primarily based on morphological characterization. However, this method is challenging because morphological differences between closely related tick species are sometimes difficult to establish, especially when the tick specimens are damaged, engorged or from non-adult instars [31, 32]. Morphological classification also requires entomological expertise which is scarce or non-existent in most of the affected countries in sub-Saharan Africa. Reliance only on morphological identification of ticks can therefore result in misidentification if the entomological personnel are not adequately trained. DNA-based methods including the sequencing of specific loci encoded in the nuclear genome such as the ribosomal internal transcribed spacer (ITS) locus, the mitochondrially-encoded cox1 gene and the mitochondrial and nuclear ribosomal small subunit RNA genes (12S and 16S) have been developed as additional tools to support identification based on morphological criteria; this is especially the case for damaged specimens, in which key anatomical features can no longer be discriminated [33–35]. Recent studies on tick identification have demonstrated cox1 to be the most reliable and suitable molecular marker for the identification of different ticks at the species level [36].
In the present study, a countrywide cross-sectional survey was undertaken with the goal of assembling baseline data on the distribution of ticks in different agro-ecological zones (AEZs) of Cameroon. The implications for the epidemiology of TBDs and tick control in the country are discussed.
Methods
Study area
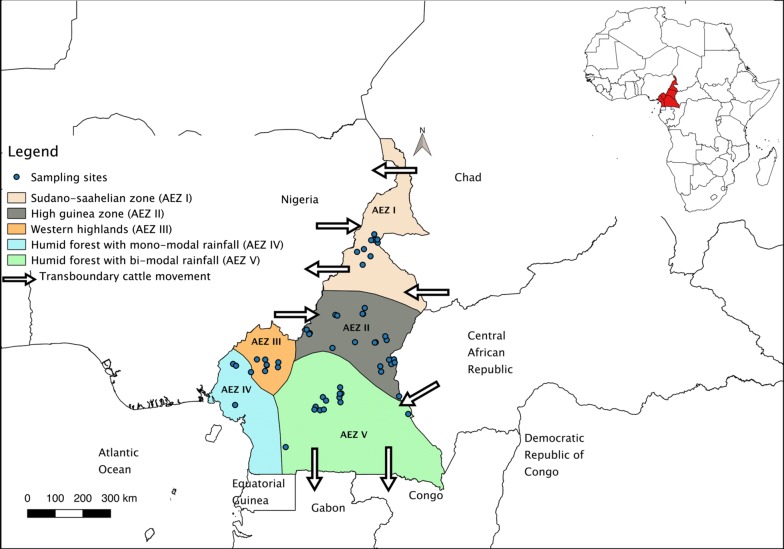
Sampling was conducted at 54 sites across five agro-ecological zones of Cameroon (Fig. 1). The number of sites sampled in each zone was determined by livestock density and willingness of farmers to participate in the survey. Each zone has distinct bioclimatic and environmental characteristics as indicated in Table 1. Briefly, AEZ I (Sudano-Sahelian zone) is characterized by the northern plain with high temperatures, dry savannah and steppes. Accurate statistics on livestock production are not easy to obtain because an appropriate data collection system is lacking [37]. The official cattle population of this zone is estimated at 1.89 million head [38]. AEZ II (High Guinea zone) is dominated by the Adamawa plateau in the center. The cattle population is estimated at 1.13 million head [38]. This region is the main destination of transhumant herders originating from neighboring countries [11]. The vegetation is characterized by savannah and degraded forest. The main physical unit of the AEZ III (Western highlands) is mountain characterized by low temperatures and high rainfall. This area mainly covers the West and the North-west region and hosts approximately 1.98 million head of cattle [38]. AEZ IV (humid forest with mono-modal rainfall) encompasses the coastal lowlands and hosts about 1472 head of cattle [38]. Temperature and annual rainfall are high. AEZ V (humid forest with bi-modal rainfall) is mainly dominated by the southern plateau. This area falls within the tsetse fly zone which constrains cattle rearing. Cattle population is estimated at 276,855 head [38]. Many farmers in this area are refugees originating from high conflict zones in C.A.R. [28].
Fig. 1.
Map showing the five AEZs of Cameroon and the sampling sites
Table 1.
Key geographical and climatic characteristics of agro-ecological zones of Cameroon
| Agro-ecological zones (AEZs) | Altitude (m) | Annual average temperature (°C) | Annual average precipitation (mm) | Rainy period | Vegetation | Cattle population |
|---|---|---|---|---|---|---|
| Sudano-sahelian zone (AEZ I) | 250–500 | 28.9 | 923.35 | June–August | Dry savannah, steppes | 1,898,890 |
| High guinea zone (AEZ II) | 500–1500 | 22.06 | 1515.3 | April–October | Savannah, degraded forest | 1,183,137 |
| Western Highlands (AEZ III) | 1500–2500 | 20.64 | 3080.5 | March–October | Savannah, degraded forest | 1,989,200 |
| Humid forest zone with mono-modal rainfall (AEZ IV) | 0–2500 | 24 | 4163.5 | March–October | Evergreen forest | 1472 |
| Humid forest zone with bimodal rainfall (AEZ V) | 400–1000 | 24.4 | 2456.8 | March–October | Humid forest-savannah mosaic | 276,855 |
Most cattle sampled in the different AEZs were of local breeds (96.67%) with a few exotic (1.83%) or crossbreed (1.5%) animals. Except for few instances where a combination of stall feeding and free grazing is practiced, most cattle are reared under an open grazing system. Small ruminants are found all over Cameroon. They are estimated to comprise 8.2 million animals, with goats outnumbering sheep [37]. Except for AEZ II, where cattle are major ruminant livestock, all the other zones are dominated by sheep and goats [38].
Sampling and morphological identification of different tick species
Ticks were collected from 601 cattle during a cross-sectional survey conducted from April to August 2016 (during the wet season) in all five agro-ecological zones, covering a total of 54 sites across the country. At each site, an average of 10 to 15 cattle was examined for the presence of ticks. Cattle were restrained and kept standing and all the body parts of the cattle were examined. Only visible adult ticks were collected. Because of their small size, immature stages were not collected. Therefore, none of the collections made from cattle in the present study were intended to be complete. Ticks were plucked using blunt steel forceps. The ticks collected were preserved in 70% ethanol and stored at 4 °C in the lab. The morphological identification of tick species was performed according to the published taxonomic keys using a standard stereomicroscope with a magnification of up to 100× [39]. This identification was conducted in the Tick Unit at the International Livestock Research Institute (ILRI) of Nairobi, Kenya.
For the spatial analysis, the exact site of each tick found attached on the cattle was recorded using a global positioning system data recorder (Garmin eTrex® 20; Garmin, Hampshire, UK). Each location was transferred into QGIS v.2.18 software and plotted on maps.
Molecular identification of different tick species
To support morphological identification, the identity of ticks was confirmed by molecular analysis of the partial sequence of the cox1 gene as described previously [36]. For each species of tick identified morphologically, two to four representative individuals were randomly selected. For specimens identified at the genus level, ticks were grouped, and representatives of each group were selected for molecular analysis (see Additional file 1: Table S1).
DNA extraction
A total of 30 adult ticks, representative of the species that were identified morphologically, were washed twice in distilled water and air dried for 30 min. Each tick was transferred into a 2 ml sterile micro-tube containing one sterile 4.5 mm glass plating bead (Rattler™ Plating Beads; ZYMO Research, California, USA). The tube was frozen in liquid nitrogen for 5 min and the tick was ground into powder using a Geno-grinder (SPEX Sample Prep; Stanmore, UK). Genomic DNA was extracted using a DNeasy Blood & Tissue Kit (Qiagen; Hilden, Germany) using a protocol recommended by the manufacturer.
Amplification and sequencing of the cox1 gene
A 710 base pair (bp) DNA fragment was generated using the forward primer LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) and the reverse primer HC02198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) as previously described [40]. PCR was performed in a final volume of 50 µl comprising 25 µl of AccuPower® Taq 2x PCR Master Mix (Bioneer; Daejeon, Korea), 50 ng of DNA template and 0.2 μM of each primer. The thermal cycling program consisted of an initial denaturation at 95 °C for 3 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 40 °C for 1 min and extension at 72 °C for 1 min. A final extension was carried out for 10 min at 72 °C. Five microliters of each PCR amplicon were run on a 1.8% agarose gel to check the quality and yield of the PCR product. The amplicons were purified using a QIAquick PCR Purification Kit (Qiagen; Hilden, Germany) following the manufacturer’s protocol. The final concentration of purified PCR product was determined using a spectrophotometer (WPA Lightwave II; Biochrom, Cambridge, UK). The purified amplicons were sequenced at Bioneer using the same forward and reverse primers used to generate the PCR products.
Sequence editing
Sequences were manually edited and assembled, and consensus sequences were generated and aligned using CLC Main Workbench software v.7.8.1 (CLC bio, Aarhus, Denmark). To confirm the identity of each tick species, the sequences were compared with those available in the GenBank database using the BLASTn program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For the BLASTn algorithm, a stringent E-value cut-off (10−6) was used as described previously [41]. The identity of the query sequence was assigned to the best hit (highest bit score) returned from BLASTn. The query ID was regarded as confirmed when the best hit (highest bit score) had an E-value below 10−6.
Phylogenetic analyses
To determine the relationships between different tick species and infer their evolutionary history, phylogenetic trees were constructed. To build these trees, reference sequences of the cox1 gene of ticks were downloaded from the GenBank database. The downloaded sequences were combined with those generated in the present study and the phylogenetic trees were built using a hierarchical likelihood ratio test based on the lowest Bayesian information criterion using MEGA v.7.0. Neighbor-Joining trees were generated and their robustness evaluated using 1000 bootstrap replicates in MEGA v.7.0.
Statistical analysis
The association between tick burden and environmental factors (agro-ecology) was assessed using generalized linear models (GLM) through the statistical software R v.3.5.3 (https://www.r-project.org). For this, a negative binomial model in which tick burden was the dependent variable and agro-ecological zone was the independent variable was used. The confidence interval (CI) for each AEZ was estimated at 95%.
Results
Tick collection and identification
A total of 7091 adult ticks were collected from 601 cattle in 54 sites distributed across the five AEZs (Fig. 1). Ticks were collected from 9, 20, 8, 3 and 14 sites in AEZs I, II, III, IV and V, respectively. On the basis of their morphology, the 7091 ticks were classified into three genera: 4210 (59.4%) Amblyomma, 1900 (26.8%) Rhipicephalus and 980 (13.8%) Hyalomma. These ticks comprised seven species: 4210 (59.4%) Amblyomma variegatum, 1112 (15.6%) R. microplus, 708 (10.0%) Rhipicephalus (Boophilus) decoloratus, 28 (0.4%) Rhipicephalus (Boophilus) annulatus, 201 (3.0%) Hyalomma rufipes, 769 (10.8%) Hyalomma truncatum and 19 (0.3%) Rhipicephalus sanguineus (see Additional file 2: Figures S1, Additional file 3: Figures S2, Additional file 4: Figures S3, Additional file 5: Figures S4, Additional file 6: Figures S5, Additional file 7: Figures S6, Additional file 8: Figures S7, Additional file 9: Figures S8, Additional file 10: Figures S9, Additional file 11: Figures S10, Additional file 12: Figures S11). Because of morphological similarities among the ticks, 36 specimens were identified to the genus level. This included three ticks of the genus Hyalomma spp. and 33 of the genus Rhipicephalus spp. The relative abundance and distribution of each tick species for each AEZ is described in Table 2.
Table 2.
Distribution of tick species per agroecological zone
| Tick genus | Tick species | No. of ticks collected (%) | Sex | AEZ I | AEZ II | AEZ III | AEZ IV | AEZ V | |
|---|---|---|---|---|---|---|---|---|---|
| Vegetation type | |||||||||
| Dry savannah, steppes (%) | Savannah, degraded forest (%) | Savannah, degraded forest (%) | Evergreen forest (%) | Humid forest-savannah mosaic (%) | |||||
| Female | Male | ||||||||
| Amblyomma | A. variegatum | 4210 (59.4) | 1077 | 3133 | 499 (29.9) | 2735 (96.5) | 243 (43.1) | 55 (34.8) | 678 (36.3) |
| Rhipicephalus | R. microplus | 1112 (15.6) | 1043 | 69 | 0 (0) | 5 (0.2) | 288 (51.1) | 103 (65.2) | 716 (38.3) |
| R. decoloratus | 708 (10.0) | 686 | 22 | 167 (10.0) | 73 (2.6) | 18 (3.2) | 0 (0) | 450 (24.1) | |
| R. annulatus | 28 (0.4) | 13 | 15 | 9 (0.5) | 0 (0) | 2 (0.4) | 0 (0) | 17 (0.9) | |
| R. sanguineus | 19 (0.3) | 9 | 10 | 5 (0.3) | 4 (0.1) | 5 (0.9) | 0 (0) | 5 (0.3) | |
| Rhipicephalus spp. | 33 (0.5) | 15 | 18 | 11 (0.7) | 13 (0.5) | 8 (1.4) | 0 (0) | 1 (0.1) | |
| Hyalomma | H. rufipes | 210 (3.0) | 59 | 151 | 207 (12.4) | 3 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| H. truncatum | 768 (10.8) | 250 | 518 | 766 (46.0) | 1 (0) | 0 (0) | 0 (0) | 1 (0.1) | |
| Hyalomma spp. | 3 (0) | 0 | 3 | 3 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 7091 (100) | 3152 | 3939 | 1667 | 2834 | 564 | 158 | 1868 | |
Analysis of sex ratio was carried out on the five most abundant species, namely A. variegatum, R. microplus, R. decoloratus, H. rufipes and H. truncatum. The sex ratio of the collected ticks varied with species and skewed towards male, except for R. microplus and R. decoloratus (Table 3).
Table 3.
Male: female sex ratio per species
| Tick species | Male (♂) | Female (♀) | Total | M:F (♂: ♀) |
|---|---|---|---|---|
| A. variegatum | 3133 | 1077 | 4210 | 2.91:1 |
| R. microplus | 69 | 1043 | 1112 | 0.07:1 |
| R. decoloratus | 22 | 686 | 708 | 0.03:1 |
| H. rufipes | 151 | 59 | 210 | 2.56:1 |
| H. truncatum | 518 | 250 | 768 | 2.07:1 |
The overall mean tick burden per animal (all tick species) fluctuated between 5.9 and 16.8 across the AEZs. Mean tick burden per infested animal was significantly high in AEZ I (16.83, 95% CI: 14.53–19.50) and AEZ V (12.58, 95% CI: 11.10–14.16) compared to other AEZs (P < 0.0001) (Fig. 2).
Fig. 2.
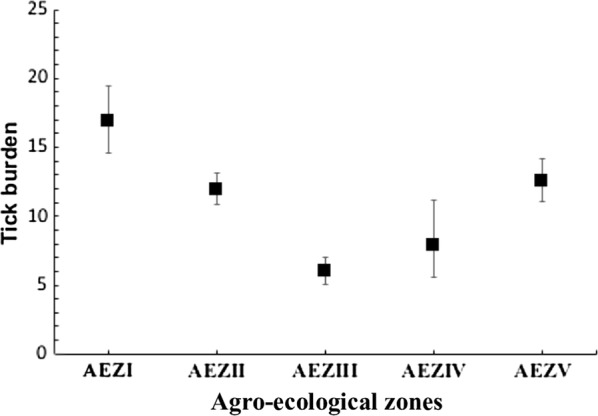
Tick burden on cattle in different agro-ecological zone (AEZ). The mean of each data set is indicated by the black centre square. The bars are confidence intervals at 95%. Values are statistically significant at P < 0.0001
To confirm results from the morphological identification, the partial cox1 gene was sequenced and analyzed. Specimens from each tick species were randomly selected for molecular analysis. Specimens of Hyalomma spp. and Rhipicephalus spp. ticks were grouped by similarity and representative individuals from each group were randomly selected for molecular analysis. A total of 30 specimens were sequenced and the BLASTn analysis was performed. The results from molecular identification are summarized in Additional file 1: Table S1. BLASTn returned best hits that matched the morphological results with identity varying between 87 and 100%. Molecular analysis revealed that among the three specimens of the genus Hyalomma spp., two were Hyalomma dromedarii and one was H. truncatum. For Rhipicephalus spp. seven specimens were analyzed among which two were identified as Rhipicephalus pusillus, one as Rhipicephalus sulcatus and four as Rhipicephalus sanguineus. Sequences were deposited in NCBI GenBank (accession numbers MK648401-MK648422, see Additional file 1: Table S1).
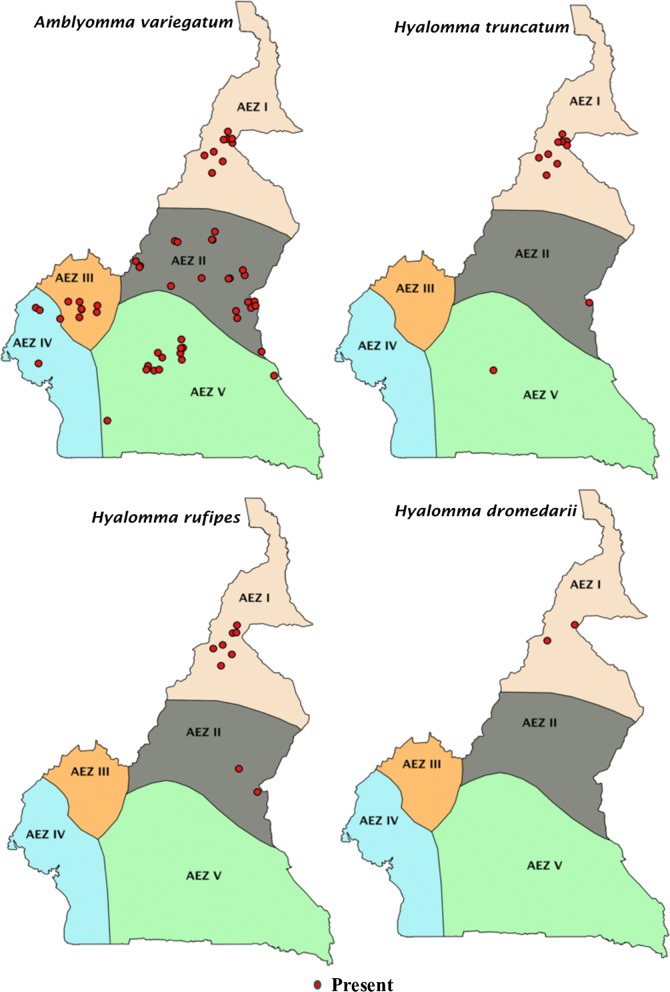
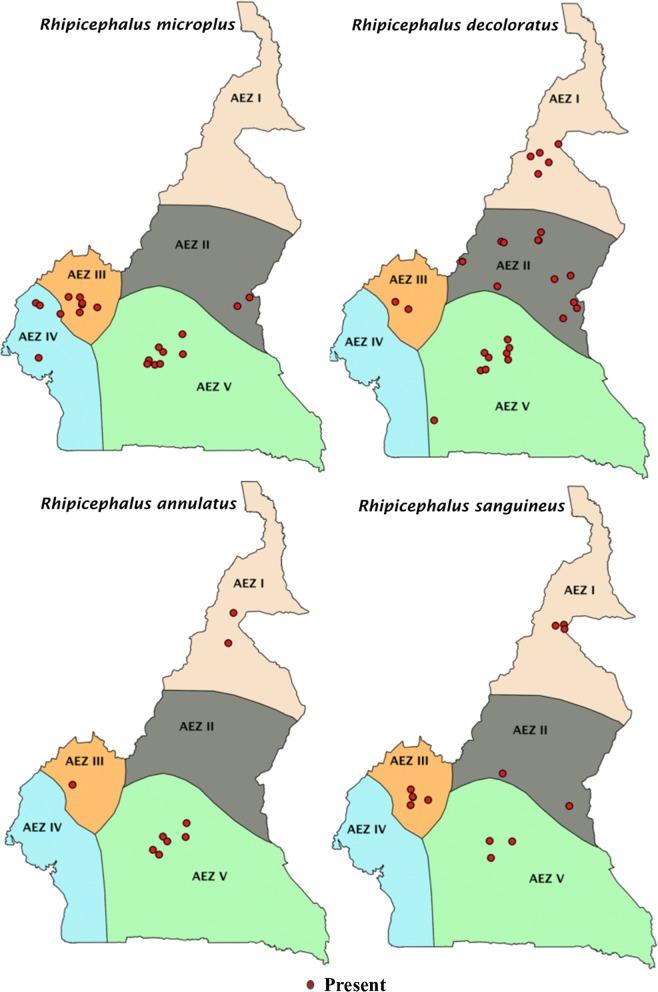
Amblyomma variegatum outnumbered all the other tick species and represented 59.4% of the total tick collection (Table 2). This tick species was found at all sampling sites (Fig. 3). The overall abundance of R. microplus was 15.6%, making it the second most frequently observed tick species. Rhipicephalus microplus represented 51.1%, 65.2% and 38.3% of ticks collected in zones III, IV and V, respectively. These three zones represent the areas invaded by R. microplus, comprising a total of 25 sampling sites. When comparing the occurrence of R. microplus and R. decoloratus species in the areas invaded by R. microplus (zones III, IV and V), R. microplus was present at 18 sites while R. decoloratus was only present in 11 sites. Interestingly, R. microplus was recorded at all the sampling localities within the humid forest zone with monomodal rainfall (zone IV) where no R. decoloratus was found (Table 4). In the Western highlands, 8 localities were sampled and R. decoloratus was recorded in fewer localities (n = 2) than R. microplus (n = 7). Overall, R. microplus and R. decoloratus were sympatric at 8 of the 25 sites. Rhipicephalus microplus was found singly in 10 sites, whereas only 3 sites had R. decoloratus but not R. microplus. Rhipicephalus annulatus was collected mainly in AEZs V and AEZ I while H. rufipes, H. dromedarii and H. truncatum were observed in AEZ I and II where annual average rainfall is low (Figs. 3, 4).
Fig. 3.
Geographical distribution of A. variegatum, H. truncatum, H. rufipes and H. dromedarii
Table 4.
Occurrence data for R. microplus and R. decoloratus from Cameroon
| AEZ | Nsite | Ncattle | Ntick | R. microplus | R. decoloratus | Sympatric | ||
|---|---|---|---|---|---|---|---|---|
| n (%) | l (%) | n (%) | l (%) | l (%) | ||||
| AEZ I | 9 | 99 | 1667 | 0 (0) | 0 (0) | 167 (10.0) | 5 (55.5) | 0 (0) |
| AEZ II | 20 | 238 | 2834 | 5 (0.2) | 2 (10) | 73 (2.6) | 12 (60) | 0 (0) |
| AEZ III | 8 | 95 | 564 | 288 (51.1) | 7 (87.5) | 18 (3.2) | 2 (25) | 2 (25) |
| AEZ IV | 3 | 20 | 158 | 103 (65.2) | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| AEZ V | 14 | 149 | 1868 | 716 (38.3) | 8 (57.1) | 450 (24.1) | 9 (64.2) | 6 (42.8) |
| Total | 54 | 601 | 7091 | 1112 (15.6) | 20 (37) | 708 (10.0) | 28 (51.8) | 8 (14.8) |
Abbreviations: Nsite, total number of sites visited; Ncattle, total number of cattle sampled; Ntick, total number of ticks collected (all species); n, number of ticks collected; l, number of sites where the tick is present
Fig. 4.
Geographical distribution of R. microplus, R. decoloratus, R. annulatus and R. sanguineus
Phylogenetic analysis of the cox1 gene
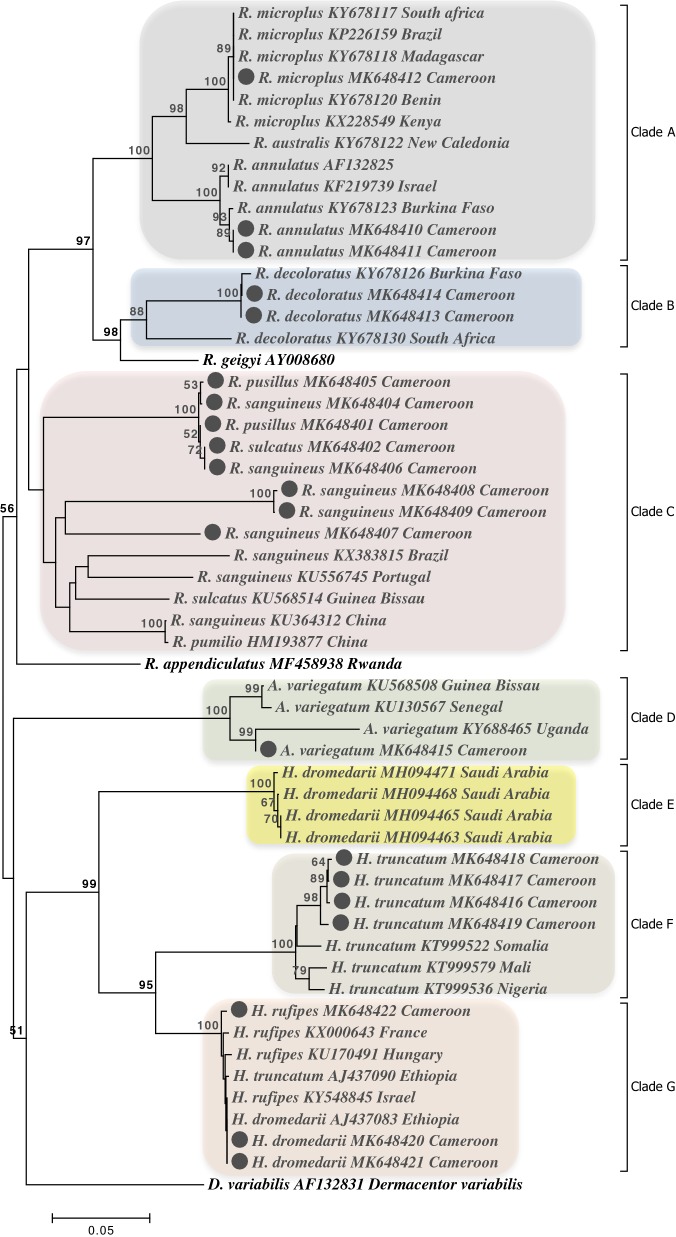
Twenty-two cox1 sequences representing all the identified tick species generated in the present study and 34 reference cox1 sequences from various tick species present in the GenBank database were used to infer phylogenetic relationships between the tick taxa. The neighbor-joining tree resulting from mitochondrial cox1 sequences derived from the Hyalomminae, Rhipicephalinea and Amblyomminae sub-families consisted of seven clades (A, B, C, D, E, F, G) that have strong bootstrap support (98–100%) at the majority of nodes (Fig. 5). Clade A constitutes the ‘R. microplus complex’. Clades B and D each contain individuals within the same species, well supported by bootstrap values of 98 and 100, respectively. Clade C comprises members of the ‘R. sanguineus group’. Clade E comprises H. dromedarii from Saudi Arabia while clade F includes H. truncatum from Cameroon collected in the present study and also H. truncatum from Nigeria, Somalia and Mali. Clade G comprises more than one species, including H. rufipes and H. dromedarii from the present study, H. rufipes from France, Hungary and Israel, and H. truncatum and H. dromedarii from Ethiopia.
Fig. 5.
Phylogenic analysis of cox1 sequences of ticks from Cameroon. The evolutionary history was inferred by using the neighbor-joining method. The percentage of trees in which the associated taxa clustered together is shown above the branches. Major clades are indicated by the letters A, B, C, D, E, F and G. Sequences from this study are highlighted
Discussion
Few studies have focused on ticks infesting cattle in Cameroon and, as a result, there is limited data on the extent of tick burden and TBDs in the country. This is particularly concerning given the recent introduction and spread into West Africa of the highly invasive tick species R. microplus [12, 14, 15]. Moreover, cattle trade in the sub-region is largely unregulated [11], creating risks of disease dissemination that require detailed investigation [11]. Therefore, accurate data from field studies is urgently needed to inform rational control strategies and establish models to predict the changing epidemiology of TBDs, and the economic impact on livestock production.
From the present study, A. variegatum, the African bont tick and the main vector of Ehrlichia ruminantium, was ubiquitous in all sampling localities spanning an extremely divergent range of climatic conditions. This finding is consistent with previous studies that reported occurrence of A. variegatum across the entire country throughout the year [20, 25]. A similar situation has been described for small ruminants [18]. Amblyomma variegatum also transmits the protozoans Theileria mutans and T. velifera causing benign bovine theileriosis [42, 43]. This tick has also been associated with dermatophilosis caused by the bacteria, Dermatophilus congolensis. The disease can affect tick-free cattle but is more severe in cattle infested by A. variegatum [44]. The role of A. variegatum in the development of dermatophilosis was demonstrated to be promoted through immunosuppression that occurs after tick-feeding and predispose entry of the bacteria into the skin [45]. Given the high prevalence and wide distribution of A. variegatum, a future survey of the impact of ehrlichiosis in the livestock sector in Cameroon would be well justified.
An immediate effect of the lack of surveillance at national borders has been the introduction of R. microplus in southern Cameroon, seemingly from Nigeria [46]. Of serious concern, R. microplus was found to be the second most abundant species. This finding contrasts with recent reports from North Central Nigeria and Cameroon which concluded that R. microplus was absent and R. decoloratus was the most abundant tick species in the areas studied [19, 47]. It is worth noting that the exotic R. microplus already outnumbers the indigenous R. decoloratus in our study, attesting to the aggressive nature of this species. Interestingly, in the areas invaded by R. microplus which were within AEZs III, IV and V, R. microplus was present in more sites than R. decoloratus (Table 4). This rapid expansion of R. microplus followed by apparent displacement of R. decoloratus in the field is likely attributable to the shorter life-cycle and higher egg production capacity of R. microplus [48]. This phenomenon has recently been reported in several African countries, including Tanzania and South Africa, both likely originating from independent introductions, and also Ivory Coast [49–51]. Moreover, the ability of R. microplus to develop resistance to most available acaricides might also have favored its expansion at the expense of more susceptible species [52]. In this scenario, the displacement of R. decoloratus by R. microplus could rather be due to the pressure exerted by acaricides on the former species. A similar situation has been described in the leafminer fly [53]. It is also the case that these two hypotheses are not mutually exclusive, and both factors may have contributed to the displacement of R. decoloratus by R. microplus. Regardless of the explanation for the changing dynamics of these two tick populations, the apparent displacement between the two species highlights the need to accurately identify species circulating in the field and adjust the management strategies accordingly.
The observed sex ratio of the tick species collected in the present study varied from one species to another. The male to female sex ratio in A. variegatum, H. truncatum and H. rufipes showed that males were present in greater number than females. These results are in agreement with previous reports [54–56]. This suggest that males remain on the host for a longer period than females which drop off to the ground to lay eggs when they are fully engorged [57]. The low male to female ratio observed for R. microplus and R. decoloratus could imply that males were not collected due to their small size, since only visible adults were picked during the sampling.
In general, the mean tick burden in the country was low. This may not reflect the reality in the field since the collection included only adult ticks. This points to a need for a quantitative longitudinal survey with complete collection that include all tick stages. The lowest tick burden in highlands (AEZIII) compared to the northern plains and the Adamawa plateau agreed with previous findings and may be attributed to the mortality of a high proportion of tick instars due to low temperatures in highlands [58].
Recently, tracking the spatial movement of transhumant herds in Cameroon, Motta et al. [9] demonstrated that seasonal migration of most of the herds originating from AEZ II occurred in the direction of the areas invaded by R. microplus (AEZ V). Therefore, it is worth noting that transhumance exposes herds to R. microplus infestation and consequently to bovine babesiosis, a serious threat to the livestock production.
Hyalomma species were commonly encountered in dry habitats (AEZ I and II), in line with the known distribution of these species [39]. The presence of a single individual of H. truncatum in AEZ V could be the result of unusual cattle migration, or bird migration as previously suggested [23, 39]. Previous studies did not report H. rufipes in Northern Cameroon [18]. The present survey reveals a relatively high abundance (12.4%) of H. rufipes in AEZ I (Table 2), which is of great public health importance since H. rufipes is one of the main competent vector of the virus causing Crimean-Congo hemorrhagic fever (CCHF) in humans [59]. This tick species also transmits Anaplasma marginale and Babesia occultans to cattle and Rickettsia conorii to humans, the latter of which being the causal agent of Mediterranean spotted fever [39, 60].
Hyalomma dromedarii was reported for the first time in Cameroon. This finding is of great veterinary and public heath importance, since the tick is an additional vector of CCHF virus and can also transmit Theileria annulata to cattle [39, 61, 62]. To the best of our knowledge, H. dromedarii has never previously been reported in Cameroon. Nevertheless, it appears in a list of ticks considered likely to occur in Cameroon published in 1958 [25].
The phylogenetic analysis of cox1 sequences from H. truncatum, H. dromedarii and H. rufipes revealed that none of these Hyalomma species form a monophyletic group according to analysis of cox1 gene sequences. This observation could be explained by introgressive hybridization where one ‘species’ incorporates genes into the gene pool of another ‘species’ as a consequence of ‘interspecific hybridization’. This lack of concordance between morphology and molecular identification has been demonstrated previously and has been attributed to hybridization between African Hyalomma taxa [63]. Such data raise fundamental issues as to what constitutes a true species and may require application of additional techniques, such as sequencing of specific genes encoded in the nuclear genome, genome-wide SNP analysis using next generation sequencing and mass spectrometry [64].
Due to the high incidence of morphological similarity and existence of cryptic species, the members of the subfamily Rhipicephalinae are often clustered within species complexes [65]. In the present study, phylogenetic analysis within this group revealed that clade C very likely contains more than one species (Fig. 5). The existence of different species classified under the generic term ‘R. sanguineus group’ has recently been reported [66]. This suggests that the members of Rhipicephalinae are often misidentified. Therefore, there is a need of further genetic and phenotypic analyses as outlined above in order to clarify the taxonomic status and genetic relationships among members of these complexes.
Comparing our results with previous studies on ticks infesting cattle in Cameroon, R. microplus and H. dromedarii have been added to the known list of tick species present in the country. This is of both veterinary and public health importance since these species are major ixodid vectors of human and livestock diseases worldwide [61, 67]. Furthermore, R. microplus is known to rapidly develop resistance to most classes of acaricides [68]. It seems that recent reports relating to increased tick infestation and acaricide application in some areas of Cameroon are the result of R. microplus resistance to locally used acaricides (manuscript in preparation). To fill this knowledge gap, further research on tick resistance or susceptibility to locally used acaricides needs to be undertaken to inform and advise farmers and stakeholders on sustainable control methods, which may differ depending on the presence of R. microplus.
This cross-sectional survey reveals the tick species circulating in Central and Eastern Cameroon (AEZ V), an understudied area as far as TBDs are concerned. Nonetheless, it is possible that the full extent of the geographical ranges of these tick species have not yet been defined. This cross-sectional survey cannot differentiate temporary tick populations from well-established populations. Rhipicephalus microplus was the most abundant tick (38.3%) in AEZ V which borders C.A.R. It is important to note that in a recent review of ticks in C.A.R., R. microplus was not reported at all [69]. These findings show the potential risk of R. microplus being introduced into C.A.R., since farmers who migrated to Cameroon with their livestock during the recent period of political instability may return to their country, resulting in further transboundary spreading of the tick.
Conclusions
The present study provides the first comprehensive overview of the current distribution of cattle ticks in Cameroon. Mapping tick occurrence provides a solid basis for identifying areas where herds are at risk of being exposed to R. microplus infestation and related TBDs. The information presented herein could help to define transhumance corridors which might potentially limit herd exposure to R. microplus. The data generated will also be useful in informing a targeted tick control strategy in a more sustainable, environmentally compatible and cost-effective manner. Further longitudinal studies are envisaged to determine whether the tick species identified in the current horizontal survey are established or transient and what factors contribute to the ongoing displacement of the native African tick species by the invasive R. microplus.
Supplementary information
Additional file 1: Table S1. Geographical coordinates, BLASTn results and GenBank accession numbers of cytochrome oxidase I (cox1) gene sequences of cattle ticks from Cameroon.
Additional file 2: Figure S1. Amblyomma variegatum and Rhipicephalus sanguineus dorsal and ventral views.
Additional file 3: Figure S2. Hyalomma truncatum and Hyalomma rufipes dorsal and ventral views.
Additional file 4: Figure S3. Rhipicephalus annulatus dorsal and ventral views.
Additional file 5: Figure S4. Rhipicephalus annulatus, adult female, hypostomal teeth.
Additional file 6: Figure S5. Rhipicephalus annulatus, adult female, palp articles.
Additional file 7: Figure S6. Rhipicephalus decoloratus dorsal and ventral views.
Additional file 8: Figure S7. Rhipicephalus decoloratus, adult female, hypostomal teeth.
Additional file 9: Figure S8. Rhipicephalus decoloratus, adult female, palp articles.
Additional file 10: Figure S9. Rhipicephalus microplus dorsal and ventral views.
Additional file 11: Figure S10. Rhipicephalus microplus, adult female, hypostomal teeth.
Additional file 12: Figure S11. Rhipicephalus microplus, adult female, palp articles.
Acknowledgements
We gratefully acknowledge the financial support provided to the Biosciences eastern and central Africa Hub at the International Livestock Research Institute (BecA-ILRI Hub, Nairobi) by the Australian Agency for International Development (AusAID) through a partnership between Australia’s Commonwealth Scientific and Industrial Research Organization (CSIRO) and the BecA-ILRI Hub; and by the Syngenta Foundation for Sustainable Agriculture (SFSA); the Bill & Melinda Gates Foundation (BMGF); and the Swedish Ministry of Foreign Affairs through the Swedish International Development Agency (SIDA), which made this work possible. We thank the Molecular Parasitology and Entomology Unit (MPEU) of the University of Dschang for providing logistics for field samples storage and the Ministry of Livestock, Fisheries and Animal Industries (MINEPIA) of Cameroon for connecting us with farmers and facilitating sampling.
Abbreviations
- cox1
cytochrome c oxidase subunit 1
- TBDs
tick-borne diseases
- C.A.R
Central African Republic
- ITS
internal transcribed spacer
- AEZs
agro-ecological zones
Authors’ contributions
BAS, GS, NF, CK, AD, JRK, FN and RP contributed to study design. BAS, RMK and FO carried out field sampling. BSA, SM and NF performed tick identification. BSA was a major contributor in writing the manuscript. RP and RB made additional input to writing the manuscript. All authors edited the final manuscript. All authors read and approved the final manuscript.
Funding
BA was a recipient of an Africa Biosciences Challenge Fund (ABCF) Fellowship under the BMGF Grant Number OPP1075938. This research was also supported by the German Academic Exchange Service (DAAD) Special Initiative SFBFR, 2015 (57220758).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from NCBI GenBank (accession numbers MK648401-MK648422).
Ethics approval and consent to participate
Permission to undertake the study was obtained from the Ministry of Livestock, Animal Husbandry and Fisheries reference number 026/L/MINEPIA/MSEG. Signed informed consent was obtained from farmers and herders for tick sampling. The collection of ticks on domestic animals did not involve national parks or other protected areas or endangered or protected species.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Barberine A. Silatsa, Email: sabarberine@yahoo.fr
Gustave Simo, Email: gsimoca@yahoo.fr.
Naftaly Githaka, Email: n.githaka@cgiar.org.
Stephen Mwaura, Email: s.mwaura@cgiar.org.
Rolin M. Kamga, Email: rolinmitterrank@yahoo.fr
Farikou Oumarou, Email: pfarikoumar@yahoo.fr.
Christian Keambou, Email: c.tiambo@cgiar.org.
Richard P. Bishop, Email: bishop5030@gmail.com
Appolinaire Djikeng, Email: appolinaire.djikeng@ctlgh.org.
Jules-Roger Kuiate, Email: jrkuiate@yahoo.com.
Flobert Njiokou, Email: njiokouf@yahoo.com.
Roger Pelle, Email: r.pelle@cgiar.org.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-019-3738-7.
References
- 1.de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Scholtz M, Spickett AM, Lombard P, Enslin C. The effect of tick infestation on the productivity of cows of three breeds of cattle. Understeeport J Vet Res. 1991;58:2. [PubMed] [Google Scholar]
- 3.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl. 1):3–14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- 4.Lew-Tabor A, Valle MR. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick Borne Dis. 2016;7:573–585. doi: 10.1016/j.ttbdis.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Mbah D. Adaptation of dairy cattle to Wakwa (Adamawa) environment, I: resistance to cattle ticks. Rev Sci Tech. 1982;2:101–106. [Google Scholar]
- 6.Bayemi P, Bryant M, Perera B, Mbanya J, Cavestany D, Webb E. Milk production in Cameroon: a review. Livest Res Rural Dev. 2005;17:6. [Google Scholar]
- 7.Volkova VV, Howey R, Savill NJ, Woolhouse ME. Sheep movement networks and the transmission of infectious diseases. PLoS ONE. 2010;5:e11185. doi: 10.1371/journal.pone.0011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fèvre EM, Bronsvoort BMDC, Hamilton KA, Cleaveland S. Animal movements and the spread of infectious diseases. Trends Microbiol. 2006;14:125–131. doi: 10.1016/j.tim.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motta P, Porphyre T, Hamman SM, Morgan KL, Ngwa VN, Tanya VN, et al. Cattle transhumance and agropastoral nomadic herding practices in Central Cameroon. BMC Vet Res. 2018;14:214. doi: 10.1186/s12917-018-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesse P, Houinato MR, Djenontin J, Dossa H, Yabi B, Toko I, et al. Transhumance en République du Bénin: états des lieux et contraintes. Int J Biol Chem Sci. 2015;9:2668–2681. doi: 10.4314/ijbcs.v9i5.37. [DOI] [Google Scholar]
- 11.Motta P, Porphyre T, Handel I, Hamman SM, Ngwa VN, Tanya V, et al. Implications of the cattle trade network in Cameroon for regional disease prevention and control. Sci Rep. 2017;7:43932. doi: 10.1038/srep43932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adakal H, Biguezoton A, Zoungrana S, Courtin F, De Clercq EM, Madder M. Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa—another three countries are affected: Burkina Faso, Mali and Togo. Exp Appl Acarol. 2013;61:383–386. doi: 10.1007/s10493-013-9706-6. [DOI] [PubMed] [Google Scholar]
- 13.Kamani J, Apanaskevich D, Gutiérrez R, Nachum-Biala Y, Baneth G, Harrus S. Morphological and molecular identification of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: a threat to livestock health. Exp Appl Acarol. 2017;73:283–296. doi: 10.1007/s10493-017-0177-z. [DOI] [PubMed] [Google Scholar]
- 14.Madder M, Adehan S, De Deken R, Adehan R, Lokossou R. New foci of Rhipicephalus microplus in West Africa. Exp Appl Acarol. 2012;56:385–390. doi: 10.1007/s10493-012-9522-4. [DOI] [PubMed] [Google Scholar]
- 15.Madder M, Thys E, Geysen D, Baudoux C, Horak I. Boophilus microplus ticks found in West Africa. Exp Appl Acarol. 2007;43:233–234. doi: 10.1007/s10493-007-9110-1. [DOI] [PubMed] [Google Scholar]
- 16.McDermott JJ, Staal SJ, Freeman H, Herrero M, Van de Steeg J. Sustaining intensification of smallholder livestock systems in the tropics. Livest Sci. 2010;130:95–109. doi: 10.1016/j.livsci.2010.02.014. [DOI] [Google Scholar]
- 17.Achukwi M, Tanya V, Messine O, Njongmeta L. Etude comparative de l’infestation des bovins Namchi (Bos taurus) et Goudali de Ngaoundéré (Bos indicus) par la tique adulte Amblyomma variegatum. Rev Elev Méd Vét Pays Trop. 2001;54:37–41. doi: 10.19182/remvt.9803. [DOI] [Google Scholar]
- 18.Awa D. Serological survey of heartwater relative to the distribution of the vector Amblyomma variegatum and other tick species in north Cameroon. Vet Parasitol. 1997;68:165–173. doi: 10.1016/S0304-4017(96)01058-8. [DOI] [PubMed] [Google Scholar]
- 19.Awa D, Adakal H, Luogbou N, Wachong K, Leinyuy I, Achukwi M. Cattle ticks in Cameroon: Is Rhipicephalus (Boophilus) microplus absent in Cameroon and the Central African region? Ticks Tick Borne Dis. 2015;6:117–122. doi: 10.1016/j.ttbdis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Bayemi P. Dynamique saisonnière de l’infestation par les tiques (Ixodoidea) des bovins commercialisés dans la région de Yaoundé, Cameroun. Rev Elev Méd Vét Pays Trop. 1991;44:309–318. [PubMed] [Google Scholar]
- 21.Mamoudou A, Nguetoum N, ZoliP A, Sevidzem S. Identification and infestation of ticks on cattle in the peri-urban area of Ngaoundere, Cameroon. J Vet Sci Med Diagn. 2016;4:2. [Google Scholar]
- 22.Merlin P, Tsangueu P, Rousvoal D. Dynamique saisonnière de l’infestation des bovins par les tiques (Ixodoidea) dans les hauts plateaux de lʼOuest du Cameroun. I. Etude de trois sites autour de Bamenda pendant un an. Rev Elev Méd Vét Pays Trop. 1986;39:367–376. [PubMed] [Google Scholar]
- 23.Merlin P, Tsangueu P, Rousvoal D. Dynamique saisonnière de lʼinfestation des bovins par les tiques (Ixodoidea) dans les hauts plateaux de lʼOuest du Cameroun. II. Elevage extensif traditionel. Rev Elev Méd Vét Pays Trop. 1987;40:133–140. [PubMed] [Google Scholar]
- 24.Ndi C, Bayemi P, Ekue F, Tarounga B. Preliminary observations on ticks and tick-borne diseases in the North West Province of Cameroon. I. Babesiosis and anaplasmosis. Rev Elev Méd Vét Pays Trop. 1991;44:263–265. [PubMed] [Google Scholar]
- 25.Morel P-C, Mouchet J. Les tiques du Cameroun (Ixodidae et Argasidae) Ann Parasitol Hum Comp. 1958;33:69–111. [PubMed] [Google Scholar]
- 26.Stachurski F, Musonge E, Achu-Kwi M, Saliki JT. Impact of natural infestation of Amblyomma variegatum on the liveweight gain of male Gudali cattle in Adamawa (Cameroon) Vet Parasitol. 1993;49:299–311. doi: 10.1016/0304-4017(93)90128-A. [DOI] [PubMed] [Google Scholar]
- 27.Ndi C, Bayemi P, Nfi A, Ekue F. Preliminary observations on ticks and tickborne diseases in the North West province of Cameroon. II. Bovine heartwater. Rev Elev Méd Vét Pays Trop. 1998;51:25–28. [PubMed] [Google Scholar]
- 28.Seignobos C. La Question mbororo. Réfugiés de la RCA au Cameroun. Yaoundé: HCR/SCAC/IRD; 2008. [Google Scholar]
- 29.Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a review. S Afr J Anim Sci. 2018;48:829–841. doi: 10.4314/sajas.v48i5.4. [DOI] [Google Scholar]
- 30.Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. Int J Parasitol Parasites Wildl. 2015;4:452–461. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guglielmone AA, Venzal JM, González-Acuña D, Nava S, Hinojosa A, Mangold AJ. The phylogenetic position of Ixodes stilesi Neumann, 1911 (Acari: Ixodidae): morphological and preliminary molecular evidences from 16S rDNA sequences. Syst Parasitol. 2006;65:1–11. doi: 10.1007/s11230-005-9024-4. [DOI] [PubMed] [Google Scholar]
- 32.Lempereur L, Geysen D, Madder M. Development and validation of a PCR-RFLP test to identify African Rhipicephalus (Boophilus) ticks. Acta Trop. 2010;114:55–58. doi: 10.1016/j.actatropica.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Mangold AJ, Bargues MD, Mas-Coma S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae) Parasitol Res. 1998;84:478–484. doi: 10.1007/s004360050433. [DOI] [PubMed] [Google Scholar]
- 34.Murrell A, Campbell NJ, Barker SC. Mitochondrial 12S rDNA indicates that the Rhipicephalinae (Acari: Ixodida) is paraphyletic. Mol Phylogenet Evol. 1999;12:83–86. doi: 10.1006/mpev.1998.0595. [DOI] [PubMed] [Google Scholar]
- 35.Song S, Shao R, Atwell R, Barker S, Vankan D. Phylogenetic and phylogeographic relationships in Ixodes holocyclus and Ixodes cornuatus (Acari: Ixodidae) inferred from COX1 and ITS2 sequences. Int J Parasitol. 2011;41:871–880. doi: 10.1016/j.ijpara.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Lv J, Wu S, Zhang Y, Chen Y, Feng C, Yuan X, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasit Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pamo E. Country pasture/forage resource profiles Cameroon. In: Suttle JM, Reynolds SG, editors. Rome: FAO; 2008. Available online at https://www.scribd.com/document/346401802/FAO-forage-profile-Cameroon-pdf. Accessed 15 Dec 2018.
- 38.MINEPIA. Enquête pastorale 2012; 2012. http://www.minepia.gov.cm. Accessed 15 Mar 2019.
- 39.Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, et al. Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh: Bioscience Reports; 2003. [Google Scholar]
- 40.Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 41.Ross HA, Murugan S, Li WL. Testing the reliability of genetic methods of species identification via simulation. Syst Biol. 2008;57:216–230. doi: 10.1080/10635150802032990. [DOI] [PubMed] [Google Scholar]
- 42.De Vos A, Roos J. Observations on the transmission of Theileria mutans in South Africa. Ondeerstepoort J Vet Res. 1981;46:1–6. [PubMed] [Google Scholar]
- 43.Uilenberg G, Camus E, Barré N. Existence en Guadeloupe (Antilles) de Theileria mutans et de Theileria velifera (Sporozoa, Theileriidae) bovin. Rev Elev Méd Vét Pays Trop. 1983;36:261–264. [PubMed] [Google Scholar]
- 44.Stachurski F, Zoungrana S, Konkobo M. Moulting and survival of Amblyomma variegatum (Acari: Ixodidae) nymphs in quasi-natural conditions in Burkina Faso; tick predators as an important limiting factor. Exp Appl Acarol. 2010;52:363–376. doi: 10.1007/s10493-010-9370-z. [DOI] [PubMed] [Google Scholar]
- 45.Walker A. Amblyomma tick feeding in relation to host health. Trop Anim Health Prod. 1996;28:26–28. doi: 10.1007/BF02310695. [DOI] [PubMed] [Google Scholar]
- 46.Silatsa BA, Kuiate J, Njiokou F, Simo G, Feussom JK, Tunrayo A, et al. A countrywide molecular survey leads to a seminal identification of the invasive cattle tick Rhipicephalus (Boophilus) microplus in Cameroon, a decade after it was reported in Côte d’Ivoire. Ticks Tick Borne Dis. 2019;10:585–593. doi: 10.1016/j.ttbdis.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorusso V, Picozzi K, de Bronsvoort BMC, Majekodunmi A, Dongkum C, Balak G, et al. Ixodid ticks of traditionally managed cattle in central Nigeria: where Rhipicephalus (Boophilus) microplus does not dare (yet?) Parasit Vectors. 2013;6:171. doi: 10.1186/1756-3305-6-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tønnesen M, Penzhorn B, Bryson N, Stoltsz W, Masibigiri T. Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol. 2004;32:199–208. doi: 10.1023/B:APPA.0000021789.44411.b5. [DOI] [PubMed] [Google Scholar]
- 49.Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, et al. Shifts in the distributional ranges of Boophilus ticks in Tanzania: evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients. Exp Appl Acarol. 2008;44:147–164. doi: 10.1007/s10493-008-9134-1. [DOI] [PubMed] [Google Scholar]
- 50.Nyangiwe N, Harrison A, Horak IG. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp Appl Acarol. 2013;61:371–382. doi: 10.1007/s10493-013-9705-7. [DOI] [PubMed] [Google Scholar]
- 51.Madder M, Thys E, Achi L, Touré A, De Deken R. Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa. Exp Appl Acarol. 2011;53:139–145. doi: 10.1007/s10493-010-9390-8. [DOI] [PubMed] [Google Scholar]
- 52.Busch JD, Stone NE, Nottingham R, Araya-Anchetta A, Lewis J, Hochhalter C, et al. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasit Vectors. 2014;7:188. doi: 10.1186/1756-3305-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Reitz SR, Wei Q, Yu W, Lei Z. Insecticide-mediated apparent displacement between two invasive species of leafminer fly. PLoS One. 2012;7:e36622. doi: 10.1371/journal.pone.0036622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser M, Sutherst R, Bourne A. Relationship between ticks and zebu cattle in southern Uganda. Trop Anim Health Prod. 1982;14:63–74. doi: 10.1007/BF02282583. [DOI] [PubMed] [Google Scholar]
- 55.Tadesse F, Abadfaji G, Girma S, Jibat T. Identification of tick species and their preferred site on cattles body in and around Mizan Teferi, Southwestern Ethiopia. J Vet Med Anim Health. 2012;4:1–5. [Google Scholar]
- 56.Dioli M, Jean-Baptiste S, Fox M. Ticks (Acari: Ixodidae) of the one-humped camel (Camel dromedarius) in Kenya and southern Ethiopia: species composition, attachment sites, sex ratio and seasonal incidence. Rev Elev Méd Vét Pays Trop. 2001;54:2. [Google Scholar]
- 57.Hoogstraal H. African Ixodoidea. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Vol 1. Research Report NM 005 050. 29.07. Washington DC: Department of the Navy, Bureau of Medicine and Surgery; 1956.
- 58.Bazarusanga T, Geysen D, Vercruysse J, Madder M. An update on the ecological distribution of Ixodid ticks infesting cattle in Rwanda: countrywide cross-sectional survey in the wet and the dry season. Exp Appl Acarol. 2007;43:279–291. doi: 10.1007/s10493-007-9121-y. [DOI] [PubMed] [Google Scholar]
- 59.Papa A, Tsergouli K, Tsioka K, Mirazimi A. Crimean-Congo hemorrhagic fever: tick-host-virus interactions. Front Cell Infect Microbiol. 2017;7:213. doi: 10.3389/fcimb.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.La Scola B, Raoult D. Diagnosis of Mediterranean spotted fever by cultivation of Rickettsia conorii from blood and skin samples using the centrifugation-shell vial technique and by detection of R. conorii in circulating endothelial cells: a 6-year follow-up. J Clin Microbiol. 1996;34:2722–2727. doi: 10.1128/jcm.34.11.2722-2727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chisholm K, Dueger E, Fahmy NT, Samaha HAT, Zayed A, Abdel-Dayem M, et al. Crimean-Congo hemorrhagic fever virus in ticks from imported livestock, Egypt. Emerg Infect Dis. 2012;18:181. doi: 10.3201/eid1801.111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazlum Z. Transmission of Theileria annulata by the crushed infected unfed Hyalomma dromedarii. Parasitology. 1969;59:597–600. doi: 10.1017/S0031182000031139. [DOI] [PubMed] [Google Scholar]
- 63.Rees DJ, Dioli M, Kirkendall LR. Molecules and morphology: evidence for cryptic hybridization in African Hyalomma (Acari: Ixodidae) Mol Phylogenet Evol. 2003;27:131–142. doi: 10.1016/S1055-7903(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 64.Rothen J, Githaka N, Kanduma EG, Olds C, Pflüger V, Mwaura S, et al. Matrix-assisted laser desorption/ionization time of flight mass spectrometry for comprehensive indexing of East African ixodid tick species. Parasit Vectors. 2016;9:151. doi: 10.1186/s13071-016-1424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skoracka A, Magalhaes S, Rector BG, Kuczyński L. Cryptic speciation in the Acari: a function of species lifestyles or our ability to separate species? Exp Appl Acarol. 2015;67:165–182. doi: 10.1007/s10493-015-9954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bock R, Jackson L, De Vos A, Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:247–269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 68.Baffi MA, de Souza GRL, de Sousa CS, Ceron CR, Bonetti AM. Esterase enzymes involved in pyrethroid and organophosphate resistance in a Brazilian population of Riphicephallus (Boophilus) microplus (Acari, Ixodidae) Mol Biochem Parasitol. 2008;160:70–73. doi: 10.1016/j.molbiopara.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Uilenberg G, Estrada-Peña A, Thal J. Ticks of the central African republic. Exp Appl Acarol. 2013;60:1–40. doi: 10.1007/s10493-012-9605-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Geographical coordinates, BLASTn results and GenBank accession numbers of cytochrome oxidase I (cox1) gene sequences of cattle ticks from Cameroon.
Additional file 2: Figure S1. Amblyomma variegatum and Rhipicephalus sanguineus dorsal and ventral views.
Additional file 3: Figure S2. Hyalomma truncatum and Hyalomma rufipes dorsal and ventral views.
Additional file 4: Figure S3. Rhipicephalus annulatus dorsal and ventral views.
Additional file 5: Figure S4. Rhipicephalus annulatus, adult female, hypostomal teeth.
Additional file 6: Figure S5. Rhipicephalus annulatus, adult female, palp articles.
Additional file 7: Figure S6. Rhipicephalus decoloratus dorsal and ventral views.
Additional file 8: Figure S7. Rhipicephalus decoloratus, adult female, hypostomal teeth.
Additional file 9: Figure S8. Rhipicephalus decoloratus, adult female, palp articles.
Additional file 10: Figure S9. Rhipicephalus microplus dorsal and ventral views.
Additional file 11: Figure S10. Rhipicephalus microplus, adult female, hypostomal teeth.
Additional file 12: Figure S11. Rhipicephalus microplus, adult female, palp articles.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from NCBI GenBank (accession numbers MK648401-MK648422).