Abstract
Background
Utilizing the linear quadratic model and the radiosensitivity index (RSI), we have derived an expression for the genomically adjusted radiation dose (GARD) to model radiation dose effect. We hypothesize GARD is associated with local recurrence and can be used to optimize individual triple negative breast cancer (TNBC) radiation dose.
Methods
TN patients from two independent datasets were assessed. The first cohort consisted of 58 patients treated at 5 European centers with breast conservation surgery followed by adjuvant radiotherapy (RT). The second dataset consisted of 55 patients treated with adjuvant radiation therapy.
Findings
In cohort 1, multivariable analysis revealed that as a dichotomous variable (HR: 2.5 95% CI 1–7.1; p = .05), GARD was associated with local control. This was confirmed in the second independent dataset where GARD was the only significant factor associated with local control (HR: 4.4 95% CI 1.1–29.5; p = .04). We utilized GARD to calculate an individualized radiation dose for each TN patient in cohort 2 by determining the physical dose required to achieve the GARD target value (GARD ≥ 21). While 7% of patients were optimized with a dose of 30 Gy, 91% of patients would be optimized with 70 Gy.
Interpretation
GARD is associated with local control following whole breast or post-mastectomy radiotherapy (RT) in TN patients. By modeling RT dose effect with GARD, we demonstrate that no single dose is optimal for all patients and propose the first dose range to optimize RT at an individual patient level in TNBC.
Keywords: Genomically adjusted radiation dose, Personalized radiotherapy, Radiotherapy, Breast cancer
Research in context.
Evidence before this study
A critical question in radiation oncology is how to shift from the current one-size-fits-all treatment paradigm to one that is biology-based and personalized. To that end, we recently developed the genomic adjusted radiation dose (GARD), which allows the customization of radiotherapy dose to match the individual genomic features of a given tumor. We hypothesize GARD can be utilized to individualize radiotherapy dosing in breast cancer.
Added value of this study
We demonstrate GARD is associated with local recurrence risk in two independent datasets of triple negative patients. We then utilized GARD to calculate an individualized radiation dose by determining the physical dose required to achieve the GARD target value.
Implications
We demonstrate the first personalized radiotherapy dose range for triple negative breast cancer. We propose GARD as a potential means to genomically tailor and personalize radiotherapy dose for triple negative breast cancer.
Alt-text: Unlabelled Box
1. Introduction
Radiotherapy (RT) is a standard component of breast cancer (BC) management. Although, there are current ongoing studies to determine whether early stage women can safely exclude RT (Clinicaltrials.gov identifier NCT02400190 and NCT01791829) according to low risk biologic subtype, a genomic approach to individualize RT dose has not been employed. Radiation dosing to the breast has been uniformly standardized to 50 Gy with long-term data for bioeffective hypofractionation doses of 40–42.56 Gy in select patients [[1], [2], [3]].
We have previously developed and validated the radiation sensitivity index (RSI) as a genomic signature to predict the inherent radiosensitivity of a given tumor [[4], [5], [6], [7], [8], [9], [10], [11]]. These findings have also been independently validated in patients undergoing breast conserving surgery with or without RT by the group at Lund University [12]. The linear quadratic model is a commonly used metric for radiation oncologists to quantify the biologic effect of radiation dose on various tumor types as well as normal tissue although the model is not patient specific [13,14]. The linear quadratic model is an equation that considers the total dose of radiation prescribed, the daily dose of radiation, as well as the general sensitivity of the tissue that is being radiated. Since the model is based on the cellular radiation survival curve, our group hypothesized RSI could be integrated into the model using a patient specific α to quantify an individual tumor's unique response to RT. The result was the genomically adjusted radiation dose or GARD, which has been published and validated in independent datasets of BC, glioblastoma, pancreatic cancer, and lung tumors [15].
GARD is a novel radiation dose prescription paradigm providing the first opportunity to optimize and personalize radiation dose to match an individual tumor's radiosensitivity. GARD has been proposed as the first opportunity for a genomically driven personalized approach in radiation oncology and a research priority for the field [16,17]. Previously, we assessed GARD for 8271 primary tumor samples in our institution's tissue biorepository and found GARD values were lowest for tumor types traditionally thought to be more radioresistant including glioblastoma and sarcoma and higher for tumor types thought to be more radiosensitive including virally associated cervical cancer as well as oropharyngeal cancer [18]. Importantly, we demonstrated large heterogeneity in GARD values achieved with uniform radiation dose, suggesting the current “one-size-fits all” treatment approach is sub-optimal for a significant proportion of patients.
The primary impact of RT in adjuvant BC management is well known to be locoregional control leading in some percentage of patients to a reduction in distant metastatic events, which over time improves BC specific survival [19]. Based on our previous analysis of GARD in BC, the current analysis explores the effect of GARD on local control in adjuvant RT management. Furthermore, we use GARD to calculate an individualized dose for triple negative (TN) patients in two independent cohorts.
2. Materials and methods
2.1. Cohort 1 patients
The presented data includes 343 BC patients treated at four Dutch centers (Netherlands Cancer Institute, Radboud University Medical Center, Erasmus Medical Center, and Ziekenyhuis Amstelland) and one French center (Institut Curie, Paris, France) [20]. Data from these 343 patients was originally compiled to develop a model for local recurrence following breast conservation surgery. In doing so, the dataset over-represents the number of local failures that would be found in a random population. The overall local recurrence rate in this cohort is 34.7%. Patients included premenopausal women with invasive primary breast carcinoma diagnosed before age 50 years and without a prior history of malignancy. Patients were treated from January 1984 to November 2002 with breast-conserving surgery. Either an axillary dissection or sentinel node procedure and postoperative radiation to the breast with or without regional lymphatic coverage was employed. Median follow-up for all patients was 10 years. A total of 58 patients were identified as triple negative and used for the cohort 1 analysis.
Tissue processing, RNA isolation, and quality assurance details have been previously published [20]. RNA was hybridized to Illumina Human Whole Genome, version 3.0 arrays. Normalized data (variance stabilization transformation and robust spline normalization in the Lumi Bioconductor package as described by the authors of the data set) was downloaded from Gene Expression Omnibus (GSE30682) and used for the present study.
2.2. Cohort 2 patients
Patients were identified from the IRB-approved Total Cancer Care (TCC) prospective observational protocol at Moffitt Cancer Center [18]. Data from a total of 643 primary breast tumors with available receptor status and genomic profiling were retrospectively identified. Patients were treated at Moffitt Cancer Center and two surrounding community hospitals. Patients ranged in age from 24 to 95 and underwent primary surgery either with TNBC negative tumors with available genomic profiling were identified with a total of 55 receiving adjuvant RT and used for the GARD analysis. Follow-up for the 55 TN patients was a median of 8.4 (0.5–17.2 years).
RNA preparation and gene expression profiling methods have been described previously [15]. Briefly, gene expression values for the samples in this study were extracted from the TCC database. These expression values were normalized against a median sample using the previously reported iterative rank order normalization method [21]. An RNA-quality related batch-effect was identified in the resulting normalized data, which was removed by training a partial least squares (PLS) model [22] to the RNA integrity number (RIN), and then subtracting the first partial least squares component.
2.3. Radiosensitivity signature
The previously tested 10 gene assay was run on tissue samples ranked according to gene expression. RSI was calculated using the previously published ranked based algorithm [4,9].
Each of the 10 genes in the assay was ranked according to gene expression [from the highest [10] to the lowest expressed gene [1]]. RSI was determined using the previously published ranked based linear algorithm:
RSI = −0.0098009*AR + 0.0128283*cJun +
0.0254552*STAT1 − 0.0017589*PKC −
0.0038171*RelA + 0.1070213*cABL −
0.0002509*SUMO1 − 0.0092431*PAK2 −
0.0204469*HDAC1 −0.0441683* IRF1
2.4. Genomically adjusted radiation dose
Methods and formulation of GARD have been previously described [15]. Briefly, GARD scores were derived using the linear quadratic model, the individual gene-expression-based RSI, and the radiation dose and fractionation schedule for each patient. The calculation for GARD is similar to the biologically effective dose, except the patient-specific α is derived by substituting the radiosensitivity index for survival (S) in S = e–nd(α+βd), where dose (d) is 2 Gy, n = 1, and β is a constant (0.05/Gy) [23]. The equation for the patient specific α thus becomes α = (−0.5lnRSI) - 0.1. A higher GARD value predicts a higher radiation therapeutic effect. We calculated GARD using a script written into Excel. The final GARD formula is GARD = nd(α + βd). GARD was modeled for the whole breast radiation dose or chest wall dose, since this is known to have a larger impact on local control than the lumpectomy cavity or chest wall scar boost [24,25] and not all patients received a boost in these cohorts.
2.5. Statistical analysis
Statistical analyses were carried out using JMP 13 (SAS Institute Inc., Cary, NC). To test differences between cohorts, the Kruskal–Wallis and Pearsons tests were used when appropriate. The local control rate was calculated using the Kaplan–Meier (KM) method, with the log-rank test used to test differences. The Cox proportional hazard model analyses were carried out using univariable analysis (UVA) and multivariable (MVA) analysis. Variables that showed significant effects on UVA (p < .1) were included in the MVA.
3. Results
3.1. Cohort 1
3.1.1. Patient characteristics
Patients were separated into TN and non-TN cohorts with details included in Table 1. A total of 58 patients were identified as TN and were used in the cohort 1 analysis. Median breast radiation dose was 50 Gy in both cohorts (p = .96). There were no differences in T stage, margin status, the presence of pathologically involved nodes, age, or LVSI. Differences were noted in the number of patients receiving adjuvant chemotherapy (p = .005), grade (p < .001), DCIS (p = .007), and the median RSI (p = .02).
Table 1.
Patient and Tumor Characteristics Cohort 1.
| Variable | Triple Negative | Not Triple Negative | P | |
|---|---|---|---|---|
| 58 | 285 | |||
| T stage | pT1 | 31 (55) | 185 (65) | 0.18 |
| pT2-3 | 25 (45) | 100 (35) | ||
| Margin status | marg− | 53 (95) | 241 (88) | 0.13 |
| marg+ | 3 (5) | 34 (12) | ||
| Node status | pN− | 36 (63) | 179 (64) | 0.91 |
| pN+ | 21 (37) | 101 (36) | ||
| Adjuvant chemotherapy | no ChT | 26 (45) | 183 (64) | 0.005 |
| ChT | 32 (55) | 101 (36) | ||
| Grade | I & II | 4 (7) | 198 (70) | <0.001 |
| III | 52 (93) | 85 (30) | ||
| DCIS | No DCIS | 28 (52) | 90 (33) | 0.007 |
| DCIS | 26 (48) | 185 (67) | ||
| GARD whole breast | 23.2 (9.2–37.2) | 17.2 (7.4–51.0) | 0.01 | |
| Age | 43 (23.00, 50.00) | 44.00 (23.00, 50.00) | 0.24 | |
| Radiation whole breast dose (Gy) | 50 (45–54) | 50 (45–55) | 0.96 | |
| Boost dose (Gy) | 15 (10–25) | 16 (6–26) | 0.36 | |
| Total radiation dose (Gy) | 65 (50–75) | 65 (50–77) | 0.77 | |
| RT boost | No boost | 15 (26) | 80 (28) | 0.73 |
| Boost | 43 (74) | 205 (72) | ||
| LVSI | No | 41 (75) | 213 (76) | 0.78 |
| Yes | 14 (25) | 66 (24) | ||
| RSI | 0.4 (0.22–0.65) | 0.51 (0.13–0.75) | 0.02 | |
*ChT = Chemotherapy; ***GARD = Genomically adjusted radiation dose; ****RT = Radiotherapy; *****LVSI = Lymphovascular space invasion; ******RSI = Radiosensitivity index.
3.1.2. Local control analysis
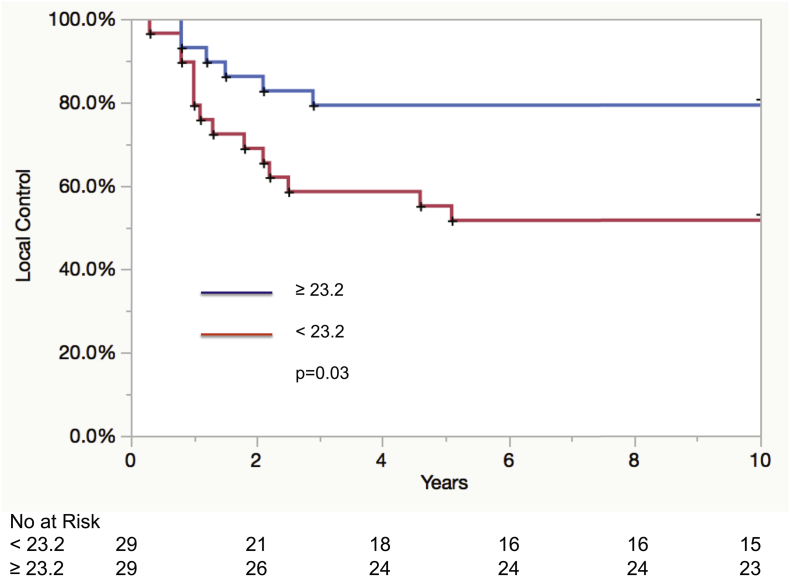
The UVA for local control is detailed in Table 2. Factors associated with local control on UVA in the TN cohort included the presence of LVSI (p = .04) and GARD as both a continuous variable (HR: 0.92 95% CI 0.84–0.99; p = .04) and a dichotomous variable at the median < 23.2 vs. ≥23.2 (HR: 2.7 95% CI 1.1–7.7; p = .03). The 5-year local control rates for GARD ≥23.2 vs. GARD < 23.2 were 79% and 55%, p = .03 (Fig. 1). MVA demonstrated that as a dichotomous variable (HR: 2.5 95% CI 1–7.1; p = .05), GARD continued to be associated with local recurrence (Table 3).
Table 2.
Univariable Analysis of Local Control Cohort 1.
| Variable | HR | 95% CI | P (log-rank) | |
|---|---|---|---|---|
| T stage (ref: pT1) | pT2-3 | 1.7 | 0.66–4.4 | 0.27 |
| Margin status (ref: marg−) | marg+ | 1.3 | 0.07–6.3 | 0.81 |
| Node status (ref: pN−) | pN+ | 1.7 | 0.68–4.2 | 0.25 |
| Adjuvant chemotherapy (ref: no Cht) | ChT | 0.62 | 0.23–1.5 | 0.29 |
| Grade (ref: I & II) | III | 1.4 | 0.29–25.4 | 0.72 |
| DCIS (ref: No DCIS) | DCIS | 1.4 | 0.5–3.6 | 0.51 |
| GARD | 0.92 | 0.84–0.99 | 0.04 | |
| GARD (discrete) (ref: ≥23.2) | <23.2 | 2.7 | 1.1–7.7 | 0.03 |
| Age | 0.97 | 0.91–1.0 | 0.44 | |
| Age (discrete) (ref: <42) | ≥42 | 1 | 0.43–2.6 | 0.94 |
| LVSI (ref: No) | Yes | 2.8 | 1.1–6.9 | 0.04 |
*ChT = Chemotherapy; ***GARD = Genomically adjusted radiation dose; ****RT = Radiotherapy; *****LVSI = Lymphovascular space invasion.
Fig. 1.
Kaplan-Meier curve for local control in triple negative patients (cohort 1) according to GARD high (≥23.2) and GARD low (<23.2) categories.
Table 3.
Multivariable Analysis of Local Control Cohort 1.
| Variable | HR | 95% CI | P-value | Variable | HR | 95% CI | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| GARD | 0.93 | 0.85–1 | 0.08 | GARD (discrete) (ref: ≥23.2) | <23.2 | 2.5 | 1–7.1 | 0.05 | |
| LVSI (ref: No) | Yes | 2.6 | 1–6.4 | 0.05 | LVSI (ref: No) | Yes | 2.7 | 1–6.6 | 0.04 |
*GARD = Genomically adjusted radiation dose; **LVSI = Lymphovascular space invasion.
3.1.3. Cohort 2 analysis
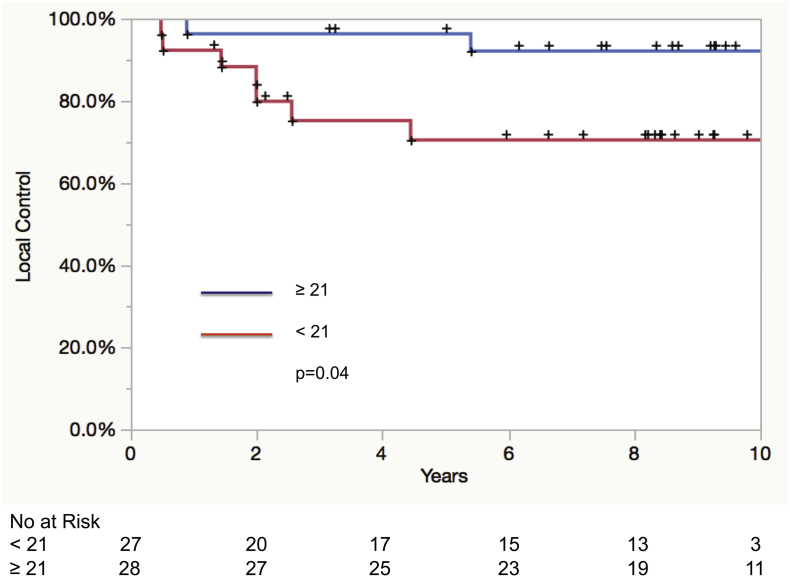
Patient characteristics for TN and non-TN patients in cohort 2 are detailed in Table 4. A total of 98 patients were identified as TN. The 55 TN patients that received radiation served as the basis of the cohort 2 analysis. The only factor found to be associated with local recurrence in this cohort was GARD dichotomized at the median whole breast/chest wall dose, GARD < 21 vs. ≥21 (HR: 4.4 95% CI 1.1–29.5; p = .04), Table 5. The 5-year KM local control rates for GARD ≥ 21 vs. GARD < 21 were 96% and 71%, p = .04 (Fig. 2).
Table 4.
Patient and Tumor Characteristics Cohort 2.
| Variable | Triple Negative | Not Triple Negative | P | |
|---|---|---|---|---|
| 98 | 545 | |||
| T stage | pT1 | 48 (49%) | 267 (50%) | 0.08 |
| pT2 | 47 (48%) | 220 (41%) | ||
| pT3 | 1 (1%) | 43 (8%) | ||
| pT4 | 1 (1%) | 6 (1%) | ||
| Margin status | marg− | 95 (97%) | 511 (94%) | 0.36 |
| marg+ | 1 (1%) | 21 (4%) | ||
| close < 2 mm | 2 (2%) | 10 (2%) | ||
| Node status | pN0 | 62 (64%) | 313 (59%) | 0.46 |
| pN1mi | 3 (3%) | 26 (5%) | ||
| pN1 | 23 (24%) | 128 (24%) | ||
| pN2 | 8 (8%) | 42 (8%) | ||
| pN3 | 1 (1%) | 25 (5%) | ||
| Surgery type | Mastectomy | 53 (55%) | 307 (57%) | 0.62 |
| Lumpectomy | 44 (45%) | 228 (43%) | ||
| Adjuvant chemotherapy | no ChT | 14 (14%) | 266 (49%) | <0.0001 |
| ChT | 84 (85%) | 279 (52%) | ||
| Grade | I | 5 (5%) | 91 (17%) | <0.0001 |
| II | 14 (15%) | 264 (50%) | ||
| III | 77 (80%) | 178 (33%) | ||
| RT | Yes | 55 (56%) | 318 (58%) | 0.68 |
| No | 43 (44%) | 227 (42%) | ||
| GARD whole breast/CW | 21 (12–58.6) | 21.9 (4.5–66.6) | 0.63 | |
| Age | 55 (25–82) | 61 (24–95) | 0.0006 | |
| Radiation breast or chest wall dose (Gy) | 46.8 (34–59.7) | 50 (9–64.8) | 0.69 | |
| Boost dose (Gy) | 14 (10−20) | 14 (3.8–20) | 0.35 | |
| Total radiation dose (Gy) | 46.8 (34–59.74) | 50 (9–64.8) | 0.7 | |
| RT boost | No boost | 60 (64%) | 359 (69%) | 0.31 |
| Boost | 34 (36%) | 160 (31%) | ||
| LVSI | No | 68 (76%) | 358 (74%) | 0.69 |
| Yes | 22 (24%) | 129 (26%) | ||
| RSI | 0.41 (0.08–0.61) | 0.38 (0.05–0.68) | 0.18 | |
*ChT = Chemotherapy; ***GARD = Genomically adjusted radiation dose; ****RT = Radiotherapy; *****LVSI = Lymphovascular space invasion; ******RSI = Radiosensitivity index.
Table 5.
Univariable Analysis Local Control Cohort 2.
| Variable | HR | 95% CI | p-Value | |
|---|---|---|---|---|
| T stage (ref pT1) | pT2-T4 | 1.7 | 0.5–6.7 | 0.4 |
| Margin status (ref: marg−) | marg+ | 8.7 | 0.5–46.6 | 0.12 |
| Node status (ref: pN0) | pN+ | 0.44 | 0.07–1.76 | 0.26 |
| Surgery type (ref: lumpectomy) | Mastectomy | 0.7 | 0.17–2.4 | 0.54 |
| Adjuvant chemotherapy (ref: no ChT) | ChT | 1.2 | 0.22–21.9 | 0.87 |
| GARD | 0.92 | 0.82–1.01 | 0.09 | |
| GARD whole breast/CW (ref: ≥21) | <21 | 4.4 | 1.1–29.5 | 0.04 |
| Age (ref: <55) | ≥55 | 1.1 | 0.29–4.4 | 0.9 |
| RT boost (ref: boost) | no boost | 1.9 | 0.51–7.8 | 0.33 |
| LVSI (ref: no) | Yes | 2.9 | 0.8–10.4 | 0.1 |
*ChT = Chemotherapy; ***GARD = Genomically adjusted radiation dose; ****RT = Radiotherapy; *****LVSI = Lymphovascular space invasion.
Fig. 2.
Kaplan-Meier curve for local control in triple negative patients (cohort 2) according to GARD high (≥21) and GARD low (<21) categories.
3.1.4. Utilizing GARD to tailor RT dose in TN patients
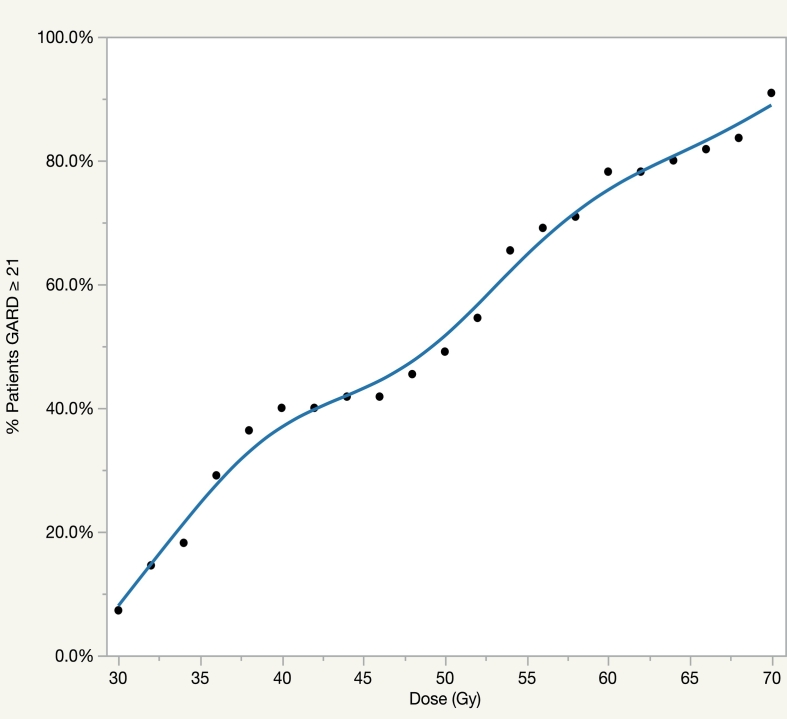
Since GARD was significant for local control and is a clinically actionable metric, we utilized GARD to calculate an individualized RT dose for each TN patient in cohort 2. In this approach, we determined the minimum radiation dose required for each patient to achieve a GARD high ≥ 21, using standard 2 Gy fractionation. In Fig. 3, we model the percentage of patients achieving the target GARD value in the dose range of 30 Gy – 70 Gy. Our model demonstrates that at uniform dosing of 30 Gy, the GARD of 7% of patients is optimized which increases to 40% at 40 Gy. The number of patients optimized rises sharply to 78% at 60 Gy. In addition, our model estimates a uniform dose of 70 Gy would optimize GARD for 91% of patients.
Fig. 3.
Model for the percentage of triple negative patients in cohort 2 achieving GARD high with doses between 30 and 70 Gy.
4. Discussion
In this manuscript, we detail outcomes using a previously developed and validated personalized radiation metric, GARD, assessing its association with local control following adjuvant RT in BC management for TN patients. In addition, we demonstrate an approach to individualized physical dose optimization for TN patients using GARD and local control as a primary endpoint. The primary outcomes of our analysis reveal GARD was a significant predictor of local control in TN patients in two independent cohorts. In addition, we demonstrate GARD can be utilized to personalize radiation dose based on individual patient tumor biology. Prospective randomized trials have demonstrated no change in local recurrence with uniform dose escalation beyond 66 Gy [24]. Our data is consistent with these observations as cumulative optimized GARD increases from 50% at 50 Gy to 81% at 66 Gy. This suggests further dose escalation above 66 Gy in unselected populations is unlikely to result in improvement in local control, consistent with current BC management.
Efforts are currently underway to omit radiation treatment to the breast in select women who are at sufficiently low risk of local recurrence, based on several large, prospective trials that have revealed the feasibility of this approach in subsets of patients based on age [26,27]. Numerous institutions are currently assessing the feasibility of RT exclusion in luminal A tumors. The University of Michigan has initiated the IDEA trial (NCT02400190), a multi-institutional trial in which women between 50 and 69 years with luminal A early stage tumors with an Oncotype DX score ≤ 18 will receive hormonal therapy alone. Other trials assessing omission of breast RT include the LUMINA study from the Ontario Clinical Oncology Group (NCT01791829), a study of women ≥ 55 years with T1 luminal A tumors receiving endocrine therapy alone. Finally, the Dana Farber Cancer Institute has initiated the PRECISION trial for women between 50 and 75 years with tumors measuring ≤2 cm with a low risk PAM-50 profile to receive hormonal therapy alone. However, a true genomic approach to personalize RT dose has not been undertaken in the prospective setting.
Several genomic signatures have shown promise in identifying patients most likely to benefit from adjuvant RT. This includes a seven-gene signature from the Danish Group, which predicted the benefit of postmastectomy RT in patients with high-risk BC in the context of the Danish 82b and 82c trials [28]. The signature was developed from data in 191 patients and validated in 112 patients identifying women with sufficiently low risk of locoregional recurrence in whom there was no benefit of post-mastectomy RT [29,30]. The University of Michigan developed a radiation sensitivity signature (RSS) using clonogenic survival assays across BC cell lines. The RSS was refined to 51 genes and validated in two independent datasets outperforming all clinical and pathologic features. The signature identified patients that would benefit from adjuvant RT [31].
However, while these efforts are critically important to more efficiently identify patients requiring RT, they assume radiation dose protocols have already been optimized at the individual patient level. In contrast, the GARD model provides a quantitative and clinically-actionable approach to account for individual differences in the radiosensitivity of a tumor by adjusting the physical dose delivered. We have previously developed RSI as a gene expression-based signature to predict the radiosensitivity of tumors treated with RT. We have shown RSI to be prognostic in several independent disease sets of patients treated with BC RT with clinical endpoints of recurrence free survival and distant metastases free survival [5]. In addition, we previously conducted an analysis with cohort 1 utilizing RSI [10]. Finally, RSI has been validated in multiple independent datasets across nine different disease sites in over 2000 patients [[4], [5], [6], [7], [8], [9], [10]].
The analysis identified cutpoints across the median GARD value to be a significant predictor of local control in TN patients. In both datasets, this was selected as the median GARD for the whole breast/chest wall dose delivered. There was an expected difference in these cutpoints for the two cohorts signifying the higher rate of local failures in cohort 1. We modeled the radiation dose necessary in order to achieve an optimized GARD in cohort 2. In this new paradigm, rather than prescribing a uniform dose of RT, we propose using a biologically informed and clinically validated prescription paradigm to adjust the dose for each patient until the GARD value is met. Interestingly, the shape of the curve is sigmoidal, a relationship predicted by tumor control probability models [32]. Our analyses provide the first proposed range for optimal RT dose at an individual patient level in TNBC and proposes a significant number of patients can be treated with lower doses of RT while still maintaining high levels of local control.
We propose the current analysis in TN patients to be the first means of delivering a personalized radiation dose based on inherent tumor radiosensitivity. A proposed actionable clinical trial may be to escalate dose in TN patients found to have an RSI that is radioresistant to a dose of radiation found to optimize GARD within safe limits [33]. Using our calculations, this would mean patients with an RSI above 0.43 up to 0.49 in cohort 2 would benefit from dose escalation beyond 50 Gy to 60 Gy, comprising 29% of cohort 2. Further dose escalation would be beneficial to 70 Gy for patients with an RSI up to 0.55 comprising a smaller percentage of patients 13% in our cohort. As we can see, this model shows that certain patients may benefit from dose escalation placing them at an acceptable risk of increased toxicity as well as a lengthened treatment course. However, this is not the majority of the cohort. Thus, through the GARD method of dose optimization, we identify appropriate TN patients for dose escalation.
We have previously shown RSI to be strongly correlated with immunogenicity of different tumor types by using the previously developed 12-chemokine (12-CK) signature for immune activation and inflammation, which has been shown to identify tertiary extra-nodal lymph node like structures within tumors [34]. In an analysis of over 10,000 unique solid tumor tissue samples, we demonstrated a significant correlation between RSI and 12-CK signatures indicating radiation sensitivity to be correlated to immunogenicity across tumor types including BC. Several of the 10 hub genes in RSI have known functions in the immune microenvironment [4] including STAT1 and IRF1. Furthermore, TN tumors are known to have the highest proportion of tumor-infiltrating lymphocytes (TILs) of the breast subtypes according to a systematic review [35]. A study from Ali et al. applying the gene expression-based CIBERSORT deconvolution algorithm to 11,000 breast tumors indicated the relative concentrations of immune cells impacted overall survival in ER- tumors; however, in ER+ tumors survival time did not differ based on the proportion of TILs [36]. In addition, it is well known TN tumors are more aggressive and prone to recurrence with fewer targeted systemic treatment options and thus the impact of radiation may be larger in this cohort of patients [37,38]. This is consistent with a recent study from the Danish Group assessing patients in the 82b and c studies revealing differing RT therapeutic impact between breast subtypes [39].
Although current efforts in the adjuvant management of BC focus on using biological subtype to exclude radiation delivery, the current analysis reveals a method by which radiation can be personalized based on the genomic profile of a tumor. GARD proposes a significant number of women can be treated with lower doses than currently utilized while still maintaining the same local control. Similarly, GARD identifies patients that would benefit from dose escalation to improve local control. As we move towards an era of oncologic personalization, radiation oncologists can consider models such as GARD to tailor RT dose.
Funding source
Morton Plant Mease Foundation, USA.
Author contributions
KAA, CLL, MNM, NBF, SAE, JGS, RD, JFT were involved in data interpretation and statistical analysis. KAA, JGS, RD and JFT were involved in the design of the study. KAA, CLL, MNM, GDG, EEH, RD, SAE, JGS, JFT were involved in preparation of the manuscript. All authors reviewed and approved the final manuscript.
Declaration of Competing Interest
Steven A. Eschrich PhD and Javier F. Torres-Roca MD report stock and leadership in Cvergenx, Inc. and royalty and patents on RSI. Javier F. Torres-Roca MD and Jacob G. Scott MD PhD report royalty and patents on GARD.
Acknowledgements
None.
Contributor Information
Roberto Diaz, Email: Roberto.diaz@moffitt.org.
Javier F. Torres-Roca, Email: javier.torresroca@moffitt.org.
References
- 1.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 3.Whelan T.J., Pignol J.P., Levine M.N., Julian J.A., MacKenzie R., Parpia S. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 4.Eschrich S., Zhang H., Zhao H., Boulware D., Lee J.H., Bloom G. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009;75(2):497–505. doi: 10.1016/j.ijrobp.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eschrich S.A., Fulp W.J., Pawitan Y., Foekens J.A., Smid M., Martens J.W. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18(18):5134–5143. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed K.A., Fulp W.J., Berglund A.E., Hoffe S.E., Dilling T.J., Eschrich S.A. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837–842. doi: 10.1016/j.ijrobp.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed K.A., Caudell J.J., El-Haddad G., Berglund A.E., Welsh E.A., Yue B. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95(5):1399–1404. doi: 10.1016/j.ijrobp.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed K.A., Chinnaiyan P., Fulp W.J., Eschrich S., Torres-Roca J.F., Caudell J.J. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget. 2015;6(33):34414–34422. doi: 10.18632/oncotarget.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eschrich S.A., Pramana J., Zhang H., Zhao H., Boulware D., Lee J.H. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489–496. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Roca J.F., Fulp W.J., Caudell J.J., Servant N., Bollet M.A., van de Vijver M. Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer. Int J Radiat Oncol Biol Phys. 2015;93(3):631–638. doi: 10.1016/j.ijrobp.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed K.A., Scott J.G., Arrington J.A., Naghavi A.O., Grass G.D., Perez B.A. Radiosensitivity of lung metastases by primary histology and implications for stereotactic body radiation therapy using the genomically adjusted radiation dose. J Thorac Oncol. 2018;13(8):1121–1127. doi: 10.1016/j.jtho.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjostrom M., Staaf J., Eden P., Warnberg F., Bergh J., Malmstrom P. Identification and validation of single-sample breast cancer radiosensitivity gene expression predictors. Breast Cancer Res. 2018;20(1):64. doi: 10.1186/s13058-018-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thames H.D., Jr., Withers H.R., Peters L.J., Fletcher G.H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8(2):219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 14.Brenner D.J. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 2008;18(4):234–239. doi: 10.1016/j.semradonc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott J.G., Berglund A., Schell M.J., Mihaylov I., Fulp W.J., Yue B. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poortmans P., Kaidar-Person O., Span P. Radiation oncology enters the era of individualised medicine. Lancet Oncol. 2017;18(2):159–160. doi: 10.1016/S1470-2045(16)30660-X. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe E.M.D.C., Agus D.B., Alexander B.M., Anderson K.C., Ashworth A., Barker A.D. Future cancer research priorities in the USA: a lancet oncology commission. Lancet Oncol. 2017;18:e653–e706. doi: 10.1016/S1470-2045(17)30698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenstermacher D.A., Wenham R.M., Rollison D.E., Dalton W.S. Implementing personalized medicine in a cancer center. Cancer J. 2011;17(6):528–536. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists' Collaborative G. Darby S., McGale P., Correa C., Taylor C., Arriagada R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Servant N., Bollet M.A., Halfwerk H., Bleakley K., Kreike B., Jacob L. Search for a gene expression signature of breast cancer local recurrence in young women. Clin Cancer Res. 2012;18(6):1704–1715. doi: 10.1158/1078-0432.CCR-11-1954. [DOI] [PubMed] [Google Scholar]
- 21.Welsh E.A., Eschrich S.A., Berglund A.E., Fenstermacher D.A. Iterative rank-order normalization of gene expression microarray data. BMC Bioinformatics. 2013;14:153. doi: 10.1186/1471-2105-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wold S.R.A., Wold H., Dunn W.J., III The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J Sci Stat Comput. 1984;5(3):735–743. [Google Scholar]
- 23.Jeong J., Shoghi K.I., Deasy J.O. Modelling the interplay between hypoxia and proliferation in radiotherapy tumour response. Phys Med Biol. 2013;58(14):4897–4919. doi: 10.1088/0031-9155/58/14/4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartelink H., Maingon P., Poortmans P., Weltens C., Fourquet A., Jager J. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 25.Mayadev J., Fish K., Valicenti R., West D., Chen A., Martinez S. Utilization and impact of a postmastectomy radiation boost for invasive breast cancer. Pract Radiat Oncol. 2014;4(6):e269–e278. doi: 10.1016/j.prro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Kunkler I.H., Williams L.J., Jack W.J., Cameron D.A., Dixon J.M., Investigators PI Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 27.Hughes K.S., Schnaper L.A., Bellon J.R., Cirrincione C.T., Berry D.A., McCormick B. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tramm T., Mohammed H., Myhre S., Kyndi M., Alsner J., Borresen-Dale A.L. Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: a study of gene expression in the DBCG82bc cohort. Clin Cancer Res. 2014;20(20):5272–5280. doi: 10.1158/1078-0432.CCR-14-0458. [DOI] [PubMed] [Google Scholar]
- 29.Mamounas E.P., Tang G., Fisher B., Paik S., Shak S., Costantino J.P. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegadeesh N.K., Kim S., Prabhu R.S., Oprea G.M., Yu D.S., Godette K.G. The 21-gene recurrence score and locoregional recurrence in breast cancer patients. Ann Surg Oncol. 2015;22(4):1088–1094. doi: 10.1245/s10434-014-4252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speers C., Zhao S., Liu M., Bartelink H., Pierce L.J., Feng F.Y. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin Cancer Res. 2015;21(16):3667–3677. doi: 10.1158/1078-0432.CCR-14-2898. [DOI] [PubMed] [Google Scholar]
- 32.Webb S., Nahum A.E. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol. 1993;38(6):653–666. doi: 10.1088/0031-9155/38/6/001. [DOI] [PubMed] [Google Scholar]
- 33.Poortmans P.M., Collette L., Horiot J.C., Van den Bogaert W.F., Fourquet A., Kuten A. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol. 2009;90(1):80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Strom T., Harrison L.B., Giuliano A.R., Schell M.J., Eschrich S.A., Berglund A. Tumour radiosensitivity is associated with immune activation in solid tumours. Eur J Cancer. 2017;84:304–314. doi: 10.1016/j.ejca.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanton S.E., Adams S., Disis M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 36.Ali H.R., Chlon L., Pharoah P.D., Markowetz F., Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med. 2016;13(12) doi: 10.1371/journal.pmed.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdulkarim B.S., Cuartero J., Hanson J., Deschenes J., Lesniak D., Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852–2858. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J., Shi M., Ling R., Xia Y., Luo S., Fu X. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100(2):200–204. doi: 10.1016/j.radonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Sjostrom M., Lundstedt D., Hartman L., Holmberg E., Killander F., Kovacs A. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol. 2017;35(28):3222–3229. doi: 10.1200/JCO.2017.72.7263. [DOI] [PubMed] [Google Scholar]