Abstract
Background
Cisplatin resistance remains a major clinical obstacle to the successful treatment of non-small cell lung cancer (NSCLC). Scribble contributes to ROS-induced inflammation and cisplatin-elevated toxic reactive oxygen species (ROS) promotes cell death. However, it is unknown whether and how Scribble is involved in the cisplatin-related cell death and the underlying mechanism of Scribble in response to chemotherapies and in the process of oxidative stress in NSCLC.
Methods
We used two independent cohorts of NSCLC samples derived from patients treated with platinum-containing chemotherapy and xenograft modeling in vivo. We analyzed the correlation between Scribble and Nox2 or Nrf2/PD-L1 both in vivo and in vitro, and explored the role of Scribble in cisplatin-induced ROS and apoptosis.
Findings
Clinical analysis revealed that Scribble expression positively correlated with clinical outcomes and chemotherapeutic sensitivity in NSCLC patients. Scribble protected Nox2 protein from proteasomal degradation. Scribble knockdown induced cisplatin resistance by blocking Nox2/ROS and apoptosis in LRR domain-dependent manner. In addition, low levels of Scribble correlated with high levels of PD-L1 via activation of Nrf2 transcription in vivo and in vitro.
Interpretations
Our study revealed that polarity protein Scribble increased cisplatin-induced ROS generation and is beneficial to chemotherapeutic outcomes in NSCLC. Although Scribble deficiency tends to lead to cisplatin resistance by Nox2/ROS and Nrf2/PD-L1, it is still possible that Scribble deficiency-induced PD-L1 may yield benefits in immunotherapy.
Fund
National Key R&D Program of China, Strategic Priority Research Program of the Chinese Academy of Sciences, National Natural Science Foundation of China, China Postdoctoral Science Foundation.
Keywords: NSCLC, Cisplatin sensitivity, Scribble, Nox2, ROS, PD-L1
Research in context.
Evidence before this study
Cisplatin resistance remains a major clinical obstacle to the successful treatment of non-small cell lung cancer (NSCLC). Chemotherapeutic agents generate reactive oxygen species (ROS) in patients undergoing chemotherapy. The only enzyme family that produces ROS as its main product is the NADPH oxidase (Nox) family. Nuclear factor, erythroid 2 like 2 (Nrf2), as an antioxidant regulator, promotes expression of PD-L1, which in turn contributes to chemoresistance in solid tumors. Scribble is considered a tumor suppressor in multiple solid tumors and mostly localizes primarily to the cell membrane, as does Nox2, this subcellular location is critical for the Scribble's function in epithelial cells. Our preliminary data suggested that Scribble contributes to Nox-induced ROS generation in inflammation. Current studies focus on the role and underlying mechanism of activity of Scribble in cisplatin resistance of NSCLC.
Added value of this study
Our clinical studies from two independent NSCLC cohorts suggested that Scribble levels were positively associated with clinical outcomes and chemotherapeutic sensitivity in NSCLC patients who had received platinum-containing first-line chemotherapy. Downregulation of Scribble increased proteasomal degradation of Nox2 to block Nox2-derived ROS signaling and apoptosis in a LRR domain-dependent fashion, subsequently inducing cisplatin-related drug resistance in NSCLC. Also, Scribble decreased PD-L1 by inhibiting Nrf2 transcription in NSCLC and cisplatin-resistant cell lines, which might reflect (in part) a response to the abnormal Scribble/Nox2/ROS signaling.
Implications of all the available evidence
Our findings demonstrated that loss of Scribble confers cisplatin resistance during NSCLC chemotherapy via Nox2/ROS and Nrf2/PD-L1 signaling. Chemotherapy is suitable for in first-line treatment in Scribblehigh patients, whereas, PD-1/PD-L1 checkpoint blockades might be the preferred choice for first-line treatment in Scribblelow patients.
Alt-text: Unlabelled Box
1. Introduction
Cisplatin (cis-diamminedichloro-platinum(II), CDDP) is one of the most commonly used chemotherapeutic agents in the treatment of cancer, in particular, in non-small cell lung cancer (NSCLC) [1,2]. Platinum-containing drugs are used as first-line chemotherapeutic agents for the treatment of human NSCLC [3]. Unfortunately, current cisplatin-based treatments for advanced NSCLC result in only modest responses [4,5]. Within the population of patients, the selection of pre-existing resistant cells and/or acquisition of resistance during treatment with chemotherapy has been proposed. Development of resistance to cisplatin is considered a primary factor in NSCLC relapse [6,7].
Scribble (Scrib) is one component of the Scribble/Discs large (Dlg)/Lethal giant larvae (Lgl) polarity complex, which localizes to the basolateral side of the epithelial cell membrane [8,9]. Loss of Scribble expression results in cancerous overgrowth of imaginal discs in Drosophila larvae, suggesting a tumor suppressor role for Scribble [8,10,11]. Human Scribble (hScrib) is a functional homologue of Drosophila Scribble [12]; mislocated or deregulated hScrib expression contributes to tumorigenesis including proliferation, invasion, metastasis and drug resistance in various of epithelial cancers [9,[13], [14], [15], [16]]. In particular, Scribble acts via the MAPK-ERK pathway to decreases the tumor burden in the KrasLSL-G12D lung cancer mouse model [14]. However, the mechanism underlying the tumor suppressor function of Scribble as wellas its potential relevance to the efficacy of chemotherapy in NSCLC is poorly understood.
Chemotherapeutic agents generate reactive oxygen species (ROS) in patients undergoing cancer therapy, in whom toxically oxidative stress is an important contributor to the response to anticancer therapies [[17], [18], [19]]. NADPH oxidases (Nox) family has been identified as one of the major sources of ROS generationin cancer cells [20,21]. Among those Nox family members, Nox2 (also referred to as gp91phox), is robustly expressed in lung alveolar epithelial cells and is involved in the regulation of epithelial cell function, a pattern that is similar to that of Scribble [20,21]. Furthermore, it has been reported that Scribble is essential for the interaction with endogenous p22phox and contributes to Nox complex activation and ROS generation in inflammation in primary myeloid cells [22]. However, it is unknown whether and how Scribble is involved in Nox2/ROS signaling in chemotherapy in NSCLC.
Nuclear factor, erythroid 2 like 2 (Nrf2) is considered an antioxidant proteins-associated transcription factor [23]. The molecular regulation of the well-known kelch like ECH associated protein 1 (Keap1)/Nrf2 system acts as a sensor responding to changes in redox homeostasis. Under homeostatic and stress-free conditions, cellular Nrf2 abundance is maintained at a very low level because the ubiquitin E3 ligase complex composed of Keap1 and Cullin 3 (Cul3) specifically promotes the ubiquitination and proteasomal degradation of Nrf2 [24,25]. In contrast, the malignant tumors, including NSCLC, exhibit constitutive activation of Nrf2 [26,27]. Interestingly, previous studies have shown that Nrf2 directly induces PD-L1 expression in melanoma [28]. Notably, conventional chemotherapy-induced PD-L1 expression was demonstrated to promote chemoresistance in NSCLC [[29], [30], [31], [32]]. Whether Nrf2/PD-L1 might serve as the master signaling to promote cancer and increase chemoresistance is not yet well known. Furthermore, are there other specific mechanisms causing sustainable high levels of Nrf2 activation? Additionally, with the approval of PD-L1/PD-1 checkpoint-based immunotherapy in lung cancer [33,34], a better understanding of the molecular basis of cisplatin resistance and the effect of cisplatin resistance on PD-L1/PD-1 checkpoint are warranted in order to elucidate the mechanisms and markers underlying this drug-resistant phenotype and to facilitate the implementation of precision medicine.
Herein, we discovered that loss of Scribble conferred cisplatin resistance in NSCLC. Scribble interacted with Nox2 in a LRR domain-dependent manner in the human bronchial epithelium cell line BEAS-2B. Mechanistically, Scribble stabilized Nox2 protein via inhibiting proteasomal degradation, thereby promoting ROS production and leading to cisplatin-induced apoptosis in NSCLC; additionally, Scribble levels negatively correlated with PD-L1 and Nrf2 levels in vivo and in vitro, a correlation that might be partially regulated by ROS in NSCLC. Together, these results suggested that Scribble might be a potential marker that can be used in chemotherapy as well as in PD-L1/PD-1-based immunotherapy.
2. Materials and methods
2.1. Tumor samples and patients
This study was approved by the Institute Research Ethics Committees of Zhongshan Hospital, Fudan University. Two validated cohorts of patients with NSCLC were enrolled in this study, in which patients must have been pathologically confirmed with NSCLC. The first cohort consisted of 115 patients who had not previously received chemotherapy. Tumor specimens from these patients were obtained at the time of surgical resection before chemotherapy. Standard cisplatin or carboplatin-based first-line chemotherapy was used for the treatment of these 115 NSCLC patients. Several types of outcome measurements were assessed in this cohort: overall response rate (ORR) (the sum of complete response [CR] and partial response [PR]), progression-free survival (PFS), and overall survival (OS). The second cohort consisted of 72 NSCLC patients from Zhongshan Hospital (Fudan University). Paired samples, embedded by formalin-fixed paraffin, were obtained from these patients who underwent operations and matched all clinicopathologic variables, with a median follow-up time of 10 years. For the two cohorts, tumor tissues were stained for Scribble protein using immuohistochemical (IHC) staining (see below).
2.2. Evaluation of clinical response
The chemotherapeutic response of the tumors were clinically evaluated according to the WHO criteria as follows: (1) CR, disappearance of all known disease; (2) PR, 50% or more decrease in the entire tumor burden; (3) stable disease (SD), <50% decrease or <25% increase in the entire tumor burden; and (4) progressive disease (PD), 25% or greater increase in the entire tumor burden or appearance of new lesions. For our analysis, subjects with CR and PR were defined as responders (sensitivityhigh); SD or PD were defined as non-responders (sensitivitylow).
2.3. IHC
IHC staining for Scribble was performed as described previously [35]. Resulting images were concurrently evaluated by two independent pathologists in the Department of Pathology, Zhongshan Hospital (Fudan University). Staining intensity was scored using a four-category grading system: I, no staining; II, weak staining; III, moderate staining; and IV, strong staining [36]. For classification of Scribble protein staining in this study, samples with moderate or strong staining (Grade III or IV) were defined as Scribblehigh; samples with no or weak staining (Grade I or II) were defined as Scribblelow.
2.4. Cell lines and reagents
The following cell culture lines were obtained as stocks from our laboratory collection: lung cancer cell lines CRL-5803, CRL-1848, A549, HTB-177, HTB-182, HCC827, CRL-5883, CRL-5866; stomach cancer cell lines MGC803 and AGS; human cervical carcinoma line HeLa and human endometrial carcinoma line HEC-1-A. CRL-1848/CDDP, MGC803/5-FU, and AGS/5-FU were CDDP- or 5-FU (5-fluorouracil)-resistant isolates selected in our laboratory from the respective parent using standard methods [37,38]. Normal human bronchial epithelium cell line BEAS-2B and NSCLC cell line CRL-5800 were kindly provided by Dr. Guohong Hu. Lines SKOV-3 and SKOV-3/CDDP were purchased from Shanghai Xuanyan Biotechnology Company. Lines SGC7901, and SGC7901/CDDP, MCF-7, and MCF-7/Adr were purchased from Shanghai Bogoo Biotechnology Company. Cisplatin, 5-FU, N-acetyl-L-cysteine (NAC), dipenylene iodonium chloride (DPI), cycloheximide (CHX), gp91ds-tat, apocynin, and MG132 were purchased from Sigma-Aldrich (St. Louis, MO, USA). FuGENE 6 was purchased from Roche (Roche, Inc., USA). Other plasmids, RNAi vectors, Scrib (WT) and Scrib (Mut) constructs, and corresponding sequences have been described previously [16].
2.5. Cell viability assays
Cells (3000/well) were seeded in 96-well plates in triplicate. Cells, seeded for 24 h, were exposed to different concentrations of drugs for another 48 h. Then MTT solution (5 mg/ml, 20 μl/well, Sigma) was added to the plate and cells were incubated for 4 h at 37 °C. The supernatant was discarded; DMSO (100 μl/well) was added to the plates and crystals were allowed to dissolve for 30 min at 37 °C. Absorbance at a wavelength of 570 nm was measured with a microplate reader (Bio-Rad, CA, USA). Results were expressed as the percentage of viable cells in drug-treated wells vs medium-treated control wells and were plotted as a drug dose-dependent cell survival curve.
2.6. Cell apoptosis assays
Cells (1 × 10 [6]/well) were seeded in 6-well dishes and treated with cisplatin for 24 h or 48 h prior to analysis. Flow cytometric analysis was performed as described previously [15].
2.7. ROS assays
Intracellular ROS levels were measured using the fluorescent dye 2′,7′- dichloro-fluorescein diacetate (DCFH-DA) (Invitrogen). Cells were plated in 6-well culture plates, incubated overnight, and treated with cisplatin for 24 h. Flow cytometric analyses (10,000 events per sample) were performed in a FACS Aria™ flow cytometer (Becton Dickinson, USA) with extinction and emission at 485 and 538 nm, respectively, and data then were evaluated with flowJo13.1 software.
2.8. Western blot
Drug-induced apoptosis was evaluated 24 h after drug treatment. Western blot analysis was performed as described previously [35]. Antibodies against PARP, caspase-3, E-cadherin, Nrf2, HSP90, β-actin, ERCC1, ATM, CHK1, TOPOII, CTR1, ATP7B, GAPDH, Flag-tag, and HA-tag were obtained from Cell Signaling Technology (Boston, MA, USA); anti-Scribble antibody (C-20) was obtained from Santa Cruz Biotechnology (CA, USA); anti-Nox2/gp91phox antibody was obtained from Abcam (Cambridge, UK); anti-PD-L1 antibody was obtained from eBioscience.
2.9. Co-immunoprecipitation (Co-IP)
Cells (3 × 106 /sample) were collected and lysed in a modified IP buffer (containing protease inhibitor cocktail). Proteins were co-IPed with anti-Scribble or anti-Flag antibody, and western blot analyses of electrophoresed proteins were performed using the indicated antibodies.
For cell membrane-related IP assay, the cell membrane was extracted using ProteoExtract Native Membrane Protein Extraction Kit (Millipore) according to the manufacturer's protocol and the cell membrane protein lysate then was used to perform the IP assay.
2.10. Immunofluorescence (IF) imaging
The IF assay was performed as described previously [35]. Anti-Scribble antibody (C-20): Santa Cruz Biotechnology (CA, USA); anti-Nox2/gp91phox antibody: Abcam (Cambridge, UK); and anti-PD-L1 antibody: eBioscience.
2.11. Animal experiments
All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute for Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All animal experiments were approved by the IACUC of the Institute for Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghia, China).
To generate Scribble conditional-knockout mice, Scribbleflox/flox mice were crossed with KrasG12D mice, yielding Scribbleflox/floxKrasG12D mice. To knockout Scribble and activate KrasLSL-G12D in the lung, 8-week-old Scribbleflox/floxKrasG12D mice were anesthetized and infected with the Adcre virus (2 × 10^ [6] pfu/mouse in 62.5 μl DMEM containing 5.8 mM CaCl2) via intranasal instillation. The resulting Scribbleflox/floxKrasLSL-G12D mice were sacrificed at 12 weeks post-virus infection, and lung tissues were collected for further analysis.
For xnograft experiments, nude mice (4 to 6 weeks old) were used for tumor cell implantation. Tumor cells (1 × 106 /mouse) in 25 μl serum-free medium (mixed with Matrigel at a 1:1 ratio) were injected into the left inguinal region using a 100-μl syringe. Tumor growth was monitored every other day. Tumor mass (weight in g) was determined by the formula for the volume of an ellipsoid sphere (V = ½(a × b [2]), where “a” is the long dimension and “b” is the perpendicular short dimension, in cm) and assuming a density of unity. When tumors achieved the desired size (about one week after tumor cell implantation), mice harboring palpable tumors were weighed and randomly sorted into control and treatment groups. Cisplatin was dissolved in physiological saline and administered (via i.p. injection; every other day for 15 days) at a doses of 2·5 mg/kg. The control group received equivalent volumes of saline only. The body weight was recorded one day after the final cisplatin dose.
2.12. Statistical analyses
All numerical data are presented as the mean ± S.D. The data were analyzed using a two-tailed non-paired Student's t-test with homoscedasticity assumed. Comparisons of patient and tumor characteristics were constructed using the χ2 test. Survival curves were constructed using the Kaplan–Meier method. All statistical analyses were performed using Prism (version 5.0; GraphPad, San Diego, CA, USA) software. P values of <0.05 were considered statistically significant.
3. Results
3.1. Low levels of Scribble are associated with poor survival and worse chemotherapeutic outcomes in human NSCLC
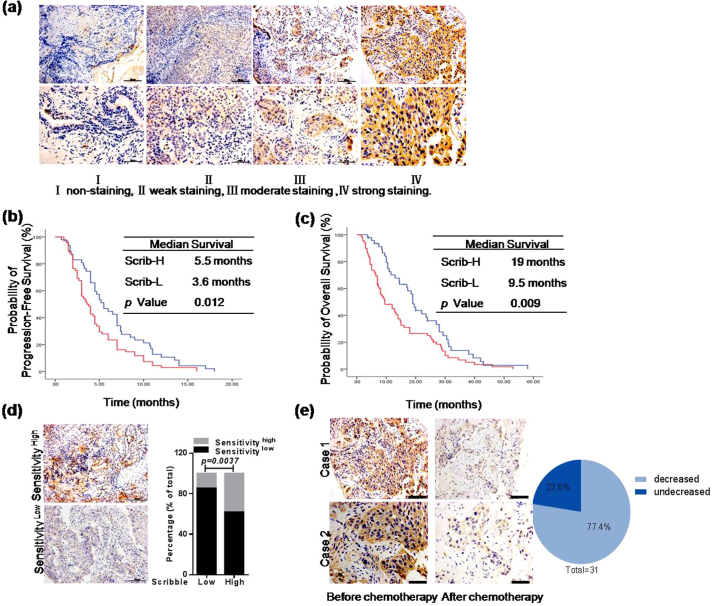
To determine the potential of Scribble as an effective clinical biomarker to predict the prognosis of chemotherapy, Scribble level was correlated to clinical outcomes in NSCLC patients who had received platinum-based chemotherapy after diagnosis. IHC staining was revealed that Scribble level was strongly positively related to progression-free survival (PFS) and overall survival (OS) in this cohort (n = 115; see Supplementary Table S1 for patients characteristics) (Fig. 1a–c and Table 1; PFS, P = 0·012; OS, P = 0·009; Kaplan-Meier analysis). Further analysis of patients' responses to the first-line platinum-containing chemotherapy showed lower sensitivity in 58 out of 68 Scribblelow patients (85·29%), which suggested that Scribble levels were positively correlated with chemotherapeutic sensitivity in NSCLC (Fig. 1d and Table 1). Consequently, it is reasonable to consider the potential of Scribble level in predicting chemotherapeutic sensitivity. Additionally, multivariate Cox regression analyses demonstrated that Scribble provided prognostic information independent of all standard clinicopathologic variables tested, including TNM stages, sex, age, pathology, EGFR-TKI status and performance status across survival types (Supplementary Table S2). To further identify the efficiency of Scribble for predicting the chemotherapeautic outcomes, another independent platinum-based chemotherapy NSCLC cohort (n = 72) was evaluated; in this second cohort patients were matched for all clinicopathologic variables and were treated with platinum-containing chemotherapy followed by tumor sampling. Kaplan–Meier analyses of this second cohort suggested that the Scribble level was significantly prognostic specifically in platinum-based chemotherapy-treated patients (Supplementary Fig.S1a and Supplementary Table S3).
Fig. 1.
Low levels of Scribble are associated with poor survival and worse chemotherapeutic outcomes in human NSCLC. (a) Immunohistochemical (IHC) staining for Scribble in 115 NSCLC specimens (scale bars: 50 μm (upper row) or 100 μm (lower row)). (b–c) Kaplan-Meier analysis of progression-free survival (PFS) (b) and overall survival (OS) (c) of 115 cases with NSCLC. Patients with NSCLC were classified based on IHC-determined Scribble protein levels (high-level expression (Scrib-H) = Grade III or IV; low-level expression (Scrib-L) = Grade I or II) (Kaplan-Meier analysis). (d) IHC staining for Scribblein NSCLC specimens from patients of the first cohort that had different responses to chemotherapy (sensitivityhigh = CR or PR; sensitivitylow = SD or PD). Scale bars, 50 μm. **P < .01, chi-square test. (e) IHC staining for Scribble in NSCLC specimens before and after chemotherapy respectively. Scale bars, 50 μm.
Table 1.
Relationship between Scribble expression and clinical responses to platinum-containing chemotherapy.
| Scribble Ha | Scribble L | P value | |
|---|---|---|---|
| PFS (months) | |||
| Median | 5.5 | 3.6 | 0.012 |
| 95%CI | 4.0–6.9 | 2.7–4.5 | |
| OS (months) | |||
| Median | 19.0 | 9.5 | 0.009 |
| 95%CI | 17.1–20.9 | 4.9–14.1 | |
| Tumor response | |||
| CR | 0 | 0 | |
| PR | 18 | 10 | |
| SD | 21 | 40 | |
| PD | 8 | 18 | |
| ORR (%) | 38.5 | 12.3 | 0.004 |
| DCR (%) | 82.3 | 73.1 | 0.234 |
Abbreviations: Scribble H, Scribble high expression; Scribble L, Scribble low expression.
Comparison between two groups with Chi-square test.
Furthermore, we obtained 31 paired samples from cisplatin-treated NSCLC patients undergoing platinum-based chemotherapy; specifically, the paired samples were obtained from biopsies performed before and after treatment. We evaluated these samples to assess the effect of platinum-containing chemotherapy on Scribble expression. The results showed that most of the surviving cells exhibited low Scribble expression in approximately 77·4% (24/31) of the patients after chemotherapy (Fig. 1e). Statistical analysis of the number of decreased metastasis sites after platinum-containing chemotherapy further suggested that patients' with decreased Scribble levels were less sensitive to chemotherapy (Supplementary Fig.S1b). Collectively, these data demonstrated that Scribble expression level may be a crucial marker for the development of clinical resistance to highly efficient chemotherapy.
3.2. Scribble is required for efficacious cisplatin-containing treatment of NSCLC in vivo
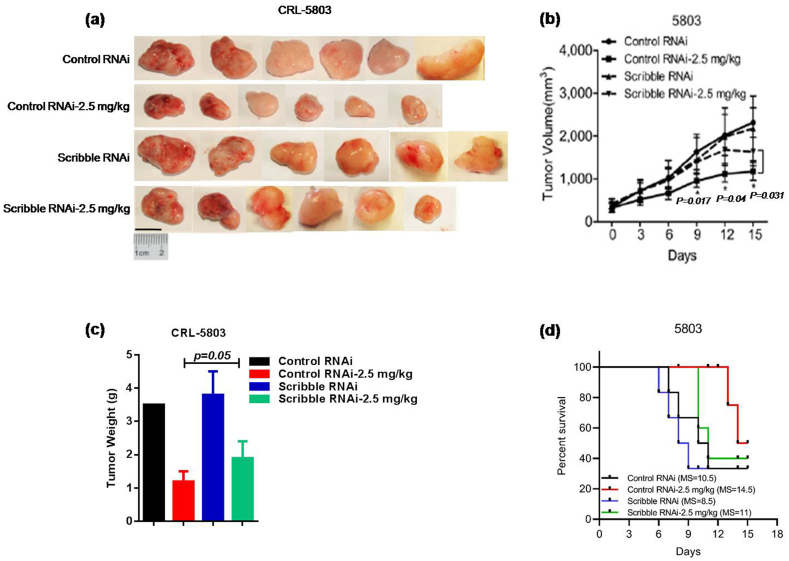
Next, to determine the influence of Scribble on the response to cisplatin treatment under physiological conditions, nude mice were implanted subcutaneously with a control cell line or with the stable KD human lung cancer cell line CRL-5803 (Scribble RNAi), and tumors were allowed to develop. The KD cell line constitutively expresses a short hairpin targeting the Scribble transcript to reduce Scribble expression; the control line expresses an RNAi that does not target this gene [16]. The expressions of Scribble of before and after xenografts were determined by western blot to confirm the knockdown efficiency of Scribble in CRL-5803 (Supplementary Fig.S2a). When the xenografts reached palpable volumes, mice were treated by intraperitoneal injection (every other day for two weeks) with saline (vehicle) or cisplatin (2·5 mg/kg). After 15 days, animals were euthanzied, and tumors were recovered and weighed. For both control cells and Scribble KD cells, cisplatin injection attenuated tumor growth compared to the saline treatment (P < 0·05 for each cell line, student's t-test). However, among the cisplatin-treated animals, residual tumors in Scribble KD-implanted mice remained significantly larger than those in control cell-implanted mice (P < 0·05, student's t-test) (Fig. 2a–c). In addition, Kaplan–Meier analysis demonstrated that mice with CRL-5803 Scribble RNAi cells exhibited shorter overall survival time after cisplatin treatment compared to the control group (Fig. 2d). Equivalent experiments using control and KD constructs in a cisplatin-sensitive cancer cell line (HEC-1-A) yielded similar results (Supplementary Fig.S2b–d). Since HEC-1-A is a human endometrial carcinoma cell line, these studies demonstrated that Scribble may mediate the efficacy of cisplatin-based treatment in NSCLC as well as other (non-NSCLC) epithelial cancers in vivo.
Fig. 2.
Scribble is required for efficacious cisplatin-containing treatment of NSCLC in vivo. (a–c) Nude mice harboring subcutaneous tumors derived from implanted Control CRL-5803 or CRL-5803 Scribble KD cells were treated by intraperitoneal injection (on alternate days for two weeks) with vehicle (saline) or 2.5 mg/kg cisplatin. Tumor dimensions were recorded on every other day and calculated tumor volumes are presented as the mean ± SD for each group (n = 6). Weight of terminal tumors was recorded on Day 15. (d) Kaplan-Meier analysis of OS of xenograft mice with or without cisplatin treatment. MS: Median Survival (Day). All assays were performed in triplicate and the results are presented as the mean ± S.D. in all panels (b, c). *P < .05, **P < .01 (Student's t-test).
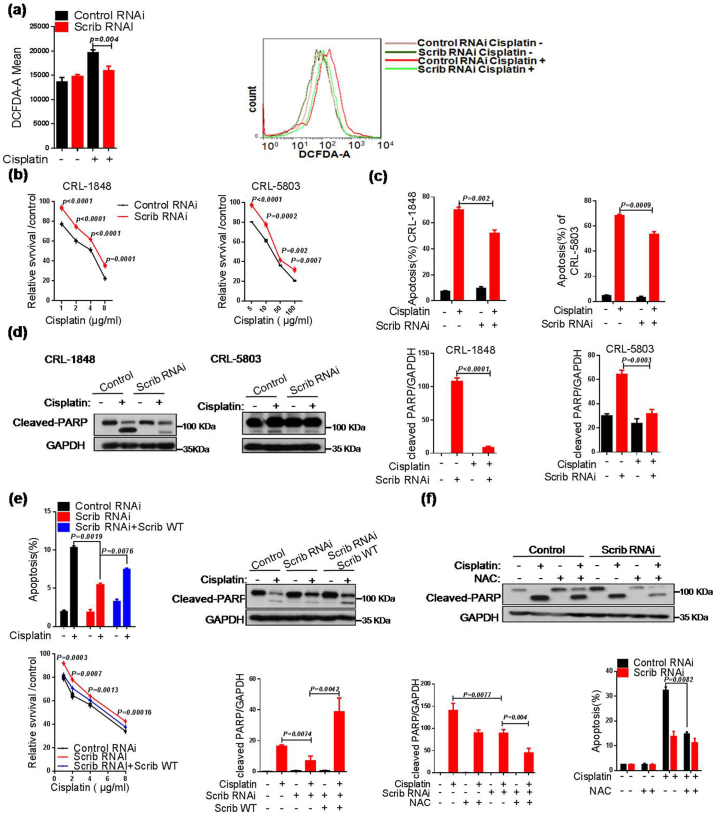
3.3. Scribble sensitizes cisplatin and induces apoptosis via elevating ROS generation in NSCLC cells
To examine the role of Scribble in response to cisplatin treatment in lung cancer cells, we firstly tested Scribble protein levels in various NSCLC cell lines (Supplementary Fig. S3a). Previous studies showed that many chemotherapeutic drugs including cisplatin dramatically elevate ROS levels to preferentially kill cancer cells, and Nox is critical to ROS production [19,39,40]. Therefore, ROS levels were measured in virous cell lines. We found that stable Scribble knockdown (KD, Scrib RNAi) attenuated the increase in cisplatin-inducing ROS signaling (Fig. 3a and Supplementary Fig. S3b). Next, to verify whether Scribble-modulated ROS affects the response to cisplatin in NSCLC, we investigated cell viability in Scribble KD cells challenged with cisplatin. Compared to control cells, Scribble downregulation increased cell viability and the IC50 (half-maximum inhibitory concentration) of cisplatin approximately 2-fold (Fig. 3b and Supplementary Fig. S3b–d). Further examination of the levels of apoptotic proteins showed that Scribble KD decreased cisplatin-resulted in apoptosis and the accumulation of the cleaved-PARP as that of cleaved-caspase-3 (Fig. 3c–d and Supplementary Fig. S3e), indicating that Scribble promotes cisplatin-induced apoptosis and elevates cisplatin sensitivity in NSCLC cells. We further introduced full-length Scribble into the CRL-5803 Scribble KD cell lines to rescue the Scribble level. Ectopic expression of Scribble potentiated the accumulation of cleaved-PARP, restored the inhibition of cisplatin in viability and counteracted the decrease in cisplatin-induced apoptosis observed in the KD cell lines lacking such ectopic expression (Fig. 3e and Supplementary Fig. S3f).
Fig. 3.
Scribble sensitizes cisplatin and induces apoptosis via elevating ROS generation in NSCLC cells. (a) Cells (CRL-1848 background) harboring control or Scrib RNAi were treated with cisplatin (2 μg/ml, 48 h), and the intracellular level of ROS was measured by DCFH-DA fluorescence using flow cytometry. (b) MTT assay was used to assess the relative survival of cells harboring the control RNAi or Scrib RNAi after treatment with cisplatin at the indicated concentrations. (c) Apoptosis was measured by monitoring annexin V-FITC and PI-double positive populations. Cells for the assay were plated into a 6-well plate and incubated with or without cisplatin (2 μg/ml) for 36 h, then subjected to flow cytometric analysis. (d) Cells harboring control or Scrib RNAi were exposed to cisplatin (2 μg/ml, 36 h) and analyzed by western blot analysis with anti-PARP antibody, and then the intensities of protein bands were quantified. (e) Ectopic expression of Scrib in CRL-5803 cells harboring the Scrib RNAi plasmid. (f) Cells (CRL-5803 background) harboring control RNAi or Scrib RNAi were pretreated with NAC (10 mM) for 30 min, then incubated with cisplatin (2 μg/ml, 48 h). Apoptosis was monitored by flow cytometry and (separately) analyzed by western blot analysis with anti-PARP antibody, and then the intensities of protein bands were quantified. All assays were performed in triplicate and the results are presented as the mean ± S.D. in all panels (a–f). *P < .05, **P < .01, ***P < .001 (Student's t-test).
Given the excess ROS production is known to trigger apoptosis [41], we further explored whether Scribble-promoted apoptosis is dependent on ROS generation. Our data suggested that exposure to a ROS scavenger (NAC) similarly attenuated cisplatin-induced apoptotic signaling in Scribble KD cells (Fig. 3f). Generally, cisplatin resistance is related to the multi-drug resistance (MDR) pathway [40]. Therefore, we assessed the effect of Scribble on the proteins' expressions involved in MDR in Scribble KD lung cancer cells, including drug transportation (CTR1, ATP7B), DNA repair (ATM, ERCC1, CHK1, TOPOII) and aborted apoptosis (p53 and Bcl2) [7]. The results showed that Scribble deficiency has no significant effect on the classic MDR pathway in NSCLC cell lines during the anti-cancer treatment of cisplatin (Supplementary Fig. S3g).
Collectively, these results demonstrated that knockdown of Scribble attenuated the response to cisplatin, an effect that was mediated by attenuation of ROS production in NSCLC cells.
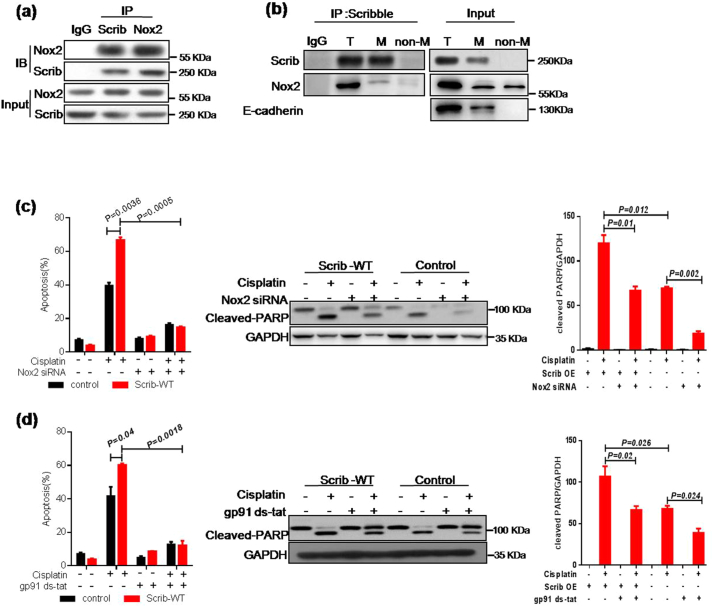
3.4. Scribble augments response to cisplatin through NADPH oxidase pathways
To identify the underlying molecular mechanism of Scribble-mediated cisplatin resistance, a DYKDDDDK (Flag)-tag affinity procedure was used to purify a Scribble-containing complex from BEAS-2B cell line; the components of the resulting complex then were identified by mass spectrometric (MS) assay. Interestingly, peptide sequences for Nox2, a member of the NADPH oxidase family, were extracted, suggesting that Nox2 was involved in Scribble-containing complex (Supplementary Fig. S4a). To identify the potential interacting partners of Scribble, we performed co-IP and IF assays. The results confirmed that Scribble interacted with Nox2 in BEAS-2B cell line and demonstrated that Scribble mainly colocalized with Nox2 on the cell membrane (Fig. 4a–b and Supplementary Fig. S4b). These observations suggested that Nox2 may contribute to Scribble regulated ROS signaling and cisplatin sensitivity in NSCLC. Therefore, we extended this analysis by investigating the effect of Nox2 KD on Scribble-related apoptosis. We found that small interfering RNA (siRNA)-mediated knockdown of Nox2 protected cells from cisplatin-induced apoptosis in CRL-1848 with ectopic Scribble expression (Fig. 4c and Supplementary Fig. S4c). Additionally, the Nox inhibitor DPI and the Nox2-specific inhibitors, gp91ds-tat [42], apocynin [43], also were used and the data confirmed that NADPH oxidase pathways pontentiated on Scribble-induced cisplatin sensitivity (Fig. 4dand Supplementary Fig. S4d–e).
Fig. 4.
Scribble augments response to cisplatin through NADPH oxidase pathways. (a) IP assay showing the interaction between endogenous Scribble and Nox2 in BEAS-2B. (b) Cell membrane proteins were extracted for an IP assay in which the Scribble is shown to specifically bind to Nox2 localized to the cell membrane. E-cadherin, a positive cell membrane protein, was included as a positive control for fractionation. T, total lysate; M, cell membrane lysate; non-M, lysate without cell membrane. (c) Cells (CRL-1848 background) harboring control or Scrib-WT contructs were transformed with Nox2 siRNA for 24 h, then treated with cisplatin (2 μg/ml, 48 h). Apoptosis and cleaved-PARP level in the treated cells were monitored by flow cytometry and western blot, respectively. (d) Cells (CRL-1848 background) harboring control or Scribble overexpression contructs were pretreated with gp91 ds-tat (1 μM), a Nox-specific inhibitor, for 30 min, then incubated with cisplatin (2 μg/ml, 48 h). Apoptosis and cleaved-PARP levelweremonitored by flow cytometry and western blot, respectively. All assays were performed in triplicate and the results are presented as the mean ± S.D. in panels (c, d). *P < .05, **P < .01 (Student's t-test).
Besides the mechanism of NADPH oxidase-mediated ROS genration, mitochondrial ROS accumulation has been reported in cisplatin-treated cells [21]. To exclude a role for mitochondrial ROS, we investigated whether Scribble affects SOD1-inhibited ROS generation and apoptosis. The results showed that Scribble elevated ROS generation and apoptosis in cells with ectopic expression of SOD1, yielding a level of ROS similar to that observed in the control group (lacking ectopic expression of SOD1) (Supplementary Fig. S5a–b). Notably, this effect was blocked by the loss of Nox2 (Supplementary Fig. S5c–d). Thus, our data suggested that the Scribble-Nox2-ROS axis was independent of SOD1-related mitochondria ROS. Together, these studies provided evidence that Scribble acts upstream of Nox2-ROS pathway in cisplatin-related apoptosis and indicated that concomitant loss of Scribble and Nox2 is associated with chemotherapy resistance.
3.5. Scribble protects Nox2 proteins from proteasomal degradation
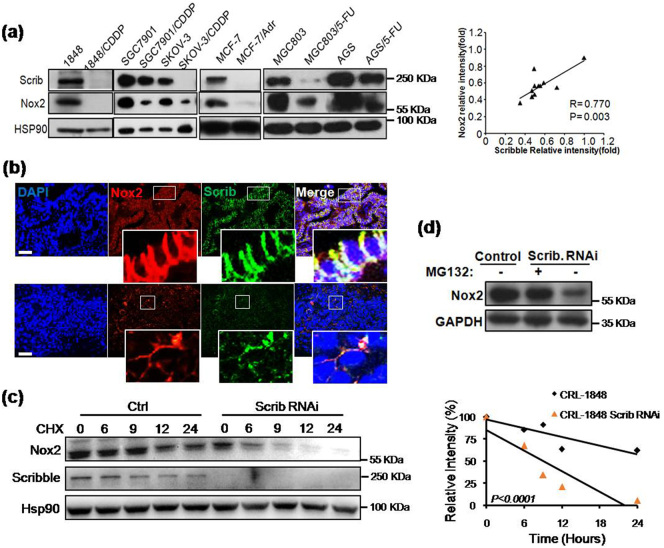
To further characterize the association between Nox2 and Scribble in chemoresistance, we examined the protein levels of Scribble and Nox2 proteins in 6 chemotherapy-resistant cancer cell lines (CRL-1848/CDDP, SGC7901/CDDP, SKOV-3/CDDP, MCF-7/Adr, MGC803/5-FU, and AGS/5-FU), in which the IC50 values of drugs were 2- to 20-fold higher than those in the respective parental lines (data not shown). Notably, the protein levels of both Scribble and Nox2 were decreased in the drug-resistant cell lines (compared to the respective parent lines) (Fig. 5a). Interestingly, IF assays showed that Scribble protein levels were positively correlated with Nox2 protein levels in NSCLC tumor samples; additionally, we observed that Scribble colocalized with Nox2 on the plasma membrane in NSCLC tumor samples (Fig. 5b). To further assess whether Scribble regulates Nox2 accumulation, Nox2 levels were tested in cells exposed to CHX, an inhibitor of translation. The data revealed that Nox2 protein had a shorter half-time in Scribble KD lung cancer cells than in control cells (Fig. 5c), suggesting that Scribble stabilized Nox2 protein. We hypothesized that the effect of Scribble KD on Nox2 protein accumulation was mediated by proteasomal degradation, an idea that we tested by treating Scribble KD cells with MG132, a proteasome inhibitor. We found that while Nox2 protein levels were lower in Scribble KD cells than in control cells. MG132 treatment significantly reversed the loss of Scribble-induced decrease in Nox2 levels (Fig. 5d). These data suggested that decreased Scribble level (in the KD cells) left Nox2 more susceptible to proteolytic degradation.
Fig. 5.
Scribble protects Nox2 proteins from proteasomal degradation. (a) Protein levels of Scribble and Nox2 in the indicated chemotherapy-resistant cell lines (and respective parent lines) were detected by western blot with anti-Scribble and anti-Nox2 antibodies, respectively. HSP90 served as a loading control. (b) The location and intensity of Scribble and Nox2 in NSCLC tissues were detected by IF assay of Scribble (red), Nox2 (green) and DAPI (blue). Scale bars, 50 μm. (c) Cells (CRL-1848 background) harboring control RNAi or Scrib RNAi were incubated with the protein synthesis inhibitor cycloheximide (CHX, 100 μg/ml); lysed after 0, 6, 9, 12, or 24 h; and Scrib and Nox2 then were detected by western blot. HSP90 served as the loading control. The right plot represents the statistical results of protein intensity. (d) Cells (CRL-1848 background) harboring control or Scrib RNAi were pre-treated with the proteasome inhibitor MG132 (10 μM, 4 h); Nox2 protein was detected by western blot assay. All assays were performed in triplicate and the results are presented as the mean ± S.D. in panels (a, c) (Student's t-test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Previous reports indicated that hScrib regulates exocytosis or cell death by directly binding to βPIX/GIT1, which can recruit Rac1 to form a functional complex [16,44,45]. Recent studies have shown that Rac participates in the activation of Nox1 and Nox2: endogenous Rac production is sufficient for Nox1 activation, whereas ectopic expression of active Rac is required for Nox2 activation [[46], [47], [48], [49]]. We also observed that Scribble KD abrogated Rac activation in cisplatin-treated cancer cells (Supplementary Fig. S6). Therefore, Rac1 may mediate Scribble-activated Nox2/ROS signaling.
3.6. Scribble binds to Nox2 in a LRR domain-dependent manner to induce ROS generation and apoptosis
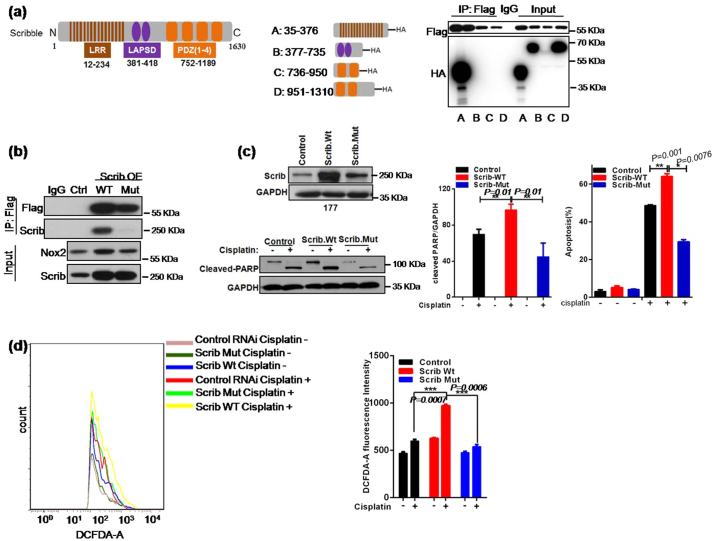
Next, to explore the mechanism by which Scribble interacts with Nox2, we performed in vitro pull-down assays using individual HA-tagged Scribble subdomains (designated here as A: LRR domain, B: LAPSD domain, C: the first two PDZ domains, or D: the last two PDZ domains) [50] in combination with a Flag-tagged Nox2 C-terminal domain (Flag-Nox2). Among the four Scribble subdomains, the LRR domain exhibited strong binding to the Nox2 C-terminus (Fig. 6a), suggesting that the interaction between Scribble and Nox2 occurrs via these respective subdomains.
Fig. 6.
Scribble binds to Nox2 in a LRR domain-dependent manner to induce ROS generation and apoptosis. (a) Schematic showing the full-length Scrib protein with Leucine Rich Repeats (LRR), LAPSD, and PDZ domains, the four separate HA-tagged Scribble subdomains (A: LRR, B: LAPSD, C: first two PDZ domains, D: last two PDZ domains) and the Flag-tagged Nox2 C-terminal domain (Flag-Nox2). HA- and Flag-tagged proteins were incubated together; IP was performed using anti-Flag antibody, and resulting precipitates were immunoblotted with anti-HA antibodies. (b) Cells harboring Control, Scrib (WT), and Scrib (P305L) (Mut) contructs were transformed with the Nox2-Flag-encoding plasmid; lysates were IPed with anti-Scrib or anti-FLAG antibody, and resulting precipitates were immunoblotted with anti-Scrib or anti-Flag antibody. (c) HTB-177 cells were transformed with constructs encoding full-length wild-type Scrib protein (Scrib (WT)) or mutant (P305L) Scrib protein (Scrib (Mut)). Apoptosis was monitored by flow cytometry and western blot, respectively. (d) Cells (HTB-177 background) harboring Control, Scrib (WT), and Scrib (Mut) were treated with cisplatin (2 μg/ml, 48 h), and intracellular levels of ROS were measured using DCFH-DA fluorescence and flow cytometry. All assays were performed in triplicate and the results are presented as the mean ± S.D. in panels (c, d), *P < .05, **P < .01, ***P < .001 (Student's t-test).
To directly investigate whether Scribble function in cisplatin sensitivity can be antagonized through blocking its binding to Nox2, we employed overexpression constructs that encode wild-type or mutant Scribble proteins, respectively. Specifically, the mutated Scribble encodes a protein in which a conserved Proin in LRR domain is replaced with a Leu residue (P305L, Mut); this substitution has been shown to disrupt membrane binding of LRR domain-containing proteins [9,45]. We further evaluated these cell lines using the IP assay. As expected, the physical interaction between Scribble and Nox2 was abolished by the P305L substitution (Fig. 6b). The plasmids encoding the WT and MutScribble were tested in NSCLC cell lines HTB-177 and HTB-182, which are known to exhibit low expression of the endogenous Scribble. Notably, the introduction of the plasmid encoding Scribble (WT) sensitized the cells to cisplatin, whereas the plasmid encoding Scribble (Mut) did not yield a similar effect (Fig. 6c–d and Supplementary Fig. S7). We also analyzed ROS generation in these cells following cisplatin exposure. The data revealed a rightward shift in the fluorescence signal (i.e., increased ROS levels) in cells harboring Scribble (WT) but not in cells harboring Scribble (Mut) (Fig. 6e). Taken together, these results suggested that Scribble binding to Nox2 via the LRR domain may be essential for cisplatin-induced apoptosis and NADPH oxidase-related ROS generation.
3.7. Loss of Scribble is responsible for elevated PD-L1 expression in NSCLC
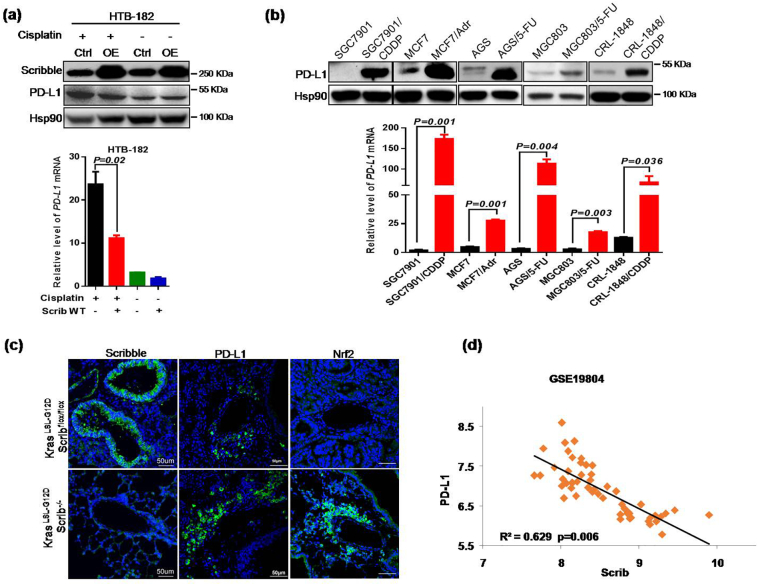
Prior studies suggested that PD-L1 expression is increased in cisplatin-resistant lung cancer cells compared to parent cells, which led us to explore whether Scribble levels correlate with PD-L1 expression in cisplatin-treated NSCLC cells lines [[30], [31], [32]]. Results from HTB-182 cells showed that Scribble overexpression resulted in decreased PD-L1 expression in HTB-182 cells challenged with cisplatin (Fig. 7a). In addition, PD-L1 expression was significantly increased in these chemotherapy-resistant cancer cell lines compared to parental cell lines, revealing that PD-L1 negatively correlated with Scribble expression (Fig. 7b). To further characterize the negative correlation in vivo, we generated a genetically engineered lung cancer mouse model, in which KrasLSL-G12DScribflox/flox mice were infected with a lentivirus encoding the Crerecombinase, resulting in lung-specific deletetion of the Scrib gene. IF assay then was performed to compare PD-L1 expression levels in lung cancer samples derived from KrasLSL-G12DScrib−/− mice and KrasLSL-G12DScribflox/flox mice (Fig. 7c). We further confirmed the negative correlation using clinic data from GEO datasets (Fig. 7d). These analyses indicated that PD-L1 levels were negatively related to Scribble levels in NSCLC (Fig. 7c–d). Generally, cancer cells exhibit higher endogenous oxidative stress than normal cells. Consistent with this observation, we demonstrated that oxidative stress were reduced in lung cancer tissues from KrasLSL-G12DScrib−/− mice compared to those from KrasLSL-G12DScribflox/flox mice (Supplementary Fig. S8a). Under homeostatic conditions, feedback regulation on Nrf2 levels upon excess ROS generation protects cells from oxidative damage. Under homeostatic and stress-free conditions, cellular Nrf2 abundance is maintained at a very low level because the ubiquitin E3 ligase complex (composed of Keap1 and Cul3) specifically promotes the ubiquitination and proteasomal degradation of Nrf2 [24,25]. Recently, Nrf2 has been reported to upregulate PD-L1 in melanoma [28] and to promote chemoresistance in diverse cancers, including NSCLC [[30], [31], [32]]. Thus, these studies led us to hypothesize that Scribble-induced Nox2/ROS signaling might affect Nrf2/PD-L1 signaling in NSCLC. Therefore, we empoloyed NAC to attenuate ROS generation in NSCLC HTB-182 cells exposed to cisplatin. The resulting data showed that interference with ROS generation markedly reversed the Scribble-associated suppression of PD-L1 expression in NSCLC cells (Supplementary Fig. S8b). Notably, Scribble prevented the accumulation of Nrf2 mRNA in a dose-dependent fashion, while ectopic expression of Nrf2 dramatically reversed the Scribble-associated suppression of PD-L1 expression in HTB-182 cells treated with cisplatin (Supplementary Fig. S8c–d). In addition, both in a mouse model and in clinical samples, Scribble levels negatively correlated with Nrf2 expression in NSCLC (Fig. 7c and Supplementary Fig. S8e–f). Taken together, our results indicated that Scribble may play a critical role in down-regulation of Nrf2/PD-L1 signaling in NSCLC, which might also contribute to loss of Scribble-mediated chemoresistance.
Fig. 7.
Loss of Scribble is responsible for elevating PD-L1 expression in NSCLC. (a) Cells harboring control or Scrib OE contructs were treated with or without cisplatin (2 μg/ml, 36 h) and analyzed by western blotand qPCR. (b) Protein and mRNA levels of PD-L1 in the indicated chemotherapy-resistant cell lines (and respective parent lines) were detected by immunoblotting and qPCR, respectively. (c) 20-week-old Kras LSL-G12DScribflox/flox and Kras LSL-G12DScrib−/− mice were euthanized lung tumor tissues then were harvested and subjected by IF staining for Scribble and PD-L1. Scale bar, 50 μm. (d) PD-L1 and Scrib mRNA expression in lung tissues from NSCLC patients from GSE19804 data set. All assays were performed in triplicate and the results are presented as the mean ± S.D. in panels (a, b). *P < .05, **P < .01, ***P < .001 (Student's t-test).
4. Discussion
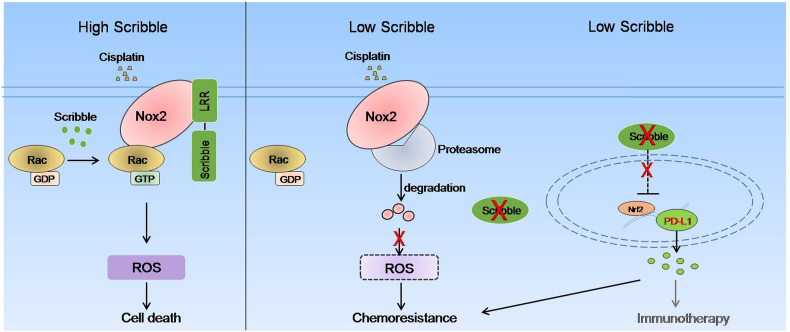
In this study, we demonstrated that Scribble is critical to the efficacy of cisplatin-based chemotherapy, an effect mediated by LRR domain-dependent protection of Nox2 from degradation, thereby promoting Nox2/Ros signaling. Interestingly, Scribble is responsible for elevated Nrf2/PD-L1 expression, which may separately contribute to cisplatin resistance in NSCLC (Fig. 8). In the present work, clinical analysis revealed that lower Scribble expression predicted poorer outcomes (shorter OS and PFS, lower chemotherapeutic sensitivity) following platinum-containing first-line chemotherapy. Additional data from in vitro and in vivo experiments demonstrated that Scribble downregulation significantly decreased sensitivity to cisplatin in NSCLC. Mechanistically, we found that Scribble correlated with Nox2 accumulation, an effect mediated by decrease in proteasomal degradation of Nox2 protein; this stabilization was critical to cisplatin-induced ROS generation and apoptosis, which is closely related to cisplatin sensitivity during chemotherapy. Notably, we showed that Scribble dramatically negatively correlated to Nrf2/PD-L1 expression in NSCLC, and that PD-L1 was expressed at higher levels in chemotherapy-resistant cancer cell lines. Thus, our study suggested a novel mechanism whereby the polarity gene Scribble influences on the cisplatin sensitivity of NSCLC cells.
Fig. 8.
Schematic figure for the proposed model of regulation of Nox2/ROS and Nrf2/PD-L1 signaling and regulation of cisplatin resistance by Scribble in NSCLC.
Accumulating evidence suggests that some chemotherapy drugs induce a high level of oxidative stress [17,51]; platinum coordination complexes and anthracyclines generate notably toxic ROS [17], which fosters cell apoptosis via diverse pathways including activation of JNK and of caspase-8 resulting in cleavage of the downstream mediators caspase-3 and PARP [21]. In the present study, Scribble boosts cisplatin-induced apoptosis via enhancing the accumulation of toxic ROS in NSCLC cell lines (Fig. 3 and Supplementary Fig. S3). Studies from other labs have suggested that, aside from effects on apoptosis, toxic ROS also triggers cell cycle arrest [21,52]. However, our prior work has shown that Scribble has no effect on cell cycle arrest in NSCLC cells treated with cisplatin [15]. Additionally, in the present study, the unchangeable MDR pathway indicated that Scribble-induced ROS is not mediated via DNA damage repair following cisplatin exposure (Supplementary Fig. S3g). However, recent reports have revealed that Scribble promotes apoptosis in breast cancer cells by ROS-dependent ERK and JNK activation [16] and the data in the present study showed that increased Scribble protein yielded elevated cleavage of caspase-3 as well as PARP. Thus, we can not excluded the possibility that Scribble/Nox2-induced ROS might promote apoptosis by direct effects on caspases function and/or by regulating JNK activation.
ROS can be generated from endogenous sources, including the mitochondrial electron transport chain and oxygen-metabolizing enzymes, but the only enzyme family that produces ROS as a main product are the members of Nox family [21]; notably the Nox enzyme, Nox2, exhibits plasma membrane-associated activities in many epithelial cell types. Coincidentally, Scribble also is localized to the cell membrane [53]. Our data confirmed that Scribble co-localized with Nox2 on the cell membrane (Figs. 4a–b, 5b, and Supplementary Fig. S4a–b). In some biological contexts, the LRR domain of Scribble is sufficient to provide Scribble function [54], however, it is relatively few known that the functional patners have been shown to bind to Scribble though the LRR domain. Nonetheless, our work revealed that Scribble interacts with Nox2 via the LRR domain, an interaction that contributes to Scribble/Nox2-promoted ROS production and apoptosis in NSCLC cell lines (Fig. 6). Thus, our finding expands the number of LRR domain-binding partners and indicates a function for LRR in cancer. Aside from Nox enzymes, the mitochondrion is another main source of ROS in cisplatin-treated cells; in response, the mitochondrial enzyme SOD1 converts superoxide anions to harmless H2O2 and antagonizes potential oxidative damage in the mitochondrial intra-membrane space [21,55,56]. Our results (Supplementary Fig. S5a–b) indicated that SOD1-correlated mitochondrional ROS did not contribute to the Scribble-mediated elevation of ROS and apoptosis.
We and others have reported that hScrib directly binds to βPIX, and its partner GIT1, which together recruit Rac1 to form a functional complex potentially regulating exocytosis or cell death [16,44,45]. We demonstrated that Scribble KD abrogates Rac activation in cisplatin-treated cancer cells (Supplementary Fig. S6). Furthermore, previous studies have shown that Rac participates in activation of the Nox1 and Nox2 [46,47]. However, there might be a difference in the demand for Rac between Nox1 and Nox2; endogenous Rac production is sufficient for Nox1 activation, whereas ectopic expression of active Rac is required for Nox2 activation [[46], [47], [48], [49]]. Hence, Scribble-activated Rac1 may be important for the Nox2-ROS pathway. However, it is notable that Scribble binds to βPIX though PDZ domain [45]; the study from our lab also revealed that ciplatin-resistant NSCLC samples encode Scribble proteins harboring mutiple mutations in the PDZ domain. Thus, we hypothesized whether any effects of the PDZ domain on Scribble/Nox2-promoted ROS and apoptosis are mediated via other proteins binding to PDZ domian, a concept that will be further explored.
Interestingly, our results showed that Scribble KD was responsible for proteosome-mediated Nox2 degradation in cancer cells. Others' work has shown that Scribble recruits PH domain and leucine rich repeat protein phosphatase 1 (PHLPP) to the cell membrane to regulate Akt activation [57]. Phosphorylation of Akt and GSK3β are required for E3 ligase β-TrCP-mediated PHLPP degradation [58,59]. Further studies will be needed to determine whether the β-TrCP-mediated ubiquitination is involved in Scribble-related Nox2 stabilization in NSCLC cells.
Nrf2 is considered an antioxidant regulator, an effect mediated by stimulation of the transcription of genes that encoding antioxidant proteins [23]. Under homeostatic and stress-free conditions, ROS levels are maintained at basal levels by Nrf2 [23,26,27]. Constitutively, activated Nrf2, as well as and the accumulation of mutagen and abundant ROS have been observed in cancers including NSCLC [26,27], which hints that the correlation between Nrf2 and ROS is more complex in a pathological context; the relation between Nrf2 and ROS levels in cancer cells will need to be further investigated. Cellular Nrf2 abundance is usually maintained at a very low level by the specifically promotion of the ubiquitination and proteasomal degradation of Nrf2 [24,25]. Markedly, our results indicated that a deficiency of Scribble correlated with Nrf2 accumulation both in vitro and in vivo. Recently, other researchers reported that Nrf2 activity yields elevated PD-L1 expression in melanoma [28], an observation that is consistent with our findings in HTB-182 cells exposed to cisplatin (Supplementary Fig. S8d and f). In our study, a negative correlation between Nrf2/PD-L1 and Scribble levels was seen both in vitro and in vivo (Fig. 7 and Supplementary Fig. S8c–f), demonstrating that Scribble might suppress PD-L1 expression via inhibition of Nrf2's transcriptional activity. However, depletion of ROS notably restored PD-L1 expression in Scribble overexpression (OE) cell line (Supplementary Fig. S8a–b), suggesting that the effect of Scribble on PD-L1 expression might depend (in part) on ROS level. Previous data have shown that Scribble (P305L) but not Scribble (WT) activates AKT/mTOR signaling in breast cancer and that AKT/mTOR is required for PD-L1 expression in NSCLC [60,61]. Combined with the fact that knockdown of Scribble increases AKT activation [57], we infer that Scribble (P305L)-activated AKT also might be involved in Scribble-mediated PD-L1 expression in NSCLC. Data from a number of clinical studies have suggested that patients with PD-L1high tumor cells experience better outcomes (compared to patients with PD-L1low tumor cells) when treated with PD-1/PD-L1 checkpoint blockade drugs [34]. In the present study, Scribblehigh patients exhibited greater sensitivity to cisplatin therapy than did Scribblelow patients; however, Scribblelow patients also showed higher PD-L1 expression. Therefore, we concluded that chemotherapy is a suitable first-line treatment for Scribblehigh patients, whereas, PD-1/PD-L1 checkpoint blockade drugs maybe a preferable first-line treatment for Scribblelow patients.
Altogether, our study revealed that loss of Scribble confers cisplatin resistance during NSCLC chemotherapy, an effect that is mediated via Nox2/ROS and Nrf2/PD-L1 signaling. Although Scribble deficiency appears to lead to the development of cisplatin resistance (by imapring Nox2/ROS and Nrf2/PD-L1), it is still possible that Scribble deficiency-elevated PD-L1 may offer benefits in immunotherapy.
Funding sources
The study was supported by grants from National Key R&D Program of China (2017YFC1600100), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12010100), the National Natural Science Foundation of China (81672965, 81872369), and the China Postdoctoral Science Foundation (45865).
Author contributions
Conception and design: LX Zhan. Development of methodology: N Wang, LL Song, Y Xu, J Guo, WW Ji, YJ Wu. Acquisition of data (provision of animals, acquisition and management of patients, provision of facilities, etc.): Yanjun Wu, LF Zhang, JY Zhao, X Zhang. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): N Wang, LL Song, Y Xu. Writing, review, and/or revision of the manuscript: LX Zhan, N Wang, LL Song, Li Li, Yanjun Wu. Administrative, technical, or material support (i.e., reporting or organizing. Data, constructing databases): LX Zhan, N Wang, LL Song, Y Xu. Study supervision: LX Zhan, X Zhang.
Declarations of interests.
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. YingyongHou, Department of Pathology, Zhongshan Hospital, for pathological diagnoses. We thank Dr. Guohong Hu, Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, CAS, for providing BEAS-2B and CRL-5803 cell lines.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.057.
Contributor Information
Xin Zhang, Email: zhang.xin@zc-hospital.sh.cn.
Lixing Zhan, Email: lxzhan@sibs.ac.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Einhorn L.H. First-line chemotherapy for non-small-cell lung cancer: is there a superior regimen based on histology. J Clin Oncol. 2008;26:3485–3486. doi: 10.1200/JCO.2008.17.2056. [DOI] [PubMed] [Google Scholar]
- 2.Schiller J.H., Harrington D., Belani C.P. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Eaton K.D., Martins R.G. Maintenance chemotherapy in non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:815–821. doi: 10.6004/jnccn.2010.0058. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R., Bergman B., Dunant A., Le Chevalier T., Pignon J.P., Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Zalcman G., Bergot E., Lechapt E. Update on nonsmall cell lung cancer. Eur Respir Rev. 2010;19:173–185. doi: 10.1183/09059180.00006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddik Z.H. Biochemical and molecular mechanisms of cisplatin resistance. Cancer Treat Res. 2002;112:263–284. doi: 10.1007/978-1-4615-1173-1_13. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L., Senovilla L., Vitale I. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 8.Bilder D., Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 9.Navarro C., Nola S., Audebert S. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 10.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 11.Wu M., Pastor-Pareja J.C., Xu T. Interaction between Ras (V12) and Scribbled clones induces tumor growth and invasion. Nature. 2010;463:545–U165. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dow L.E., Brumby A.M., Muratore R. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22:9225–9230. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- 13.Halaoui R., McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene. 2015;34:939–950. doi: 10.1038/onc.2014.59. [DOI] [PubMed] [Google Scholar]
- 14.Elsum I.A., Yates L.L., Pearson H.B. Scrib heterozygosity predisposes to lung cancer and cooperates with KRas hyperactivation to accelerate lung cancer progression in vivo. Oncogene. 2014;33:5523–5533. doi: 10.1038/onc.2013.498. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Chang R., Ji W. Loss of Scribble promotes snail translation through translocation of HuR and enhances cancer drug resistance. J Biol Chem. 2016;291:291–302. doi: 10.1074/jbc.M115.693853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan L.X., Rosenberg A., Bergami K.C. Deregulation of Scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conklin K.A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 18.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 19.Tickner J., Fan L.M., Du J., Meijles D., Li J.M. Nox2-derived ROS in PPARgamma signaling and cell-cycle progression of lung alveolar epithelial cells. Free Radic Biol Med. 2011;51:763–772. doi: 10.1016/j.freeradbiomed.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pommier Y., Sordet O., Antony S., Hayward R.L., Kohn K.W. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 21.Moloney J.N., Cotter T.G. ROS signaling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W., Umitsu M., Jagan I. An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function. Nat Cell Biol. 2016;18:1244–1252. doi: 10.1038/ncb3413. [DOI] [PubMed] [Google Scholar]
- 23.DeNicola G.M., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi A., Kang M.I., Okawa H. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeddi F., Soozangar N., Sadeghi M.R., Somi M.H., Samadi N. Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair (Amst) 2017;54:13–21. doi: 10.1016/j.dnarep.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi K., Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B., Tang L., Chen S. Targeting the upstream transcriptional activator of PD-L1 as an alternative strategy in melanoma therapy. Oncogene. 2018;37:4941–4954. doi: 10.1038/s41388-018-0314-0. [DOI] [PubMed] [Google Scholar]
- 29.Meng X., Liu Y., Zhang J., Teng F., Xing L., Yu J. PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: new development and challenges. Cancer Lett. 2017;405:29–37. doi: 10.1016/j.canlet.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Yan F., Pang J., Peng Y., Molina J.R., Yang P., Liu S. Elevated cellilar PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells. Plos One. 2016;11 doi: 10.1371/journal.pone.0162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wangpaichitr M., Li Y.Y., Wu C. Relationship of metabolic alterations and PD-L1 expression in cisplatin resistance lung cancer. Cell Dev Biol. 2017;6 doi: 10.4172/2168-9296.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P., Ma Y., Lv C. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107:1563–1571. doi: 10.1111/cas.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 34.Aguiar P.N., Jr., De Mello R.A., Hall P., Tadokoro H., Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9:499–506. doi: 10.2217/imt-2016-0150. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y., Chang R., Peng Z. Loss of polarity protein AF6 promotes pancreatic cancer metastasis by inducing snail expression. Nat Commun. 2015;6:7184. doi: 10.1038/ncomms8184. [DOI] [PubMed] [Google Scholar]
- 36.Yi E.S., Boland J.M., Maleszewski J.J. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol. 2011;6:459–465. doi: 10.1097/JTO.0b013e318209edb9. [DOI] [PubMed] [Google Scholar]
- 37.Tegze B., Szallasi Z., Haltrich I. Parallel evolution under chemotherapy pressure in 29 breast cancer cell lines results in dissimilar mechanisms of resistance. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L.Y., Trujillo J.M. Biological characterization of multidrug-resistant human colon carcinoma sublines induced/selected by two methods. Cancer Res. 1990;50:3218–3225. [PubMed] [Google Scholar]
- 39.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 40.Bedard K., Krause K.H. The Nox family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 41.Casares C., Ramirez-Camacho R., Trinidad A., Roldan A., Jorge E., Garcia-Berrocal J.R. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol. 2012;269:2455–2459. doi: 10.1007/s00405-012-2029-0. [DOI] [PubMed] [Google Scholar]
- 42.Higgins C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 43.Patel N.R., Pattni B.S., Abouzeid A.H., Torchilin V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliv Rev. 2013;65:1748–1762. doi: 10.1016/j.addr.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahuna O., Quellari M., Achard C. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J. 2005;24:1364–1374. doi: 10.1038/sj.emboj.7600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Audebert S., Nourry C., Chasserot-Golaz S. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14:987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 46.Cheng G., Diebold B.A., Hughes Y., Lambeth J.D. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 47.Miyano K., Sumimoto H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie. 2007;89:1133–1144. doi: 10.1016/j.biochi.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Miyano K., Ueno N., Takeya R., Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 49.Ueyama T., Geiszt M., Leto T.L. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeitler J., Hsu C.P., Dionne H., Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167:1137–1146. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itoh T., Terazawa R., Kojima K. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic Res. 2011;45:1033–1039. doi: 10.3109/10715762.2011.591391. [DOI] [PubMed] [Google Scholar]
- 52.Moon D.O., Kim M.O., Choi Y.H., Hyun J.W., Chang W.Y., Kim G.Y. Butein induces G2/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010;288:204–213. doi: 10.1016/j.canlet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 53.von Löhneysen K., Noack D., Wood M.R., Friedman J.S., Knaus U.G. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonello T.T., Peifer M. Scribble: master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J Cell Biol. 2019;218:742–756. doi: 10.1083/jcb.201810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goncalves R.L.S., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem. 2015;290:209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X., Yang H.H., Liu J.Y., Schmidt M.D., Gao T.Y. Scribble-mediated membrane targeting of PHLPP1 is required for its negative regulation of Akt. EMBO Rep. 2011;12:818–824. doi: 10.1038/embor.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warfel N.A., Niederst M., Stevens M.W., Brennan P.M., Frame M.C., Newton A.C. Mislocalization of the E3 ligase, beta-transducin repeat-containing protein 1 (beta-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt. J Biol Chem. 2011;286:19777–19788. doi: 10.1074/jbc.M111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X., Liu J., Gao T. beta-TrCP-mediated ubiquitination and degradation of PHLPP1 are negatively regulated by Akt. Mol Cell Biol. 2009;29:6192–6205. doi: 10.1128/MCB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feigin M.E., Akshinthala S.D., Araki K., Rosenberg A.Z., Muthuswamy L.B., Martin B. Mislocalization of the cell polarity protein Scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res. 2014;74:3180–3194. doi: 10.1158/0008-5472.CAN-13-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lastwika K.J., Wilson W., 3rd, Li Q.K., Norris J., Xu H., Ghazarian S.R. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material