Abstract
Introduction
Obesity and psychosocial stress (PS) co-exist in individuals of Western society. Nevertheless, how PS impacts cardiac and hippocampal phenotype in obese subjects is still unknown. Nor is it clear whether changes in local brain-derived neurotrophic factor (BDNF) account, at least in part, for myocardial and behavioral abnormalities in obese experiencing PS.
Methods
In adult male WT mice, obesity was induced via a high-fat diet (HFD). The resident-intruder paradigm was superimposed to trigger PS. In vivo left ventricular (LV) performance was evaluated by echocardiography and pressure-volume loops. Behaviour was indagated by elevated plus maze (EPM) and Y-maze. LV myocardium was assayed for apoptosis, fibrosis, vessel density and oxidative stress. Hippocampus was analyzed for volume, neurogenesis, GABAergic markers and astrogliosis. Cardiac and hippocampal BDNF and TrkB levels were measured by ELISA and WB. We investigated the pathogenetic role played by BDNF signaling in additional cardiac-selective TrkB (cTrkB) KO mice.
Findings
When combined, obesity and PS jeopardized LV performance, causing prominent apoptosis, fibrosis, oxidative stress and remodeling of the larger coronary branches, along with lower BDNF and TrkB levels. HFD/PS weakened LV function similarly in WT and cTrkB KO mice. The latter exhibited elevated LV ROS emission already at baseline. Obesity/PS augmented anxiety-like behaviour and impaired spatial memory. These changes were coupled to reduced hippocampal volume, neurogenesis, local BDNF and TrkB content and augmented astrogliosis.
Interpretation
PS and obesity synergistically deteriorate myocardial structure and function by depleting cardiac BDNF/TrkB content, leading to augmented oxidative stress. This comorbidity triggers behavioral deficits and induces hippocampal remodeling, potentially via lower BDNF and TrkB levels.
Fund
J.A. was in part supported by Rotary Foundation Global Study Scholarship. G.K. was supported by T32 National Institute of Health (NIH) training grant under award number 1T32AG058527. S.C. was funded by American Heart Association Career Development Award (19CDA34760185). G.A.R.C. was funded by NIH (K01HL133368-01). APB was funded by a Grant from the Friuli Venezia Giulia Region entitled: “Heart failure as the Alzheimer disease of the heart; therapeutic and diagnostic opportunities”. M.C. was supported by PRONAT project (CNR). N.P. was funded by NIH (R01 HL136918) and by the Magic-That-Matters fund (JHU). V.L. was in part supported by institutional funds from Scuola Superiore Sant'Anna (Pisa, Italy), by the TIM-Telecom Italia (WHITE Lab, Pisa, Italy), by a research grant from Pastificio Attilio Mastromauro Granoro s.r.l. (Corato, Italy) and in part by ETHERNA project (Prog. n. 161/16, Fondazione Pisa, Italy). Funding source had no such involvement in study design, in the collection, analysis, interpretation of data, in the writing of the report; and in the decision to submit the paper for publication.
Keywords: Brain-heart axis, Brain-derived neurotrophic factor (BDNF), Obesity, Psychosocial stress, Left ventricle, Hippocampus, Tropomyosin receptor kinase B (TrkB), Oxidative stress
Research in context.
Evidence before this study
Obesity and PS often inhabit the same individual, owing to the Western society lifestyle. Yet these stressors are often modeled separately, precluding a full grasp of their potentially adverse synergy on organ or system function, and of the pathogenic mechanisms triggered by their combination. Of note, when models have been harnessed to study obesity and PS, the priority has been always given to the central nervous system (CNS), or lipid metabolism, with little attention to heart function. Studies, for instance, have evaluated how high-fat diet and stress induced by the alternation of damp sawdust, cold, swim, and restraint alters the autonomic control of heart rate. However, no investigation has portrayed yet how obesity and stress (of social or psychological nature) affect myocardial structure and function. Moreover, the hippocampus is often targeted by cardiac diseases, and yet no studies have evaluated to what extent PS in obese subjects affects this brain region.
Added value of this study
Our study demonstrates that psychological and social stress impinging on an obese pathological substrate alters myocardial and hippocampal phenotype more than each condition alone. Accordingly, two diffuse and often coexisting environmental risk factors, such as diet-induced obesity and PS act synergistically to deteriorate heart structure and function, triggering a marked decline in both systolic and diastolic function. These phenotypes, at least in part, stem from the loss of viable cardiomyocytes, cardiac matrix and vascular remodeling, increased oxidative stress, and depletion of cardiac BDNF and TrkB pool. Alterations of the cardiac phenotype are associated with a substantial hippocampal remodeling due to lower levels of local BDNF and TrkB, thus accounting for major behavioral disorders, such as extreme anxiety and loss of spatial memory. Therefore, BDNF signaling impairment is a common feature of the brain-heart axis dysfunction following obesity and exposure to environmental stressors.
Implications of all the available evidence
The present study shows, for the first time, that psychosocial stress makes systo-diastolic and hippocampal dysfunction manifest in obese mice. Notably, both cardiac and hippocampal BDNF/TrkB levels are markedly reduced in PS-challenged obese mice. These findings suggest that measures apt to rescue BDNF signaling at both levels, i.e., hippocampus and myocardium, should improve the brain-heart communication, attenuating the behavioral and cardiac abnormalities found in PS-challenged obese mice.
Alt-text: Unlabelled Box
1. Introduction
Obesity is becoming so epidemic that the World Health Organization (WHO) has coined the term “globesity” to design its relentless rise worldwide [1]. Psychosocial stress (PS), i.e., the aversive social and psychological conditions that tax or exceed the physiological resources of the organism to cope with them [2,3], is another fast and pandemically growing issue of the industrialized societies [4,5]. Burn-out caused by chronic stress at work (an example of widespread psychosocial stress condition) has been recognized as an official medical diagnosis by the WHO in 2019 (WHO. 28 May 2019. Retrieved 2019-06-01). Obesity and PS often inhabit the same individual, owing to the Western society lifestyle [5,6]. Yet, very often, they are modeled separately, precluding a full grasp of their potentially adverse synergy on organ or system function, as well as of a deeper understanding of the disease mechanisms triggered by these concomitant stressors.
Obesity and PS have widespread targets in the body. However, the cardio- and neural systems are especially vulnerable to their acute or chronic adverse effects [[7], [8], [9], [10], [11]]. What is more, affecting one compartment may have negative reverberation on the other, resulting in an altered brain-heart communication. Models have been harnessed to study the interactive effects triggered by these two risk factors. However, priority has always been assigned to the central nervous system (CNS), or lipid metabolism [12,13]. To the best of our knowledge, no investigation so far has focused on whether and how obesity and stress of social and psychological nature influence myocardial contractility and structure, in principle. Only one study, in fact, evaluated how high-fat diet and stress induced by the alternation of damp sawdust, cold, swim, and restraint alter the autonomic control of heart rate [14].
Brain-derived neurotrophic factor (BDNF), with its associated signaling, acts as a proxy for optimal brain health and neuroplasticity [15], helping neuronal cells to cope with stress conditions, including the metabolic ones [16]. Obesity and PS both exact a heavy toll on BDNF bioavailability [[17], [18], [19]]. Of relevance, BDNF and its associated receptor Tropomyosin receptor kinase B (TrkB) are required for normal myocardial function in adulthood [20,21]. In light of these facts, BDNF and TrkB are potential primary targets of the adverse influence of obesity and PS, particularly when combined. Moreover, BDNF could shuttle beneficial effects between the brain and the heart, both ways.
Here, we report that obesity (via high-fat diet, HFD) or psychosocial stress (triggered by the resident-intruder paradigm, RIP) does not significantly alterin vivo myocardial performance of adult mice. Conversely, when obese mice are PS-challenged, cardiac contractility and relaxation are markedly impaired, and considerably more than the sum of the effects produced by obesity and PS, each taken alone. This evidence is consistent with the histological findings showing that myocyte cell death, fibrotic tissue deposition, oxidative stress are increased, while cardiac BDNF and TrkB levels are impoverished in obese/PS-treated mice, in a synergistic manner. In a similar, consistent manner, this comorbidity deteriorates the hippocampal structure, reducing its volume and in situ neurogenesis, while increasing astrogliosis. Again, these phenotypes are coupled to a marked depletion in local BDNF and TrkB pool.
2. Materials and methods
2.1. Animals and experimental protocol
Forthy healthy male C57BL/6 J mice (12 weeks old; 23–26 g body weight) were housed under controlled 12/12 h light/dark cycle, temperature (21 ± 0·5 °C) and relative humidity (55% ± 2%) in animal rooms and fed with standard diet and water ad libitum until the beginning of the experimental protocol, which lasted 18 weeks (Fig. 1a). The animals were randomly assigned to one of the following groups: Control (n = 10), mice fed a standard diet (2700 kCal/kg; Table 1) for 18 weeks; Ob (n = 10) mice fed a high-fat diet (HFD; 5000 kCal/kg; Table 1, provided by Mucedola S.R.L., Settimo Milanese, MI, Italy) for 18 weeks; PS (n = 10) mice fed a standard diet for 18 weeks and subjected to the resident-intruder paradigm for the last two weeks of the protocol; Ob + PS (n = 10) mice fed HFD for 18 weeks and submitted to the resident-intruder model for the last two weeks of the protocol. Additional experiments were performed in cardiac-selective TrkB−/− male mice (cTrkB KO), (12 weeks old; 24–26 g body weight) previously characterized by us [20]. Our cTrkB KO mice were generated deleting the exon 14, which leads to complete ablation of the ectodomain of all TrkB isoforms. The animals were randomly assigned to Control (n = 4), Ob (n = 5), PS (n = 5) and Ob + PS (n = 5) groups. Thirty healthy male CD1 mice (retired breeders) were employed for the resident-intruder model. All animal procedures were approved by the Italian Ministry of Health and by the Johns Hopkins University Animal Care and Use Committee, and conducted in conformity with the guidelines from Legislative Decree n°26/2014 of Italian Ministry of Health and Directive 2010/63/EU of the European Parliament, and with the guidelines for the Care and Use of Laboratory Animals (NIH publication No. 85–23) on the protection of animals used for scientific purposes.
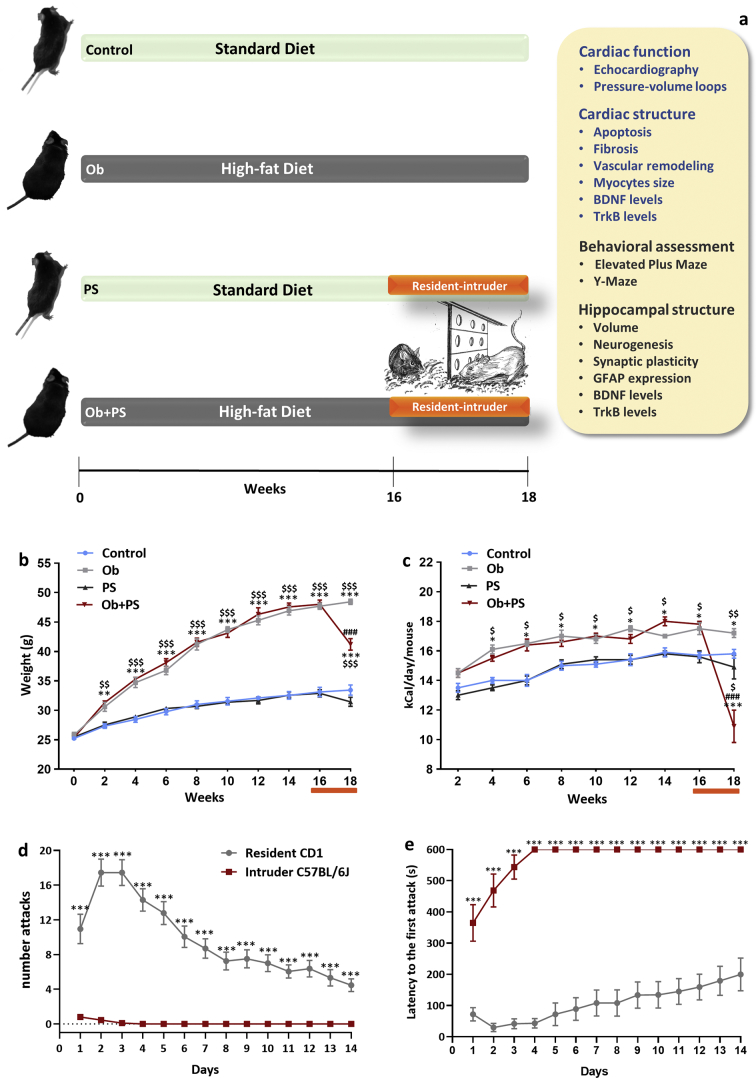
Fig. 1.
PS in obese mice induces hypophagia and body weight loss. (a) Simplified cartoon of experimental design. The experimental groups are represented from top to bottom as follow: Control (n = 10), mice fed with a standard diet (SD) for 18 weeks; Ob (n = 10) mice fed with a high fat diet (HFD) for 18 weeks; PS (n = 10) mice fed with a standard diet for 18 weeks and subjected to the resident-intruder paradigm from week 16th to week 18th of the protocol; Ob + PS (n = 10) mice fed a high fat diet for 18 weeks and subjected to the resident-intruder paradigm from week 16th to week 18th. At the end of the protocol the analysis indicated on the right panel was performed. (b) Time-dependent effects of standard diet (control, n = 10; PS, n = 10) or high fat diet (Ob, n = 10; Ob + Ps, n = 10) on murine body weight and (c) caloric (kcal) intake. (d) Number attacks and (e) latency to the first attack of mice exposed to the resident-intruder paradigm. One-way ANOVA was performed for each temporal point of the curves represented in the panels b and c (*p < 0·05, **p < 0·01, and ***p < 0·001 Vs Controls; #p < 0·05, ### p < 0·001 Vs Ob; $p < 0·05, $$$p < 0·001 Vs PS). Two-tailed Student's t-test was performed for each temporal point of the curves represented in d and e (***p < 0·001). All measurements are shown as mean ± SEM.
Table 1.
Composition of High-fat diet and Standard diet.
| Component | High-fat diet | Standard diet |
|---|---|---|
| Metabolizable energy, kcal/kg | 5000 | 2700 |
| Metabolizable energy from fat, % | 60 | 10 |
| Total protein, % | 20 | 18.5 |
| Total fat, % | 34 | 3 |
| Total ash, % | 5.5 | 7 |
| Total fiber, % | 1 | 6 |
2.2. Resident–intruder paradigm
We employed a modified version of the previously validated models of the resident-intruder paradigm (RIP) [[22], [23], [24]].The Resident-Intruder test is a behavioral translational model of psychosocial stress able to induce offensive behavior (aggression of the resident mouse) and defensive/submissive behavior (avoidance and the social defeat of the intruder mouse). Male CD1 mice, higher by weight and previously selected for their aggression, were placed individually in a single cage for seven days, allowing for the creation of an individual territory (Supplementary Fig. 1a). Each resident CD1 mouse received an intruder in its cage (a C57BL/6 J mouse from the experimental groups PS and Ob + PS) and the two animals were allowed to interact freely for 10 min (Supplementary Fig. 1b). After the interaction, the mice were separated through a perforated plexiglass partition halving the cage, which maintained them in continuous sensory contact (visual, auditory and olfactory) for 24 h (Supplementary Fig. 1c). The entire procedure has been repeated for 14 consecutive days without changing the resident mice each day in order to maintain social hierarchy throughout the experiment in accord with previous studies [25,26]. During the 10 min of interaction the main behavioral parameters linked to aggression and social defeat were recorded (i.e. the frequencies, durations, latencies of the attacks). To prevent physical injury the procedure was interrupted in case of physical damages.
2.3. Cardiac function assessment
2.3.1. Echocardiography
The cardiac morphology and function were assessed by transthoracic echocardiography using a high-frequency and high-resolution echocardiography system (Vevo 2100, FUJIFILM VisualSonics Inc., Toronto, Canada) equipped with a 40- MHz ultrasound probe (MS550, FUJIFILM VisualSonics Inc., Toronto, Canada) in conscious mice as described previously [27]. In stressed animals, the echocardiogram was performed the day after the last day of the entire stress period in a different room/context. An experienced cardiologist and an echocardiography expert blinded to experimental groups performed all measurements. More details are described in Supplemental information.
2.3.2. Pressure-volume loops
In vivo, the left ventricular (LV) load-independent contractile function and general hemodynamics were assessed by analysis of pressure-volume (PV) loops, as described previously [27]. PV loops were analyzed by a blinded investigator using dedicated software [20]. More details are provided in Supplemental information.
2.4. Electron paramagnetic resonance
Fresh LV tissue was collected to detect superoxide levels by electron paramagnetic resonance spectroscopy (EPR) as previously described [28]. More details are described in Supplemental information.
2.5. Behavioral tests
Previously validated methods were used to assess hippocampus-dependent behavior. An expert in behavioral test, blinded to experimental groups performed all measurements.
2.5.1. Elevated plus maze
Elevated plus maze (EPM) is an established test to study anxiety-like behavior in rodents as described previously [29]. More details are described in Supplemental information. The analysis of the behavioral parameters has been performed by means of ANY-maze software.
2.5.2. Y-maze
Y-Maze is a well-recognized behavioral test used to study spatial memory in rodents [30]. More details are described in Supplemental information. The analysis of the behavioral parameters has been performed by means of ANY-maze software.
2.6. Enzyme-linked immune assay
Tissues from the hippocampus and LV were dissected and frozen in isopentane. Whole hippocampus and ~10 mg of LV tissue were homogenized in 2–3 ml of lysis buffer (100 mM PIPES (pH 7), 500 mM NaCl, 0·2% Triton X-100, 2 mM EDTA, 200 μM PMSF, and protease inhibitor cocktail (Sigma-Aldrich), followed by centrifugation for 30 min at 16000g at 4 °C. BDNF protein levels were determined by Enzyme-Linked Immune Assay (ELISA) (BDNF Immuno Assay System, Promega, Madison, WI). Lysates, after dilution, were loaded into 96-well plates. BDNF concentration (pg/ml) was normalized to total soluble protein previously measured trough BCA Protein Assay Kit (Pierce) for each sample and data were expressed as percent change of WT (pg BDNF/mg total protein) [30].
2.7. Western blotting
Proteins were extracted from snap-frozen left ventricular and hippocampal samples. We loaded fourty microgram of total protein lysate per lane on 4–20% precast polyacrylamide gel (Mini-PROTEAN® TGX™, Bio-Rad Laboratories, Inc., Hercules, CA, USA) and blotted electrophoretically. The membrane including LV samples was probed with a specific rabbit polyclonal antibody raised against all TrKB isoforms (#07–225; dilution 1:1000; EMD Millipore), and then re-probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with rabbit polyclonal antibody (#ABS16; dilution1:1000; EMD Millipore). Moreover, the membrane including hippocampal sampleswas probed with a anti-TrKB rabbit polyclonal antibody (#18987; dilution 1:1000; Abcam), and then re-probed for beta-tubulin with rabbit polyclonal antibody (#6046; dilution 1:500; Abcam). Anti-GAPDH and beta-tubulin antibodies were used to verify the uniformity of protein loading. The protein bands were developed in a chemiluminescence substrate solution (Pierce SuperSignal Chemiluminescents Substrate). Analysis of protein bands was performed using ImageJ software (National Institute of Healt, USA). We checked the predicted molecular weight trough the use of Precision Plus Protein™ Dual Colour Standards (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.8. Histological analyses
2.8.1. Heart
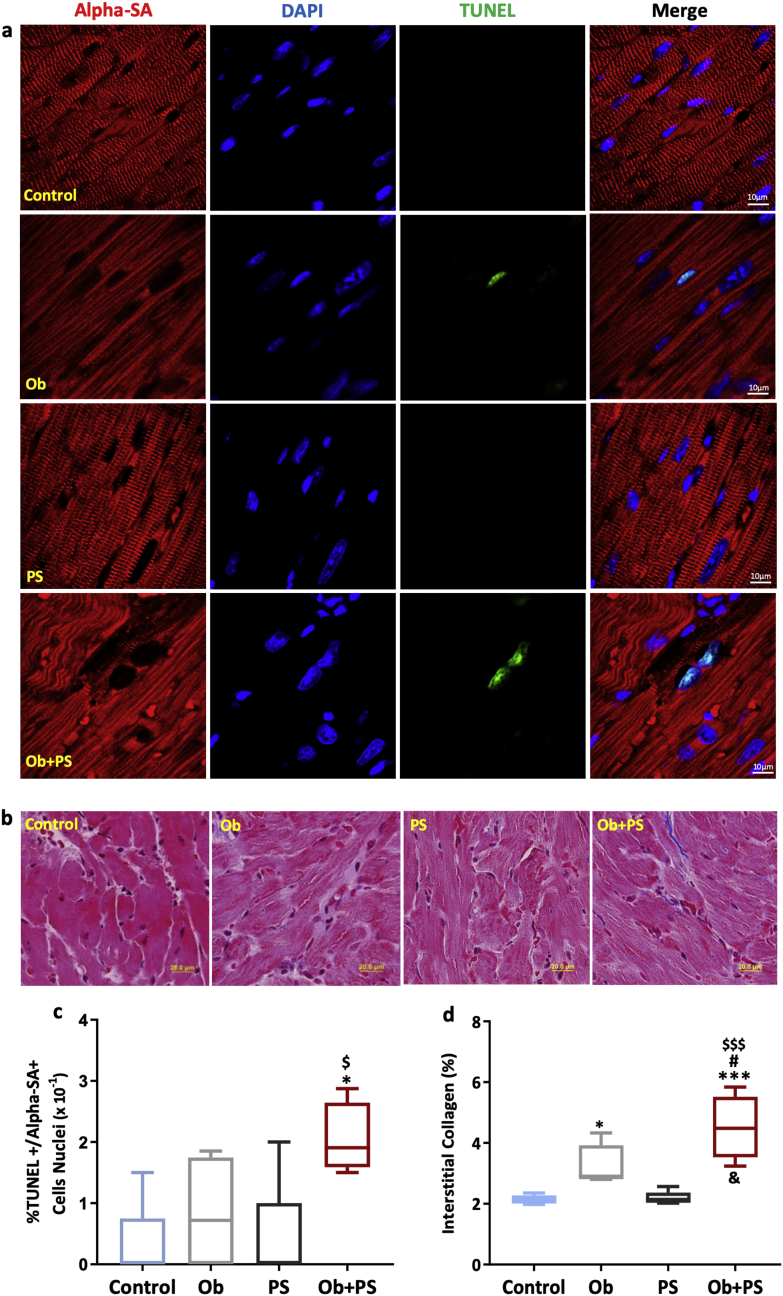
Myocardium was formalin-fixed and paraffin-embedded (FFPE). 5 μm thick tissue sections were stained as described in the Supplementary material and methods, employing the antibody concentrations indicated in the Supplementary Table 1. TUNEL staining was used to detect myocardial apoptosis. Apoptotic cardiac cells were detected after staining with anti-cardiac troponin T antibody, which may even mark cardiac interstitial cells [31]. Moreover, apoptotic cardiomyocytes, were identified by anti-alpha-Sarcomeric Actin (alpha-SA) antibody, a well-established staining of cardiomyocytes [[32], [33], [34]]. Percent apoptotic cardiac cells and cardiomyocytes (% ×10−1) were calculated via the number of TUNEL positive nuclei over the total number of cardiac or cardiomyocyte nuclei per field (at a magnification of 100×), respectively [34]. Additionally, interstitial collagen was determined via Masson Trichrome and H&E-stained sections (HT15 Trichrome Stain kit; Sigma). To assess cardiomyocyte hypertrophy, we employed a cross-sectional area–CSA, a surrogate parameter [35]. To evaluate artery morphology, we counted the number of vessels positive to smooth muscle actin antibody on an entire transverse section of the mid-myocardium and measured both their thickness and relative caliber. Artery dimensions and cardiomyocyte cross-sectional areas were computed employing ImageJ software [36]. Vessel morphology was evaluated on ≈15 images of Isolectin B4 stained sections of mid-myocardium, taken at 400× magnification, employing the AngioTool free software [37].
2.8.2. Hippocampus
Animals were anesthetized with an overdose of chloral hydrate (10·5%, in saline) and transcardially perfused with PBS, followed by paraformaldehyde (PFA) 4% solution. To evaluate adult hippocampal neurogenesis, BrdU (Sigma-Aldrich) was administered intraperitoneally at 50 mg/kg body weight 2 h before the perfusion. Brains were post-fixed for 2 h and then cryoprotected in sucrose (30% in phosphate buffer). Brain coronal sections (50 μm) were cut and stained as described in supplementary methods. The number of Parvalbumin and BrdU positive cells was estimated in serial coronal sections covering the complete rostrocaudal extension of the dentate gyrus (DG), CA1 and CA2/3 as previously described [38]. To quantify the DG volume sections were stained with Hoechst 33258 (Sigma-Aldrich, USA). The hippocampal region of interest was contoured using Stereo Investigator software (MBF Bioscience, USA) with a 10× objective and its area measured. The volume for each animal was calculated by summing up all the areas traced and multiplying the number by section thickness and by 6 (the spacing factor). The quantification of GFAP expression was performed offline using Fiji (ImageJ software). Images were first converted to 8 bit and 3 ROIs of DG, CA1 or CA2/3 per section (200 μm wide) were selected. For the 3 ROIs the mean grey value was calculated and normalized using the value taken from the same ROI in a control image acquired in the thalamus of the same slice were only background labeling was present. Normalized values of the 3 ROIs of the single animal were then averaged to obtain the mean GFAP expression value of the single animal. The total amount of GFAP staining was then given as the mean ± standard error for each experimental group.
2.9. Statistics
Results are presented as box-and-whisker plots (The box extends from the 25th to 75th percentiles, the line in the middle of the box is plotted at the median, the whiskers go down to the 1thpercentile and up to the 99th) with the only exception of Figs. 2, 8c (data presented as mean ± SEM). All data were analyzed by two-way ANOVA to identify a possible synergistic effect (source of variation due to interaction) between the variables obesity and PS. Post hoc multiple comparisons between the groups were performed trough Sidak's test. 95% CIs were calculated for each comparison. Correlations between groups of values were evaluated calculating the best fit, based on least-squares regression analysis. P value <0·05 was considered statistically significant.
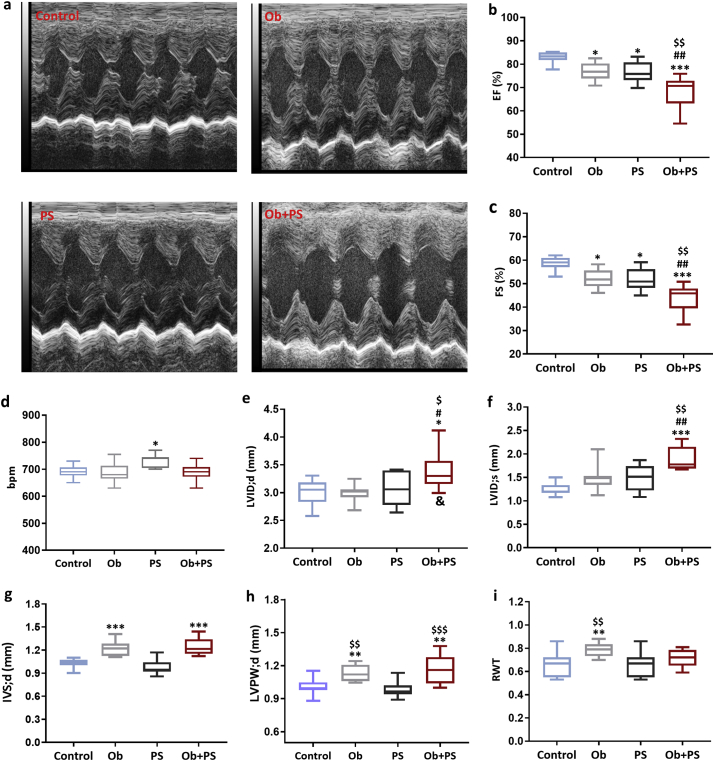
Fig. 2.
Subjecting obese mice to PS jeopardizes cardiac function. (a) Representative left ventricular (LV) M-Mode echocardiograms of each experimental group. Global LV function (b-d) and structure (e-i) of conscious mice. EF, ejection fraction; FS, fractional shortening; HR, heart rate; LVID;d, LV end-diastolic internal diameter; LVID;s, LV end-systolic internal diameter; IVS;d, end-diastolic interventricular septum thickness; LVPW;d, end-diastolic LV posterior wall thickness; RWT, relative wall thickness. All values are represented trough box-and-whisker plots (n = 10, two-way ANOVA, *p < 0·05, **p < 0·01, and ***p < 0·001 Vs Controls; #p < 0·05, ## p < 0·01,### p < 0·001 Vs Ob; $p < 0·05,$$p < 0·01, $$$p < 0·001 Vs PS; &p < 0·05 interaction between Ob and PS).
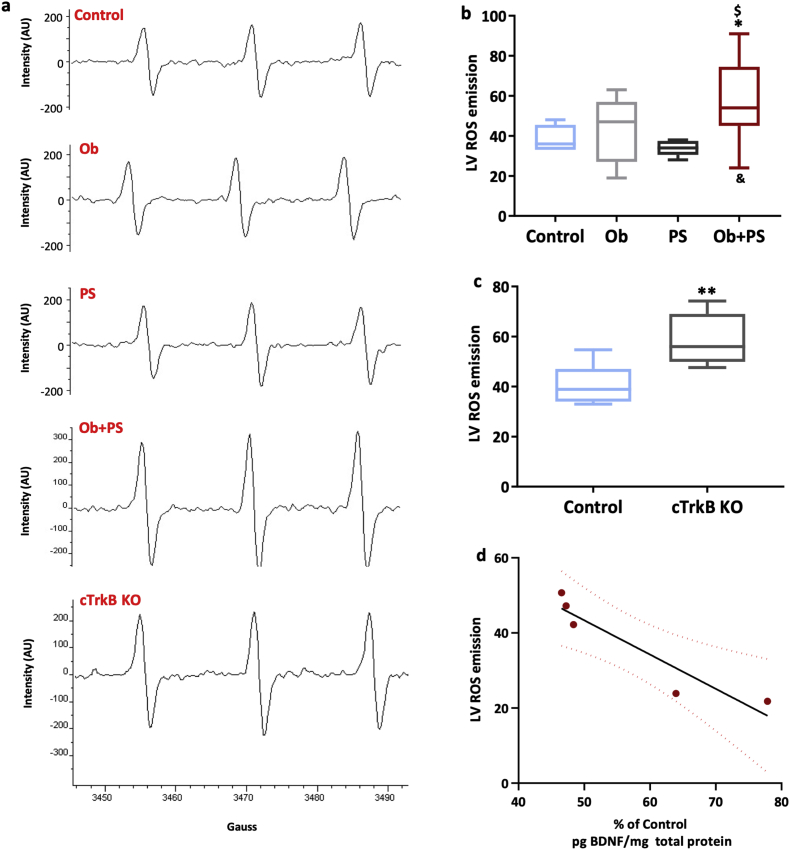
Fig. 8.
HFD/PS combination enhances cardiac oxidative stress. (a) Representative images of electron paramagnetic resonance spectra at room temperature of left ventricular (LV) myocardium of each experimental group. (b) Measure of LV reactive oxygen species (ROS) emission (n = 6–10 per group). (c) LV ROS emission in Control wild type and cTrkB knockout (KO) murine left ventricle (n = 4–5per group). (d) Relationship between LV ROS emission and myocardial BDNF content. All values are represented trough box-and-whisker plots (n = 4–8, two-way ANOVA, *p < 0·05, **p < 0·01 Vs Controls; ## p < 0·01 Vs Ob; $p < 0·05, $$p < 0·01 Vs PS; &&p < 0·01 interaction between Ob and PS).
3. Results
3.1. High-fat diet combined to resident-intruder test efficiently model obesity and psychosocial stress in mice
First, we confirmed that sixteen weeks of HFD are sufficient to markedly increase the body weight (Fig. 1b) of healthy mice, data in accord with an increased caloric intake (Fig. 1c), which is maintained up to 18 weeks of diet (Fig. 1c). These features are all compatible with a condition of diet-induced obesity [39]. Conversely, Control and PS mice did not gain any weight (Fig. 1b, c), consistent with lower caloric intake. However, superimposing RIP in obese mice promoted a significant reduction of body weight related to reduced caloric intake (Fig. 1b, c) in accordance with a previous study [40]. We also checked that, during the fourteen days of RIP, irrespective of the experimental cohort, all the intruders (C57BL6/J) mice were immediately subdued to the resident (CD1) mice. As shown in Fig. 1d for the entire test duration, the intruders suffered from an average of 10 attacks per day perpetrated by the resident CD1 mice. Of note, they ceased to react to these physical challenges almost immediately (latency to the first attack shown in Fig. 1e). Thus, administering RIP for fourteen days instigates a condition compatible with social defeat and chronic psychological stress, as previously documented [41,42]. Interestingly, the number of attacks was significantly higher in obese rather than lean mice during the entire stress period (Supplementary Fig. 2). The number of attacks occurring during the second week of stress was significantly reduced compared to first week in both lean and obese mice by −60% and −49.38%, respectively.
3.2. PS primarily jeopardizes myocardial function in already obese mice
PS is a significant fomite or cofactor for cardiovascular diseases [43], with or without concomitant obesity. Although previous studies have almost exclusively focused on heart rate and autonomic control as the primary endpoint to evaluate how PS influences the cardiovascular function [44], so far no study was performed to assess its direct effect on cardiac phenotype. On the other hand, when obesity is concerned, HFD alone may not be incisive enough to alter myocardial function, at least in a relatively short/medium timeframe [45,46]. Therefore, this evidence is particularly valuable to determine whether PS synergizes with pre-existing obesity to deteriorate myocardial and/or vascular performance, as intended here. We observed that both obesity and PS slightly but significantly reduced echo-derived ejection fraction (EF %) and fractional shortening (FS %) (Fig. 2b, c), yet heart rate was significantly increased only in awake PS mice (Fig. 2d). However, subjecting obese mice to PS led to a much more marked decline in both EF% and FS% (Fig. 2b, c) without increasing heart rate. Of note, the outcome of the Ob + PS group was significantly different vs. controls (p < 0·001) but also vs. PS or Ob group (p < 0·01), each taken alone. Besides, when considering both LV end-diastolic and systolic diameter (LVID;d and LVID;s) only in the Ob + PS group is present significant deterioration respect to all the other groups and this difference arises from a synergistic effect (Fig. 2e, f). Even though, Ob and Ob + PS hearts displayed an increased thickness of both septa (IVS;d) (Fig. 2g) and LV posterior wall (LVPW;d) (Fig. 2h). Of relevance, RWT (ratio between LVPW;d + IVS;d and LVID;d), an established index of cardiac hypertrophy, was significantly higher only in the Ob animals (Fig. 2i). This finding is not surprising considering that LV hypertrophy is a well-documented feature of HFD-induced obesity [47,48].
3.3. PS reduces myocardial inotropy in obese mice without affecting peripheral vascular loading conditions
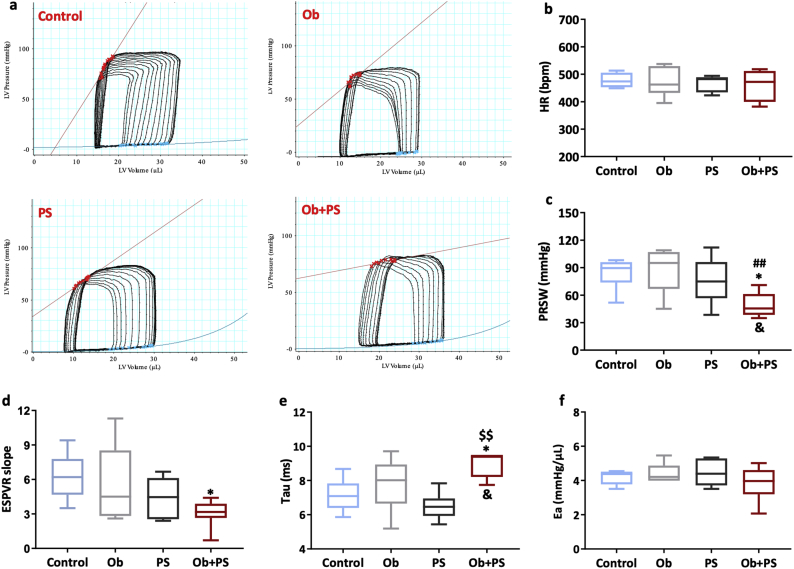
Theoretically, PS and obesity can affect cardiac function by directly targeting the myocardium, or indirectly, i.e. by modifying pre-or afterload conditions, or both. However, no studies have posed these questions yet. Here, we used a pressure-volume approach that allows discerning the impact of HFD or PS, alone or in combination, on intrinsic myocardial performance, from changes in cardiac function secondary to peripheral load [49]. Preload recruitable stroke work (PRSW) is a highly-linear, pre- and afterload insensitive index of myocardial contractility [49]. This parameter was not different between Control, Ob, and PS group (Fig. 3c). In stark contrast, PRSW dropped by 44%in the Ob + PS group (p < 0·05 vs. Control group, P < 0·01 vs. Ob group). The synergistic nature of the obesity/PSinteraction is proved by the interaction between the two independent variables (two-way ANOVA p < 0·05). Consistently, another load-independent index of myocardial contractility, i.e. LV elastance, declined by ~50% in the Ob + PS group compared the Control group, while no effect was present in the Ob or PS group(Fig. 3d). Finally, neither HFD nor PS per se were incisive enough to prolong tau, an index of isovolumic LV relaxation (Fig. 3e). However, their combination resulted in a marked prolongation of tau, attesting that Ob and PS act synergistically to induce diastolic dysfunction (two-way ANOVA, interaction between the two independent variables p < 0·05). Finally, arterial elastance (EA), a valid measure of arterial load [50] was unchanged across all experimental groups (Fig. 3f), thus showing that present experimental conditions and timing were not intense or prolonged enough to alter peripheral vascular load. In essence, this set of data demonstrates that HFD per se (for 18 weeks) have a little-to-null impact on LV systolic and diastolic function, measured in a load-independent manner. Likewise, PS triggered by RIP may not be enough to affect LV performance primarily. In stark contrast, superimposing PS to existing obesity primarily and severely affects myocardial performance, i.e., independently or in addition to altered vascular loading conditions that, however, did not manifest under the experimental conditions adopted herein.
Fig. 3.
Superimposing PS to obesity primarily affects myocardial performance. (a) Representative left ventricular Pressure-Volume loops of each experimental group. (b)HR, heart rate; (c) PRSW, preload recruitable stroke work; (d) ESPVR, end-systolic pressure-volume relationship; (e) Tau, LV relaxation time constant; (f) Ea, arterial elastance. All values are represented trough box-and-whisker plots (n = 6–8, two-way ANOVA, *p < 0·05, Vs Controls; #p < 0·05, ## p < 0·01 Vs Ob; $p < 0·05, $$p < 0·01Vs PS; &p < 0·05 interaction between Ob and PS).
3.4. PS in obese mice triggers myocardial apoptosis, fibrosis and vascular remodeling
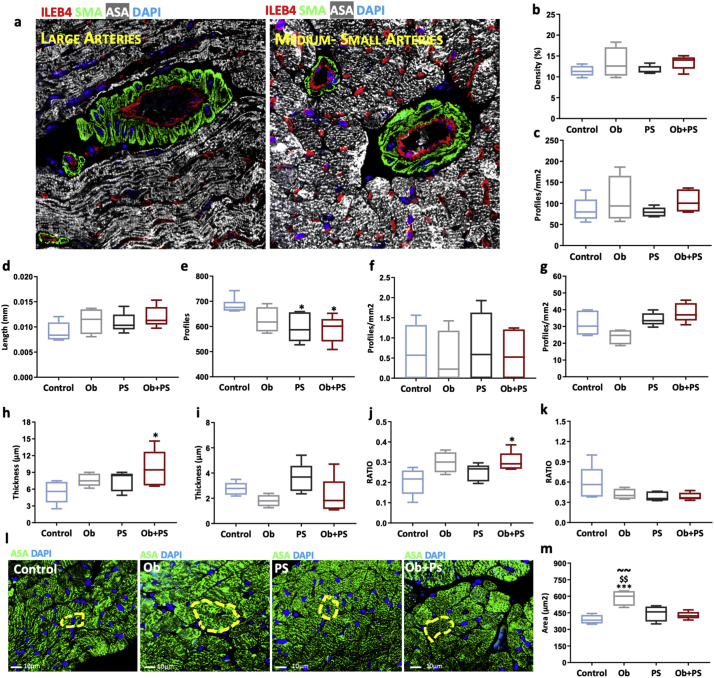
Even very low levels of cardiomyocyte apoptosis are sufficient to endanger LV structure and function [51]. Accordingly, here we sought to determine whether, and to what extent increased myocyte cell death accounts for myocardial dysfunction after obesity, PS, or their combination. HFD slightly augmented interstitial fibrosis, without increasing levels of total apoptotic cardiac cells (Supplementary Fig. 3) and, in particular, cardiomyocyte apoptosis (Fig. 4a, c). Conversely, PS did not trigger any rise in these parameters. However, subjecting obese mice to PS resulted in a marked increment in both, reaching values that were four and two times higher than those found in the control group and significantly different from all groups (Fig. 4a, c). Again, when testing for the nature of obesity/PS interaction, a synergistic effect on both apoptosis and fibrosis emerged (two-way ANOVA, interaction between the two independent variables p < .05). Since coronary microvascular remodeling contributes to cardiomyocyte death [52], we assessed the status of the coronary vessels at histology level. Morphometric analysis of coronary tree revealed that vessel density, branching index (branch points/unit area), a measure of angiogenic sprouting activity [37] and average vessel length, an important determinant of myocardial flow distribution [53], did not change among groups (Fig. 5b, c, d), but total number of endpoints, a measureof open-ended segments of arterioles causing coronary redistribution among different myocardial regions [37], was significantly reduced in PS and Ob + PS animals (Fig. 5e). Moreover, the number of large size and medium-small size of arterioles was similar among groups (Fig. 5f, g). Otherwise, wall thickness and the wall-to-lumen ratio of large arterioles were significantly increased only in Ob + PS group (Fig. 5h, j), yet these parameters were similar in medium-small arterioles of both groups (Fig. 5I, k). Finally, as shown in Fig. 5l, m, cross-sectional area of cardiomyocyte, an index of cardiac hypertrophy, was significantly increased only in Ob mice, in accordance with RWT values.
Fig. 4.
PS in obese mice increases myocardial apoptosis and interstitial collagen deposition. (a) Representative images of the detection of left ventricular apoptotic cardiomyocytes in each experimental group (a white arrow indicates an example of an apoptotic interstitial cell, a yellow arrow an example of an apoptotic cardiomyocyte). DAPI, 4′,6-diamidino-2-phenylindole; TUNEL,terminal deoxynucleotidyl transferase dUTP nick end labeling. (b) Representative images of histological Masson's trichrome-stained sections to detect left ventricle interstitial collagen in each experimental group. (c) LV fraction of alpha-sarcomeric actin positive cells that are TUNEL positive (%). (d) LV % Interstitial collagen amount(n = 4–5 per group). All values are represented trough box-and-whisker plots (n = 4–5 per group, two-way ANOVA, **p < 0·01 Vs Controls; ## p < 0·01 Vs Ob; $$p < 0·01, $$$p < 0·001 Vs PS; &p < 0·05 interaction between Ob and PS). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
PS in obese mice induces arteriolar remodeling without affecting vascular density. (a) Representative images of large and medium-small coronary arteries. DAPI, 4′,6-diamidino-2-phenylindole; ILEB4, isolectin B4; SMA, alpha-smooth muscle actin. (b) Vascular density. (c) Branching index. (d) Average vessel length. (e) Total number of endpoints. (f) Total number of large coronary arteries. (g) Total number of medium-small arteries. (h) Wall thickness of large arteries. (i) Wall thickness of medium-small arteries. (j) Wall-to-lumen ratio of large arteries. (k) Wall-to-lumen ration of medium-small arteries. (l) Representative images of the cross-sectional area of left ventricular cardiomyocytes in each experimental group. Control, mice fed with standard diet; Ob, high-fat diet-induced obese mice; PS, mice fed with standard diet and exposed to psychosocial stress; Ob + PS, high-fat diet-induced obese mice exposed to psychosocial stress. ASA, alpha-sarcomeric actin. (m) Cardiomyocyte cross-sectional area. All values are represented trough box-and-whisker plots (n = 4–5per group, two-way ANOVA, *p < 0·05 Vs Controls, ***p < 0·001 Vs Controls; ## p < 0·01 Vs Ob; $p < 0·05, $$p < 0·01 Vs PS; ~~p < 0·01 Vs Ob + PS).
3.5. Cardiac BDNF and TrkB depletion accounts for myocardial dysfunction induced by PS in obese mice
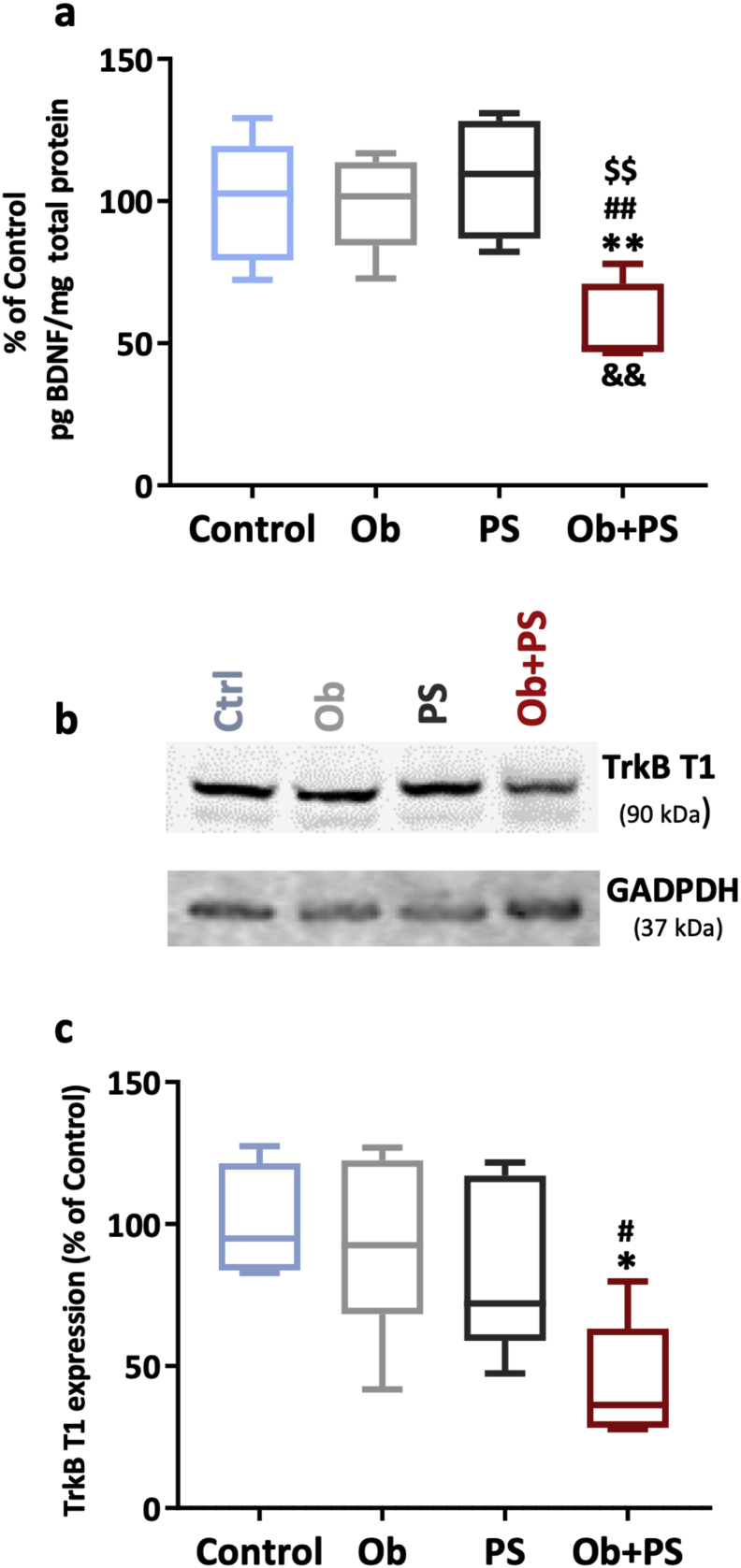
Cardiac BDNF-TrkB signaling is central for heart growth/development [54], myocardial function in adulthood [20], and protection against myocardial apoptosis [55]. Accordingly, we asked the original question that is whether either obesity, PS, or their combination alter cardiac BDNF and TrkB content. Of note, neither HFD-induced obesity nor PS via RIP significantly affected the cardiac content of this neurotrophin and its receptor (Fig. 6a–c). However, PS on the top of obesity halved cardiac BDNF and TrkB.T1 content with a significant interaction between the two independent variables (two-way ANOVA, p < 0·01). TrkB.T1 is the most important BDNF receptor in adult cardiomyocytes [21]. Thus, impairing cardiac BDNF signaling accounts, at least in part, for the cardiac mechanical dysfunction and myocardial remodeled structure prompted by PS in obese mice.
Fig. 6.
HFD/PS combination depletes myocardial BDNF levels while decreasing left ventricular (LV) TrkB.T1 expression. (a) LV brain-derived neurotrophic factor (BDNF) levels of each experimental group. (b) representative densitometric bands of LV TrkB.T1 expression in each experimental group. (c) LV TrkB.T1 levels of each experimentl group. All values are represented trough box-and-whisker plots (n = 6–8per group, two-way ANOVA,*p < 0·05, **p < 0·01 Vs Controls; ## p < 0·01 Vs Ob; $p < 0·05, $$p < 0·01 Vs PS; &&p < 0·01 interaction between Ob and PS).
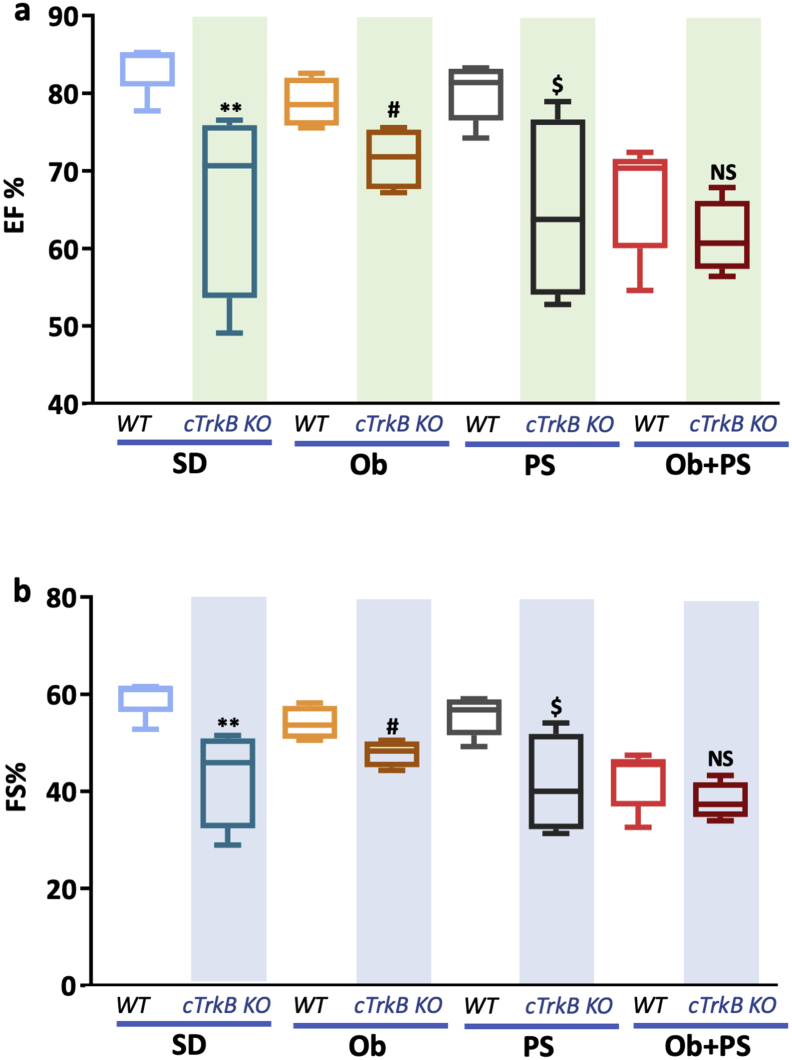
3.6. Obesity/PS combination mimics the cardiac phenotype of cTrkB KO mice
An intact BDNF/TrkB signaling is required for maintaining normal LV function, as previously shown by us [20] and others [21]. In accord with previous evidence, marked drop in cardiac BDNF and TrkB content in our WT obese/PS mice (Fig. 6) hints at the intriguing possibility that cardiac BDNF/TrkB is altered during obesity/PS. Here, testing this new hypothesis directly, we found that the extent of LV function decline due to Ob + PS (and expressed in terms of EF% and FS%) was superimposable in WT and TrkB KO mice (Fig. 7a, b). This fact suggests that in WT mice the synergy enacted by obesity and PS disrupts TrkB-dependent myocardial function in a manner reminiscent of that seen after genetic deletion of cardiac TrkB itself. Consistent with this interpretation are the additional, current data attesting that both WT Ob and WT PS mice have preserved cardiac BDNF and TrkB level and better-maintained LV function compared to unstressed TrkB KO mice (Fig. 7a, b).
Fig. 7.
The reduction of left ventricular (LV)ejection fraction (EF,%) and fractional shortening (FS,%) is similar in wild type (WT) and cTrkB knockout (KO) obese mice experiencing psychosocial stress. (a) Changes of LVEF in each experimental group of WT and KO mice. (b) Changes of LV FS in each experimental group of WT and KO mice.All values are represented trough box-and-whisker plots (n = 4–8, **p < .01 Vs corresponding WT standard diet (SD) mice; # p < .05 Vs corresponding WT obese (Ob) mice; $p < 0·05 Vs corresponding WT mice experiencing psychosocial stress (PS); NS, not signifcant).
3.7. PS in obese mice increases cardiac ROS emission
Oxidative stress is a hallmark of both obesity [56] and psychosocial stress [57]. Notwithstanding, to the best of our knowledge, no attempts have been made so far to measure myocardial ROS emission (more specifically from the LV) during stress such as chronic HFD or RIP using a quantitative, real-time ROS assessment via EPR. Using the latter approach we found that neither HFD nor RIP increased LV ROS emission above control levels (Fig. 8b). However, their combination synergistically augmented LV ROS emission (two-way ANOVA, p < 0·05).
3.8. TrkB-dependent signaling contributes to maintaining a proper myocardial redox balance
Whether cardiac TrkB signaling plays a role in maintaining myocardial redox balance is currently unclear. To fill this gap in our knowledge, we measured ROS emission from freshly isolated LVs of unstressed cTrkB KO via electron paramagnetic resonance (EPR). As shown Fig. 8c,overall ROS emission was more elevated in cTrkB KO than in their WT littermates already at baseline, i.e., under non-stress condition. This finding advances the new concept that an intact cardiac TrkB-dependent signaling contributes to limit basal ROS emission from the heart, more specifically from the LV. Consistent with this is the present data showing that average LV ROS emission is similar in unstressed cTrkB KO mice and in the WT Ob + PS group that harbors severely reduced TrkB receptor density (Fig. 6c). Thus, at least to some extent, an intact TrkB signaling maintains LV function by limiting excessive ROS production/emission, among other possible salutary actions. In agreement with this possibility is the fact that a strong negative correlation exists between LV ROS expression measured by EPR and LV BDNF levels measured by ELISA in each Ob/PS mouse (Fig. 8d, r = 0.88, p < .05).
3.9. PS in obese mice prompts severe anxiety and spatial memory impairment in otherwise healthy mice
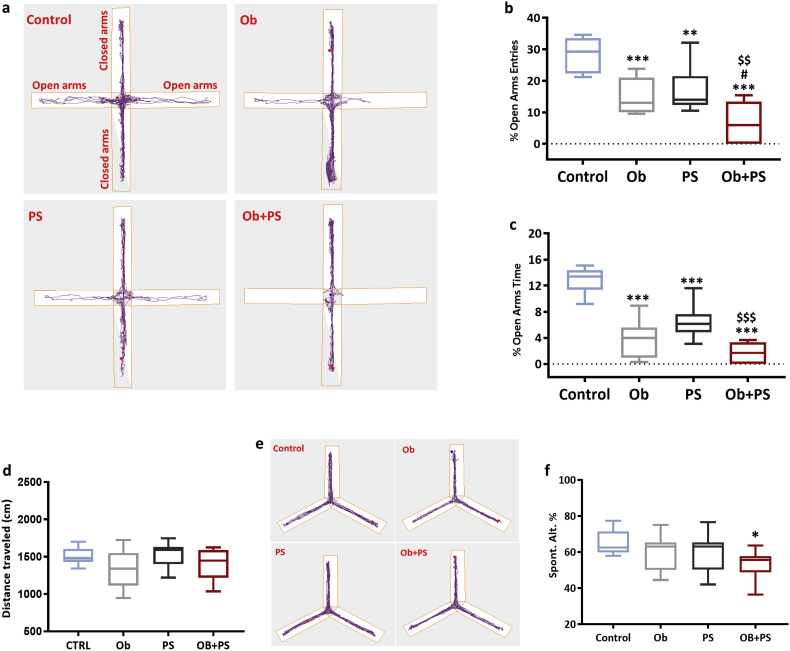
Both obesity and PS can separately generate anxiety [58,59]. Therefore, using the elevated plus maze (EPM) test specifically designed for this purpose [60], we next determined how effective obesity and PS are in triggering anxiety, when administered alone or in combination. Both HFD and PS made mice manifestly anxious. Accordingly, obese mice displayed a significant reduction in both the % entries (Fig. 9b) and % time spent in the open arms of the EPM (Fig. 9c). A similar phenotype was observed in the PS-challenged mice. Notably, superimposing PS in obese mice exacerbated anxiety-like behavior. Indeed, % entries in open arms was four times lower in HFD/PS mice with respect to the control group, and more than two-time lower respect to Ob and PS groups (Ob + PS Vs Control p < 0·001; Ob + PS Vs PS p < 0·01; Ob + PS Vs Ob p < 0·05) (Fig. 9b). A similar outcome emerged from the % time spent in open arms (Fig. 9c). When HFD and PS were combined, the behavioral parameters verged on zero, thus denoting maximal levels of anxiety and almost complete lack of open arms entries. More specifically, 44% of Ob + PS mice never egressed from the closed into the open arms (thus reaching the minimum score attainable in the test). This phenomenon was absent in all other groups. Of relevance, these drastic changes in behavior were not due to a physical impediment. In fact, locomotor activity was similar across all experimental groups (Fig. 9d).
Fig. 9.
PS in obese mice triggers severe anxiety while impairing spatial memory. (a) Representative examples of mice exploratory activity in the elevated plus maze apparatus. (b) % of entries of mice into open arms (n = 10 per group). (c) % of time spent in open arms (n = 10 per group). (d) The average distance traveled in elevated plus maze apparatus (n = 10 per group). (e) Representative images show typical examples of mice trajectories in the Y-maze apparatus. (f) % of spontaneous alternation in the Y-maze. All values are represented trough box-and-whisker plots (n = 10, two-way ANOVA, *p < 0·05, **p < 0·01, and ***p < 0·001 Vs Controls; #p < 0·05 Vs Ob; $$p < 0·01, $$$p < 0·001 Vs PS).
Next, we analyzed the spatial memory domain and found that neither obesity nor PS altered the mouse performance (% spontaneous alternations) in the one-trial Y-maze test. In stark contrast, their combination manifestly impaired spatial memory as witnessed by the 20% decrement in spontaneous alternation parameter (p < 0·05, Fig. 9f). Hence, challenging otherwise healthy mice with HFD/RIP is a reliable and affordable way to start dissecting the mechanisms by which obesity and PS conjure up to alter behavioral patterns in mice.
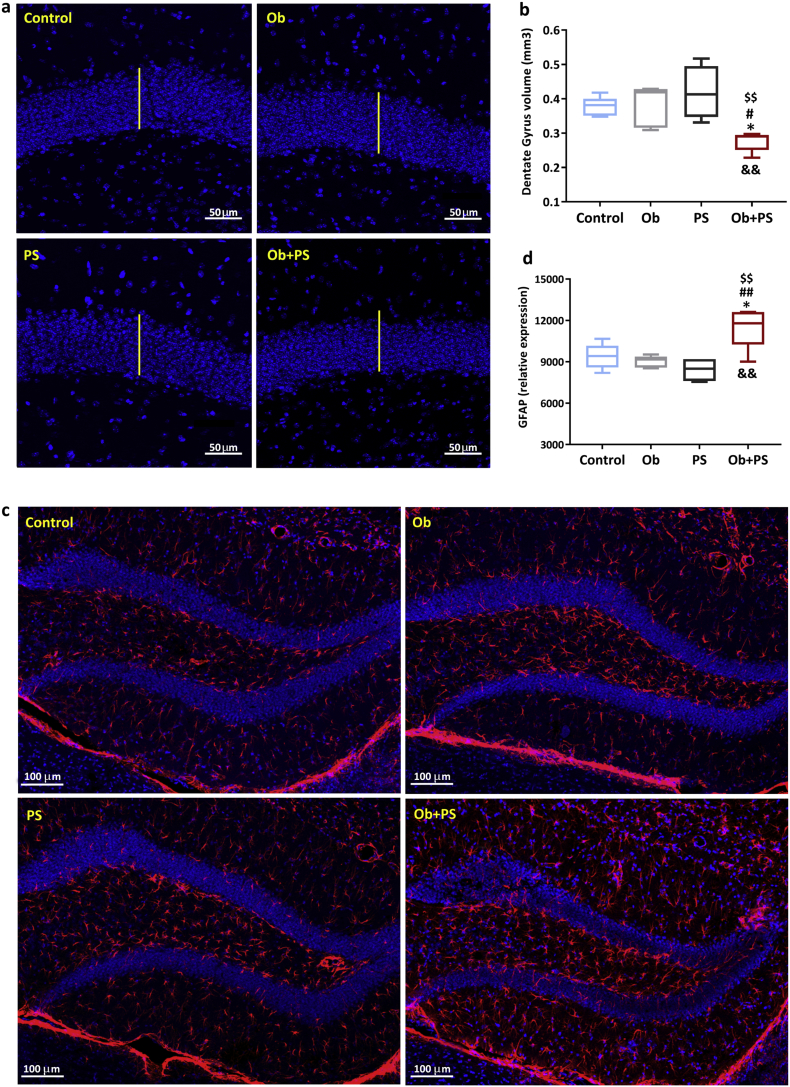
3.10. PS in obese mice alters hippocampal morphology
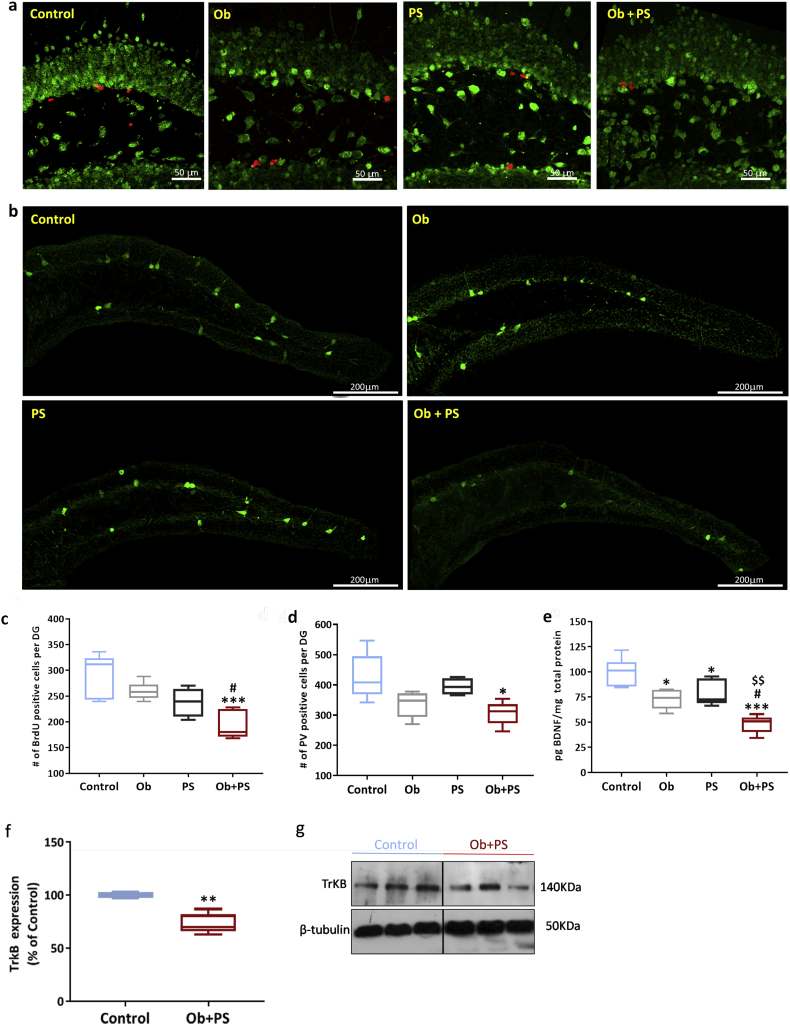
In mammals, the hippocampal formation is a critical structure for mood balance and memory consolidation [61]. Furthermore, the hippocampus is a crucial hub for the brain-heart communication, both under normal and pathological conditions. Accordingly, studies have attested that the hippocampal formation is exquisitely sensitive to cardiac disease conditions [[62], [63], [64]]. What is more, the hippocampus is very sensitive to exogenous risk factors, such as unhealthy diets and social stress [65,66]. In the wake of this evidence and of the above myocardial-related data, we next sought to determine what, and to what extent hippocampal morpho-functional traits are compromised by obesity, PS, or their combination. In particular, we focused on the DG, the main gateway of the hippocampal formation [67]. Neither obesity nor PS modified the volume of the DG. Conversely, their co-presence reduced overall DG size by 27% (p < .05 vs. both Control and Ob group; p < 0·01 vs. PS group), and in a synergistic manner as revealed by the analysis of the interaction between the two factors (two-way ANOVA, p < 0·01) (Fig. 10b). Moreover, the DG shrinkage visible in the Ob + PS group was paralleled by increased astrogliosis as shown by the augmented expression of the glial fibrillary acidic protein, GFAP (Fig. 10d). This structural alteration was evident only in the Ob + PS group. Since the DG is a site of adult neurogenesis, we next examined cell proliferation in the subgranular zone. We found a significant decrease in newborn, BrdU-labeled cells only in Ob + PS mice (Fig. 11c). Consistent with the possibility that the hippocampus is severely affected during Ob + PS, the expression of one of the main markers of GABAergic interneurons, i.e., Parvalbumin (PV) was markedly lessened in the Ob + PS vs. Control (p < 0·05, Fig. 11d). Finally, we have performed the analysis of volume, number of PV-interneurons and GFAP expression in CA1 and CA2/3 regions of the hippocampus. As shown in Supplementary Fig. 4e,f, we did not find any difference in the number of PV cells of abovementioned hippocampal regions among each experimental group, yet GFAP expression was significantly increased in CA1 (Supplementary Fig. 4c) and CA2/3 (Supplementary Fig. 4d) regions even if the corresponding volume was unchanged in both regions (Supplementary Fig. 4 a,b).
Fig. 10.
PS in obese mice decreases DG volume, while inducing astrogliosis. (a) Representative images of measurement of dentate gyrus (DG) volume in each experimental group. The white bar denotes normal thickness of the DG in controls. (b) Average measure of DG volume. (c) Representative images of detection of glial fibrillary acidic protein (GFAP)-positive cells in the DG for each experimental group. (d) Relative expression of GFAP. All values are represented trough box-and-whisker plots (n = 5, two-way ANOVA, *p < 0·05 Vs Controls; ## p < 0·05 Vs Ob; $$p < 0·01 Vs PS; &&p < 0·01 interaction between Ob and PS).
Fig. 11.
HFD/PS mice display impaired hippocampal neurogenesis, reduced number of parvalbumin− + interneurons,and halved BDNF levels. (a) Representative images of BrdU-positive cells (red) in the DG per each group. Green, NeuN counterstaining. (b) Representative images of detection of parvalbumin (PV)-positive cells resident in the DG per each experimental group. (c) Number (#) of BrdU-positive cells per DG. (d) Number (#) of PV-positive cells per DG. (e) Hippocampal brain-derived neurotrophic factor (BDNF) protein levels. (f) Hippocampal TrkB protein levels. (g) Representative images of cropped densitometric bands of hippocampal TrkB expression in Control and Ob + PS mice. The full-length blots/gels are presented in Supplementary Fig. 5. All values are represented trough box-and-whisker plots (n = 5, two-way ANOVA, *p < .05, **p < .01, ***p < .001 Vs Controls; #p < .05 Vs Ob; $$p < 0·01, Vs PS). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.11. PS in obese mice reduces hippocampal BDNF and TrKB levels
BDNF plays a crucial role in the hippocampus physiology, promoting neuronal survival, synaptic plasticity, and neurogenesis linked to different behavioral domains, such as mood, and memory [68,69]. Accordingly, we measured hippocampal BDNF levels through ELISA assay. As reported in Fig. 11e, the hippocampal content of BDNF was dramatically reduced (−52%) in the Ob + PS mice with respect to the Control group (p < 0·001). BDNF content was slightly lower in the Ob and PS groups (p < 0·05 and PS p < 0·01 vs. Control, respectively). Altogether, the present data set reveals that when PS is imposed onto obese subjects, the DG structure is primarily targeted and local pools of BDNF are severely depleted, likely accounting for hippocampal functional perturbations. Alterations of murine hippocampal BDNF/TrkB signaling result in a remarkably increased anxiety-like behavior [70] similarly to our Ob + PS mice. In our model of brain-heart axis dysfunction, TrkB hippocampal levels were significantly reduced compared to Control group (Fig. 11f).
4. Discussion
Despite their proximity and increasingly evident clinical association, how superimposing psychological or social stress condition on an obese phenotype alters myocardial and hippocampal phenotype remains poorly understood. Our study adds new evidence in order to dissect the effects of a single causal factor or their combinationon both organs in the same animal. Obesity has been long associated with a broad spectrum of cardiovascular alterations, spanning from a hyperdynamic circulation –via subclinical cardiac structural changes - to overt heart failure (HF) [71]. Although its comorbid conditions, such as hypertension, dyslipidemia, diabetes, or insulin resistance may lead to systolic and/or diastolic dysfunction overt, no clinical systolic failure is usually noticed in the context of isolated obesity [72]. Our present findings that regular intake of HFD increases body weight without inducing severe decay of ejection fraction of left ventricle with mild hypertrophy dovetails nicely in with this existing clinical evidence. On the other hand, PS per se, a recognized independent risk factor for CVD [73], contributes to the onset of adverse pathophysiological events such as thrombosis/plaque rupture, lethal arrhythmias, atherosclerosis or myocardial ischemia [9,74]. Studying how isolated PS alters human cardiovascular homeostasis is hampered by the minimal forms of stress that can be ethically delivered to human beings, and by the difficulty in controlling multiple external variables [44]. Therefore, modeling PS via the resident-intruder paradigm (RIP) is extremely helpful to explore how eventually PS jeopardizes heart function.
So far, previous studies have mostly focused on the autonomic response to PS, without characterizing myocardial phenotype [75,76] that is, instead, the main aim of our investigation. Our study reveals, for the first time, that only superimposing PS to obesity precipitates a condition of overt systolic-diastolic dysfunction, while triggering apoptosis of cardiomyocytes, interstitial deposits of collagen, remodeling of large coronary arterioles in normotrophic LV tissue, and oxidative stress in the LV. Our data suggest that obesity/PS synergy primarily concerns the myocardium itself since arterial elastance (Ea), an index of peripheral arterial load, is similar to the control group. Of note, it is conceivable that additional cardiomyocyte apoptosis is the critical determinant of most functionally relevant damage of mildly hypertrophic myocardium in our stressed obese mice. Indeed, the increased apoptosis is a critical mechanism mediating the transition from compensated hypertrophy to heart failure during inflammation [77]. Long-term HFD alone, however, causes interstitial fibrosis and mild cardiac hypertrophy without leading both cardiomyocyte apoptosis and remodeling of coronary arterioles in the presence of a slight reduction of LV ejection fraction in conscious mice. Consistent with our present data, a previous study showed that sustained HFD induces myocardial hypertrophy and fibrosis without promoting cardiomyocytes apoptosis [78]. Finally, hearts explanted from lean mice exposed to PS do not display any abovementioned features of cardiac remodeling even if heart rate and global systolic function measured by echocardiography are slightly altered in conscious mice. Since alterations of cardiac functionin both Ob and PS mice disappear under anesthesia, we cannot exclude that chronic sub-clinical inflammation linked to HFD [79] or PS [76] may induce mild alterations of cardiovascular parameters due to autonomic imbalance, which is counteracted by anesthetics [80]. Conversely, additional hit by PS exerted on inflamed myocardium of obese mice recapitulates ischemic microenvironment through rising myocardial ROS levels and would lead over the long-term to a self-fostering gradual process of cardiomyocyte apoptosis that is functionally detrimental. Indeed, large coronary arterioles with increased wall-to-lumen ratio, a typical vascular hallmark of the ischemic heart [81], are detected only in obese mice exposed to PS. Interestingly, the heart of PS-challenged obese mice does not show the histological hallmark of myocardial hypertrophy in accordance with echocardiographic RWT values, even if the end-diastolic thickness of LV walls is higher than that in control mice and similar to that in unstressed obese mice. In stressed obese mice, we speculate that the more prominent wall thickness without compensatory ventricular myocyte hypertrophy may depend on the increased interstitial myocardial fibrosis, whereas LV enlargement is due to side-to-side slippage of cardiac myocytes [82].
From a mechanistic point of view previous evidence attests that BDNF/TrkB signaling is required to maintain normal cardiac contraction and relaxation in adult mice [20,21]. Moreover, local depletion of this neurotrophin renders the heart more prone to apoptosis during ischemia-reperfusion injury [83]. Since increased oxidative stress is a significant driver of apoptosis [84], it is very plausible that reduced levels of cardiac BDNF and/or TrkB increase the vulnerability of cardiomyocytes to oxidative stress, thus exacerbating myocardial cell loss and LV dysfunction in PS-challenged obese mice. This eventuality is supported by present and previous evidence. In fact, our study reveals that lack of cardiac TrkB receptors per se leads to a marked rise in ROS production/emission in the LV, even in the absence of any stress condition. Moreover, it shows, for the first time, that obesity/PS tackle TrkB receptors, curtailing their cardiac density. Although the mechanisms underlying the latter effect remain to be investigated in-depth, our study provides a mechanistic bridge, i.e., reduced TrkB bioavailability, explaining previous evidence showing that a mutual influence exists between BDNF and magnitude of oxidative stress in the heart [85,86] and other organs/tissues [87,88] as well. In essence, our data suggest a scenario in which PS/Obesity alters both cardiac BDNF and TrkB levels, prompting cardiac cells apoptosis that is, at least in part, due to the highly oxidizing conditions emanating from the lack of functioning TrkB, as in the case of obese/PS-treated mice. This pro-oxidizing conditions may, in turn, affect receptors and other structures within the myocyte, including TrkB receptors, in a vicious loop.
This conceptual framework could be also applied to the central nervous system. The Hippocampal formation plays a pivotal role in the regulation of the so-called brain-heart axis (i.e. the bidirectional crosstalk between the nervous system and the cardiovascular system) [89]. The dentatus gyrus (DG), in particular, is the structure centrally involved in memory consolidation and mood homeostasis, representing the first station in the hippocampal trisynaptic system, one of the few neurogenic sites in the adult brain, thus a primary regulator of memory formation. Therefore, it is not so surprising that psychiatric and neurologic disorders are associated with DG injury, and, given the bidirectionality of the brain-heart axis, in cardiovascular diseases as well [[62], [63], [64]]. When examining the hippocampus, we found that HFD per se does not modify the morphology of the hippocampal formation, and of the DG in particular whose volume, extent of adult neurogenesis and parvalbumin-+ interneurons remain uncompromised, while in presence of normal GFAP levels. These findings are consistent with the fact that HFD per se augments anxiety-like behavior, as previously reported [58], yet it does not impair spatial memory. Similarly, PS per se, another stressing factor implicated in the pathogenesis of psychological/psychiatric disorders [90,91], induces anxiety-like behavior without altering spatial memory and DG morphology. Thus, at least under present experimental conditions, HFD and PS, when administered alone, induce anxiety without causing structural damages but - unlike the heart – lead to slight reduction of hippocampal BDNF levels. In both groups, it is conceivable that mild hippocampal BDNF downregulation [92] leads to perturbations at the level of the synthesis and/or release/reuptake of neurotransmitters controlling emotional states, and this hypothetical explanation fits very well with the long-known modulating effects that BDNF exerts, for example, on the release of crucial neurotransmitters such as serotonin [93]. When considering the obesity/PS scenario, it is very plausible that these stressors generate an intense vicious circle fueled by a common psychosocial background that is characteristic of the Western society lifestyles [94]. More specifically, here we reveal that obesity/PS aggravates anxiety well beyond the effects evoked by each abovementioned single hit. Nearly half of the PS-challenged obese mice never egressed from the closed arms (phenomenon utterly absent in the other groups), totalizing the minimum score obtainable in the EPM test. This floor effect prevented the appreciation of any potentially worse condition of this cohort of mice, thus blinding a possible synergy between the two risk factors. Furthermore, obese/PS animals displayed and impairment in spatial memory. These alterations can be explained, at least in part, by the marked reduction in both hippocampal BDNF and TrkB levels, and the DG volume with a concomitant increase of astrogliosis (as indexed by higher GFAP expression). Interestingly, hippocampal CA1 and CA2/3 regions showed increased GFAP expression as consequence of inflammatory response without changing in volume and number of PV cells. Therefore, we can assume an hippocampal damage at level of DG region in our murine model of brain-heart dysfunction. Although previous studies have already established the causal relationship between reduced BDNF pathway, increased oxidative stress and impairment of hippocampal function in mice [95,96], the precise molecular mechanisms underlying such alterations remain to be defined in more detail, particularly with respect to the status/contribution of hippocampal BDNF signaling. Altogether, our data suggest hippocampal damage accompanied by reactive astrogliosis [97]. In the same vein, both neurogenesis and parvalbumin expression in fast-spiking interneurons decreased significantly in Ob + PS mice compared to control mice. Our murine model recapitulates morphological and biochemical hippocampal alterations within the DG already observed in patients with mood disorders [98]. Thus, obesity/PS triggers major behavioral disorders and severe decay of hippocampal BDNF/TrkB signaling that account, at least in part, for this structural and functional aberrations. Accordingly, the opposite condition, i.e., environmental enrichment can counteract the outcome of adverse experiences by promoting BDNF expression and synaptophysin in the hippocampus [99].
4.1. Limitations and studies in perspective
One limitation of the present study is the lack of a measure of circulating BDNF levels in order to assess the neurotrophic cross-talk among heart and brain since there is some evidence that exogenous BDNF crosses the blood-brain barrier in mice [100]. Unfortunately, murine blood BDNF was undetectable in accord with other studies [101]. Another limitation of our study is the lack of a measure of BDNF in other cardiac chambers besides the left ventricle as a parallel analysis of ROS emission in different heart compartments revealed no significant differences in ROS production at baseline, and we were worried about delaying the harvesting and processing of the freshly isolated tissue. The present study opens several appealing perspectives around murine model of obese experiencing PS. However, further investigations focused on the direct mechanicistic link between cardiac and hippocampal TrkB/BDNF signaling are mandatory. It has already been reported that lack of TrkB signaling in the adult hippocampal newborn cells affects anxiety behavior of mice [70]. These findings, together with our results, could suggest that obesity/PS can trigger vicious loop of reduced TrkB, oxidative stress and cell loss also in the hippocampal formation.
Different preventative or therapeutic measures can bolster BDNF expression in specific brain areas, such as the hippocampus, thus improving cognitive and/or other functions. Among them, intermitted fasting/dietary restriction [102] or endurance exercise [103,104] appear quite useful. Future, in-depth studies should test whether any of these - pharmacological, dietary, or exercise-centered - interventions improve both cardiac and cognitive function in obese subjects exposed to PS, and whether, and to what extent these presidia bolster both hippocampal and cardiac BDNF/TrkB signaling. At the same time, the reason(s) (genetic, posttranslational, or both) accounting for local BDNF decay deserve dedicated additional studies investigating underlying regulatory mechanisms.
5. Conclusions
Our study reveals that two highly diffused and often coexisting environmental stress factors, such as HFD-induced obesity and PS act synergistically to deteriorate heart function and structure, marking a profound decline in both systolic and diastolic feature that originates from loss of viable cardiomyocytes, depletion of cardiac BDNF pool, matrix and vascular remodeling under oxidative burst. At the central level, PS impinging on an obesity condition leads to a substantial hippocampal remodeling, accounting for major behavioral disorders, such as extreme anxiety and loss of cognitive capacity. Hippocampal depletion of BDNF can explain both the mood alterations and spatial memory impairment. Thus, the present study suggests that BDNF-centered enriching therapies may benefit peripheral (myocardium) and central (hippocampus) structures, at the same time, improving the brain-heart axis function, thus improving the well-being of subjects at risk of cerebral-cardiac disorders such as obese individuals affected by psychosocial stress.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Literature search: Jacopo Agrimi, Cristina Spalletti, Vincenzo Lionetti. Study design: Vincenzo Lionetti. Animal data collection: Jacopo Agrimi, Carlotta Baroni, Nicole Di Lascio, Gizem Keceli, Cristina Spalletti, Genaro Ramirez-Correa, Guanshuo Zhu. Molecular and histological data collection: Jacopo Agrimi, Djahida Bedja, Angela Caragnano, Stephen Chelko, Cristina Spalletti, Arianna Scalco. Data analysis: Valentina Casieri, Antonio Paolo Beltrami, Marco Matteucci, Arianna Scalco. Data interpretation: Jacopo Agrimi, Matteo Caleo, Vincenzo Lionetti, Nazareno Paolocci, Cristina Spalletti. Drafting of the manuscript: Jacopo Agrimi, Cristina Spalletti. Figures: Jacopo Agrimi, Angela Caragnano, Cristina Spalletti. Critical revision of the manuscript: Antonio Paolo Beltrami, Matteo Caleo, Vincenzo Lionetti, Nazareno Paolocci.
Declaration of Competing Interest
The authors have declared that no competing interests exist.
Acknowledgments
We thank Nicholas Bruno, Massimo Giannoni, Seungho Jun and Francesca Mastorci for their valuable technical support. The content is solely the responsability of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.042.
Appendix A. Supplementary data
Supplementary material
References
- 1.Lifshitz F., Lifshitz J.Z. Globesity: the root causes of the obesity epidemic in the USA and now worldwide. Pediatr Endocrinol Rev. 2014;12(1):17–34. [PubMed] [Google Scholar]
- 2.McEwen B.S., Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011;101(Suppl. 1):S131–S139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogler L., Muller V.I., Chang A., Eickhoff S.B., Fox P.T., Gur R.C. Psychosocial versus physiological stress - meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan M., Tannahill C., Petticrew M., Thomas S. Psychosocial risk factors in home and community settings and their associations with population health and health inequalities: a systematic meta-review. BMC Public Health. 2008;8:239. doi: 10.1186/1471-2458-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siervo M., Wells J.C., Cizza G. The contribution of psychosocial stress to the obesity epidemic: an evolutionary approach. Horm Metab Res. 2009;41(4):261–270. doi: 10.1055/s-0028-1119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwarteng J.L., Schulz A.J., Mentz G.B., Israel B.A., Perkins D.W. Independent effects of neighborhood poverty and psychosocial stress on obesity over time. J Urban Health. 2017;94(6):791–802. doi: 10.1007/s11524-017-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 8.Minguez-Olaondo A., Irimia P., Fruhbeck G. Obesity and the nervous system: more questions. Lancet Neurol. 2017;16(10):773. doi: 10.1016/S1474-4422(17)30292-2. [DOI] [PubMed] [Google Scholar]
- 9.Bairey Merz C.N., Dwyer J., Nordstrom C.K., Walton K.G., Salerno J.W., Schneider R.H. Psychosocial stress and cardiovascular disease: pathophysiological links. Behav Med. 2002;27(4):141–147. doi: 10.1080/08964280209596039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piirainen S., Youssef A., Song C., Kalueff A.V., Landreth G.E., Malm T. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer's disease: the emerging role for microglia? Neurosci Biobehav Rev. 2017;77:148–164. doi: 10.1016/j.neubiorev.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Agrimi J., Baroni C., Anakor E., Lionetti V. Perioperative heart-brain axis protection in obese surgical patients: the nutrigenomic approach. Curr Med Chem. 2018 doi: 10.2174/0929867325666181015145225. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Alzoubi K.H., Abdul-Razzak K.K., Khabour OF, Al-Tuweiq G.M., Alzubi M.A., Alkadhi K.A. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behav Brain Res. 2009;204(1):117–123. doi: 10.1016/j.bbr.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 13.de Sousa Rodrigues M.E., Bekhbat M., Houser M.C., Chang J., Walker D.I., Jones D.P. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav Immun. 2017;59:158–172. doi: 10.1016/j.bbi.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simas B.B., Nunes E.A., Crestani C.C., Speretta G.F. Cardiovascular and metabolic consequences of the association between chronic stress and high-fat diet in rats. Stress. 2018;21(3):247–256. doi: 10.1080/10253890.2018.1437413. [DOI] [PubMed] [Google Scholar]
- 15.Rothman S.M., Mattson M.P. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228–240. doi: 10.1016/j.neuroscience.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marosi K., Mattson M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motamedi S., Karimi I., Jafari F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): kill two birds with one stone. Metab Brain Dis. 2017;32(3):651–665. doi: 10.1007/s11011-017-9997-0. [DOI] [PubMed] [Google Scholar]
- 18.Gattere G., Stojanovic-Perez A., Monseny R., Martorell L., Ortega L., Montalvo I. Gene-environment interaction between the brain-derived neurotrophic factor Val66Met polymorphism, psychosocial stress and dietary intake in early psychosis. Early Interv Psychiatry. 2018;12(5):811–820. doi: 10.1111/eip.12371. [DOI] [PubMed] [Google Scholar]
- 19.Aleisa A.M., Alzoubi K.H., Gerges N.Z., Alkadhi K.A. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22(3):453–462. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Feng N., Huke S., Zhu G., Tocchetti C.G., Shi S., Aiba T. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc Natl Acad Sci U S A. 2015;112(6):1880–1885. doi: 10.1073/pnas.1417949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulgenzi G., Tomassoni-Ardori F., Babini L., Becker J., Barrick C., Puverel S. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J Cell Biol. 2015;210(6):1003–1012. doi: 10.1083/jcb.201502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartolomucci A., Pederzani T., Sacerdote P., Panerai A.E., Parmigiani S., Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29(7):899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pryce C.R., Fuchs E. Chronic psychosocial stressors in adulthood: studies in mice, rats and tree shrews. Neurobiol Stress. 2017;6:94–103. doi: 10.1016/j.ynstr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartolomucci A., Palanza P., Gaspani L., Limiroli E., Panerai A.E., Ceresini G. Social status in mice: behavioral, endocrine and immune changes are context dependent. Physiol Behav. 2001;73(3):401–410. doi: 10.1016/s0031-9384(01)00453-x. [DOI] [PubMed] [Google Scholar]
- 26.Moles A., Bartolomucci A., Garbugino L., Conti R., Caprioli A., Coccurello R. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31(5):623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Kaludercic N., Mialet-Perez J., Paolocci N., Parini A., Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belmonte F., Das S., Sysa-Shah P., Sivakumaran V., Stanley B., Guo X. ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309(8):H1271–H1280. doi: 10.1152/ajpheart.00517.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sestakova N., Puzserova A., Kluknavsky M., Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdisc Toxicol. 2013;6(3):126–135. doi: 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maynard K.R., Hill J.L., Calcaterra N.E., Palko M.E., Kardian A., Paredes D. Functional role of BDNF production from unique promoters in aggression and serotonin signaling. Neuropsychopharmacology. 2016;41(8):1943–1955. doi: 10.1038/npp.2015.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omatsu-Kanbe M., Nishino Y., Nozuchi N., Sugihara H., Matsuura H. Prion protein- and cardiac troponin T-marked interstitial cells from the adult myocardium spontaneously develop into beating cardiomyocytes. Sci Rep. 2014;4:7301. doi: 10.1038/srep07301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross P., Honnorat N., Varol E., Wallner M., Trappanese D.M., Sharp T.E. Nuquantus: Machine learning software for the characterization and quantification of cell nuclei in complex immunofluorescent tissue images. Sci Rep. 2016;6:23431. doi: 10.1038/srep23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avolio E., Meloni M., Spencer H.L., Riu F., Katare R., Mangialardi G. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116(10) doi: 10.1161/CIRCRESAHA.115.306146. (e81–94) [DOI] [PubMed] [Google Scholar]
- 34.Lionetti V., Matteucci M., Ribezzo M., Di Silvestre D., Brambilla F., Agostini S. Regional mapping of myocardial hibernation phenotype in idiopathic end-stage dilated cardiomyopathy. J Cell Mol Med. 2014;18(3):396–414. doi: 10.1111/jcmm.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseliou E., de Couto G., Terrovitis J., Sun B., Weixin L., Marban L. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zudaire E., Gambardella L., Kurcz C., Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi C., Angelucci A., Costantin L., Braschi C., Mazzantini M., Babbini F. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24(7):1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 39.Hariri N., Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23(2):270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 40.Balsevich G., Uribe A., Wagner K.V., Hartmann J., Santarelli S., Labermaier C. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J Endocrinol. 2014;222(1):15–26. doi: 10.1530/JOE-14-0129. [DOI] [PubMed] [Google Scholar]
- 41.Bartolomucci A., Palanza P., Sacerdote P., Panerai A.E., Sgoifo A., Dantzer R. Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev. 2005;29(1):67–81. doi: 10.1016/j.neubiorev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Hammels C., Pishva E., De Vry J., van den Hove D.L., Prickaerts J., van Winkel R. Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–140. doi: 10.1016/j.neubiorev.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Dimsdale J.E. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51(13):1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgoifo A., Carnevali L., Grippo A.J. The socially stressed heart. Insights from studies in rodents. Neurosci Biobehav Rev. 2014;39:51–60. doi: 10.1016/j.neubiorev.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Nascimento A.F., Luvizotto R.A., Leopoldo A.S., Lima-Leopoldo A.P., Seiva F.R., Justulin L.A., Jr. Long-term high-fat diet-induced obesity decreases the cardiac leptin receptor without apparent lipotoxicity. Life Sci. 2011;88(23–24):1031–1038. doi: 10.1016/j.lfs.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Brainard R.E., Watson L.J., Demartino A.M., Brittian K.R., Readnower R.D., Boakye A.A. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng H., Vaka V.R., He X., Booz G.W., Chen J.X. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19(8):1847–1856. doi: 10.1111/jcmm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceylan-Isik A.F., Kandadi M.R., Xu X., Hua Y., Chicco A.J., Ren J. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol. 2013;63:4–13. doi: 10.1016/j.yjmcc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Cingolani O.H., Kass D.A. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301(6):H2198–H2206. doi: 10.1152/ajpheart.00781.2011. [DOI] [PubMed] [Google Scholar]
- 50.Chirinos J.A. Ventricular-arterial coupling: Invasive and non-invasive assessment. Artery Res. 2013;7(1) doi: 10.1016/j.artres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wencker D., Chandra M., Nguyen K., Miao W., Garantziotis S., Factor S.M. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111(10):1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibbons G.H., Dzau V.J. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 53.VanBavel E., Spaan J.A. Branching patterns in the porcine coronary arterial tree. Estimation of flow heterogeneity. Circ Res. 1992;71(5):1200–1212. doi: 10.1161/01.res.71.5.1200. [DOI] [PubMed] [Google Scholar]
- 54.Donovan M.J., Lin M.I., Wiegn P., Ringstedt T., Kraemer R., Hahn R. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127(21):4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 55.Kermani P., Hempstead B. BDNF actions in the cardiovascular system: roles in development, adulthood and response to injury. Front Physiol. 2019;10:455. doi: 10.3389/fphys.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niemann B., Rohrbach S., Miller M.R., Newby D.E., Fuster V., Kovacic J.C. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol. 2017;70(2):230–251. doi: 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegrist J., Sies H. Disturbed redox homeostasis in oxidative distress: a molecular link from chronic psychosocial work stress to coronary heart disease? Circ Res. 2017;121(2):103–105. doi: 10.1161/CIRCRESAHA.117.311182. [DOI] [PubMed] [Google Scholar]
- 58.Jeong M.Y., Jang H.M., Kim D.H. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci Lett. 2019;698:51–57. doi: 10.1016/j.neulet.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Prabhu V.V., Nguyen T.B., Cui Y., Oh Y.E., Lee K.H., Bagalkot T.R. Effects of social defeat stress on dopamine D2 receptor isoforms and proteins involved in intracellular trafficking. Behav Brain Funct. 2018;14(1):16. doi: 10.1186/s12993-018-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komada M., Takao K., Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rauramaa T., Pikkarainen M., Englund E., Ince P.G., Jellinger K., Paetau A. Cardiovascular diseases and hippocampal infarcts. Hippocampus. 2011;21(3):281–287. doi: 10.1002/hipo.20747. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki H., Sumiyoshi A., Matsumoto Y., Duffy B.A., Yoshikawa T., Lythgoe M.F. Structural abnormality of the hippocampus associated with depressive symptoms in heart failure rats. Neuroimage. 2015;105:84–92. doi: 10.1016/j.neuroimage.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 64.Munoz-Lopez M., Hoskote A., Chadwick M.J., Dzieciol A.M., Gadian D.G., Chong K. Hippocampal damage and memory impairment in congenital cyanotic heart disease. Hippocampus. 2017;27(4):417–424. doi: 10.1002/hipo.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeomans M.R. Adverse effects of consuming high fat-sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc. 2017;76(4):455–465. doi: 10.1017/S0029665117000805. [DOI] [PubMed] [Google Scholar]
- 66.Laine M.A., Sokolowska E., Dudek M., Callan S.A., Hyytia P., Hovatta I. Brain activation induced by chronic psychosocial stress in mice. Sci Rep. 2017;7(1):15061. doi: 10.1038/s41598-017-15422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kesner R.P. An analysis of dentate gyrus function (an update) Behav Brain Res. 2018;354:84–91. doi: 10.1016/j.bbr.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 68.Deltheil T., Guiard B.P., Guilloux J.P., Nicolas L., Delomenie C., Reperant C. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: studies in adult mice hippocampus. Pharmacol Biochem Behav. 2008;90(2):174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Aarse J., Herlitze S., Manahan-Vaughan D. The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus. 2016;26(6):739–751. doi: 10.1002/hipo.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergami M., Rimondini R., Santi S., Blum R., Götz M., Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105(40):15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasan R.S. Cardiac function and obesity. Heart. 2003;89(10):1127–1129. doi: 10.1136/heart.89.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pascual M., Pascual D.A., Soria F., Vicente T., Hernandez A.M., Tebar F.J. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89(10):1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rozanski A., Blumenthal J.A., Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 74.Hagstrom E., Norlund F., Stebbins A., Armstrong P.W., Chiswell K., Granger C.B. Psychosocial stress and major cardiovascular events in patients with stable coronary heart disease. J Intern Med. 2018;283(1):83–92. doi: 10.1111/joim.12692. [DOI] [PubMed] [Google Scholar]
- 75.Wideman C.H., Cierniak K.H., Sweet W.E., Moravec C.S., Murphy H.M. An animal model of stress-induced cardiomyopathy utilizing the social defeat paradigm. Physiol Behav. 2013;120:220–227. doi: 10.1016/j.physbeh.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Finnell J.E., Lombard C.M., Padi A.R., Moffitt C.M., Wilson L.B., Wood C.S. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engel D., Peshock R., Armstong R.C., Sivasubramanian N., Mann D.L. Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression. Am J Physiol Heart Circ Physiol. 2004;287(3):H1303–H1311. doi: 10.1152/ajpheart.00053.2004. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z., Li L., Zhao H., Peng S., Zuo Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism. 2015;64(8):917–925. doi: 10.1016/j.metabol.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan Y., Zeng L., Zheng C., Song B., Li F., Kong X. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9:2649. doi: 10.3389/fimmu.2018.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Constantinides C., Mean R., Janssen B.J. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 2011;52(3):e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 81.Abuderman A., Abbas M. Morphological changes evaluation of left atrial appendage in patients with ischaemic heart disease. Biom J. 2016;39(4):277–282. doi: 10.1016/j.bj.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto S., Yang G., Zablocki D., Liu J., Hong C., Kim S.J. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111(10):1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okada S., Yokoyama M., Toko H., Tateno K., Moriya J., Shimizu I. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler Thromb Vasc Biol. 2012;32(8):1902–1909. doi: 10.1161/ATVBAHA.112.248930. [DOI] [PubMed] [Google Scholar]
- 84.Kannan K., Jain S.K. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 85.Zeng N., Xu J., Yao W., Li S., Ruan W., Xiao F. Brain-derived neurotrophic factor attenuates septic myocardial dysfunction via eNOS/NO pathway in rats. Oxid Med Cell Longev. 2017;2017:1721434. doi: 10.1155/2017/1721434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hang P., Zhao J., Sun L., Li M., Han Y., Du Z. Brain-derived neurotrophic factor attenuates doxorubicin-induced cardiac dysfunction through activating Akt signalling in rats. J Cell Mol Med. 2017;21(4):685–696. doi: 10.1111/jcmm.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behl T., Kotwani A. Downregulated brain-derived neurotrophic factor-induced oxidative stress in the pathophysiology of diabetic retinopathy. Can J Diabetes. 2017;41(2):241–246. doi: 10.1016/j.jcjd.2016.08.228. [DOI] [PubMed] [Google Scholar]
- 88.Yoo J.M., Lee B.D., Sok D.E., Ma J.Y., Kim M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/antioxidant enzyme in neuronal cells. Redox Biol. 2017;11:592–599. doi: 10.1016/j.redox.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Templin C., Hanggi J., Klein C., Topka M.S., Hiestand T., Levinson R.A. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J. 2019;40(15):1183–1187. doi: 10.1093/eurheartj/ehz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zorn J.V., Schur R.R., Boks M.P., Kahn R.S., Joels M., Vinkers C.H. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36. doi: 10.1016/j.psyneuen.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 91.Quinlan E.B., Cattrell A., Jia T., Artiges E., Banaschewski T., Barker G. Psychosocial stress and brain function in adolescent psychopathology. Am J Psychiatry. 2017;174(8):785–794. doi: 10.1176/appi.ajp.2017.16040464. [DOI] [PubMed] [Google Scholar]
- 92.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murinova J., Hlavacova N., Chmelova M., Riecansky I. The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: a systematic review. Front Behav Neurosci. 2017;11:101. doi: 10.3389/fnbeh.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Razzoli M., Pearson C., Crow S., Bartolomucci A. Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76(Pt A):154–162. doi: 10.1016/j.neubiorev.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu M., Zou W., Wang C.Y., Chen X., Tan H.Y., Zeng H.Y. Hydrogen sulfide protects against chronic unpredictable mild stress-induced oxidative stress in hippocampus by upregulation of BDNF-TrkB pathway. Oxid Med Cell Longev. 2016;2016:2153745. doi: 10.1155/2016/2153745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan T., He B., Xu M., Wu B., Xiao F., Bi K. Kaempferide prevents cognitive decline via attenuation of oxidative stress and enhancement of brain-derived neurotrophic factor/tropomyosin receptor kinase B/cAMP response element-binding signaling pathway. Phytother Res. 2019;33(4):1065–1073. doi: 10.1002/ptr.6300. [DOI] [PubMed] [Google Scholar]
- 97.Watanabe H., Iqbal M., Zheng J., Wines-Samuelson M., Shen J. Partial loss of presenilin impairs age-dependent neuronal survival in the cerebral cortex. J Neurosci. 2014;34(48):15912–15922. doi: 10.1523/JNEUROSCI.3261-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson Ray M., Weickert C.S., Wyatt E., Webster M.J. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36(3):195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dandi E., Kalamari A., Touloumi O., Lagoudaki R., Nousiopoulou E., Simeonidou C. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int J Dev Neurosci. 2018;67:19–32. doi: 10.1016/j.ijdevneu.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 101.Klein A.B., Williamson R., Santini M.A., Clemmensen C., Ettrup A., Rios M. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 102.Mattson M.P., Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16(3):129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Alomari M.A., Khabour OF, Alzoubi K.H., Alzubi M.A. Combining restricted diet with forced or voluntary exercises improves hippocampal BDNF and cognitive function in rats. Int J Neurosci. 2016;126(4):366–373. doi: 10.3109/00207454.2015.1012587. [DOI] [PubMed] [Google Scholar]
- 104.Gomez-Pinilla F., Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3(1):403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.