Abstract
Background
Talimogene laherparepvec (T-VEC) is an intralesionally delivered, modified herpes simplex virus type-1 oncolytic immunotherapy. The biodistribution, shedding, and potential transmission of T-VEC was systematically evaluated during and after completion of therapy in adults with advanced melanoma.
Methods
In this phase 2, single-arm, open-label study, T-VEC was administered into injectable lesions initially at 106 plaque-forming units (PFU)/mL, 108 PFU/mL 21 days later, and 108 PFU/mL every 14 (±3) days thereafter. Injected lesions were covered with occlusive dressings for ≥1 week. Blood, urine, and swabs from exterior of occlusive dressings, surface of injected lesions, oral mucosa, anogenital area, and suspected herpetic lesions were collected throughout the study. Detectable T-VEC DNA was determined for each sample type; infectivity was determined for all swabs with detectable T-VEC DNA.
Findings
Sixty patients received ≥1 dose of T-VEC. During cycles 1–4, T-VEC DNA was detected in blood (98·3% of patients, 36·7% of samples), urine (31·7% of patients, 3·0% of samples) and swabs from injected lesions (100% of patients, 57·6% of samples), exterior of dressings (80% of patients,19·5% of samples), oral mucosa (8·3% of patients, 2·5% of samples), and anogenital area (8·0% of patients, 1·1% of samples). During the safety follow-up period, T-VEC DNA was only detected on swabs from injected lesions (14% of patients, 5.8% of samples). T-VEC DNA was detected in 4/37 swabs (3/19 patients) of suspected herpetic lesions. Among all samples, only those from the surface of injected lesions tested positive for infectivity (8/740 [1·1%]). Three close contacts reported signs and symptoms of suspected herpetic origin; however, no lesions had detectable T-VEC DNA.
Interpretation
Using current guidelines, T-VEC can be administered safely to patients with advanced melanoma and is unlikely to be transmitted to close contacts with appropriate use of occlusive dressings.
Fund
This study was funded by Amgen Inc.: ClinicalTrials.gov, NCT02014441.
Keywords: Talimogene laherparepvec, Oncolytic immunotherapy, Biodistribution, Shedding, Transmission, Melanoma, T-VEC
Research in context.
Evidence before this study
T-VEC is the first intratumoral oncolytic virus approved for the treatment of unresectable melanoma recurrent after initial surgery. During the clinical development of T-VEC, data on shedding of T-VEC DNA were collected in several clinical trials. T-VEC DNA was detected in blood and urine and on the surfaces of injected tumours of some patients, and there was no evidence of transmission of live T-VEC virus from patients to close contacts. While other genetically modified or naturally occurring oncolytic viruses have been investigated for treatment of cancer (including, but not limited to HSV-1, coxsackie, vaccinia, adenovirus, and poliovirus-based agents) a Pub Med review of English literature on the biodistribution, shedding and transmissibility of these agents in human subjects revealed only limited data for one HSV-1-derived virus and one genetically engineered vaccinia virus. Like T-VEC, these oncolytic viruses are designed to selectively infect, replicate within, and lyse tumour cells and are, therefore, less infectious or virulent than the parent strain of the virus. Nevertheless, given that these agents are derived from infectious viruses, there are concerns regarding the potential for transmission of therapeutic oncolytic viruses to untreated individuals (close contacts or health care providers). The FDA and EMA have provided guidance for industry on collecting viral shedding data from the target patient population during development of oncolytic viruses to provide an assessment of the risk of transmission to untreated individuals.
Added value of this study
Our study was designed to thoroughly and systematically evaluate T-VEC DNA biodistribution, shedding of both T-VEC DNA and infectious virus (per FDA and EMA clinical oncolytic virus shedding study guidance), and potential transmission of T-VEC to exposed healthcare workers and close contacts during and after completion of therapy in patients with melanoma treated according to the current approved dosage and handling guidelines. Not only do the results of our study support data from prior T-VEC studies, they provide extensive information on the potential risk of transmission of T-VEC to close contacts or health care providers. To our knowledge, this is the most extensive dataset published to date on the biodistribution and transmissibility of an oncolytic virus.
Implications of all the available evidence
The results of this and previous T-VEC clinical trials indicate that with proper handling, administration, and post-injection care, T-VEC can be administered safely to patients with advanced melanoma, with minimal risk of transmission to close contacts and health care providers. The methodological approach and results of our study should be of value in the development of other intralesionally injected oncolytic viruses for the treatment of cancer.
Alt-text: Unlabelled Box
1. Introduction
The past decade has seen rapid advances in the treatment of advanced-stage melanoma, with the availability of new therapies that induce anti-cancer immune reactions or target oncogenic mutations driving tumour growth [1]. Talimogene laherparepvec (T-VEC) is an intralesionally delivered oncolytic virus approved to treat advanced-stage melanoma. T-VEC was engineered by deleting the ICP34·5 and ICP47 genes from the highly oncolytic JS1 strain of herpes simplex virus type 1 (HSV-1), resulting in selective viral replication within tumour cells and enhanced systemic antitumor responses [2,3]. In addition, insertion of the human granulocyte-macrophage colony-stimulating factor gene enhances immune response to tumour antigens released during tumour-cell lysis.
In previous studies, T-VEC DNA was detected in blood and urine and on the surfaces of injected tumours of some patients, with no evidence of transmission of live T-VEC virus from patients to close contacts [[4], [5], [6]]. The objective of the current study was to systematically evaluate T-VEC DNA biodistribution, shedding of DNA and infectious virus, and potential transmission of T-VEC to exposed healthcare workers and close contacts during and after completion of therapy in patients with melanoma treated according to the current approved dosage and handling guidelines [[7], [8], [9]].
2. Materials and methods
2.1. Study design
This phase 2, single-arm, open-label study was conducted at 11 centres in North America. T-VEC was administered intralesionally into injectable metastatic melanoma lesions (cutaneous, subcutaneous, and nodal tumours) at an initial dose of 106 plaque-forming units (PFU)/mL (up to 4 mL) on study day 1, followed by 108 PFU/mL (up to 4 mL) 21 days after the initial dose and every 14 (±3) days thereafter. Injected lesions were covered with occlusive dressings for ≥1 week.
Patients received T-VEC until achievement of a complete response, disappearance of all injectable tumours, intolerance to treatment, or clinically relevant disease progression. Patients were encouraged to receive ≥6 months of treatment to allow for delayed responses. Adverse events (AEs) were assessed throughout the study and up to 60 (+7) days after the last dose of T-VEC.
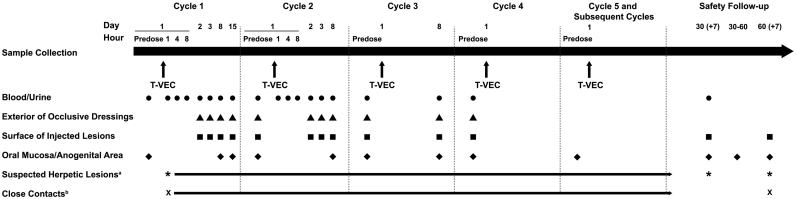
Samples from blood, urine, exterior of occlusive dressings, and surface of injected lesions were collected at multiple time points up to day 1 of cycle 4. Except for injected lesions, samples were also taken during the 30- and/or 60-day follow-up visit. Samples from the oral mucosa and anogenital area were collected during every cycle and follow-up visit (Fig. 1). Swabs of suspected herpetic lesions were collected throughout the study within 3 days of their occurrence. Suspected exposure of close contacts to T-VEC was reported by the investigator within 24 h, and swabs were taken with the individual's consent. The sampling schedule is outlined in the Supplementary Material.
Fig. 1.
Sampling schedule. aSwab taken in clinic within 3 days of the occurrence of reportable lesion. bAny potential or known unintended exposure to T-VEC was reported within 24 h of the investigator's knowledge of the event of exposure. Swabs were taken with the consent of the individual. Abbreviations: T-VEC, talimogene laherparepvec.
Patients were trained to take swabs from the oral mucosa and anogenital area at home (with the help of a service provider, if required) during the 31–60-day follow-up period; kits were provided for swab collection for central laboratory testing.
Radiographic imaging (computed tomography [CT], positron emission tomography [PET]–CT, magnetic resonance imaging [MRI], or ultrasound) of the chest, abdomen, and pelvis was conducted at screening, weeks 12 and 24, and at least every 3 months until disease progression or end of treatment. Tumour response was assessed using modified World Health Organization (WHO) response criteria.
AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3·0.
2.2. Patients
Patients were ≥ 18 years old with a histologically confirmed diagnosis of stage IIIB, IIIC, IVM1a, IVM1b, or IVM1c melanoma (per the American Joint Committee on Cancer 7th Edition Melanoma Staging System [10]) and cutaneous, subcutaneous, or nodal tumours of ≥10 mm diameter that were accessible for intralesional injection by direct or ultrasound-guided approaches. Additional inclusion criteria were serum lactate dehydrogenase ≤1·5 x upper limit of normal, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate hematologic, hepatic, and renal function. Patients were excluded if they had >3 visceral metastases (not including lung metastases or nodal metastases associated with visceral organs); bone or clinically active cerebral metastases (patients with ≤3 asymptomatic, treated cerebral metastases with no evidence of progression or requirement for steroids for ≥2 months prior to enrolment were allowed); human immunodeficiency virus, hepatitis B, or hepatitis C infection; evidence of immunosuppression; participated in another clinical trial <1 month before enrolment; or were pregnant or lactating. The protocol was approved by the Institutional Review Board at each site, and all patients provided written informed consent before the start of any study-related procedures.
2.3. Endpoints
Biodistribution and shedding endpoints were incidence of detectable T-VEC DNA in blood and urine and clearance from the blood and urine; patient and sample incidence of T-VEC DNA and viral infectivity on swabs collected from the exterior of occlusive dressings, surface of injected lesions, oral mucosa, and anogenital area during and after treatment (only during treatment for occlusive dressings); and incidence of T-VEC DNA in lesions suspected to be of herpetic origin in treated patients and close contacts. Collection of anogenital swabs was included after 2 protocol amendments, the first requiring swabs for patients with injected lesions below the waist, and the second requiring swabs for all patients.
Efficacy endpoints included best overall response, objective response rate (complete response or partial response, according to modified WHO criteria), and durable response rate (complete or partial response for ≥6 months).
Safety endpoints were patient incidence of treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs).
2.4. Precautionary measures to reduce the risk of T-VEC virus transmission
Consistent with the current T-VEC US prescribing information [7], patients and their close contacts were advised to avoid direct contact with injection sites, dressings, and body fluids; wear gloves when changing dressings; keep injection sites covered for ≥1 week after each treatment visit; replace the dressing if it fell off; and dispose of used dressings and cleaning materials in household waste in a sealed plastic bag. Patients were advised to use barrier contraception methods while receiving T-VEC. Patients were also advised that pregnant or immunocompromised close contacts should not change dressings or clean injection sites. In the event of accidental exposure, patients and caregivers were advised to clean the area with soap and water and/or a disinfectant. If a patient or close contact developed herpetic lesions, they were encouraged to have follow-up testing and had the option to receive antiviral agents. Study staff wore gowns, gloves, and eye protection while preparing and administering T-VEC, consistent with biosafety level 1 (BSL-1) live virus containment practices [11], as described in the T-VEC prescribing information [7]. Although not required, BSL-2 practices could have been implemented in accordance with individual institutional protocols.
2.5. Detection of T-VEC DNA and infectivity of virus
Samples were tested for T-VEC DNA using a validated T-VEC–specific quantitative polymerase chain reaction (qPCR)–based assay (no detection of non-target nucleic acids, including wild-type HSV-1 and HSV-2). The test was considered positive if DNA was detectable with results above the assay cutoff values, even if the level was too low to be quantified. The lower limit of quantification (LLOQ) of the assay (copies of T-VEC DNA/μg total DNA) is 1·76 for blood, 24 for urine, and 18 for swabs.
qPCR-positive swab samples were tested for infectious virus in the validated 50% tissue culture infective dose (TCID50) assay, which quantifies the amount of virus required to create a cytopathic effect in 50% of inoculated tissue culture cells. In brief, Vero cells (ATCC No. CCL-81) in 96-well plates were incubated with qPCR-positive samples at 37 °C for 66–80 h, and the cytopathic effect was recorded. Virus titre was calculated as TCID50/mL; the LLOQ for the TCID50 assay is 5,940 TCID50/mL in swab samples.
2.6. Statistical methods
Data were summarized for patients who received ≥1 dose of T-VEC and had ≥1 postdose blood or urine sample collected. The amount of quantifiable DNA measured by qPCR was analysed as a continuous variable, and summary statistics of DNA counts over time are provided. Clearance of T-VEC was measured as the number of patients with undetectable T-VEC DNA.
Summary statistics are presented for patient and sample incidence (overall and by time) of detectable T-VEC DNA in each sample type as well as virus in swabs from the exterior of occlusive dressings, surface of injected lesions, oral mucosa, anogenital area, and lesions of suspected herpetic origin.
Objective response rate was analysed as a binary variable with 95% confidence intervals (CIs) using exact method. Duration of response was analysed for responders using the Kaplan-Meier method.
Patient incidence of AEs after the initiation of therapy through the 30-day safety follow-up visit is summarized.
2.7. Role of the funding source
The funder contributed to study design, data collection, data analysis, and data interpretation, and funded a professional medical writer to assist with writing the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient disposition and baseline characteristics
Sixty-one patients were enrolled (57 in the US, 4 in Canada), 60 (98%) received ≥1 dose of T-VEC, 49 (80%) completed the study, and 12 (20%) withdrew consent and discontinued the study.
All patients were white, 55% were men, the median (range) age was 65 (19–93) years, and 75% had an ECOG performance status of 0 (Table 1). Most patients had stage III melanoma; 30% had stage IV. At baseline, 40 patients (67%) were seropositive for HSV-1, and 20 (33%) were seropositive for HSV-2. Most patients (68%) had received previous treatment for melanoma. The most frequently reported prior therapies were immunotherapy (58%, including ipilimumab, pembrolizumab, and nivolumab), radiotherapy (17%), and chemotherapy (15%).
Table 1.
Baseline demographics and clinical characteristics.
| All patientsa (N = 60) | |
|---|---|
| White/Caucasian, n (%) | 60 (100) |
| Men, n (%) | 33 (55) |
| Median (range) age, years | 65 (19–93) |
| Age ≥ 65 years, n (%) | 31 (52) |
| ECOG PS, n (%) | |
| 0 | 45 (75) |
| 1 | 15 (25) |
| Disease stage at screening,b n (%) | |
| IIIB | 10 (17) |
| IIIC | 32 (53) |
| IVM1a | 11 (18) |
| IVM1b | 3 (5) |
| IVM1c | 4 (7) |
| HSV-1 baseline serostatus, n (%) | |
| Positive | 40 (67) |
| Negative | 17 (28) |
| Unknown | 3 (5) |
| HSV-2 baseline serostatus, n (%) | |
| Negative | 36 (60) |
| Positive | 20 (33) |
| Equivocal | 1 (2) |
| Unknown | 3 (5) |
| BRAF V600 mutation status, n (%) | |
| Mutant | 20 (33) |
| Wild type | 38 (63) |
| Unknown or missing | 1 (2) |
| Otherc | 1 (2) |
| Prior anti-cancer therapy, n (%) | 41 (68) |
| Immunotherapy | 35 (58) |
| External beam radiotherapy | 10 (17) |
| Chemotherapy | 9 (15) |
| Targeted small molecules | 6 (10) |
| Other | 5 (8) |
| Stereotactic radiosurgery | 1 (2) |
| Chemoradiotherapy | 1 (2) |
| Prior therapy for melanoma, n (%) | 41 (68) |
| First line | 36 (60) |
| Second line | 19 (32) |
| Third line | 9 (15) |
| Fourth line | 4 (7) |
| Other | 11 (18) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HSV, herpes simplex virus.
Patients who received ≥1 dose of talimogene laherparepvec.
American Joint Committee on Cancer, 7th edition [10].
BRAF D594N mutation reported.
3.2. Biodistribution and shedding of T-VEC DNA
3.2.1. Blood
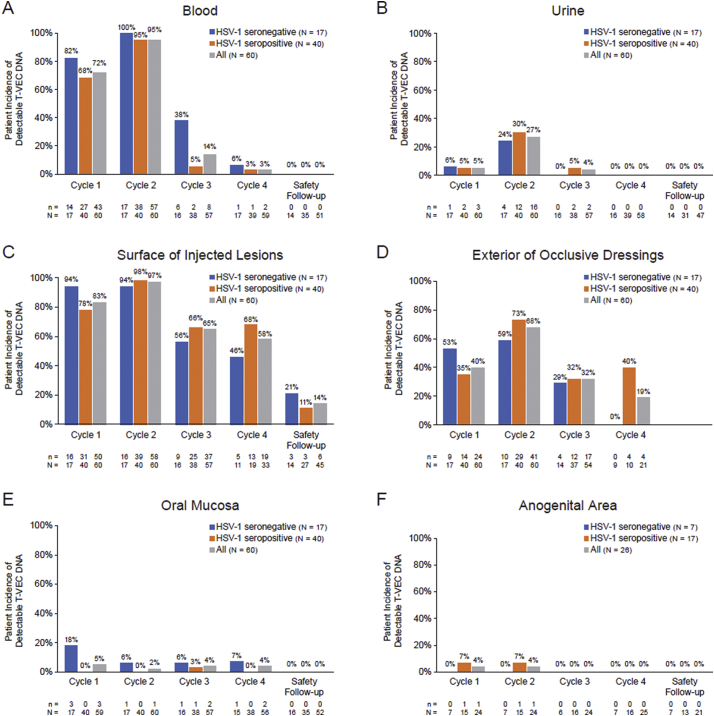
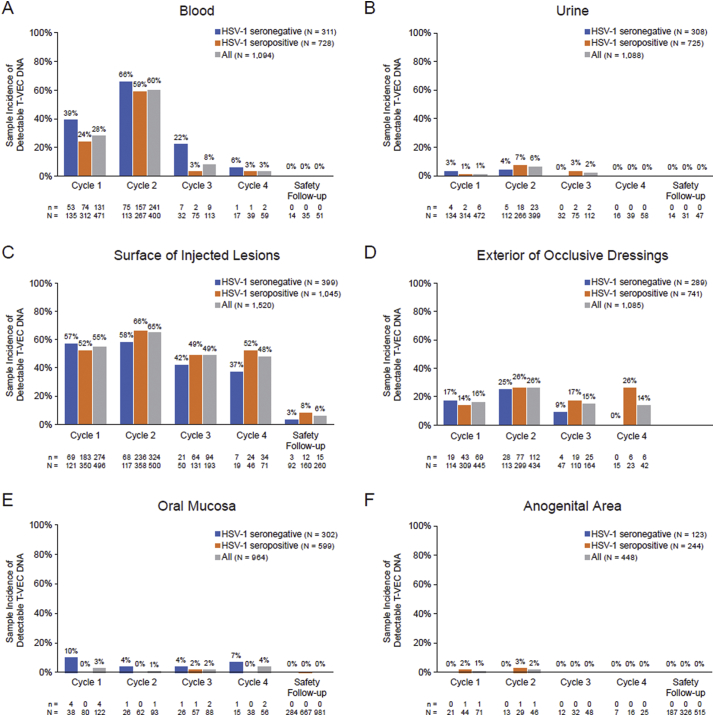
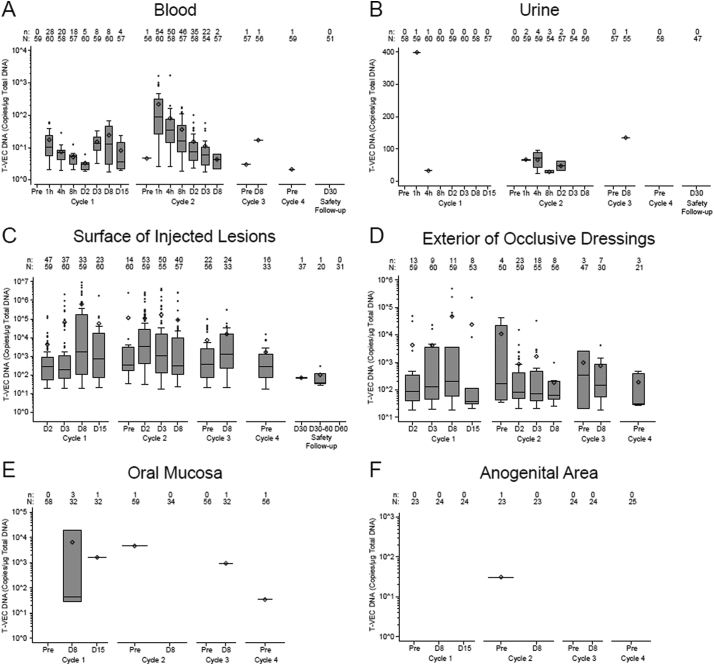
T-VEC DNA in blood during cycles 1–4 was detected in 98·3% of patients (59/60) and 36·7% of samples (383/1043), with a higher incidence among patients who were HSV-1 seronegative at baseline (Table 2). The incidence of T-VEC DNA was highest during cycle 2 and decreased to zero by the safety follow-up visit (Fig. 2A, 3A); DNA copy number was highest during cycle 2 (Fig. 4A). Within cycle 2, the DNA copy number was highest 1 h after injection (mean of 219·4 copies/μg DNA; maximum of 1650 copies/μg DNA) and lower during subsequent measurements; DNA copy number was largely independent of baseline HSV-1 serostatus (data not shown).
Table 2.
Patient and sample incidence of T-VEC DNA during cycles 1–4.
| Patient incidence of T-VEC DNA |
Sample incidence of T-VEC DNA |
|||||
|---|---|---|---|---|---|---|
| Baseline HSV-1 seronegative |
Baseline HSV-1 seropositive |
Overall |
Baseline HSV-1 seronegative |
Baseline HSV-1 seropositive |
Overall |
|
|
N = 17 |
N = 40 |
N = 60 |
n3/n4 (%) |
n3/n4 (%) |
n3/n4 (%) |
|
| n1/n2 (%) | n1/n2 (%) | n1/n2 (%) | ||||
| Blood | 17/17 (100·0) | 39/40 (97·5) | 59/60 (98·3) | 136/297 (45.8) | 234/693 (33.8) | 383/1043 (36.7) |
| Urine | 5/17 (29·4) | 14/40 (35·0) | 19/60 (31·7) | 9/294 (3.1) | 22/694 (3·2) | 31/1041 (3·0) |
| Surface of injected lesions | 17/17 (100·0) | 40/40 (100·0) | 60/60 (100·0) | 165/307 (53·7) | 507/885 (57·3) | 726/1260 (57·6) |
| Exterior of occlusive dressings | 13/17 (76·5) | 33/40 (82·5) | 48/60 (80·0) | 51/289 (17·6) | 145/741 (19·6) | 212/1085 (19·5) |
| Oral mucosa | 3/17 (17·6) | 1/40 (2·5) | 5/60 (8·3) | 7/105 (6.7) | 1/237 (0·4) | 9/359 (2·5) |
| Anogenital area | 0/7 (0·0) | 2/16 (12·5) | 2/25 (8·0) | 0/53 (0·0) | 2/121 (1·7) | 2/190 (1·1) |
N = Number of patients in the analysis set; n1 = number of patients with positive qPCR testing result; n2 = number of patients with samples collected; n3 = number of samples with positive qPCR testing result; n4 = number of samples collected.
Abbreviations: HSV, herpes simplex virus; qPCR, quantitative polymerase chain reaction; T-VEC, talimogene laherparepvec.
Fig. 2.
Patient incidence of detectable T-VEC DNA in (A) blood, (B) urine, (C) surface of injected lesions, (D) exterior of occlusive dressings, (E) oral mucosa, and (F) anogenital area. HSV-1 serostatus at baseline was missing for 3 patients. Samples were taken on day 30 and/or 60 of the safety follow-up period; no swabs from occlusive dressings were taken during the safety follow-up period. N = number of patients with samples collected; n = number of patients positive for T-VEC DNA. Abbreviations: HSV-1, herpes simplex virus type 1; T-VEC, talimogene laherparepvec.
Fig. 3.
Sample incidence of detectable T-VEC DNA in (A) blood, (B) urine, (C) surface of injected lesions, (D) exterior of occlusive dressings, (E) oral mucosa, and (F) anogenital area. HSV-1 serostatus at baseline was missing for 3 patients. Samples were taken on days 30–60 of the safety follow-up period; no swabs from occlusive dressings were taken during the safety follow-up period. A number of samples taken throughout the study contained detectable T-VEC DNA below the lower limit of quantification: 79/383 (21%) samples from blood, 17/31 (55%) samples from urine, 77/741 (10%) swabs from the surface of injected lesions, 52/212 (25%) swabs from the exterior of occlusive dressings, 3/12 (25%) swabs from the oral mucosa, and 5/7 (71%) swabs from the anogenital area. N = number of samples collected; n = number of samples positive for T-VEC DNA. Abbreviations: HSV-1, herpes simplex virus type 1; T-VEC, talimogene laherparepvec.
Fig. 4.
Quantification of T-VEC DNA by qPCR in patient samples. (A) blood, (B) urine, (C) surface of injected lesions, (D) exterior of occlusive dressings, (E) oral mucosa, and (F) anogenital area. Samples were collected throughout the treatment period and at the 30- and 60-day safety follow-up visits. N = number of patients tested; n = number of patients with T-VEC DNA equal to or above the lower limit of quantification. Upper and lower edges of the box represent Q3 and Q1, respectively. Median is presented as a horizontal bar in the box, and mean is presented as a diamond. Upper and lower whiskers represent the maximum and minimum, excluding outliers. Abbreviations: D, day; Pre, predose; qPCR, quantitative polymerase chain reaction; T-VEC, talimogene laherparepvec.
3.2.2. Urine
T-VEC DNA was detected in urine during cycles 1–3 in 31·7% of patients (19/60) and 3·0% of samples (31/1041) (Table 2) and was highest during cycle 2, zero during cycle 4 and the safety follow-up visits, and independent of HSV-1 serostatus (Fig. 2B, 3B). There were 24–399 copies/μg T-VEC DNA in urine during cycles 1–3; DNA was not detected after cycle 3 (Fig. 4B).
3.2.3. Surface of injected lesions
All patients (60/60) and 57·6% of samples (726/1260) had detectable T-VEC DNA in swabs from the surface of injected lesions at some point during cycles 1–4, independent of HSV-1 serostatus (Table 2). The incidence of T-VEC DNA was highest during cycles 1 and 2 (Fig. 2C, 3C). DNA was detected on the surface of injected lesions in 14% of patients (5.8% of samples) during the safety follow-up period. In cycle 1, mean DNA copy number peaked 8 days postdose for seronegative patients (867,764 copies/μg DNA) and seropositive patients (562,377 copies/μg DNA) and was slightly lower in cycle 2 (Fig. 4C). Eight patients (in cycles 1 and 2) had DNA levels >1,000,000 copies/μg DNA. Two samples taken during the safety follow-up period had quantifiable DNA (mean of 71 copies/μg DNA).
3.2.4. Exterior of occlusive dressings
T-VEC DNA was detectable in swabs of the exterior of occlusive dressings during cycles 1–4 in 80% of patients (48/60) and 19·5% of samples (212/1085) with no consistent trends based on HSV-1 serostatus (Table 2). The incidence of DNA was slightly higher during cycle 2 compared with cycles 1, 3, and 4 (Fig. 2D, 3D). DNA copy number was highest in cycle 1, peaking at day 8 for seropositive (mean 61,313 copies/μg DNA) and seronegative (mean 17,652 copies/μg DNA) patients (Fig. 4D).
3.2.5. Oral mucosa
The incidence of T-VEC DNA in swabs taken from the oral mucosa at any time during cycles 1–4 was 8·3% of patients (5/60) and 2·5% of samples (9/359), with a higher incidence among HSV-1 seronegative patients (Table 2; Fig. 2E, 3E). Three patients with detectable DNA had received injections to lesions on the face and/or neck. After cycle 4 (cycles 5–47), DNA was detectable on mucosal swabs in 5·4% of patients (3/56) and 0·5% of samples (3/596). No DNA was detected at the safety follow-up visits 30 and 60 days after completion of injections. DNA copy number was highest on day 8 of cycle 1 (6491 copies/μg DNA) in a sample from a seronegative patient (Fig. 4E).
3.2.6. Anogenital area
The incidence of T-VEC DNA in swabs taken from the anogenital area during cycles 1–4 was 8·0% of patients (2/25, both HSV-1 seropositive) and 1·1% of samples (2/190) (Table 2; Fig. 2F, 3F). After cycle 4 (cycles 5–50), DNA was detectable on anogenital swabs in 13·0% of patients (3/23, all HSV-1 seropositive) and 2·1% of samples (5/238). No DNA was detected in swabs taken during the safety follow-up period. T-VEC DNA was above the LLOQ in one sample (31 copies/μg DNA) taken predose in cycle 2 from a seropositive patient (Fig. 4F).
3.3. Clearance of T-VEC from blood and urine
Almost all patients with detectable T-VEC DNA in blood or urine during cycles 1 and 2 had cleared the virus before the next dose (Supplementary Table 1). Further details regarding the clearance of T-VEC are provided in the Supplementary Material.
3.4. T-VEC DNA in patient lesions of suspected herpetic origin
Of 19 patients with lesions of suspected herpetic origin, four of 37 swabs taken from three patients had detectable T-VEC DNA: from the left nipple (124 copies/μg DNA; shared a dressing with an injected lesion) and right arm (85 copies/μg DNA; not near injected lesion) of a seronegative patient, from the right arm (below the LLOQ; close to injected lesion) of a patient with unknown serostatus, and from the oral cavity (9070 copies/μg DNA; not near injected lesion) of a seropositive patient. None of these lesions had been injected.
3.5. Transmissibility of T-VEC
The TCID50 assay for infectivity was performed on all swabs with detectable T-VEC DNA: 211 swabs from the exterior of occlusive dressings, 740 from the surface of injected lesions, 12 from the oral mucosa, seven from the anogenital area, and four from lesions of suspected herpetic origin. Only swabs from the surface of injected lesions tested positive for infectivity (8/740 [1·1%]): seven samples from cycle 1 and one from cycle 2.
Three close contacts reported signs and symptoms of suspected herpetic origin. One had sores on the inside of the lip with no detectable T-VEC DNA, one had cold sores/fever blisters with no detectable T-VEC DNA, and one had cold sores/fever blisters and declined testing. One investigator was exposed to T-VEC (unprotected skin) but had no signs or symptoms, and one developed a lesion on the lower lip with no detectable T-VEC DNA.
3.6. Safety
All patients had ≥1 TEAE, and 95% had ≥1 AE that was considered related to study treatment. The most common TEAE was chills (65%) (Table 3). Grade ≥ 3 TEAEs and TRAEs were reported by 12 (20%) and 6 (10%) patients, respectively; serious TEAEs and TRAEs were reported by 13 (22%) and 8 (13%) patients, respectively. Serious TRAEs were delirium and pyrexia (two patients each) and upper abdominal pain, atrial fibrillation, increased body temperature, cellulitis, influenza-like illness, posterior reversible encephalopathy syndrome, and skin infection in one patient each. One patient who had lymphopenia and thrombocytopenia at study baseline experienced grade 4, non-serious, TRAE of lymphopenia.
Table 3.
Safety summary.
| All patients |
|
|---|---|
| N = 60 | |
| Treatment-emergent AEs, n (%) | 60 (100·0) |
| Grade ≥ 3 | 12 (20·0) |
| Serious AEs | 13 (21·7) |
| Leading to permanent discontinuation of T-VEC | 5 (8·3) |
| AEs in ≥20% of patients, n (%) | |
| Chills | 39 (65·0) |
| Fatigue | 34 (56·7) |
| Headache | 27 (45·0) |
| Nausea | 27 (45·0) |
| Pyrexia | 24 (40·0) |
| Injection-site pain | 15 (25·0) |
| Pain | 15 (25·0) |
| Vomiting | 15 (25·0) |
| Diarrhoea | 13 (21·7) |
| Influenza-like illness | 13 (21·7) |
| Myalgia | 12 (20·0) |
| Rash | 12 (20·0) |
| T-VEC–related AEs, n (%) | 57 (95·0) |
| Grade ≥ 3 | 6 (10·0) |
| Serious AEs | 8 (13·3) |
| Leading to permanent discontinuation of T-VEC | 3 (5·0) |
Abbreviations: AE, adverse event; T-VEC, talimogene laherparepvec.
Fifty-nine patients (98%) had AEs of interest: four had serious events of flu-like symptoms, one had a serious event of cellulitis (occurred in the anatomical area of injections but not specifically in injected lesions), and one had a serious event of deep vein thrombosis (unrelated to treatment).
AEs leading to discontinuation of T-VEC were reported by five patients (8%): confusion, pyrexia, and atrial fibrillation (in the same patient and treatment related), anaemia (related), posterior reversible encephalopathy syndrome (related), haemorrhage (unrelated), and sepsis (unrelated). There were no fatal AEs; one patient died of disease progression 91 days after their last dose of T-VEC.
3.7. Efficacy
The ORR was 35%, including nine patients (15%) with a complete response and 12 patients (20%) with a partial response (Table 4). Median time to response was 3·1 months (95% CI:
Table 4.
Best overall response to T-VEC.
| All patients |
|
|---|---|
| N = 60 | |
| Response, n (%) | |
| Complete response | 9 (15) |
| Partial response | 12 (20) |
| Stable disease | 10 (17) |
| Progressive disease | 26 (43) |
| Not determined/not evaluable | 3 (5) |
| Overall response rate,a n (%; 95% CI) | 21 (35; 23–48) |
| Median time to response in responders (95% CI),b months | 3·1 (2·6–5·3) |
Response was assessed per modified WHO response criteria [21].
Abbreviations: CI, confidence interval; T-VEC, talimogene laherparepvec; WHO, World Health Organization.
Defined as the proportion of patients with a complete or partial response.
Kaplan-Meier estimate of the probability of objective response and the point-wise 95% CI based on the Greenwood formula.
2·6–5·3). As of the date of the final analysis, median duration of response was not reached.
4. Discussion
Our in-depth investigation of the biodistribution, shedding, and transmissibility of T-VEC in this target population confirms observations from previous clinical trials [[4], [5], [6]] that T-VEC is unlikely to be transmitted from treated patients to their close contacts. Instructions for the preparation, handling, and administration of T-VEC, precautions to avoid accidental exposure to the virus, and information provided to patients and close contacts who reported exposure or signs and symptoms of suspected herpetic origin reflected those in the current T-VEC prescribing information [7]. Although some differences in the management of patients in a clinical trial cannot be ruled out, we believe that the results of our study are relevant to patients with melanoma treated with T-VEC in the real-world clinical practice setting.
Approximately one-third of blood samples contained detectable T-VEC DNA during treatment, with a higher incidence in HSV-1 seronegative versus seropositive patients. The amount of DNA in blood was highest during cycle 2, potentially due to the higher dose of T-VEC administered during this cycle, and then declined rapidly, suggesting a humoral response to repeated injections, leading to clearance of injected virus. The overall incidence of T-VEC DNA in urine and in swabs of the oral mucosa and anogenital area was low (1·2%–3·0% of samples) and did not appear to be substantially impacted by baseline HSV-1 serostatus; the amount of DNA in these samples was, again, highest during cycle 2. T-VEC DNA was cleared from blood and urine by end of treatment.
As expected, the incidence of detectable T-VEC DNA was highest in samples from the surface of injected lesions. Fourteen percent of patients and 6% of samples tested positive for T-VEC DNA during the safety follow-up period; however, each sample was negative in the TCID50 assay. Live virus was detected from injected lesions (1·1% of samples), but only during the first 2 cycles of treatment. Live virus was not detected on the exterior surface of occlusive dressings, demonstrating that when applied according to the T-VEC prescribing information [7], they serve as an effective barrier against viral transmission. Preparation and administration of commercial T-VEC should be consistent with BSL-1 live virus containment practices, using personal protective equipment, such as eye protection, gloves, and a lab coat [11]; BSL-2 protocols are not required [12].
One-third of patients developed mucosal or skin lesions while on treatment; however, only three had detectable T-VEC DNA and none were positive for infectivity. Of three close contacts and two investigators who reported exposure or signs and symptoms of suspected herpetic origin, none had detectable T-VEC DNA. A limitation of our study is that detailed descriptions of the lesions were not required, and we were unable to ascertain whether they were consistent with typical herpetic mucosal or skin lesions. Also, none of these lesions were tested for wild-type HSV-1 or HSV-2. Lastly, the study design did not allow extensive investigation of the occurrence of live T-VEC virus transmission to close contacts. T-VEC is an infectious HSV-1 virus; therefore, it is advised to follow current published guidelines [7,11,12] to reduce risks for healthcare providers and close contacts especially for immunocompromised persons, pregnant women, and newborns. Ongoing postmarketing studies collecting real-world transmission data are expected to provide a better understanding of these potential risks.
Our study provides additional data on the efficacy and safety of T-VEC in patients with advanced-stage melanoma. Thirty-five percent of patients achieved an objective response, which was higher than the 26% observed in a phase 2 study and the registrational OPTiM study of patients with advanced-stage melanoma [6,13]. The difference in objective response may be attributable to the smaller sample size, the descriptive nature of the analysis, and the higher proportion of patients with stage IIIB, IIIC, or IVM1a metastatic melanoma in our study (88% compared to 55% in OPTiM) [13]. The spectrum and incidence of AEs reported in our study were similar to those reported previously [6,13], with a majority of patients reporting flu-like symptoms.
While a number of other intratumorally administered oncolytic viruses have been investigated [14,15], published studies of biodistribution and transmissibility are scarce: limited data on viral shedding have been reported for an engineered vaccinia virus administered to patients with solid tumours as well as an HSV-1–derived oncolytic virus administered to young patients with extracranial cancers [16,17]. Our clinical study appears to be the most extensive to date on the biodistribution and transmissibility of an intratumoral oncolytic virus.
In summary, with proper handling, administration, and post-injection care [7,[18], [19], [20]], T-VEC can be administered safely in patients with advanced melanoma, with minimal risk of transmission to close contacts. The methodological approach and results of our study may be of value in the development of future oncolytic viruses for the treatment of melanoma and other cancers.
Acknowledgments
Acknowledgments
This study was funded by Amgen Inc.: ClinicalTrials.gov, NCT02014441. The authors acknowledge the patients and their families, friends, and caregivers; study staff; and study investigators for their participation in this study. Medical writing support was funded by Amgen Inc. and provided by Kathryn Boorer, PhD (KB Scientific Communications, LLC).
Contributors
RA, TA, JN, JSZ, JW, JC, and JMM contributed to study design. RA, TA, JN, JSZ, JW, JC, KL, C-PH, CP, and JMM contributed to data acquisition, data analysis, or data interpretation. RA, TA, JN, JSZ, JW, JC, KL, C-PH, CP, and JMM wrote the draft report. RA, TA, JN, JSZ, JW, JC, KL, C-PH, CP, and JMM revised the report. All authors approved the final version to be published.
Data sharing statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Declaration of Competing Interest
RA has received research grants and consulting fees from Amgen. TA has provided editorial consultation for Amgen. JC is a consultant for Amgen and RepIimune; has received research funding from Amgen, Bristol-Myers Squibb, and Merck Sharp & Dohme; has intellectual property interests at the University of Louisville; and has received travel expenses from Replimune. CP-H, KL, and CP are employees of and own stock in Amgen. JMM has received research grants from Amgen, AstraZeneca, Bristol-Myers Squibb, EMD Serono, Immunocore, Incyte, Macrogenics, Merck Sharp & Dohme, Novartis, Polynoma, and Sanofi; received consulting fees from Amgen, Array BioPharma, Boehringer Ingelheim, and Merck Sharp & Dohme; received honoraria from EMD Serono and Pfizer; and participated on an advisory board for Array BioPharma, Bristol-Myers Squibb, EMD Serono, and Merck Sharp & Dohme. JN is employed by, owns stock in, has partnership/ownership in, and holds a nonremunerative position of influence in Gradalis, Inc., and has served on speakers' bureaus for Amgen, AstraZeneca, and Foundation Medicine. JW has nothing to disclose. JSZ has received research grants and consulting fees from Amgen and served on speakers' bureaus for Amgen.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.066.
Contributor Information
Thomas Amatruda, Email: thomas.amatruda@usoncology.com.
Cheryl A. Pickett, Email: cpickett@amgen.com.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 2.Liu B.L., Robinson M., Han Z.Q. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman H.L., Ruby C.E., Hughes T., Slingluff C.L., Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11. doi: 10.1186/2051-1426-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J.C., Coffin R.S., Davis C.J. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 5.Harrington K.J., Hingorani M., Tanay M.A. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 6.Senzer N.N., Kaufman H.L., Amatruda T. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 7.IMLYGIC® (talimogene laherparepvec) prescribing information. BioVex, Inc., a subsidiary of Amgen Inc.; 2018. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/imlygic/imlygic_pi.ashx (accessed 16 May 2019) [Google Scholar]
- 8.U. S. Food and Drug Administration: vaccine and related biological product guidances. 2019. https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/vaccine-and-related-biological-product-guidances (accessed 1 June 2019)
- 9.European medicines agency: oncolytic viruses. 2009. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-22.pdf (accessed 1 June 2019)
- 10.Balch C.M., Gershenwald J.E., Soong S.J. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services . 5th ed. 2009. Biosafety in microbiological and biomedical laboratories (BMBL)https://www.cdc.gov/labs/pdf/CDC-BiosafetyMicrobiologicalBiomedicalLaboratories-2009-P.PDF (Section IV—laboratory biosafety level criteria). (accessed 16 May 2019) [Google Scholar]
- 12.IMLYGIC® (talimogene laherparepvec) patient information. BioVex, Inc., a subsidiary of Amgen Inc.; 2019. https://www.imlygic.com/ (accessed 16 May 2019) [Google Scholar]
- 13.Andtbacka R.H., Kaufman H.L., Collichio F. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy M.L., Bommareddy P.K., Boland G., Kaufman H.L. Oncolytic immunotherapy. Surg Oncol Clin N Am. 2019;28:419–430. doi: 10.1016/j.soc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Reale A., Vitiello A., Conciatori V., Parolin C., Calistri A., Palu G. Perspectives on immunotherapy via oncolytic viruses. Infect Agent Cancer. 2019;14:5. doi: 10.1186/s13027-018-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeh H.J., Downs-Canner S., McCart J.A. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23:202–214. doi: 10.1038/mt.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streby K.A., Geller J.I., Currier M.A. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin Cancer Res. 2017;23:3566–3574. doi: 10.1158/1078-0432.CCR-16-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seery V. Intralesional therapy: consensus statements for best practices in administration from the melanoma nursing initiative. Clin J Oncol Nurs. 2017;21:76–86. doi: 10.1188/17.CJON.S4.76-86. [DOI] [PubMed] [Google Scholar]
- 19.Harrington K.J., Michielin O., Malvehy J. A practical guide to the handling and administration of talimogene laherparepvec in Europe. Onco Targets Ther. 2017;10:3867–3880. doi: 10.2147/OTT.S133699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBride A., Valgus J., Parsad S., Sommermann E.M., Nunan R. Pharmacy operationalization of the intralesional oncolytic immunotherapy talimogene laherparepvec. Hosp Pharm. 2018;53:296–302. doi: 10.1177/0018578717749926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO handbook for reporting results of cancer treatment. World Health Organization; Geneva, Switzerland: 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2