Abstract
Background
Metastatic bladder cancer (BLCA) is a lethal disease with an unmet need for study. Transgelin (TAGLN) is an actin-binding protein that affects the dynamics of the actin cytoskeleton indicating its robust potential as a metastasis initiator. Here, we sought to explore the expression pattern of TAGLN and elucidate its specific functioning and mechanisms in BLCA.
Methods
A comprehensive assessment of TAGLN expression in BLCA was performed in three cohorts with a total of 847 patients. The potential effects of TAGLN on BLCA were further determined using clinical genomic analyses that guided the subsequent functional and mechanistic studies. In vitro migration, invasion assays and in vivo metastatic mouse model were performed to explore the biological functions of TAGLN in BLCA cells. Immunofluorescence, western blot and correlation analysis were used to investigate the molecular mechanisms of TAGLN.
Findings
TAGLN was highly expressed in BLCA and correlated with advanced prognostic features. TAGLN promoted cell colony formation and cell migration and invasion both in vitro and in vivo by inducing invadopodia formation and epithelial-mesenchymal transition, during which a significant correlation between TAGLN and Slug was observed. The progression-dependent correlation between TGF-β and TAGLN was analysed at both the cellular and tissue levels, while TGF-β-mediated migration was abolished by the suppression of TAGLN.
Interpretation
Overall, TAGLN is a promising novel prognosis biomarker of BLCA, and its metastatic mechanisms indicate that TAGLN may represent a novel target agent that can be utilized for the clinical management of invasive and metastatic BLCA.
Fund
This work was supported by the National Natural Science Foundation of China [81772703, 81672546, 81602253]; the Natural Science Foundation of Beijing [71772219, 7152146]. and Innovative Fund for Doctoral Students of Peking University Health Science Center (BUM2018BSS002). Funders had no role in the design of the study, data collection, data analysis, interpretation, or the writing of this report.
Keywords: Bladder cancer, Transgelin, Metastasis, EMT, Invadopodia, TGF-β
Research in context.
Evidence before this study
Metastatic bladder cancer remains a complex disease with poor survival outcomes and limited treatment. Better and deeper understanding of metastatic bladder cancer is definitely needed. Transgelin (TAGLN) is conserved cytoplasmic protein that primarily participates in remodeling of actin cytoskeleton. TAGLN was implicated in cancer development in a series of controversial studies. Decreased levels of TAGLN have been observed in some cancerous tissues, while increased levels have been linked with poor prognosis and metastasis, including bladder cancer. The specific expression pattern, functioning and mechanisms of TAGLN in bladder cancer remain largely unknown.
Added value of this study
According to our results, TAGLN was highly expressed in bladder cancer and correlated with advanced prognostic features, including stage, grade and overall survival. In line with the functional prediction, the inhibition of TAGLN repressed cell migration and invasion in vitro and led to a decrease in the number and sizes of lung metastases in vivo. Mechanistically, we found that TAGLN promotes metastasis by inducing invadopodia formation and EMT. The progression-dependent correlation between TGF-β and TAGLN was found at both the cellular and tissue levels. The higher correlation was determined for the subgroups with advanced clinicopathological features. Notably, TGF-β-mediated migration would be totally abolished by the suppression of TAGLN.
Implications of all the available evidence
Our study demonstrated that TAGLN, via its promotion of metastasis, may serve as a promising biomarker and new target agent for follow-up and treatment.
Alt-text: Unlabelled Box
1. Introduction
Bladder cancer (BLCA) is a complex disease with high morbidity and mortality rates despite improvements in its management [1]. Roughly 25% of newly diagnosed BLCA patients are diagnosed with muscle-invasive or metastatic disease. Non-muscle-invasive BLCA patients, meanwhile, continue to suffer from high recurrence (50%–70%) and progression (10%–30%) rates [[2], [3], [4]]. Advanced and metastatic BLCA is a lethal disease that produces poor survival outcomes. Almost all patients who succumb to BLCA have suffered from distant failure and/or symptomatic metastasis at the time of death [5]. Once cancer spreads, systemic cisplatin-based chemotherapy is the only standard treatment that can be used and usually only yields a 50% initial response rate, which results in 5-year overall and progression-free survival of <15% [6,7]. Meanwhile, patients nearly always suffer side effects resulting from chemotherapy. Although surgical resection is also an optional treatment that can be used, only a very select group of patients with limited metastatic burden can benefit [8]. Rapid developments over the past few years have shed light on the use of immunotherapy and target therapy in the treatment of BLCA [9]. An improved understanding of invasive BLCA has emerged due a substantial increase in molecular research that allows for the identification of the chemotherapeutic sensitivity of invasive BLCA based on its molecular subtype [10]. Increased research of invasive and metastatic BLCA will have important implications for future clinical target development and disease management.
Transgelin (TAGLN) is a well-known actin-binding protein that affects the dynamics of the actin cytoskeleton [11,12]. Owing to progress in the field of cancer biomarker discovery, studies have been conducted that TAGLN expression is decreased and dysfunctional in various cancers and that, at first, resulted in its being considered as a tumour suppressor [13]. However, subsequent studies of TAGLN and the optimization of its tissue detection produced results that were contradictory, even for the same type of tumour. Notably, its robust potential as a metastasis initiator has also been highlighted [14]. Decreased levels of TAGLN have been observed in some cancerous tissues, while increased levels have been linked with poor prognosis and metastasis other types of cancer, such as oesophageal cancer [15,16], pancreatic cancer [17,18] and colorectal cancer [19,20]. The ambiguous role of TAGLN in BLCA has also been reported. TAGLN was shown to be downregulated in BLCA tissues [21,22], while its expression level was observed to be upregulated during the transition to muscle-invasive cancer [21]. Recently, some RNA-level bioinformatics analyses have also highlighted the vital role of TAGLN in BLCA [23,24]. However, none of these studies explains the controversial expression patterns of TAGLN that have been observed. The precise expression pattern and molecular function of TAGLN in BLCA remain unknown.
Herein, we first demonstrated that TAGLN was overexpressed in BLCA. The high expression level of TAGLN markedly contributed to advanced clinicopathological features and poor prognosis in three patient cohorts. Consistent with the functional prediction performed for TAGLN, TAGLN promotes migration and invasion of BLCA cells both in vitro and in vivo. Importantly, inhibition of TAGLN suppressed invadopodia formation and epithelial-mesenchymal transition (EMT). Additionally, a progression-dependent correlation between TGF-β and TAGLN was observed in BLCA. TAGLN was also essential for the development of TGF-β-mediated BLCA metastasis. By combining bioinformatics analyses with functional and mechanistic studies, the complete picture of the role of TAGLN in BLCA has been drawn, which contributes to an improved understanding of cancer metastasis and provides a novel target agent for the purposes of follow-up and treatment.
2. Materials and methods
2.1. Collection of patient tissues and data
Paraffin-embedded BLCA tissues and adjacent noncancerous tissues were obtained from patients who underwent radical cystectomy at Peking University First Hospital between 2007 and 2012 (PKU-cohort, Supplementary Table S1). All the post-operative patients were followed up every three months for the first two years and then at least once year thereafter by phone or visit at our institute. The median follow-up was 42 months (range: 4–90). 79 patients received intravesical chemotherapy before radical cystectomy and 59 patients received adjuvant chemotherapy. Total 62 patients had cancer metastasis. Twenty-seven paired, freshly isolated BLCA tissues and adjacent noncancerous tissues were obtained from patients who underwent radical cystectomy at Peking University First Hospital between 2015 and 2017; all fresh tissues were immediately snap-frozen in liquid nitrogen. All samples were verified by at least two pathologists. Tumour stage was classified based on the 2002 UICC TNM classification of malignant tumours, while histological grade was assessed based on the WHO classification of 1973. The studies were conducted in accordance with the approved guidelines. Written informed consent was obtained from all subjects and all experimental protocols were approved by the ethics committee at Peking University First Hospital.
2.2. Antibodies and immunohistochemistry analysis
The antibodies used in this study are listed in Supplementary Table S2. All paraffin-embedded tissues were cut into 5 μm sections and placed on slides. After deparaffinization, the tissue sections were treated with citrate buffer (10 mM, pH 6.0) at 95 °C for 2.5 min for antigen retrieval. Subsequently, the slides were immersed in 3% H2O2 for 20 min to block endogenous peroxidases and were then incubated with 10% normal goat serum at room temperature for 60 min to reduce nonspecific binding. The samples were subsequently incubated in the presence of anti-TAGLN or anti-Slug primary antibody at 4 °C overnight and were then processed using the Power-VisionTM two-step histostaining reagent and a 3,3-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-Bio, China) according to the manufacturer's protocol. Each sample was graded as – (negative), + (weak), ++ (moderate) or +++ (strong) based on the staining intensity by two independent pathologists.
2.3. RNA extraction and quantitative reverse-transcription PCR (RT-qPCR)
Total RNA from tissues or cells was extracted using TRIzol reagent (Invitrogen, USA) and reverse-transcribed using a Fastking RT-PCR kit (Tiangen, China) according to the manufacturer's instructions. Quantitative PCR was conducted using SYBR Green Premix reagent (Tiangen, China) and an ABI PRISM 7000 Fluorescent Quantitative PCR System (Applied Biosystems, USA) according to the manufacturer's instructions. The sequences of the primers used in this study are listed in Supplementary Table S3. The data analysis was performed using the ΔΔCt method, and expression was normalized to that of β-actin.
2.4. In silico analysis of TAGLN using online datasets
A transcriptome dataset obtained from BLCA tissues (TCGA-BLCA) from the Cancer Genome Atlas (TCGA) was downloaded using the UCSC Xena browser in June 2018; data from the GSE13507-BLCA cohort was obtained from the gene expression omnibus (GEO) dataset GSE13507. The patients in each cohort were sorted into high and low groups based on the median of TAGLN expression level. Differentially expressed genes (DEGs) were selected using the TwoClassDif method [25,26]. All enrichment analyses were performed using Metascape (http://metascape.org) [27]. The assessment and integration of protein–protein interactions were conducted using the STRING database [28] and modelled using Cytoscape [29]. The minimum-required interaction score generated using STRING was set to that corresponding to a medium confidence level (0.400). The IHC images in Supplementary Fig. S4b were obtained from Human Protein Atlas (www.proteinatlas.org) [30].The prognostic analyses shown in Supplementary Fig. S4c–h were obtained from GEPIA (http://gepia.cancer-pku.cn/index.html) [31].
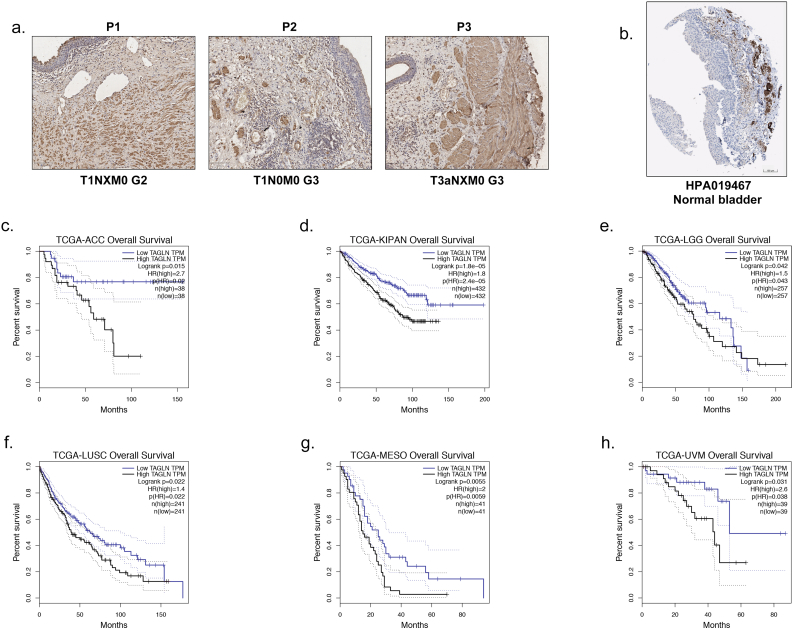
Supplementary Fig. S4.
IHC staining of TAGLN in normal bladder tissues and prognosis analyses of TAGLN in other cancers. a. Representative IHC staining images of TAGLN in normal bladder tissues in PKU-BLCA cohort. Scale bars, 100 μm. b. Representative IHC staining images of TAGLN in normal bladder tissues from Human Protein Atlas (HPA019467). Scale bars, 100 μm. c-h. The negative correlation between TAGLN expression and overall survival of various cancer in TCGA cohorts estimated by Kaplan-Meier curves. Cutoff values of TAGLN in TCGA cohort were defined by medium.
2.5. Cell lines and cell culture
Authenticated SV-HUC-1, SW780, 5637, T24, and 293 T cells were obtained from the American Type Culture Collection (ATCC). The SV-HUC-1 cells were cultured in F-12 K (Gibco, USA), the SW780, T24 and 293 T cells were cultured in DMEM (Gibco, USA) and the 5637 cells were cultured in RPMI 1640 (Gibco, USA). All media were supplemented with 10% foetal bovine serum and 1% penicillin-streptomycin. All cells were cultured in a humified atmosphere containing 5% CO2 at 37 °C and were maintained in low passage cultures throughout the experiments.
2.6. Western blotting
The total cellular protein was extracted using RIPA (Millipore, USA) and 1 mM phenylmethylsulfonyl fluoride (PMSF) and was quantified via a bicinchoninic acid assay (Sigma, USA). The lysates were then separated using SDS–PAGE and electroblotted onto polyvinylidene difluoride membranes. After incubation with the primary and secondary antibodies in sequence, the bands were visualized via enhanced chemiluminescence using an ImmobilonTM Western Kit (Millipore, USA). β-actin served as a loading control.
2.7. Immunofluorescence assay
Cells were cultured on glass coverslips for 24 h and subsequently fixed with 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.2% Triton. The coverslips were then probed with the primary antibody and the corresponding fluorescent secondary antibody. The labelled cells were then stained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted using antifade mounting medium. Phalloidin-tetramethylrhodamine B isothiocyanate (Sigma, USA) was used to label actin. All slides were examined using multiphoton lifetime confocal laser-scanning microscopy (TCS SP8; Leica, Germany).
2.8. Cell transfection
The plasmids used in this study were purchased from Beijing Syngentech. The shRNA sequences that were used are shown in Supplementary Table S3. The plasmids of interest and the packaging plasmids into were co-transfected into 293 T cells for the purposes of lentiviral production, and subsequently the viral supernatants were titrated using RT-qPCR for which the WPRE (woodchuck hepatitis virus posttranscriptional regulatory element) was used as the primer. All BLCA cells were infected with lentivirus at a MOI (multiplicity of infection) of 10. The stable cells were selected using puromycin.
2.9. Cell viability, cell cycle, and cell colony formation assays
The cell viability assays were performed using the CellTiter 96® AQ One Solution Reagent (Promega, USA). The cell cycle assays were performed using a Cell Cycle Detection Kit (KeyGEN BioTECH, China). All procedures were performed according to the manufacturer's instructions. For the cell colony formation assay, 1000 cells/well were seeded in a 6-well plate and cultured for 10 days. The colonies were then fixed and stained with a 0.5% crystal violet methanol solution for 30 min and scanned to generate images.
2.10. Cell migration and invasion assays
Cell migration was first assessed via wound healing assays. After seeding the cells in a 6-well plate for 24 h, straight vertical and horizontal lines were scratched using a 200 μl pipette tip. Images were obtained using the digital camera that was attached to the microscope immediately after the scratches were made and 48 h later. Cell migration was also measured via transwell migration assays using chambers with membranes containing 8 μm pores (Corning, USA). The cells were suspended in 100 μl of serum-free media and seeded in the upper chamber, and the lower chamber was filled with 500 μl of complete media. After incubation at 37 °C for 24 h, the transwell membranes were fixed and stained with 0.5% crystal violet methanol solution for 30 min. The un-migrated cells were removed using cotton swabs and photographed with the digital camera. The cell invasion assays were performed similarly to the transwell migration assays with transwell membranes that were coated with Matrigel (BD Biosciences) and were incubated for 48 h.
2.11. Tail vein injection metastatic mouse model
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals with the approval of the Institutional Animal Care and Use Committee of Peking University First Hospital. T24-Luc cells, which are T24 cells that stably express luciferase, were used in this model. One hundred microliters of cell suspension (2 × 107/ml) was injected into the lateral tail vein of each 6-week-old B-NDG mouse (Biocytogen, China). Six weeks after injection, the mice were anesthetized with isoflurane (Yipin Pharmaceutical, China), and then D-luciferin sodium salt (Biovision, USA) was injected intraperitoneally according to the manufacturer's instructions. The metastatic lesions were visualized using a Xenogen IVIS system (PerkinElmer, USA) and were then harvested and prepared for H&E staining and IHC.
2.12. Statistical analysis
All experiments were performed independently at least three times except for the experiments shown in Fig. 1, Fig. 2, Fig. 3f–g, 4e, 5a–f, Supplementary Figs. S1, S2c, and S3–4. The cut-off values used for TAGLN in Supplementary Fig. S1f and 1 g were defined using X title [32]. Univariate survival analyses were performed by Kaplan-Meier estimator (Log-rank test) and multivariate analyses used backward method (Wald test). All meta-analyses were performed with Stata12.0 (StataCorp LP, USA). All statistical tests were performed using SPSS software version 22.0 (SPSS, USA). A P value <.05 was considered to be statistically significant.
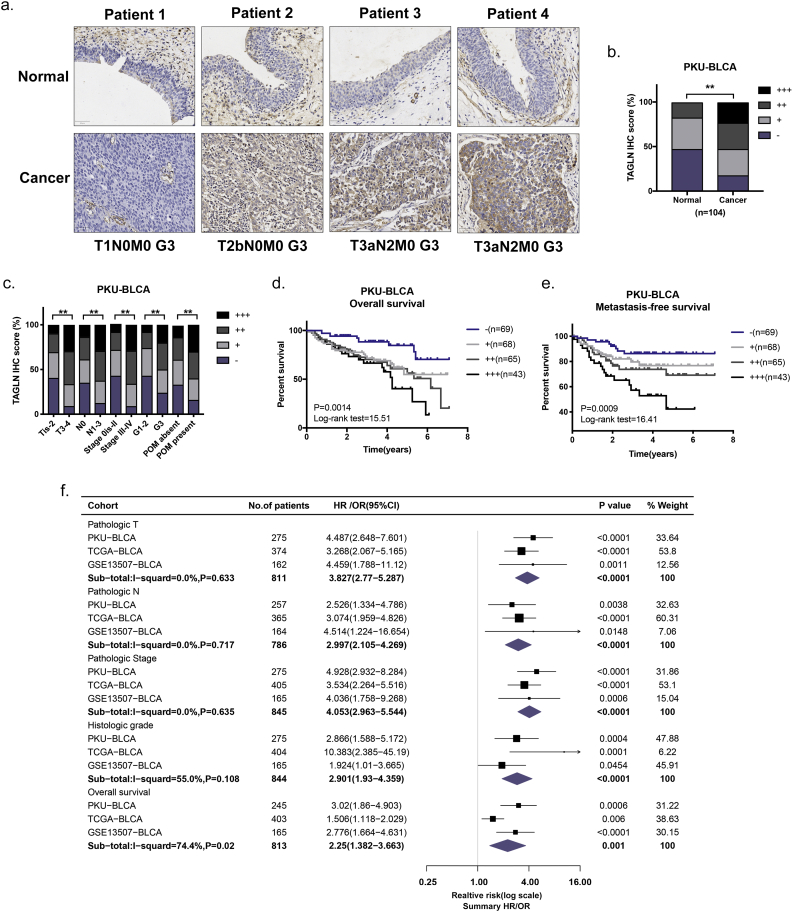
Fig. 1.
TAGLN overexpression correlates with the presence of poor prognostic features in BLCA. a. Representative immunohistochemical staining images of TAGLN protein in cancer tissues and paired normal tissues. P1-P4 were four representative patients with different clinicopathological features. Scale bar, 50 μm. b. Percentage distribution of TAGLN IHC intensity in cancer versus normal tissues. The staining intensity was graded as – (negative), + (weak), ++ (moderate) or +++ (strong). c. Percentage distribution of TAGLN IHC intensity in BLCA with various clinicopathological features. POM, postoperative cancer metastasis. d. Kaplan-Meier curves of the TAGLN IHC intensity on OS in PKU-BLCA cohort. e. Kaplan-Meier curves of the TAGLN IHC intensity on MFS in PKU-BLCA cohort. f. General prognostic signatures and meta-analyses of TAGLN in three cohort. All of the prognostic events of TAGLN are illustrated in forest plots, with corresponding P value and 95% CI. The estimated effect size of each event is presented as a black square that is proportional in size to the weight of the study. The pooled effect size is presented as a red rhombus that is sized in the center for summary effect size and whose width depicts the confidence interval. The confidence interval of effect size appears as a horizontal. The n values indicate the number of patients. *, P < .05; **, P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
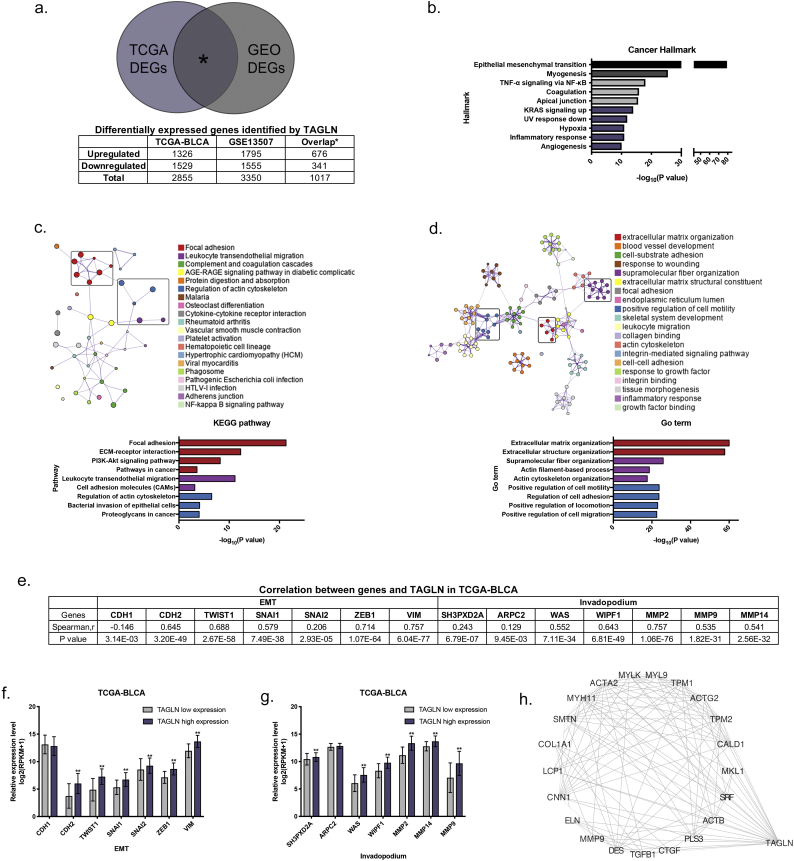
Fig. 2.
Functional prediction of TAGLN based on the online dataset. a. A Venn diagram of consensus differentially expressed genes (DEGs) across TCGA-BLCA cohort and GSE13507-BLCA cohort. b. Cancer hallmark enrichment analysis results for consensus upregulated DEGs. c-d. KEGG pathway (c) and GO term (d) enrichment analysis results for consensus upregulated DEGs. Individual gene ontology terms with similar gene members are clustered by categories (node colour) and labelled using a representative member, where terms with a Kappa similarity score > 0.3 are connected by edges. Node size is proportional to statistical significance (enrichment P value). The clusters highlighted by the rectangular region were further annotated. e. Correlation between EMT- and invadopodium-associated genes and TAGLN in TCGA-cohort assessed by Spearman's rho. f-g. The expression level of EMT- (f) and Invadopodium- (g) associated genes in low- and high- TAGLN expression groups in TCGA cohort. Error bars represent SD (standard deviation) of the mean. *, P < .05; **, P < .01. h. The protein-protein interaction network of TAGLN and 676 consensus upregulated DEGs.
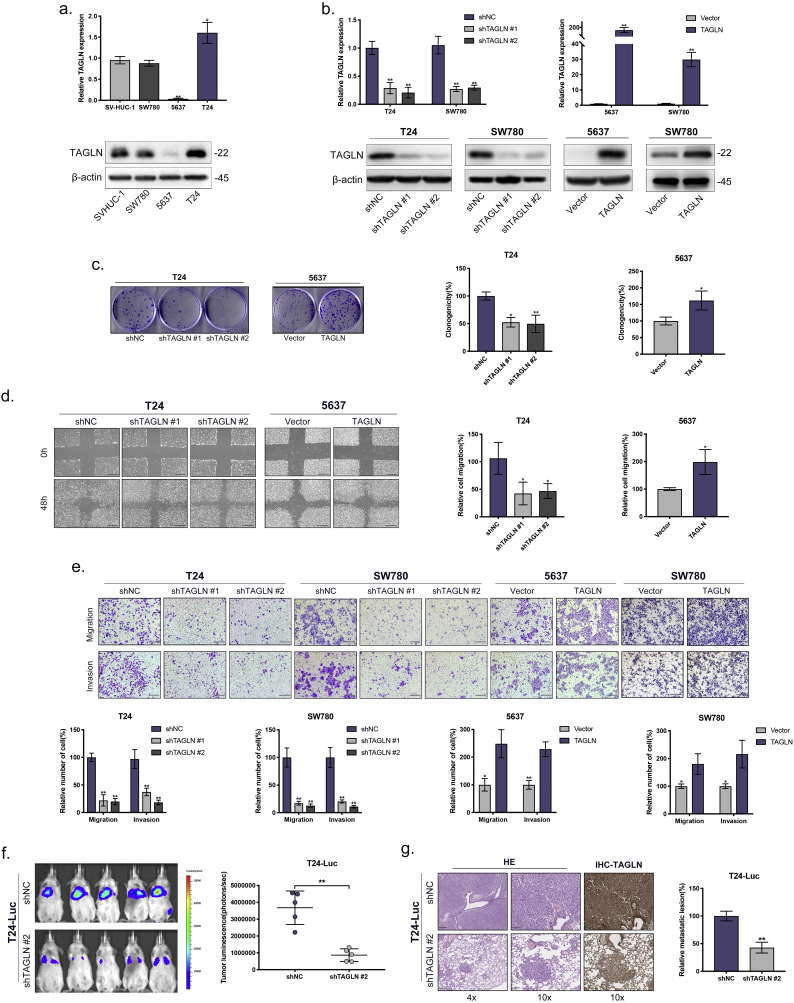
Fig. 3.
TAGLN promotes the migration and invasion of BLCA cells in vitro and in vivo. a. RT-qPCR and western blot analyses of TAGLN in the normal uroepithelium cell line (SV-HUC-1) and BLCA cells (SW780, 5637 and T24). b. RT-qPCR and western blot analyses of BLCA cells infected with a lentivirus-mediated TAGLN-overexpressing vector or TAGLN shRNAs. c. Clone formation assay. Representative images of plates seeded with BLCA cells and quantification of colony formation after 10 day. d. Wound-induced migration assay. Representative images of wound-induced cell migration by the T24-shTAGLN, 5637-TAGLN and control cells. Scale bars, 100 μm. And quantification of migration by the described cells. e. Transwell migration and invasion assay. Representative images of transwell migration and invasion assay of TAGLN-silenced cells and TAGLN-overexpressed cells. Scale bars, 100 μm. And quantification of transwell migration and invasion by the described cells. f-g. Tail vein-injected T24-luc metastasis model. Representative IVIS images of mice injected TAGLN-silenced or control cells and analysis of tumour luminescence representing lung metastasis measured on day 42. Five mice in each group (f). Lung metastasis was confirmed by H&E and IHC-TAGLN staining. Scale bars, 250 μm and 100 μm. And quantification of the metastatic lesion(g). Error bars represent SD of the mean. *, P < .05; **, P < .01.
Fig. 4.
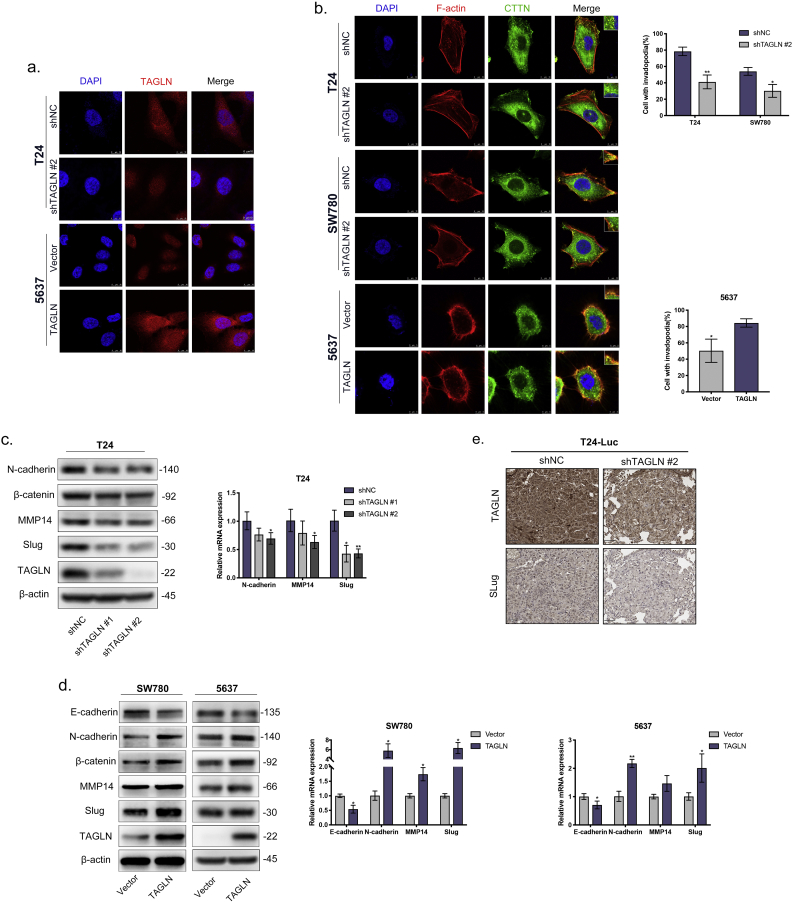
TAGLN promotes invadopodia formation and EMT. a. Representative anti-TAGLN immunofluorescence images of TAGLN-silenced cells and TAGLN-overexpressed cells. Cells were counterstained with DAPI; combined images were indicated. Scale bar, 10 μm. b. Representative invadopodia immunofluorescence images in BLCA cells. TAGLN-silenced cells, TAGLN-overexpressed cells and control cells stained for nuclei (blue), F-actin (red), and. CTTN (green). Quantification of the percentage of cells with invadopodia. N = 50 cells/sample. Scale bars, 10 μm in T24 and SW780(a); 5 μm in 5637(b). c-d. Western blot and RT-qPCR analyses of EMT marker and transcription factors after TAGLN depletion in T24 (c) and TAGLN overexpression in SW780 and 5637 (d). e. Representative IHC staining images of TAGLN and Slug in lung metastatic of tail vein-injected T24-Luc metastasis model. Scale bars, 50 μm. Error bars represent SD of the mean. *, P < .05; **, P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
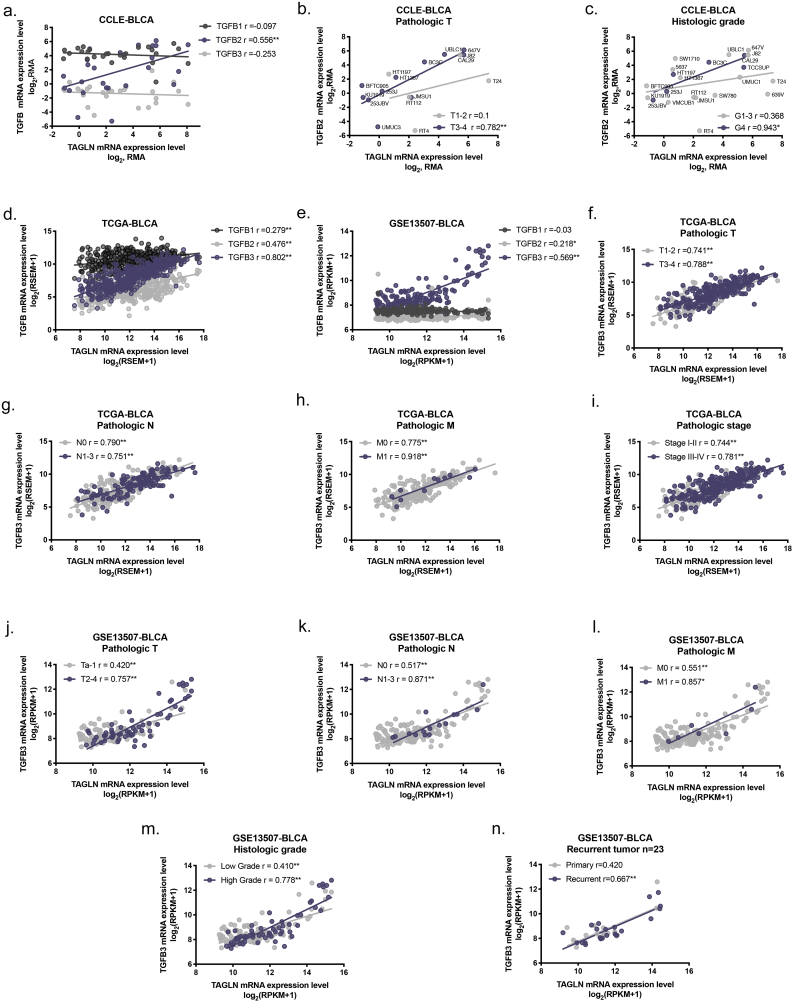
Fig. 5.
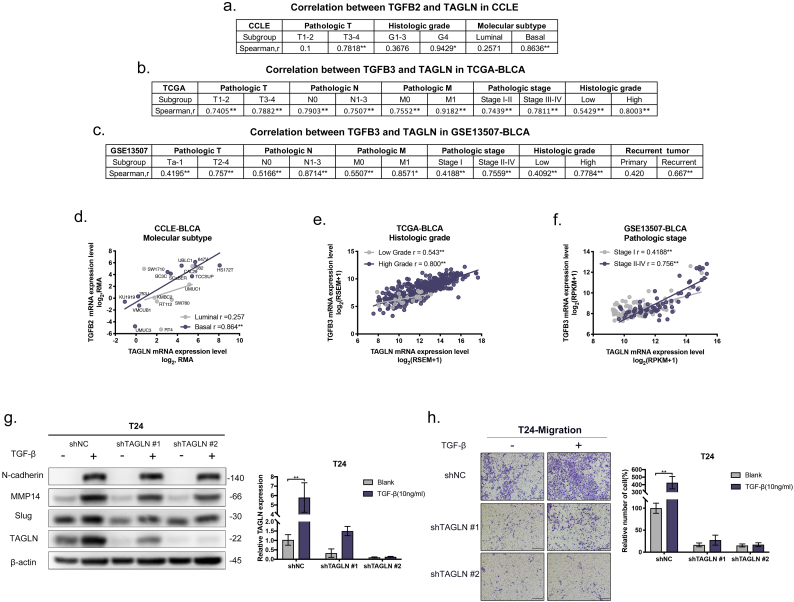
TALGN is essential for the promotion of BLCA metastasis by TGF-β. a. Summary of correlation between TGFB2 and TAGLN of different featured subgroups in CCLE. b-c. Summary of correlation between TGFB3 and TAGLN of different clinicopathological subgroups in TCGA-BLCA cohort(b)and GSE13507-BLCA cohort (c). d-f. Representative correlation graphs of TGFB and TAGLN in different featured subgroups. Molecular subtype in CCLE-BLCA(d). Histologic grade in TCGA-BLCA(e). Pathologic stage in GSE13507-BLCA(f). g. Western blot analyses of N-cadherin, MMP14, Slug, and TAGLN of T24-shTAGLN and control cultured in 10 nM TGF-β for 72 h. And RT-qPCR analyses of TAGLN expression in T24-shTAGLN and control after culturing in 10 nM TGF-β for 72 h. h. Representative images of transwell migration assay of T24-shTAGLN and control with and without 10 nM TGF-β stimulation. Scale bars, 100 μm. And quantification of transwell migration by the described cells. Error bars represent SD of the mean. *, P < .05; **, P < .01.
Supplementary Fig. S1.
TAGLN mRNA expression level in PKU-BLCA, TCGA-BLCA and GSE13507-BLCA cohort. a. RT-qPCR analysis of TAGLN expression in 27 cancer-normal paired frozen tissues. b-c. TAGLN mRNA expression between normal bladder tissues and BLCA tissues in TCGA-BLCA(b) and GSE13507-BLCA cohort(c). *, P < .05; **, P < .01. d-e. TAGLN expression level of clinicopathological features in TCGA-BLCA cohort (d) and GSE13507-BLCA cohort(e). f-g. Kaplan-Meier curves of the TAGLN expression level on OS in TCGA-BLCA cohort (f) and GSE13507-BLCA cohort(g). Cutoff values of TAGLN in TCGA-BLCA cohort and GSE13507-BLCA cohort were defined by X title.
Supplementary Fig. S2.
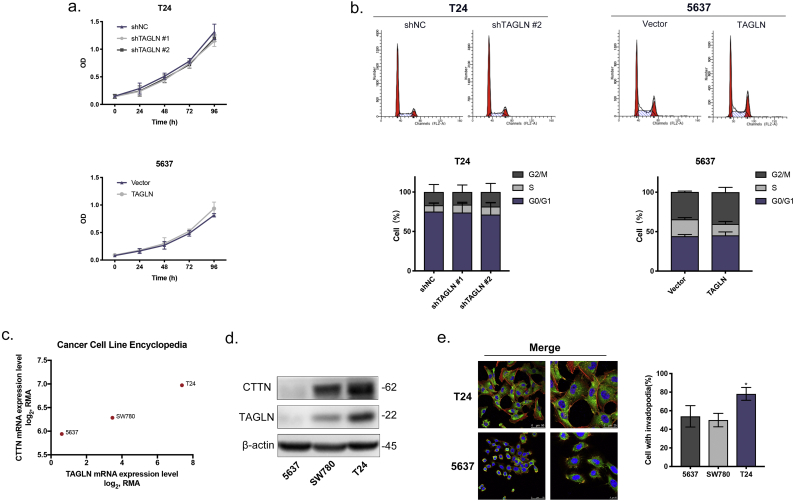
Function and mechanism studies of in BLCA cell lines. a. Cell viability assay showed cell count in replicate cultures cells for five days. b. Cell cycle assay. Representative images of cells stained with PI measured by flow cytometer and percentage distribution of cells in different phases of the cell cycle. c. TAGLN and CTTN expression level of 5637, SW780 and T24 in CCLE. d. Western blot analyses of CTTN and TAGLN of 5637, SW780 and T24. e. Representative merged immunofluorescence images of invadopodia in T24 and 5637. Scale bars, 50 μm and 25 μm in T24; 50 μm and 10 μm in 5637. And quantification of the percentage of BLCA cells with invadopodia. N = 50 cells/sample. Error bars represent SD of the mean. *, P < .05. d.
Supplementary Fig. S3.
Expression of TGF-β and TAGLN are tightly linked during the tumour development and progress in BLCA. a. The relationship of expression between TAGLN and TGFB1, TGFB2, TGFB3 in 26 BLCA cell lines included in CCLE by Spearman's correlation. The x-axis represents TAGLN expression level and the axis represents expression level of TGFB1 or TGFB2 or TGFB3. b-c. Correlation between TGFB2 and TAGLN in BLCA cell lines in various featured subgroups. Pathologic T(b). Histologic grade(c). d-e. The relationship of expression between TAGLN and TGFB1, TGFB2, TGFB3 in TCGA-BLCA cohort(d) and GSE13507-BLCA cohort(e). f-n. Correlation between TGFB3 and TAGLN in two cohort in various featured subgroups. *, P < .05; **, P < .01.
3. Results
3.1. TAGLN overexpression correlates with the presence of poor prognostic features in BLCA
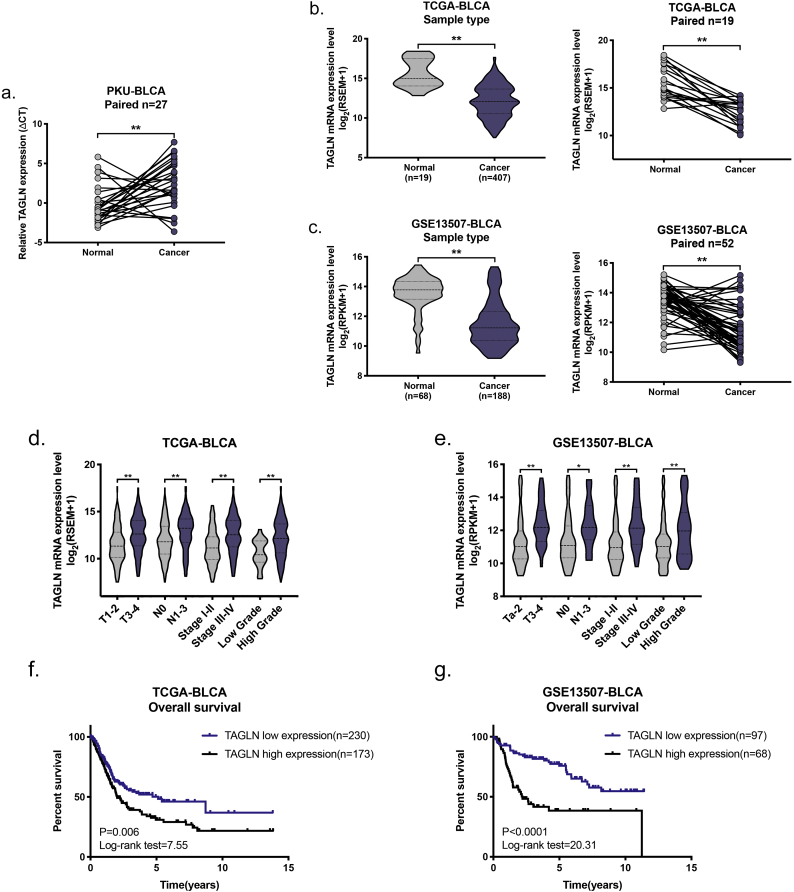
To validate the differences in the levels of TAGLN transcription in BLCA and normal tissue, TAGLN mRNA was measured using RT-qPCR in 27 paired frozen tissues. Consistent with the results of previous studies [21,22], TAGLN mRNA was downregulated in the BLCA tissues (Supplementary Fig. S1a). A similar result was also observed in both the TCGA-BLCA and GSE13507-BLCA cohorts (Supplementary Fig. S1b–c). To further verify the expression of TAGLN in BLCA and normal tissues, 104 paired paraffin-embedded tissue sections were examined using immunohistochemistry (IHC) staining. Unlike the RT-qPCR results, the IHC results showed that TAGLN was significantly upregulated in BLCA tissues compared with normal bladder tissues (Fig. 1a–b). Consistent with previous findings, TAGLN was mainly localized in the cytosol in both normal and cancerous tissues [14].
To confirm the relationship between TAGLN and BLCA, we next evaluated the correlation between the TAGLN expression level and clinicopathological features in three cohorts (Supplementary Table S1). In the PKU-BLCA cohort, statistical analysis of a total of 275 BLCA patients revealed that the TAGLN protein level was correlated with the pathologic T, pathologic N, pathologic stage and histologic grade (Fig. 1c, Table 1). Notably, higher TAGLN expression level contributed to higher rate of postoperative cancer metastasis. These results were confirmed in the other cohorts. The TAGLN mRNA level was significantly correlated with the pathologic T, pathologic N, pathologic stage and histologic grade in both the TCGA-BLCA and GSE13507-BLCA cohorts (Supplementary Fig. S1d–e). There was no significant correlation between TAGLN expression and the pathologic M due to the limited number of M1 patients in the cohorts. However, the number of patients with high TAGLN expression was twice as high as the number with low TAGLN expression in both two pathologic M1 cohorts (Supplementary Table S4). Furthermore, both univariate and multivariate analyses showed that patients with BLCA that expressed high levels of TAGLN had a shorter overall survival (OS) than patients with cancers that expressed low levels of TAGLN in all three cohorts (Fig. 1d, Table 2; Supplementary Fig. S1f–g, Supplementary Table S5). Crucially, we also found that high TAGLN level was significantly correlated with poor metastasis-free survival (MFS) of BLCA patients in PKU-BLCA cohort (Fig. 1e).
Table 1.
Correlation between TAGLN immunostaining intensity and clinicopathological features in PKU-BLCA cohort.
| Cohort characteristics | TAGLN immunostaining intensity |
P value | ||||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | |||
| Gender | Female | 12 | 12 | 9 | 6 | 0.9221 |
| Male | 69 | 63 | 64 | 40 | ||
| Age (median 67, year) | ≦67 | 48 | 43 | 30 | 25 | 0.1103 |
| >67 | 33 | 32 | 43 | 21 | ||
| Pathologic T | Tis-T1 | 45 | 30 | 10 | 6 | <0.0001 |
| T2 | 27 | 21 | 27 | 11 | ||
| T3 | 5 | 12 | 15 | 18 | ||
| T4 | 4 | 12 | 21 | 11 | ||
| Pathologic N | N0 | 73 | 55 | 52 | 28 | 0.0146 |
| N1 | 3 | 7 | 5 | 4 | ||
| N2 | 3 | 6 | 10 | 10 | ||
| N3 | 0 | 0 | 0 | 1 | ||
| Pathologic stage | I | 44 | 28 | 8 | 5 | <0.0001 |
| II | 27 | 19 | 24 | 9 | ||
| III | 4 | 14 | 23 | 18 | ||
| IV | 6 | 14 | 18 | 14 | ||
| Histologic grade | G1–2 | 32 | 23 | 13 | 6 | 0.0021 |
| G3 | 49 | 52 | 60 | 40 | ||
| Postoperative cancer metastasis | Absent | 33 | 28 | 25 | 13 | <0.0001 |
| Present | 16 | 14 | 30 | 30 | ||
Table 2.
Univariate and multivariate analyses of clinicopathological characteristics and TAGLN expression with overall survival in PKU-BLCA cohort.
| Cohort characteristics | Univariate analysisa |
Multivariate analysisb |
||
|---|---|---|---|---|
| HR(95% CI) | P value | HR(95% CI) | P value | |
| TAGLN | ||||
| +/++/+++ vs.– | 3.02(1.86–4.903) | 0.001 | 2.477(1.016–6.041) | 0.046 |
| Gender | ||||
| Female vs. Male | 1.171(0.602–2.28) | 0.642 | ||
| Age | ||||
| >median vs. ≤median | 1.654(1.046–2.616) | 0.031 | ||
| Pathologic T | ||||
| T3–4 vs. Tis-2 | 3.554(2.241–5.637) | <0.001 | 2.158(1.18–3.948) | 0.013 |
| Pathologic N | ||||
| N1–3 vs. N0 | 3.632(2.202–5.991) | <0.001 | 2.396(1.307–4.393) | 0.005 |
| Histologic grade | ||||
| High grade vs. Low grade | 2.172(1.171–4.03) | 0.014 | ||
| Postoperative chemotherapy | ||||
| Yes vs. No | 2.194(1.283–3.753) | 0.004 | ||
Univariate analysis were performed by Kaplan-Meier estimator (Log-rank test).
Multivariate analysis used backward method (Wald test).
To summarize the correlation between the TAGLN expression level and clinicopathological features in all three cohorts, meta-analyses were performed for each feature (Fig. 1f). Based on these meta-analyses, TAGLN, which showed high consistency for all features, was significantly associated with unfavourable prognosis for all four clinical features (T, OR, odds ratios: 3.827; N, OR: 2.997; stage, OR: 4.053; grade, OR: 2.901; P < .0001) and one survival feature (OS, HR, hazard ratio: 2.25; P = .001). All of the results demonstrated that increased TAGLN expression contributes to poor prognosis in BLCA patients, which suggests a potential link between TAGLN and BLCA progression.
3.2. Functional prediction of TAGLN in silico analysis
In order to reveal the potential function of TAGLN in BLCA, the patients were separated into low- and high- TAGLN expression groups based on the median expression level in the TCGA and GSE13507 cohorts, respectively. The differentially expressed genes (DEGs) in the two cohorts were determined (Fig. 2a, Supplementary Table S6-1). Additional enrichment analyses were performed using the consensus upregulated genes in the high TAGLN group. EMT-related genes were significantly highlighted during the cancer hallmark enrichment analysis (Fig. 2b, Supplementary Table S6-2). KEGG pathway and GO term enrichment analyses were also performed. Genes involved in focal adhesion, leukocyte trans-endothelial migration, regulation of the actin cytoskeleton and relevant member pathways were enriched (Fig. 2c, Supplementary Table S6-3). Terms related to extracellular matrix organization, supramolecular fibre organization, the positive regulation of cell motility and relevant member terms were also enriched (Fig. 2d, Supplementary Table S6-4). All enrichment analyses indicated that TAGLN is involved in actin cytoskeleton regulation, cell adhesion, cell motility and EMT in BLCA.
Actin is a ubiquitous and essential protein involved in tumour cell motility, structure and integrity that is also involved in the formation, stabilization and maturation of invadopodia in cancer [33,34]. Additionally, the role of TAGLN in the processes of cell migration and metastasis has been described in both smooth muscle cells and cancer cells, in which it contributes to the formation of podosomes [[35], [36], [37]]. Thus, we speculated that TAGLN may participate in invadopodia formation. Additional verification analyses were performed in the TCGA-BLCA cohort. Based on the Spearman correlation coefficient, both the EMT- and invadopodium- associated genes showed a significant correlation with TAGLN (Fig. 2e). The expression level of these genes also showed significant differences in the low- and high-TAGLN expression groups, which confirms the potential relationship of TAGLN with EMT and invadopodia (Fig. 2f–g). Next, the potential interaction targets of TAGLN in the protein-protein interaction network were determined (Fig. 2h), including actin-associated genes (ACTA2, ACTB, ACTG2) and EMT-associated genes (TGFB1, MMP9, COL1A1). Overall, these results indicated that TAGLN may be involved in cell migration and metastasis resulting from invadopodia formation and EMT in BLCA.
3.3. TAGLN promotes the clonogenicity of BLCA cells
To validate the predicted function of TAGLN, we performed a series of functional assays using the relevant cell lines. The expression levels of TAGLN in BLCA cells (SW780, 5637 and T24) and a normal uroepithelium cell line (SV-HUC-1) were examined using RT-qPCR and western blotting (Fig. 3a). Compared with SV-HUC-1, TAGLN was upregulated in T24 cells and downregulated in 5637 cells. BLCA cell lines were then established that stably overexpressed either TAGLN or an shRNA that targeted TAGLN (Fig. 3b). To assess the effects of TAGLN on proliferation, cell viability and cell cycle assays were performed on the T24-shTAGLN and 5637-TAGLN cells. Both assays showed that TAGLN expression had essentially no effect on cell growth or the distribution of cells in the cell cycle (Supplementary Fig. S2a–b). Interestingly, there were significant changes in colony formation (Fig. 3c). Both the number and size of the colonies were affected by the expression level of TAGLN. These results demonstrated that TAGLN was involved in the clonogenicity of BLCA cells rather than cell proliferation.
3.4. TAGLN promotes the migration and invasion of BLCA cells
To characterize the role of TAGLN in cell migration, a wound healing assay was performed in T24-shTAGLN and 5637-TAGLN cells. Their migratory capabilities were significantly decreased in T24-shTAGLN cells and increased in 5637-TAGLN cells (Fig. 3d). These results were also confirmed using transwell migration assays (Fig. 3e). Similarly, invasive capability was dramatically reduced in TAGLN-silenced cells and enhanced in TAGLN-overexpressed cells (Fig. 3e). To confirm the positive effects of TAGLN on cell metastasis, a tail-vein injection metastasis model was established using luciferase-labelled T24 (T24-Luc). A significant reduction in tumour metastasis in the TAGLN-silenced group compared with the control group was observed (Fig. 3f). Based on H&E and IHC staining, the number and sizes of the metastatic lesions in the TAGLN-silenced group were significantly decreased (Fig. 3g). Overall, these results indicated that TAGLN promotes the invasiveness of BLCA cells both in vitro and in vivo, which is consistent with the earlier functional prediction.
3.5. TAGLN promotes invadopodia formation and EMT
It is well-known that tumour cell invadopodia play a vital role in tumour metastasis [38]. Notably, TAGLN may promote cell motility by regulating the formation of invadopodia, which is in line with the results of the functional prediction analyses. Invadopodia are actin-based dynamic protrusions that utilize the actin regulators cortactin, N-WASP, TKS4, TKS5, and MMP14 [39]. Thus, we evaluated the correlation between the presence of invadopodia and the co-localization of F-actin with cortactin (CTTN) in bladder cell lines. Based on immunofluorescence staining, both the up- and downregulated TAGLN was distributed primarily in the cytoplasm, which indicates its association with actin in BLCA (Fig. 4a). Also, we found that the cell lines with greater expression of TAGLN also showed higher expression of cortactin (Supplementary Fig. S2c–d). In T24 cells, which exhibited a high metastatic capability and a high level of TAGLN, over 75% of the cells contained invadopodia, while only 40–60% of 5637 or SW780 cells, both of which have a low metastatic capability and lower level of TAGLN, contained invadopodia (Supplementary Fig. S2e). Next, we examined the effects of TAGLN on invadopodia formation. Suppression of TAGLN significantly reduced the number of invadopodia in T24-shTAGLN (approximately 40% of cells) and SW780-shTAGLN (30% of cells) cells. Meanwhile, the number of cells containing invadopodia was increased to 80% in the TAGLN-overexpressed cell line 5637-TAGLN, and the invadopodia contained a greater number of puncta as well as larger puncta (Fig. 4b). These results strongly demonstrate that TAGLN is involved in invadopodia formation.
Reactivation of EMT in cancer cells enhances the metastatic phenotype [40]. As suggested by the functional prediction as well as the positive effect of TAGLN on cancer metastasis, there was a potential reliable connection between TAGLN and EMT. EMT markers were detected in both the TAGLN-silenced and TAGLN-overexpressed cells. The expression of the mesenchymal markers/transcription factors N-cadherin, β-catenin and Slug were significantly decreased in TAGLN-silenced cells and increased in TAGLN-transduced cells, while the expression of the epithelial marker E-cadherin was downregulated in TAGLN-overexpressed cells (Fig. 4c–d). At the same time, the expression level of MMP14, which is also involved in the EMT process [40] and invadopodia formation [41], was decreased via the downregulation of TAGLN and increased via the upregulation of TAGLN. These results were also confirmed using RT-qPCR. We also further confirmed the correlation between TAGLN and Slug expression in the metastatic animal model. The expression level of Slug, which is distributed primarily in the nucleus, was dramatically decreased by the inhibition of TAGLN (Fig. 4e). All of these results were also highly consistent with the earlier functional prediction analysis, which demonstrated that TAGLN promotes metastasis by inducing invadopodia formation and EMT.
3.6. TALGN is essential for the promotion of BLCA metastasis by TGF-β
The TGF-β signalling pathway is one of the most well-characterized and vital pathways involved in the induction of cancer metastasis and EMT [42]. Based on the involvement of TAGLN in cancer metastasis and EMT in BLCA cells and the regulation of TAGLN by TGF-β that was observed in previous studies [43,44], we further analysed the expression patterns of TGF-β and TAGLN at both the tissue and cellular levels. In the 26 BLCA cell lines included in the CCLE (Supplementary Fig. S3a), a significant positive correlation between TGFB2 and TAGLN was found (Spearman's correlation r = 0.556, P < .01). Based on the clinicopathological features of each cell line [45], correlation tests were performed in each of the various subgroups. Significant correlations between TGFB2 and TAGLN were only found in relation to advanced clinicopathological features, such as advanced T and G (Fig. 5a, Supplementary Fig. S3b–c). Our results also showed that the expression of TGFB2 and TAGLN was closely associated in basal subtype BLCA cells, which were more aggressive and resulted in reduced survival compared with luminal cancers (Fig. 5d) [10,46]. To confirm the correlation between TGF-β and TAGLN at the tissue level, similar analyses were performed in the TCGA-BLCA cohort and the GSE13507-BLCA cohort. Interestingly, TAGLN was more closely correlated with TGFB3 than TGFB2 and TGFB1 in BLCA tissues (Supplementary Fig. S3d–e). In line with the cell analyses, higher correlation coefficients were determined for the subgroups with advanced clinicopathological features (Fig. 5b–c,e–f, Supplementary Fig. S3f–n). In particular, high correlation coefficients were obtained for the pathologic M1 subgroups in both cohorts. Additionally, analysis of 23 primary-recurrent paired BLCA tissues from the GSE13507-BLCA cohort indicated that a significant positive correlation between TGFB3 and TAGLN existed only in the recurrent group rather than the primary tumour group. These results provide support for the presence of a tight functional correlation between TGF-β and TAGLN during tumour development and metastasis in BLCA.
Given the strong association between TGF-β and TAGLN that is observed during the progression of BLCA, confirmatory tests were performed in BLCA cell lines. The expression levels of TAGLN and mesenchymal markers/transcription factors were measured in the presence of TGF-β. As expected, the TAGLN and mesenchymal markers/transcription factors expression levels were upregulated by TGF-β stimulation (Fig. 5g). Notably, the stimulating effect of TGF-β was attenuated by MMP14 and Slug in the TAGLN-silenced group (Fig. 5g). Functionally, the migratory ability of BLCA cells was dramatically increased after TGF-β stimulation. However, TGF-β-induced migration was completed abolished by the inhibition of TAGLN, especially in T24-shTAGLN#2 cells. Meanwhile, the effects of TAGLN silencing on migration was slightly rescued by TGF-β in T24-shTAGLN#1 cells, which indicates that the migratory ability of BLCA cells depends greatly on the TAGLN expression level (Fig. 5h). Overall, the results show that TAGLN is essential for TGF-β-induced metastasis in BLCA.
4. Discussion
In this study, we explored the expression patterns and molecular function of TAGLN in BLCA using specimen detection analysis, bioinformatics analysis and a series of functional assays both in vitro and in vivo. The results demonstrated that TAGLN contributes to poor prognosis in BLCA. TAGLN overexpression may promote the migration and invasion of BLCA cells via invadopodia formation and the induction of EMT. The strong relationship between TGF-β and TAGLN was demonstrated during the progression of BLCA. Additionally, TGF-β-mediated migration was shown to be abolished by the inhibition of TAGLN. These results provide further insight regarding the metastasis of BLCA, which implies that TAGLN may serve as a potential treatment target for BLCA metastasis.
Based on immunohistochemical staining of 104 paired BLCA tissues, TAGLN is upregulated in cancer tissues. However, our RT-qPCR results from 27 paired BLCA tissues as well as an online dataset and previous studies [21,22] have indicated that the TAGLN mRNA expression level is decreased in BLCA tissues compared with normal bladder tissues. It is important to note that all of the normal bladder tissues that were used in this study were complex tissues that were not subject to microdissection and consisted not only of bladder epithelial cell but also stromal cells and smooth muscle cells (SMCs). It is well known that TAGLN is an abundant protein in SMCs [47,48]. According to the IHC results obtained from the PKU-BLCA cohort, SMCs were found in almost all of the normal bladder samples (Supplementary Fig.S4a). This was also confirmed using the online database Human Protein Atlas (Supplementary Fig. S4b). Although the BLCA tissues also consist SMCs, especially muscle-invasive BLCA, the limited bladder epithelium might more easily be distorted by the unmeasured smooth muscle cells comparing to the bladder cancer tissue. Ambiguous results, such as those that have been found for TAGLN, have also been found for other cancers that affect SMC-rich tissues, including oesophageal cancer [15,16] and colorectal cancer [19,20]. Therefore, it is important to consider the rationale design of the experiments, such as applying tissues microdissection and multiomics analysis to the validation. Upon the integration of clinicopathological features, the contribution of TAGLN to poor prognosis has been observed in all three cohorts. As a benefit of the meta-analyses, the combined effects of each prognostic feature were determined in all three cohorts, which all demonstrated the unfavourable effects of TAGLN on BLCA. Collectively, the overall unfavourable expression pattern of TAGLN in BLCA indicates that TAGLN plays an important role in the development and progression of BLCA. Additionally, aberrant expression of TAGLN has also been shown to be involved in other cancers; for example, unfavourable prognostic effects have been noted for colorectal cancer. TAGLN upregulation has been correlated with submucosal invasiveness [49], lymph node invasiveness [19] and poor prognosis [50] in colorectal cancer. According to prognostic analysis of the TCGA dataset, the unfavourable effects of TAGLN on overall survival have been noted for adrenocortical carcinoma, kidney cancer, lower grade gliomas, squamous cell carcinoma of the lung, mesothelioma and uveal melanoma (Supplementary Fig. S4c–h). In contrast, TAGLN has been shown to be decreased in lymph node metastatic prostate cancer [51] and triple-negative breast cancer [52], which indicates the favourable prognostic effects of TAGLN. Differences in the cancer of origin may account for the various effects of TAGLN; for example, the bladder and colorectum are tract organs and of endodermic origin, while the prostate and breast are sex hormone-targeted glands. It is important to elucidate the role of TAGLN in each type of cancer separately.
To better understand the oncogenic role of TAGLN in BLCA, step-forward functional prediction and verification were performed. As indicated by the enrichment analyses, TAGLN may be involved in actin regulation and cell motility in BLCA. Actin serves as a key player in cell migration [33]. As an actin-binding protein, TAGLN shows great potential in altering motility via its interactions with actin. The verification assays proved that TAGLN promotes the migration and invasion of BLCA cells both in vitro and in vivo. Interestingly, TAGLN promotes the clonogenicity of BLCA cells rather than cell proliferation, which indicates the potential role of TAGLN in metastatic colonization. All of these functions of TAGLN in BLCA were correlated with the above-mentioned unfavourable prognostic effects of TAGLN. It was demonstrated that TAGLN levels significantly affect metastatic potential. To explain the role of TAGLN in BLCA metastasis more clearly, the relationships between TAGLN, EMT and invadopodia were documented. First, the involvement of TAGLN in EMT was highlighted in the cancer hallmark enrichment analysis. Our study demonstrated that the TAGLN expression level was significantly associated with EMT markers in terms of both perdition and verification. EMT status affects the metastatic potential [40]. During EMT in myofibroblasts (EMyT), the transcription factor serum response factor (SRF) and myocardin family transcription factors (MRTFs) are able to activate TAGLN transcription [53]. TAGLN is normally expressed in mesenchymal cells [48], especially in smooth muscle cells, and serves as an early marker of smooth muscle differentiation [54]; its appearance in epithelial-derived tumour cells is related to EMT, whereby tumour cells acquire greater metastatic potential. Indeed, the BLCA cell line T24, which is characterized by mesenchymal tumour cells that express extremely low levels of E-cadherin level and have elongated shapes, also expresses a high level of TAGLN and exhibits an aggressive metastatic potential in comparison to the epithelial tumour cell line 5637. Thus, TAGLN may serve as a type of EMT indicator in BLCA. Notably, we found that the TAGLN expression level also affect EMTs. The expression of mesenchymal state markers and transcription factors was decreased after the inhibition of TAGLN and were increased by the overexpression of TAGLN. Likewise, an EMT-regulatory function of TAGLN was identified during a previous colorectal cancer study [19]. Therefore, the metastatic modulation of TAGLN should influence EMT. Next, we also showed that TAGLN participates in invadopodia formation, which supports the role of TAGLN in the promotion of metastasis. During podosome formation in SMCs, TAGLN is recruited and TAGLN-decorated actin bundles undergo rapid reorganization into podosomes [36,37]. Since podosomes and invadopodia are highly similar and nearly indistinguishable, we applied rules that state that ‘normal cells have podosomes’ and ‘cancer cells have invadopodia’ [39]. Previous studies as well as our prediction analyses have revealed the strong interaction of actin and TAGLN. Actin dynamics are a key element of the assembly and maintenance of invadopodia [34]. Due to the integration of the involvement of TAGLN in podosomes with the vital role of actin in invadopodia formation, we hypothesized that TAGLN also plays important role in invadopodia formation in BLCA. The positive correlation between TAGLN and invadopodium-associated genes in BLCA was documented during the prediction analysis. Our results also showed that a reduction in TAGLN inhibits invadopodia formation, while invadopodia formation is promoted by TAGLN overexpression in BLCA cells. Invadopodium maturation results from matrix metalloproteinase (MMP) recruitment, which involves the delivery of MMP14 (MT1-MMP) to invadopodia and the activation of local matrix degradation [41]. In this context, MMP14 was significantly reduced by the depletion of TAGLN and increased by TAGLN overexpression in BLCA cells. TAGLN was shown to play specific roles in invadopodia formation in BLCA. The association between EMT and in invadopodia formation appears to be inseparable during the systemic spread of tumours [55,56]. Overall, the involvement of TAGLN in EMT and invadopodia formation contribute to its robust metastatic potential. Nevertheless, the precise involvement of TAGLN in EMT and invadopodia formation will require further evaluation.
The question of the factors involved in the regulation of the expression of TAGLN during the progression of BLCA was raised. Serum response factor (SRF)–binding CArG boxes, a Smad-binding element (SBE) and a TGF-β control element (TCE) are included in the TAGLN promoter [43,44], which suggests the vital roles played by SRF and TGF-β in TAGLN regulation. Although many studies have shown that the TGF-β signalling pathway regulates TAGLN expression in various systems [[57], [58], [59]], this regulation is rarely studied in cancer. TGF-β signalling is undoubtedly a central player in cancer progression due to its dual functioning [42]. Not to mention, TGF-β acts as a lymph node metastatic biomarker [60] and is involved in the invasion and metastasis of BLCA [61,62]. Furthermore, TGF-β reprograms the tumour microenvironment to attenuate anti-tumour immunity in metastatic bladder cancer [63]. Therefore, we analysed the expression patterns of TGF-β and TAGLN in both cells (26 cell lines) and tissues (572 patients) in BLCA. As expected, a tight positive correlation between TGF-β and TAGLN was found in BLCA. Notably, only subgroups with advanced clinicopathological features reflected a significant relationship via increased correlation coefficients, which implies that TGF-β promotes TAGLN expression during cancer progression. TGF-β signalling induces EMT [42] and promotes invadopodia formation [55,56]. This supports the previous assumption that TAGLN promotes metastasis via EMT and invadopodia formation. Additionally, the TGF-β-stimulated upregulation of Slug and MMP14 were attenuated by TAGLN inhibition, while cell migration induced by TGF-β was totally abolished, which indicated the essential role of TAGLN in the promotion by TGF-β of BLCA metastasis. Collectively, TAGLN may serve as a powerful treatment target for TGF-β-mediated metastasis resulting from BLCA.
Although there is no selective TAGLN inhibitor yet, quite a few drugs show available potential. Since the progression-dependent correlation between TGF-β and TAGLN was highlighted in bladder cancer, TGF-β inhibitors could serve as potential drugs. Virtually, several TGF-β pathway targeting drugs have been applied into clinical trials and many more have been tested through numerous design strategies [64]. SB-431542, a TβRI kinase inhibitor in preclinical development, could effective downregulate TAGLN expression level indicating it promising application potential in clinic [58]. Recently, Zhong et.al reported that salvianolic acid A enhances vasoconstriction by targeting the transgelin-actin complex [65]. Salvianolic acid, a well-known treatment compound for cardiovascular-related diseases, was discovered as novel and effective drugs for cancer treatment by promoting cells apoptosis and inhibiting EMT transistion [66]. TAGLN2 (transgelin-2), one of TAGLN's homologs with 64% amino acid sequence homology to TAGLN [14], was supressed by salvianolic acid A resulting in inhibition of breast cancer cells migration and invasion [67]. Taken together, these perspectives on the correlation between salvianolic acid A and TAGLN highlight the robust therapeutic potential of salvianolic acid A as TAGLN-targeting drug. However, all the therapeutic potential still need being well studied in order to be better applied to the treatment.
In summary, TAGLN has been identified as a novel prognosis predictor due to its unfavourable prognostic effects and its function as a metastatic driver via EMT and invadopodia formation and was also shown to be induced by TGF-β in BLCA. Future studies of the specific roles played by TAGLN in EMT and invadopodia formation are critical to increase our knowledge of BLCA metastasis. TAGLN, via its promotion of metastasis, may serve as a promising biomarker and new target agent for follow-up and treatment.
The following are the supplementary data related to this article.
Clinicopathological characteristics of patients with BLCA in three cohorts.
Characteristics of the corresponding antibodies included in the context.
Characteristics of the corresponding qPCR primer and shRNA included in the context.
Correlation between TAGLN mRNA expression level and clinicopathological features in TCGA-BLCA and GSE13507-BLCA cohorts.
Univariate and multivariate analyses of clinicopathological characteristics and TAGLN expression with overall survival in TCGA-BLCA and GSE13507-BLCA cohorts.
Consensus genes of differentially expressed genes by TAGLN in TCGA-BLCA and GSE13507-BLCA cohorts and enrichment analyses.
Data accessibility
The transcriptome dataset of bladder cancer from The Cancer Genome Atlas (TCGA), TCGA-BLCA cohort, was downloaded from UCSC Xena browser in June 2018, while GSE13507-BLCA cohort was obtained from gene expression omnibus (GEO) dataset: GSE13507.The other data generated or analysed during this study are included in this published article and its Additional file information files.
Authors' contributions
Conception and design: Z.C, S.H Y.G, X.L, and L.Z; Methodology development: Z.C, S.H, and Y.Z; Acquisition of clinical specimens and information: Z.C, Y.Z, A.H, and D.F; Experiment implementation: Z.C and Y.Z; Analysis and interpretation of data: Z.C and S.H; Drafting of the manuscript: Z.C; Review and revision of the manuscript: Y.G, S.H, X.L, and L.Z; Administrative support, obtaining funding, supervision: S.H, Y.G, X.L, and L.Z. All authors read and approved the submitted manuscript.
Declaration of Competing Interest
All authors declare that there are no conflicts of interest.
Acknowledgements
The authors greatly appreciate the help from Qihua He for her help in confocal microscope application, Hounan Wu for her help in flow cytometry, Jieshan Chi and Ding Peng for their help in bioinformatics analysis, Yifan Li for his help in cell experiments. All mentioned individuals approved the submitted manuscript.
Contributor Information
Yanqing Gong, Email: yqgong@bjmu.edu.cn.
Xuesong Li, Email: pineneedle@sina.com.
Liqun Zhou, Email: zhoulqmail@sina.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Li K., Lin T., Chinese Bladder Cancer C., Xue W., Mu X., Xu E. Current status of diagnosis and treatment of bladder cancer in China - analyses of Chinese bladder cancer consortium database. Asian J Urol. 2015;2(2):63–69. doi: 10.1016/j.ajur.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comperat E., Larre S., Roupret M., Neuzillet Y., Pignot G., Quintens H. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015;466(5):589–594. doi: 10.1007/s00428-015-1739-2. [DOI] [PubMed] [Google Scholar]
- 4.Cambier S., Sylvester R.J., Collette L., Gontero P., Brausi M.A., van Andel G. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guerin. Eur Urol. 2016;69(1):60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Abufaraj M., Gust K., Moschini M., Foerster B., Soria F., Mathieu R. Management of muscle invasive, locally advanced and metastatic urothelial carcinoma of the bladder: a literature review with emphasis on the role of surgery. Transl Androl Urol. 2016;5(5):735–744. doi: 10.21037/tau.2016.08.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von der Maase H., Hansen S.W., Roberts J.T., Dogliotti L., Oliver T., Moore M.J. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H., Sengelov L., Roberts J.T., Ricci S., Dogliotti L., Oliver T. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 8.Abufaraj M., Dalbagni G., Daneshmand S., Horenblas S., Kamat A.M., Kanzaki R. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. 2018;73(4):543–557. doi: 10.1016/j.eururo.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamat A.M., Hahn N.M., Efstathiou J.A., Lerner S.P., Malmström P.-U., Choi W. Bladder cancer. The Lancet. 2016;388(10061):P2796–P2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 10.Choi W., Porten S., Kim S., Willis D., Plimack E.R., Hoffman-Censits J. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y., Liu H.W., Forsythe S.M., Kogut P., McConville J.F., Halayko A.J. Mutagenesis analysis of human SM22: characterization of actin binding. J Appl Physiol. 2000;89(5):1985–1990. doi: 10.1152/jappl.2000.89.5.1985. 1985. [DOI] [PubMed] [Google Scholar]
- 12.Han M., Dong L.H., Zheng B., Shi J.H., Wen J.K., Cheng Y. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci. 2009;84(13–14):394–401. doi: 10.1016/j.lfs.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Assinder S.J., Stanton J.A., Prasad P.D. Transgelin: an actin-binding protein and tumour suppressor. Int J Biochem Cell Biol. 2009;41(3):482–486. doi: 10.1016/j.biocel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Dvorakova M., Nenutil R., Bouchal P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert Rev Proteomics. 2014;11(2):149–165. doi: 10.1586/14789450.2014.860358. [DOI] [PubMed] [Google Scholar]
- 15.Qi Y., Chiu J.F., Wang L., Kwong D.L., He Q.Y. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics. 2005;5(11):2960–2971. doi: 10.1002/pmic.200401175. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Wang K., Zhang J., Liu S.S., Dai L., Zhang J.Y. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10(6):2863–2872. doi: 10.1021/pr200141c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitek B., Luttges J., Marcus K., Kloppel G., Schmiegel W., Meyer H.E. Application of fluorescence difference gel electrophoresis saturation labelling for the analysis of microdissected precursor lesions of pancreatic ductal adenocarcinoma. Proteomics. 2005;5(10):2665–2679. doi: 10.1002/pmic.200401298. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L., Zhang R., Zhang L., Sun Y., Yao W., Zhao A. Upregulation of transgelin is an independent factor predictive of poor prognosis in patients with advanced pancreatic cancer. Cancer Sci. 2013;104(4):423–430. doi: 10.1111/cas.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y., Buckhaults P.J., Lee J.R., Xiong H., Farrell C., Podolsky R.H. Association of the actin-binding protein transgelin with lymph node metastasis in human colorectal cancer. Neoplasia. 2009;11(9):864–IN5. doi: 10.1593/neo.09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo M., Park H.J., Kim D.K., Kim Y.B., Cheong J.Y., Lee K.J. Loss of SM22 is a characteristic signature of colon carcinogenesis and its restoration suppresses colon tumorigenicity in vivo and in vitro. Cancer. 2010;116(11):2581–2589. doi: 10.1002/cncr.25003. [DOI] [PubMed] [Google Scholar]
- 21.Chen R., Feng C., Xu Y. Cyclin-dependent kinase-associated protein Cks2 is associated with bladder cancer progression. J Int Med Res. 2011;39(2):533–540. doi: 10.1177/147323001103900222. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami K., Enokida H., Tachiwada T., Gotanda T., Tsuneyoshi K., Kubo H. Identification of differentially expressed genes in human bladder cancer through genome-wide gene expression profiling. Oncol Rep. 2006;16(3):521–531. [PubMed] [Google Scholar]
- 23.Liu Y., Wu X., Wang G., Hu S., Zhang Y., Zhao S. CALD1, CNN1, and TAGLN identified as potential prognostic molecular markers of bladder cancer by bioinformatics analysis. Med (Baltimore) 2019;98(2) doi: 10.1097/MD.0000000000013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning X., Deng Y. Identification of key pathways and genes influencing prognosis in bladder urothelial carcinoma. Onco Targets Ther. 2017;10:1673–1686. doi: 10.2147/OTT.S131386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright G.W., Simon R.M. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19(18):2448–2455. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]
- 26.Clarke R., Ressom H.W., Wang A., Xuan J., Liu M.C., Gehan E.A. The properties of high-dimensional data spaces: implications for exploring gene and protein expression data. Nat Rev Cancer. 2008;8(1):37–49. doi: 10.1038/nrc2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathi S., Pohl M.O., Zhou Y., Rodriguez-Frandsen A., Wang G., Stein D.A. Meta- and orthogonal integration of influenza "OMICs" data defines a role for UBR4 in virus budding. Cell Host Microbe. 2015;18(6):723–735. doi: 10.1016/j.chom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doerks T., Copley R.R., Schultz J., Ponting C.P., Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12(1):47–56. doi: 10.1101/gr.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlen M., Zhang C., Lee S., Sjostedt E., Fagerberg L., Bidkhori G. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 31.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camp R.L., Dolled-Filhart M., Rimm D.L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 33.Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94(1):235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 34.Albiges-Rizo C., Destaing O., Fourcade B., Planus E., Block M.R. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122(Pt 17):3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson O., Moghraby J.S., Ayscough K.R., Winder S.J. Depletion of the actin bundling protein SM22/transgelin increases actin dynamics and enhances the tumourigenic phenotypes of cells. BMC Cell Biol. 2012;13(1) doi: 10.1186/1471-2121-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaverina I., Stradal T.E., Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–4924. doi: 10.1242/jcs.00818. Pt 24. [DOI] [PubMed] [Google Scholar]
- 37.Gimona M., Kaverina I., Resch G.P., Vignal E., Burgstaller G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol Biol Cell. 2003;14(6):2482–2491. doi: 10.1091/mbc.E02-11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddy R.J., Weidmann M.D., Sharma V.P., Condeelis J.S. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27(8):595–607. doi: 10.1016/j.tcb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy D.A., Courtneidge S.A. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12(7):413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastushenko I., Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2018;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Jacob A., Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. 2015;3:4. doi: 10.3389/fcell.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colak S., Ten Dijke P. Targeting TGF-beta signaling in cancer. Trends Cancer. 2017;3(1):56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Qiu P. Interaction of Smad3 and SRF-associated complex mediates TGF-β1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35(12):1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen S. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res. 2003;31(4):1302–1310. doi: 10.1093/nar/gkg224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuiverloon T.C.M., de Jong F.C., Costello J.C., Theodorescu D. Systematic review: characteristics and preclinical uses of bladder cancer cell lines. Bladder Cancer. 2018;4(2):169–183. doi: 10.3233/BLC-180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seiler R., Ashab H.A.D., Erho N., van Rhijn B.W.G., Winters B., Douglas J. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017;72(4):544–554. doi: 10.1016/j.eururo.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Camoretti-Mercado B., Forsythe S.M., LeBeau M.M., Espinosa R., 3rd, Vieira J.E., Halayko A.J. Expression and cytogenetic localization of the human SM22 gene (TAGLN) Genomics. 1998;49(3):452–457. doi: 10.1006/geno.1998.5267. [DOI] [PubMed] [Google Scholar]
- 48.Lawson D., Harrison M., Shapland C. Fibroblast transgelin and smooth muscle SM22 alpha are the same protein, the expression of which is down-regulated in many cell lines. Cell Motil Cytoskeleton. 1997;38(3):250–257. doi: 10.1002/(SICI)1097-0169(1997)38:3<250::AID-CM3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Song M.Q., Zhu J.S., Zhou Z., Xu Z.P., Chen W.X. Identification of differentially-expressed proteins between early submucosal non-invasive and invasive colorectal cancer using 2d-Dige and mass spectrometry. Int J Immunopathol Pharmacol. 2011;24(4):849–859. doi: 10.1177/039463201102400404. [DOI] [PubMed] [Google Scholar]
- 50.Kim H.J., Kang U.B., Lee H., Jung J.H., Lee S.T., Yu M.H. Profiling of differentially expressed proteins in stage IV colorectal cancers with good and poor outcomes. J Proteomics. 2012;75(10):2983–2997. doi: 10.1016/j.jprot.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Pang J., Liu W.P., Liu X.P., Li L.Y., Fang Y.Q., Sun Q.P. Profiling protein markers associated with lymph node metastasis in prostate cancer by DIGE-based proteomics analysis. J Proteome Res. 2010;9(1):216–226. doi: 10.1021/pr900953s. [DOI] [PubMed] [Google Scholar]
- 52.Sayar N., Karahan G., Konu O., Bozkurt B., Bozdogan O., Yulug I.G. Transgelin gene is frequently downregulated by promoter DNA hypermethylation in breast cancer. Clin Epigenetics. 2015;7:104. doi: 10.1186/s13148-015-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S., Crawford M., Day R.M., Briones V.R., Leader J.E., Jose P.A. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem. 2006;281(3):1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pignatelli J., Tumbarello D.A., Schmidt R.P., Turner C.E. Hic-5 promotes invadopodia formation and invasion during TGF-beta-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197(3):421–437. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eckert M.A., Lwin T.M., Chang A.T., Kim J., Danis E., Ohno-Machado L. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19(3):372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maleszewska M., Gjaltema R.A., Krenning G., Harmsen M.C. Enhancer of zeste homolog-2 (EZH2) methyltransferase regulates transgelin/smooth muscle-22alpha expression in endothelial cells in response to interleukin-1beta and transforming growth factor-beta2. Cell Signal. 2015;27(8):1589–1596. doi: 10.1016/j.cellsig.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Elsafadi M., Manikandan M., Dawud R.A., Alajez N.M., Hamam R., Alfayez M. Transgelin is a TGFbeta-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016;7(8):e2321. doi: 10.1038/cddis.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldeiri B., Roostalu U., Albertini A., Wong J., Morabito A., Cossu G. Transgelin-expressing myofibroblasts orchestrate ventral midline closure through TGFbeta signalling. Development. 2017;144(18):3336–3348. doi: 10.1242/dev.152843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shariat S.F., Kim J.H., Andrews B., Kattan M.W., Wheeler T.M., Kim I.Y. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer. 2001;92(12):2985–2992. doi: 10.1002/1097-0142(20011215)92:12<2985::aid-cncr10175>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 61.Fan Y., Shen B., Tan M., Mu X., Qin Y., Zhang F. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20(6):1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 62.Yang H.J., Liu G.L., Liu B., Liu T. GP73 promotes invasion and metastasis of bladder cancer by regulating the epithelial-mesenchymal transition through the TGF-beta1/Smad2 signalling pathway. J Cell Mol Med. 2018;22(3):1650–1665. doi: 10.1111/jcmm.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akhurst R.J., Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong W., Sun B., Gao W., Qin Y., Zhang H., Huai L. Salvianolic acid a targeting the transgelin-actin complex to enhance vasoconstriction. EBioMedicine. 2018;37:246–258. doi: 10.1016/j.ebiom.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L., Tang L., Yi Q. Salvianolic acids: potential source of natural drugs for the treatment of fibrosis disease and cancer. Front Pharmacol. 2019;10:97. doi: 10.3389/fphar.2019.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng X., Chen S., Yang Q., Cai J., Zhang W., You H. Salvianolic acid a reverses the paclitaxel resistance and inhibits the migration and invasion abilities of human breast cancer cells by inactivating transgelin 2. Cancer Biol Ther. 2015;16(9):1407–1414. doi: 10.1080/15384047.2015.1070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathological characteristics of patients with BLCA in three cohorts.
Characteristics of the corresponding antibodies included in the context.
Characteristics of the corresponding qPCR primer and shRNA included in the context.
Correlation between TAGLN mRNA expression level and clinicopathological features in TCGA-BLCA and GSE13507-BLCA cohorts.
Univariate and multivariate analyses of clinicopathological characteristics and TAGLN expression with overall survival in TCGA-BLCA and GSE13507-BLCA cohorts.
Consensus genes of differentially expressed genes by TAGLN in TCGA-BLCA and GSE13507-BLCA cohorts and enrichment analyses.
Data Availability Statement
The transcriptome dataset of bladder cancer from The Cancer Genome Atlas (TCGA), TCGA-BLCA cohort, was downloaded from UCSC Xena browser in June 2018, while GSE13507-BLCA cohort was obtained from gene expression omnibus (GEO) dataset: GSE13507.The other data generated or analysed during this study are included in this published article and its Additional file information files.