Abstract
Background
Antibody-dependent cellular cytotoxicity (ADCC) has been associated with improved infant outcome in mother-to-child transmission (MTCT) of HIV-1. Epitopes of these ADCC-mediating antibodies remain unidentified. CD4-inducible (CD4i) epitopes on gp120 are common ADCC targets in natural infection and vaccination. We tested whether CD4i epitope-specific ADCC mediated by maternal antibodies or passively-acquired antibodies in infants is associated with reduced MTCT and improved infant survival.
Methods
We used variants of CD4i cluster A-specific antibodies, A32 and C11, and a cluster C-specific antibody, 17b, with mutations abolishing Fc–Fc receptor interactions as inhibitors in a competition rapid and fluorometric ADCC assay using gp120-coated CEM-nkr target cells with plasma from 51 non-transmitting and 21 transmitting breastfeeding mother-infant pairs.
Findings
Cluster A-specific ADCC was common. Individually, neither A32-like nor C11-like ADCC was statistically significantly associated with risk of MTCT or infected infant survival. In combination, total maternal cluster A-specific ADCC was statistically significantly associated with decreased infected infant survival in a log-rank test (p = 0·017). There was a non-significant association for infant passively-acquired total cluster A-specific ADCC and decreased infected infant survival (p = 0·14). Surprisingly, plasma ADCC was enhanced in the presence of the defective Fc 17b competitor. Defective Fc 17b competitor-mediated maternal ADCC enhancement was statistically significantly associated with reduced infected infant survival (p = 0·011). A non-significant association was observed for passively-acquired infant ADCC enhancement and decreased survival (p = 0·19).
Interpretations
These data suggest that ADCC targeting CD4i epitopes is not associated with protection against breast milk HIV transmission but is associated with decreased survival of infected infants.
Fund
This study was funded by NIH grant R01AI076105 and NIH fellowship F30AI136636.
Keywords: HIV, ADCC, Mother-to-child transmission, CD4-inducible epitopes
Research in context.
Evidence before this study
ADCC was implicated in protection in the modestly efficacious RV144 HIV-1 vaccine trial. This trial showed that pre-existing HIV-specific antibodies were associated with reduced HIV infection, but the specific nature of protective ADCC-mediating antibodies has not been defined. Breastfeeding transmission of HIV is a natural setting in which HIV-specific antibodies are present at the time of HIV exposure and is therefore an ideal context in which to study the effect of pre-existing antibodies on HIV transmission risk and clinical outcome. ADCC has been associated with protection and improved outcome in the setting of breastfeeding HIV transmission.
A number of epitopes of ADCC-mediating antibodies have been identified, including the commonly targeted CD4-inducible epitopes on gp120, but the epitopes of ADCC-mediating antibodies that correlate with protection and improved clinical outcome are largely undefined. A better understanding of the epitopes targeted by protective antibodies could improve vaccine efficacy.
Added value of this study
This study was designed to directly assess whether ADCC targeting several common CD4-inducible epitopes on gp120 is associated with improved outcomes among breastfeeding infants born to HIV-infected mothers. These infants have HIV-specific antibodies present prior to breastfeeding exposure. The data presented here indicate that ADCC targeting the CD4-inducible epitopes is not associated with reduced risk of MTCT or improved infected infant survival. Rather, our results suggest that ADCC specific for the CD4-inducible epitopes may be associated with decreased infant survival.
Implications of all the available evidence
CD4-inducible epitope-specific ADCC-mediating antibodies are a dominant ADCC response; however, it is unclear whether these responses are protective or beneficial. Other studies by Richard, Lee, and colleagues have shown that ADCC-mediating antibodies targeting CD4-inducible epitopes predominantly kill uninfected bystander cells that have become coated with shed gp120 and are not effective at clearing virally infected cells. Our data suggest that CD4i epitope-specific ADCC-mediating antibodies are not protective and may be associated with worse infant disease progression. Whether this association is a direct effect of ADCC targeting CD4i epitopes or a marker of another aspect of the response needs to be examined.
Alt-text: Unlabelled Box
1. Introduction
Designing a preventive HIV vaccine remains a major, yet elusive, goal. While there has been some success in preventing infection in macaque vaccination studies, where the challenge is often a single defined viral strain [[1], [2], [3]], success in the more complex setting of human vaccination has been much more modest [4]. In the RV144 trial, the only human vaccine trial showing any efficacy to date, non-neutralizing antibodies appeared to play a protective role, while the neutralizing antibody response was weakly elicited and not correlated with protection [[5], [6], [7]]. In a subsequent analysis, antibody-dependent cellular cytotoxicity (ADCC), a non-neutralizing Fc-mediated antibody function that targets infected cells for destruction by innate effector cells, was correlated with protection in vaccinees that had low circulating IgA [6]. However, the RV144 trial was only modestly efficacious [5]; thus, an improved knowledge of the correlates of protection from HIV transmission and disease progression is necessary to improve rational vaccine and therapeutic design.
Mother-to-child transmission (MTCT) is a natural setting in which correlates of protection from HIV transmission as well as impact on disease progression and outcome can be investigated. MTCT can occur in utero, peripartum, or via breastfeeding [8]. Mothers passively transfer antibodies to their infants in utero via placental transfer, and these antibodies remain in infant circulation for months after birth [[9], [10], [11], [12], [13], [14], [15], [16]]. Breastfeeding infants who are uninfected at birth have pre-existing HIV-specific antibodies circulating prior to HIV breastmilk exposure, analogous to what is expected as a result of vaccination. Importantly, while breastmilk contains antibodies that act in the infant gut, breastmilk antibodies generally do not cross the gut barrier [17]. Therefore, pre-existing passively-acquired maternal antibodies present in infant circulation were acquired via placental transfer.
Our lab has previously reported that ADCC activity, but not neutralization, correlated with improved infant outcomes in the setting of breastfeeding. In a cohort of high-risk mother-infant pairs, breast milk ADCC activity was correlated with reduced risk of MTCT [18]. In a larger cohort of breastfeeding mother-infant pairs, passively-acquired ADCC in infant plasma was significantly associated with improved survival of infected infants and associated with trends toward protection from MTCT and reduced set point viral load [9]. Studies from other groups have also shown ADCC to be associated with improved infant outcomes, such as reduced disease progression to AIDS or death [[19], [20], [21]]. These studies measured total ADCC; however, the epitopes of the ADCC-mediating antibodies that correlate with improved outcomes have not been investigated in the context of MTCT.
Major targets of ADCC-mediating antibodies are CD4-inducible (CD4i) epitopes, which are epitopes in the HIV envelope (Env) protein that are typically not well exposed in the native Env trimer [22,23]. CD4i epitopes become exposed as a result of a conformational change in Env after CD4 binding that is necessary to complete subsequent steps in entry, including co-receptor binding [22,24,25]. CD4i epitopes have been further divided into clusters based on the specific epitope target in the CD4-induced state of Env, with cluster A, which targets the gp41-interactive region of gp120, and cluster C, which targets the co-receptor binding site, being the subject of intensive study [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. ADCC activities targeting these epitopes are both common and potent [[26], [27], [28], [29],33,41,43,44]. In multiple studies that directly measured the amount of CD4i epitope-specific ADCC in plasma from small cohorts of infected individuals (N = 9–14), over 90% of the participants had detectable cluster A-specific ADCC [27,28,43]. The epitopes of two cluster A-defining antibodies, A32 and C11, are nearby each other but do not overlap [23]. In the aforementioned studies, plasma ADCC specific for the A32 and C11 epitopes was dominant and ranged from 14–87% and 18–78% of total plasma ADCC from each participant, respectively [27,28]. The majority of the participants also had some detectable plasma ADCC activity to the CD4i cluster C co-receptor binding site epitope, as measured by ADCC specific for the epitope of a prototypical cluster C antibody, 17b [26,27,43]. While less dominant than cluster A-specific ADCC responses, ADCC targeting the 17b epitope accounted for 0–53% of total plasma ADCC in a cohort of infected individuals [27].
While CD4i epitope-specific ADCC is common and potent, especially cluster A-specific activity, the role of CD4i epitope-specific ADCC in protection or clinical outcome in humans has not been defined. A handful of non-human primate (NHP) studies have shown that antibodies targeting CD4i epitopes can provide protection from SHIV challenge, control viremia, and reduce transmitted/founder variants providing proof-of-concept that CD4i epitope-specific ADCC-mediating antibodies have the potential to play an important role in HIV vaccines and antibody-based therapies [[45], [46], [47]]. We hypothesized that maternal and passively-acquired ADCC activity targeting cluster A (A32 and C11) and/or cluster C (17b) CD4i epitopes is correlated with reduced transmission and slower disease progression in the setting of MTCT. We utilized samples from a unique cohort of 72 antiretroviral (ART)-naïve breastfeeding mother-infant pairs from the Nairobi Breastfeeding Clinical Trial in which passively-acquired ADCC was significantly associated with improved survival of infected infants and a trend toward protection from transmission [9,48]. In the present study, we investigated whether ADCC targeting the cluster A and C CD4i epitopes is associated with reduced risk of MTCT and/or improved infected infant outcome in this cohort.
2. Materials and methods
2.1. Study design and plasma samples
Plasma samples were from the Nairobi Breastfeeding Clinical Trial, conducted in Nairobi between 1992 and 1998, prior to the use of ART [48]. HIV-1 positive mothers were enrolled during the third trimester, at which time maternal blood samples were collected. Infant blood samples were collected at birth and at regular intervals thereafter until 2 years of age. Infant PBMC samples were tested for HIV infection by single copy detection DNA PCR [49]. Infants testing positive for HIV DNA, were retrospectively tested for HIV RNA using a prototype Gen-Probe/Hologic HIV viral load assay that detects diverse subtypes from samples collected from previous timepoints [50]. For this study, estimated time of infection was defined as the midpoint between the last HIV-negative and first HIV DNA and/or RNA-positive test [9]. The Kenyan Ministry of Health gave permission for the Nairobi Breastfeeding Clinical Trial to be conducted, and the Institutional Review Boards of the University of Nairobi, University of Washington, and the Fred Hutchinson Cancer Research Center approved the current study.
Plasma or cord blood samples from 72 mother-infant pairs from the Nairobi Breastfeeding Clinical Trial meeting selection criteria described previously were utilized in the present study [9]. Briefly, mother-infant pairs were included if the following criteria were met: the infant was HIV RNA and DNA negative at birth, breastfed for a minimum of three months, HIV-exposed uninfected (HEU) infants remained HIV-negative for a minimum of six months and at each follow up timepoint, and an infant plasma or cord blood sample was available from the first week of life (prior to estimated time of infection). Infant cord blood (N = 60) or neonatal plasma from delivery (N = 10) or week 1 (N = 1) were tested in this study along with paired maternal plasma samples from the third trimester of pregnancy (N = 68) or delivery (N = 3). We verified that cord blood and plasma samples from infants in this cohort gave similar results by testing matched samples from infants in the Trial where both were collected. Of note, 1 infected infant and 1 non-transmitting mother from the 72 pairs studied by Milligan et al. had no more plasma available [9]; their corresponding transmitting maternal and HEU infant plasma samples were available and included in this study. The final cohort included 70 paired maternal and infant samples (50 non-transmitting pairs and 20 transmitting pairs), 1 unpaired transmitting maternal sample, and 1 unpaired HEU infant sample. All plasma and cord blood samples were heat inactivated at 56 degrees Celsius for 1 h. All experiments were performed in an unblinded fashion.
2.2. Antibodies
A32, C11, and 17b antibodies were produced by cloning A32 (light chain PDB: 3TNM_L; heavy chain PDB: 3TNM_A), C11 (light chain PDB: 4FZ8_L; heavy chain PDB: 4FZ8_H), and 17b (light chain PDB: 2NY1_C; heavy chain PDB: 2NY1_D) variable regions into IgG1 expression vectors (kindly provided by Michel Nussenzweig). Fc receptor binding defective variants called LALA variants were generated by mutagenesis, introducing two leucine to alanine changes, at L234A and L235A into the IgG1 expression vectors as described previously [28,51,52]. All antibodies were expressed and purified as described previously [28,53].
17b fab was produced by papain digestion of functional 17b using the Fab Preparation Kit (Pierce catalog#: 44985) according to the manufacturer's protocol as described previously [28].
Negative control influenza antibody FI6v3 was produced by stably transfected 293F cells kindly provided by Jesse Bloom.
2.3. Competition ADCC assay
The competition ADCC assay was performed by using a modified version of the rapid and fluorometric ADCC assay (RFADCC assay) with LALA variants as competitive inhibitors as described previously [28,54]. Briefly, CEM-NkR cells (NIH AIDS Reagent Program, catalog #: 458; RRID: CVCL_X622) were double stained with PKH26 cell linker (Sigma Aldrich), a cell membrane dye, and CFSE (Vybrant CFDA SE Cell Tracer Kit, Life Technologies), a cytoplasmic dye, and coated with either clade A/D BL035.W6M.ENV.C1 gp120 protein (Immune Tech) or clade B SF162 gp120 (Immune Tech) for 1 h at room temperature (RT). In the A32-LALA and C11-LALA competitive RFADCC assays, the target cells were coated with clade A/D BL035 gp120, the antigen used by Milligan et al., which was cloned from an infant in this cohort [9,55]. Functional 17b did not mediate measurable ADCC against BL035 gp120-coated target cells reproducibly. Because 17b consistently mediates ADCC against clade B SF162 gp120-coated target cells, target cells in the 17b-LALA competitive RFADCC assays were coated with clade B SF162 gp120. Cells were coated with 1.5μg gp120 per 100,000 cells. Coated target cells were washed and added at a concentration of 5000 cells/well to 96-well plates containing 50ul LALA antibody (at a concentration of 25μg/ml for A32-LALA and C11-LALA or 5μg/ml for 17b-LALA, empirically determined to completely abolish ADCC mediated by the appropriate wildtype antibody while avoiding non-specific inhibition of the other wildtype antibodies) or an equivalent volume of media and incubated for 15 min in the dark to allow the LALA antibody to bind gp120. Following this pre-incubation, 50ul of plasma at a 1:5000 dilution or 100 ng/ml of the monoclonal control antibodies were added to the plate and incubated for another 15 min in the dark at RT to allow the plasma to bind to available sites on gp120. HIV-negative donor PBMCs (Bloodworks Northwest) were added at a 50:1 effector:target ratio to each well. The plates were incubated for 4 h at 37 degrees Celsius and fixed with 150 ul of 1% paraformaldehyde in PBS (Affymetrix). 100 ul of fixed cells were analyzed by flow cytometry using an LSR II (BD) (for assays with maternal samples against BL035 gp120-coated target cells) or a Symphony (for all other assays) (BD). PKH was detected in the PE channel and CFSE was detected in the FITC channel. ADCC was measured as the percent PE+, FITC- cells of total PE+ cells after subtracting out background (ADCC against uncoated target cells), which was set to 3–5% as analyzed by FlowJo (Treestar). ADCC was normalized to killing mediated by pooled anti-HIV immune globulin (HIVIG, NIH AIDS Reagent Program, catalog # 3957), which was set to 100%. Results are averaged from two biological replicates. Each biological replicate contained two technical replicates. We used a negative control influenza antibody (FI6v3) as an isotype control to measure non-HIV-specific RFADCC activity. We pre-specified the limit of detection of the competition RFADCC assay as twice the percent RFADCC activity mediated by FI6v3 in the absence of the LALA variants. This allowed us to measure LALA antibody-mediated inhibition of RFADCC activity in samples that had measurable HIV-specific RFADCC activity. This strategy also allowed us to avoid skewing the results by calculating inhibition of very low RFADCC activity, which is likely not biologically relevant. Therefore, within each biological replicate, samples with total ADCC below our limit of detection (pre-specified as 2*total ADCC mediated by negative control influenza FI6v3 antibody) were excluded from this analysis. Seven maternal samples (six non-transmitters, one transmitter) and six infant samples (five HEU, one HIV-infected) met this exclusion criteria for at least one biological replicate. Data from the biological replicate above the limit of detection for those samples was included in the analysis.
Relative ADCC was defined as:
CD4i epitope-specific ADCC, referred to as CD4i antibody-like ADCC activity, is defined as: 100%- relative ADCC.
Enhancement of ADCC is defined as: relative ADCC-100%.
Total cluster A-specific ADCC is defined as: A32-like ADCC + C11-like ADCC.
Negative values were treated as zeros.
All cells were cultured in RPMI complete (RPMI (Gibco) supplemented with 10% FBS (Gibco), 1% PSF antibiotic-antimycotic (Life Technologies), and 1% 4.0 mM Glutamax (Gibco)). All PBMCs were stored frozen and thawed overnight before the day of the competition RFADCC assay. All PBMCs were prescreened for RFADCC activity. Ten PBMC donors were utilized for these experiments (one donor per biological replicate). RFADCC activity mediated by the positive control HIVIG ranged from 15% RFADCC activity to 46% RFADCC activity across all ten donors. All antibodies and plasma samples were diluted in RPMI complete.
2.4. Statistical analysis
Statistical analyses were performed with Stata15SE (StataCorp, College Station, TX) and GraphPad Prism7 (GraphPad Software, Inc., San Diego, CA). All graphs were generated by GraphPad Prism7. To determine whether CD4i-specific ADCC affected risk of MTCT, relative ADCC or CD4i antibody-like ADCC activity of HEU vs HIV-infected infant plasma or non-transmitting vs transmitting maternal plasma were compared using a Mann-Whitney U test and logistic regression analysis adjusted for maternal plasma HIV RNA viral load. The effect of ADCC targeting CD4i epitopes on survival of HIV-infected infants was assessed by a Cox-proportional hazards model and a log-rank test comparing Kaplan-Meier survival curves of HIV-infected infants with CD4i-specific ADCC at/above vs. below the HIV-infected infant cohort median as noted in the figure legends. While these survival analyses are similar, they are measuring different outcomes: Cox-proportional hazards models measure the effect of CD4i epitope-specific ADCC (as a continuous variable) on the overall risk of death, and log-rank tests measure the effect of high or low CD4i epitope-specific ADCC (categorical variable) on length of survival of infected infants. Statistical significance was defined as p < 0·05.
3. Results
3.1. Quantification of CD4i epitope-specific ADCC activity
To measure ADCC activity that targets known CD4i-specific epitopes, we used variants of A32, C11, and 17b, containing L234A and L235A (LALA) mutations that abrogate binding to the Fc gamma receptors (FcγR) as competitive inhibitors in the RFADCC assay [51]. Each LALA variant inhibited at least 90% of the ADCC activity mediated by its fully functional counterpart (Fig. S1) but did not inhibit ADCC activity of the unmatched antibodies, demonstrating their specificity.
We utilized samples from a cohort of 72 breastfeeding mother-infant pairs of which all infants tested HIV-negative at birth [9]. We measured ADCC of infant cord blood or plasma from the first week of life, which captures passively acquired maternal antibodies, as well as paired maternal plasma from the third trimester in the presence or absence of A32-LALA, C11-LALA, or 17b-LALA (Fig. S2). For all maternal and infant samples in the cohort, we normalized the ADCC activity in the presence of the LALA variant to ADCC activity in the absence of the LALA variant to calculate the percent relative ADCC for each sample in the presence of the LALA competitor variant (Fig. S2 D-F). Both A32-LALA and C11-LALA inhibited plasma ADCC for the majority of plasma samples (Fig. S2 D, E, orange points below dotted line). Conversely, 17b-LALA weakly inhibited plasma ADCC in only a small number of samples (Fig. S2 F, orange points below dotted line). Of note, relative RFADCC activity was strongly correlated between paired mothers and their infants (A32-LALA Spearman r: 0.72, p < 0.0001; C11-LALA Spearman r: 0.57, p < 0.0001; 17b-LALA Spearman r: 0.70, p < 0.0001).
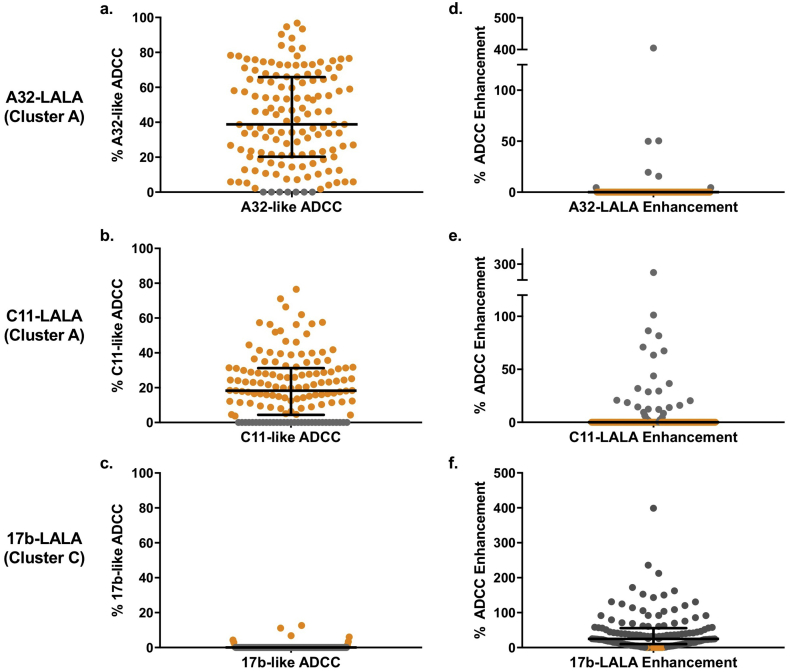
Relative ADCC is a measure of the ADCC activity targeting epitopes other than that of the LALA competitor variant, as it is the activity that is not blocked by the LALA variant. Therefore, we calculated the percent reduction in ADCC activity in the presence of the LALA variant, as described in the materials and methods (referred to as CD4i antibody-like ADCC: i.e. A32-like ADCC, C11-like ADCC, or 17b-like ADCC) to define the fraction of activity due to that antibody specificity in each sample (Fig. 1A-C). The cohort average for A32-like ADCC, C11-like ADCC and 17b-like ADCC was 42·0% (maximum 96·8%), 20·8% (maximum 76·5%), and 0·36% (maximum 12·7%) respectively (Fig. 1 A-C). Surprisingly, in the case of 17b-LALA, the majority of plasma ADCC was enhanced in the presence of 17b-LALA (Fig. 1F, grey points). The cohort average ADCC enhancement mediated by 17b-LALA was 43·0% (maximum 398·7%) (Fig. 1F)
Fig. 1.
CD4i epitope-specific ADCC in MTCT cohort.
Results of the competition RFADCC assay performed on 142 plasma samples from breastfeeding Kenyan mother-infant pairs. a-c: Percent CD4i epitope-specific (referred to as CD4i antibody-like) ADCC activity (100% - relative ADCC) was calculated for each plasma sample in the presence of A32-LALA (a), C11-LALA (b), and 17b-LALA (c). Negative values were treated as zeros (grey points). d-f: Percent LALA-mediated ADCC enhancement (relative ADCC - 100%) was calculated for each plasma sample in the presence of A32-LALA (d), C11-LALA (e), and 17b-LALA (f) Negative values were treated as zeros (orange points). Results are averaged from two biological replicates. Error bars represent median + interquartile range. Data from individual biological replicates from seven maternal samples and six infant samples that were below the limit of detection were excluded from the analysis as described in the Materials and Methods.
3.2. Effect of ADCC activity targeting CD4i epitopes on risk of MTCT
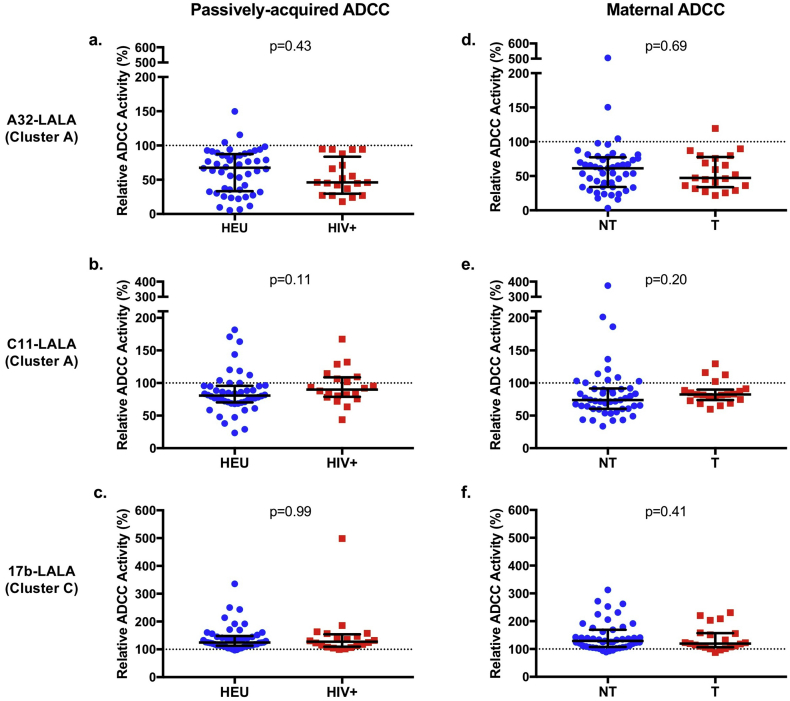
To measure the association of CD4i epitope-specific ADCC with risk of MTCT, we compared relative ADCC between 51 HIV-exposed uninfected infants (HEU) and 20 infants who acquired HIV over the course of the follow up period, or between their paired 50 non-transmitting and 21 transmitting mothers (Fig. 2). There was no statistically significant difference in relative ADCC activity in the presence of any of the three competitor LALA variants between the HEU and HIV-infected infants or between the corresponding non-transmitting and transmitting mothers (Fig. 2). Because maternal viral load is a known risk factor for MTCT, we also performed a logistic regression adjusting for maternal plasma HIV RNA viral load (Table 1). We found no statistically significant association between passively-acquired or maternal A32-like or C11-like ADCC and MTCT. Because A32-like and C11-like antibodies are common and have close but non-overlapping epitopes [23,[26], [27], [28], [29],33,41,43,44], we also examined their combined effect on outcome. To do so, we summed A32-like activity + C11-like activity to calculate “total cluster A-like ADCC activity”, as each LALA variant inhibits activity targeting its specific epitope (Fig. S1). There was no statistically significant association between total cluster A-like ADCC activity (sum of A32-like ADCC + C11-like ADCC) with MTCT risk (Table 1, top).
Fig. 2.
Relative ADCC among transmission groups.
Results of the competition RFADCC assay for passively acquired ADCC in 71 infant plasma samples (a-c) and maternal ADCC in 71 corresponding maternal plasma samples (d-f). Relative ADCC in the presence of A32-LALA (a, d), C11-LALA (b, e), or 17b-LALA (d, f) is shown for HIV-exposed uninfected infants (HEU) compared to HIV-infected (HIV+) infants or non-transmitting (NT) compared to transmitting (T) mothers. Relative ADCC was compared between the infection or transmission groups by a Mann-Whitney U test. Statistical significance was defined as p < 0·05 (*). Error bars represent median + interquartile range. Results are averaged from two biological replicates. Negative values were treated as zeros. Data from individual biological replicates from seven maternal samples and six infant samples that were below the limit of detection were excluded from the analysis as described in the Materials and Methods.
Table 1.
Association of CD4i antibody-like ADCC or ADCC enhancement with risk of MTCT.
| Passively-acquired ADCC |
Maternal ADCC |
|||||
|---|---|---|---|---|---|---|
| Entire cohort | Adjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value |
| A32-like ADCC | 1.005 | 0.985–1.026 | 0.61 | 1.013 | 0.989–1.038 | 0.29 |
| C11-like ADCC | 0.967 | 0.929–1.006 | 0.096 | 0.977 | 0.943–1.013 | 0.21 |
| Total cluster A-specific ADCC | 0.995 | 0.977–1.013 | 0.60 | 1.001 | 0.982–1.021 | 0.90 |
| 17b-LALA-mediated enhancement | 0.997 | 0.988–1.007 | 0.58 | 0.989 | 0.977–1.002 | 0.11 |
| Excluding transmissions after 6 months | Adjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value |
| A32-like ADCC | 1.003 | 0.979–1.029 | 0.80 | 1.016 | 0.988–1.045 | 0.26 |
| C11-like ADCC | 0.962 | 0.915–1.011 | 0.12 | 0.993 | 0.954–1.033 | 0.71 |
| Total cluster A-specific ADCC | 0.992 | 0.968–1.016 | 0.49 | 1.008 | 0.986–1.031 | 0.48 |
| 17b-LALA-mediated enhancement | 0.987 | 0.965–1.009 | 0.25 | 0.990 | 0.975–1.005 | 0.18 |
The association of CD4i epitope-specific ADCC (A32-like ADCC, C11-like ADCC, or total cluster A-specific ADCC (sum of A32-like ADCC + C11-like ADCC)) and 17b-LALA-mediated enhancement of plasma ADCC with odds of MTCT was measured using a logistic regression analysis adjusted for maternal plasma viral load. Adjusted odds ratios (OR), 95% confidence intervals (CI), and p-values are shown for infant samples (passively-acquired ADCC, left) and maternal samples (maternal ADCC, right). Statistical significance was defined as p < 0·05 (*). The top rows show results from the entire cohort (71 maternal samples and 71 infant samples). The bottom rows show results from the cohort after excluding the seven mother-infant pairs in which transmission occurred after six months of age of the infant (64 maternal samples and 64 infant samples). Data from individual biological replicates from seven maternal samples and six infant samples that were below the limit of detection were excluded from the analysis as described in the Materials and Methods.
Because 17b-like ADCC as measured by LALA competition was only detected rarely, we could not assess whether 17b-like ADCC was associated with risk of MTCT. Since 17b-LALA enhanced ADCC activity in a majority of the cohort samples (91·2% of maternal samples and 95·7% of infant samples showed enhancement of ADCC), we assessed the association between 17b-LALA-mediated enhancement of ADCC and MTCT adjusting for maternal HIV RNA viral load. We did not find a statistically significant association between 17b-LALA-mediated ADCC enhancement and risk of MTCT (Table 1, top).
Passively-acquired ADCC is often undetectable in infants by six months of age [9]. When the analysis was restricted to infants who were infected prior to six months (N = 14/21), associations between ADCC and MTCT remained non-significant (Table 1, bottom).
3.3. Effect of overall ADCC on infant survival in this cohort
In this cohort, which includes 21 infants who went on to acquire HIV after birth, there was an association of passively-acquired ADCC activity and improved survival of infants who subsequently acquired HIV [9]. The present study was conducted with data from 20 of these 21 HIV-infected infants who had remaining samples available, of whom seven died during the two year follow-up. In this independent evaluation of ADCC activity and infant survival, the results from our prior study showing an association between passively-acquired ADCC activity against infant-derived, clade A/D BL035 gp120 antigen with improved HIV-infected infant survival were confirmed (hazard ratio (HR) = 0·948, p = 0·031; log-rank Χ2 = 5·05, p = 0·025; Fig. S3). We also tested a clade B gp120 antigen in this study, SF162 gp120, which demonstrated an association between passively-acquired ADCC activity and improved HIV-infected infant survival with a different antigenic variant (HR = 0·965, p = 0·044; Χ2 = 1·70, p = 0·19; Fig. S3).
3.4. Effect of CD4i Cluster A-specific ADCC on survival of HIV-infected infants
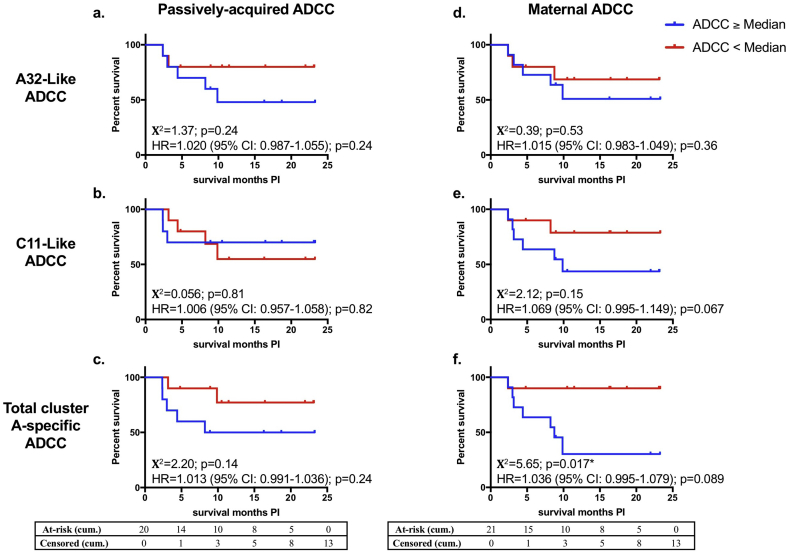
We next measured the association between cluster A-specific (A32-like or C11-like) ADCC and survival of infected infants (Fig. 3). There was no statistically significant association of A32-like ADCC (maternal or infant) with risk of infected infant mortality in Cox-proportional hazards models. Consistent with this result, there was no difference in length of infected infant survival between HIV-infected infants who had high or low passively-acquired A32-like ADCC, nor was there a difference in length of infected infant survival between infants born to mothers who had high versus low A32-like ADCC, as measured by log-rank tests (Fig. 3 A, D). There was no statistically significant association between passively-acquired C11-like ADCC in infants and infected infant mortality (Fig. 3B). However, higher maternal C11-like activity was associated with a trend toward higher risk of mortality in HIV-infected infants (HR: 1·069, p = 0·067) (Fig. 3 E). Interestingly, higher maternal total cluster A-specific ADCC was statistically significantly associated with shorter infected infant survival (Χ2 = 5·65, p = 0·017) and associated with a trend for increased risk of infected infant mortality (HR: 1·036, p = 0·089) (Fig. 3 F). Higher passively-acquired total cluster A-specific ADCC showed a similar relationship with shorter HIV-infected infant survival but this association was not statistically significant (Χ2 = 2·20; p = 0·14) (Fig. 3 C). When these analyses were restricted to infants who acquired HIV prior to six months of life, results were similar with maternal total cluster A-specific ADCC being statistically significantly associated with poorer infant outcome (HR = 1·108 (95% CI: 1·019–1·205), p = 0·016; Χ2 = 5·84, p = 0·016) and maternal C11-like ADCC showing trends toward poorer infant outcome (HR = 1·083 (95% CI: 0·999–1·174), p = 0·052; Χ2 = 3·54, p = 0·060). Additionally, it is possible that maternal viral load could be associated with infant survival by being a marker for maternal health and/or by affecting maternal antibody levels. After adjusting for maternal viral load in the Cox proportional hazards models, similar results with stronger associations between cluster A-specific ADCC with reduced infected infant survival were seen for both maternal and passively-acquired ADCC (Table S1).
Fig. 3.
Effect of Cluster A-specific ADCC on HIV-infected infant survival.
a-c: Kaplan-Meier survival curves between infants (N = 20) that had passively-acquired cluster A CD4i epitope-specific ADCC at/above the HIV-infected infant cohort median (blue lines) or below the HIV-infected infant cohort median (red lines) were compared by a log-rank test for A32-like ADCC (a), C11-like ADCC (b), or total cluster A-specific ADCC (sum of A32-like ADCC + C11-like ADCC, c). d-f: Kaplan-Meier survival curves between infants whose mothers (N = 21) had maternal cluster A CD4i epitope-specific ADCC at/above the transmitting mothers cohort median (blue lines) or below the transmitting mothers cohort median (red lines) were compared by a log-rank test for A32-like ADCC (d), C11-like ADCC (e), or total cluster A-specific ADCC (A32-like ADCC + C11-like ADCC, f). Χ2 values and p-values are shown. The x-axis shows months survival post infection (PI). The association of passively-acquired or maternal cluster A CD4i epitope-specific ADCC with risk of HIV+ infant mortality was measured by a Cox-proportional hazards model. Hazard ratios (HR), 95% confidence intervals (CI), and p-values are shown on the graphs. Statistical significance was defined as p < 0·05 (*). Cumulative (cum.) number of infants at-risk or censored by the end of each month on the x-axis are shown in the tables. Data from individual biological replicates from one maternal sample and one infant sample that were below the limit of detection were excluded from the analysis as described in the Materials and Methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Effect of ADCC enhancement on survival of HIV-infected infants
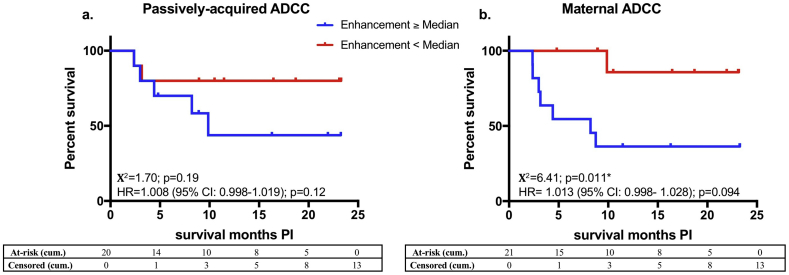
We also assessed the association of 17b-LALA-mediated ADCC enhancement with survival in the HIV-infected infants (Fig. 4). 17b-LALA-mediated enhancement of maternal ADCC was statistically significantly associated with reduced survival of HIV-infected infants and associated with a trend for increased risk of mortality (Χ2 = 6·41, p = 0·011; HR = 1·013, p = 0·094; Fig. 4B). Similarly, we found non-significant associations between 17b-LALA-mediated enhancement of infant passively-acquired ADCC and increased mortality of HIV-infected infants (Χ2 = 1·70, p = 0·19; HR = 1·008, p = 0·12 Fig. 4A). These associations were similar after adjusting for maternal viral load (Table S1).
Fig. 4.
Effect of 17b-LALA-mediated ADCC enhancement on HIV-infected infant survival.
a: Kaplan-Meier survival curves between infants (N = 20) that had 17b-LALA-mediated enhancement of passively-acquired ADCC at/above the HIV-infected infant cohort median (blue line) or below the HIV-infected infant cohort median (red line) were compared by a log-rank test. b: Kaplan-Meier survival curves between infants whose mothers (N = 21) had 17b-LALA-mediated enhancement of maternal ADCC at/above the transmitting mothers cohort median (blue line) or below the transmitting mothers cohort median (red line) were compared by a log-rank test. Χ2 values and p-values are shown. The x-axis shows months survival post infection (PI). The association of 17b-LALA-mediated enhancement of passively-acquired or maternal ADCC with risk of HIV-infected infant mortality was measured by a Cox-proportional hazards model. Hazard ratios (HR), 95% confidence intervals (CI), and p-values are shown on the graphs. Statistical significance was defined as p < 0·05 (*). Cumulative (cum.) number of infants at-risk or censored by the end of each month on the x-axis are shown in the tables. Data from individual biological replicates from one maternal sample and one infant sample that were below the limit of detection were excluded from the analysis as described in the Materials and Methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
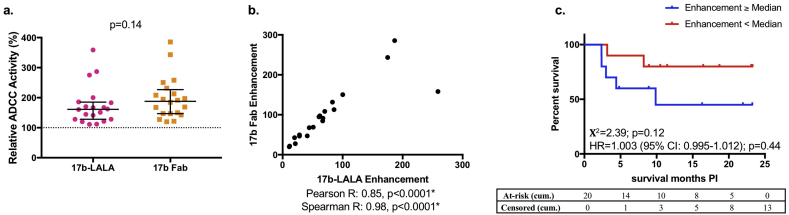
To further explore the mechanism of the association between 17b-LALA-mediated ADCC enhancement and infant outcome, and to rule out possible indirect steric effects or an artifactual increase in avidity due to pre-incubation with 17b-LALA, we compared plasma ADCC activity of the HIV-infected infants in the presence of 17b-LALA compared to 17b Fab (Fig. 5). Plasma ADCC was enhanced to a similar degree by both 17b-LALA and 17b Fab (mean LALA enhancement: 73·7%; mean Fab enhancement: 97·5%) and the enhancement observed in the presence of the Fab competitor correlated with the enhancement mediated by the 17b LALA variant (Pearson R = 0·85, p < 0·0001; Spearman R = 0·98, p < 0·0001). Similar to the results with 17b-LALA, enhancement mediated by 17b Fab was associated with shorter survival of HIV-infected infants, although this association was not statistically significant (Χ2 = 2·39, p = 0·12).
Fig. 5.
Enhancement of plasma ADCC by 17b Fab compared to 17b-LALA.
a: Either 5μg/ml 17b-LALA or 10μg/ml 17b Fab was used as the competitive inhibitor in the competition RFADCC assay with HIV-infected infant plasma (N = 20). Relative ADCC in the presence of 17b-LALA or 17b-Fab is shown and was compared by a Mann-Whitney U test. Error bars represent median + interquartile range. Relative ADCC in the presence of 17b-LALA is averaged from four biological replicates, relative ADCC in the presence of 17b-Fab is averaged from two biological replicates. b: Correlation between enhancement mediated by 17b-LALA and enhancement mediated by 17b-Fab is shown. Pearson and Spearman correlation coefficients and corresponding p-values are shown. c: Kaplan-Meier survival curves between HIV-infected infants (N = 20) that had 17b Fab-mediated passively-acquired ADCC enhancement at/above the HIV-infected infant cohort median (blue lines) or below the HIV-infected infant cohort median (red lines) were compared by a log-rank test. Χ2 values and p-values are shown. The x-axis shows months survival post infection (PI). The association of 17b Fab-mediated enhancement of passively-acquired ADCC with risk of HIV+ infant mortality was measured by a Cox-proportional hazards model. The hazard ratio (HR), 95% confidence intervals (CI), and p-values are shown on the graph. Cumulative (cum.) number of infants at-risk or censored by the end of each month on the x-axis are shown in the tables. Statistical significance was defined as p < 0·05 (*). Data from an individual biological replicate from one infant sample that was below the limit of detection was excluded from the analysis as described in the Materials and Methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
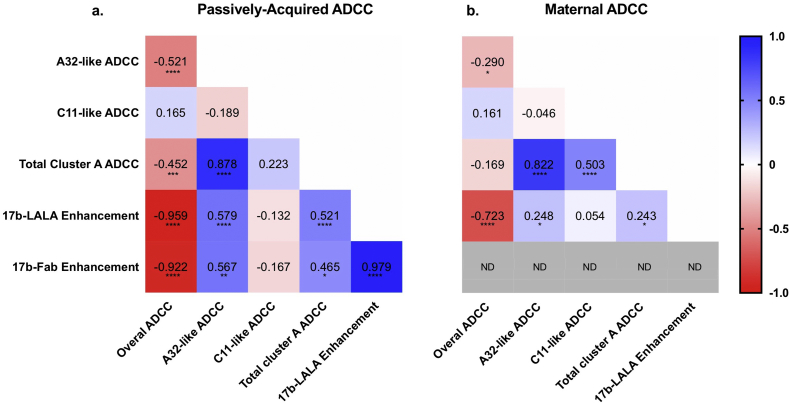
3.6. Correlations between various ADCC activities
To gain insight into interactions among the ADCC activities to specific epitopes and the overall ADCC activities in the absence of competitor, we compared correlations of A32-like ADCC, C11-like ADCC, total cluster A-specific ADCC, 17b-LALA-mediated ADCC enhancement, and 17b-Fab-mediated ADCC enhancement with overall ADCC (without the competitor) and with each other (Fig. 6). Interestingly, passively-acquired A32-like ADCC, total cluster A-specific ADCC, 17b-LALA-mediated ADCC enhancement, and 17b-Fab-mediated ADCC enhancement are statistically significantly inversely correlated with total plasma ADCC and directly correlated with each other, with a similar but weaker pattern for maternal plasma. C11-like ADCC showed a different pattern and was not statistically significantly correlated with overall ADCC or with ADCC enhancement. The inverse relationship between 17b-LALA mediated ADCC enhancement and overall ADCC was quite striking and strongly statistically significant for both infant and maternal plasma (infant spearman R = -0·959, p < 0·0001; maternal spearman R = -0·723, p < 0·0001).
Fig. 6.
Correlations between various ADCC activities.
Correlation matrix for ADCC activities of passively-acquired infant plasma (a) or maternal plasma (b). Spearman rank correlations are shown on the heatmap. Red indicates a negative correlation, white indicates no correlation, and blue indicates a positive correlation. ND = not done. Statistical significance levels are shown as:
p < 0·05 = *.
p < 0·01 = **.
p < 0·001 = ***.
p < 0·0001 = ****. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
ADCC has been shown to correlate with reduced risk of infection, reduced disease progression, and improved clinical outcome in MTCT, chronic infection, and vaccination [6,9,[18], [19], [20], [21],[56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]]. However, there are limited data on which epitopes of ADCC mediating antibodies are correlated with protection or improved clinical outcome in humans [57,60,69]. This study aimed to address this question in the context of breastfeeding MTCT in a cohort where passively-acquired ADCC activity has been reported as a correlate of improved infant outcome [9,18]. To our knowledge, this is the first study designed to measure the association of ADCC targeting CD4i epitopes with infection risk and disease outcome in humans. We did not find any associations between these responses and HIV transmission risk. Unexpectedly, rather than explaining the overall beneficial effect of ADCC-mediating antibodies, we found that commonly elicited anti-cluster A-activities were associated with increased mortality among those infants who contracted HIV infection.
Both A32-like and C11-like ADCC responses targeting the gp41-interactive region of gp120 were common and dominant in mothers and their infants, consistent with non-MTCT human studies [[26], [27], [28], [29],33,41,43,44]. While these specificities were common, there was no detectable association of A32-like ADCC, C11-like ADCC, or total cluster A-specific ADCC with risk of infant infection. This lack of association with transmission is perhaps not surprising given our previous finding of a weak and non-significant association between high total plasma ADCC and reduced risk of infant infection in this cohort [9].
In this study, we extended prior findings showing that passively-acquired ADCC in infants, but not maternal ADCC, was associated with improved survival of HIV-infected infants using two different antigens [9]. Neither passively-acquired nor maternal A32-like or C11-like ADCC were significantly associated with HIV-infected infant survival; rather, there was evidence from multiple analyses that high levels of total cluster A-specific ADCC was associated with reduced HIV-infected infant survival. These data indicate that ADCC-mediating antibodies targeting the cluster A CD4i epitopes are not driving the association of passively-acquired ADCC with improved survival of HIV-infected infants, and that ADCC targeting cluster A CD4i epitopes may be associated with increased mortality among those infants who acquire HIV-infection.
There was little detectable 17b-like ADCC activity in this cohort, perhaps reflecting the fact that cluster C-specific antibodies are less potent than cluster A [26,27,31]. Our lack of detection of 17b-like ADCC is in contrast to cohorts of chronically infected individuals in which cluster C-specific plasma ADCC was readily detectable and common [27,43]. However, this difference in detection of cluster C-specific ADCC may be due to differences in methods between our study and those conducted by Ferrari et al. and Alsahafi et al., which used the chromium release ADCC assay and a FACS-based assay against infected target cells, respectively [27,43]. Instead of detecting cluster C-specific plasma ADCC, we saw a surprising effect of preincubation of the target cells with 17b-LALA in which plasma ADCC was enhanced compared to preincubation with no competitor antibody. This was also observed when we used 17b Fab fragment as the competitor. Due to this enhancement phenomenon, we cannot rule out that the enhancement of non-17b-like ADCC was masking the 17b-like ADCC activity.
Interestingly, 17b-LALA-mediated enhancement of plasma ADCC measured in this assay was associated with decreased survival among the HIV-infected infants. Notably, 17b-LALA-mediated enhancement of ADCC was strongly inversely correlated with total plasma ADCC, raising the possibility that 17b-LALA-mediated ADCC enhancement may be a proxy for low overall ADCC, which was correlated with poorer infant outcome in this cohort, as shown here and in the prior study of this cohort conducted by Milligan et al. [9]
We hypothesized the following mechanism to explain the unexpected enhancement of ADCC mediated by 17b-LALA: during the preincubation step with 17b-LALA, binding of a 17b Fab arm to gp120 increases exposure of other epitopes of ADCC-mediating antibodies. This hypothesis is consistent with a number of other studies that have shown co-receptor binding site antibodies, such as 17b, to enhance the binding and ADCC activity of cluster A antibodies, such as A32 and C11 [30,40,43,70]. Of note, enhancement of plasma ADCC by 17b-Fab and 17b-LALA were significantly directly correlated with A32-like ADCC and total cluster A-specific ADCC, but not with C11-like ADCC, suggesting that 17b binding to its co-receptor binding site epitope leads to further exposure of the A32 epitope. This model is consistent with structure studies conducted by Tolbert et al. in the context of trimer, showing that after 17b antibody or 17b fab binding, A32 binds more efficiently to envelope than C11 does, and the authors suggest that the A32 epitope is exposed earlier in the viral entry process compared to the C11 epitope [40]. These findings were replicated by Alsahafi et al. [43] Taken together these data support a model that antibody binding to the co-receptor binding site leads to differential exposure of the non-overlapping cluster A A32 and C11 epitopes, with the A32 epitope becoming better exposed upon 17b binding. Thus, the 17b enhancement data, which may be indicative of increased cluster A-specific activity, is consistent with the finding that cluster A-specific activity is associated with worse infant outcome. However, we cannot rule out that the 17b fab was forming bivalent complexes after long-term storage [71], in which case bivalent 17b fab would resemble 17b f(ab)’2, and be expected to act similarly to 17b-LALA.
These findings add to limited studies of ADDC epitopes in relation to clinical outcomes [29,44,57,60,69,72]. A handful of studies have identified ADCC epitopes associated with elite controller or long-term non-progressor (LTNP) status compared to viremic progressors [57,60,69]. Additionally, CD4i-specific antibodies have been isolated from natural viral suppressors and RV144 vaccinees [26,29], but these studies did not compare epitopes between controllers vs progressors or HIV-infected vs uninfected vaccinees. In contrast to these promising data, our data in the setting of MTCT suggests that ADCC targeting the cluster A CD4i epitopes was not associated with protection from infection and was associated with accelerated HIV progression in infants who acquired HIV infection.
Cluster A-specific antibodies, while common, also have some unusual properties and have been shown to poorly mediate ADCC against cells infected with primary isolates due to the reduction of CD4 and Env on the cell membrane upon productive infection [[30], [31], [32], [33],35,37,38,70,[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83]]. Because they can recognize the open conformation of Env and thus the gp120 monomer, cluster A-specific antibodies can also target uninfected cells that have bound gp120 that has been shed from virus or infected cells [32,33,84]. As suggested by our data that cluster A-specific ADCC is associated with reduced infected infant survival, it is possible that the killing of uninfected bystander cells by cluster A-specific antibodies could contribute to accelerated disease progression among infected infants. It is interesting that A32-like ADCC measured in the assay used here, which uses target cells with bound gp120, was inversely correlated with total ADCC. This could suggest that A32-dominant responses typically arose when ADCC responses overall were weak due to the high immunogenicity of the A32 epitope, and that the positive association between A32-like activity and poor infant survival actually reflects a relatively weak overall ADCC response. In this scenario, the presence of A32 responses per se are not accelerating HIV progression but represent a misdirection of the immune response away from more protective antibody epitopes. Interestingly, ADCC responses in vivo are often polyclonal [27,28,33,41], and combinations of monoclonal antibodies have been shown to be important for ADCC [30,42,43,70]. Since most plasma samples had some A32-like activity, an alternative, plausible explanation for the negative correlation between A32-like activity and overall ADCC is that the strongest ADCC responses associated with improved infant outcome are polyclonal, where A32-like activity would be detectable, but would make up a lower percentage of the overall ADCC response compared to a monoclonal response, which could have a high percentage of A32-specific activity, but weak total activity.
Interestingly, while cluster A epitope-specific ADCC and 17b-LALA-mediated ADCC enhancement were associated with worse infant outcome, these associations were stronger for maternal ADCC activities compared to passively-acquired ADCC activities in infant plasma. This may suggest that the mechanism by which these antibodies are acting is occurring in the mother. Another possibility is based on placental transfer of antibodies. A recent study by Martinez and colleagues showed that even among the same mother-infant pair, antibody transfer rates across the placenta can vary between antibodies targeting different epitopes, even among antibodies targeting various HIV epitopes [85]. This variation in antibody transfer rate could explain why maternal ADCC activities were more strongly associated with worse infant outcome compared to passively-acquired ADCC activities in infant plasma for cluster A epitope-specific ADCC and 17b-LALA-mediated ADCC enhancement.
Our study has a number of limitations. Our study is limited by relatively low statistical power because of the small number of infants that acquired HIV. Additionally, of the 21 infants that acquired HIV, 7 had an estimated time of infection after six months of age, by which time passively-acquired antibody levels may have waned in the infant. Therefore, the relevance of passively-acquired ADCC in these late transmissions is unclear. Notably, when our data analyses were restricted to the 14 infants who acquired HIV prior to six months of age, results were similar to those of the entire cohort. Another caveat to this study is that we used the RFADCC assay with coated target cells. The RFADCC assay has been shown to predominantly measure a “monocyte-mediated ADCC” or trogocytosis process [[86], [87], [88]]. It has also been argued that coated cells are not as biologically relevant as infected cells. While the exact activity captured in this assay remains controversial, results of this assay have repeatedly shown a correlation between activity and outcome in humans [9,18,58,61,68]. Specifically, we chose to take this approach to replicate the methods used by Milligan et al. that showed a correlate of RFADCC activity and outcome [9]. The aim of the current study was to identify the epitopes of plasma ADCC initially measured in that prior study that are associated with the observed clinical outcome as these associations can help inform mechanisms of protection.
Although CD4i epitopes are highly conserved and immunodominant, our findings indicate that ADCC targeting these epitopes by themselves may not be beneficial, as CD4i epitope-specific ADCC was not associated with either reduced risk of infant infection or improved HIV-infected infant survival. These findings highlight a need for further research to clarify the role of CD4i epitope-specific ADCC-mediating antibodies in protection and clinical outcomes and whether they are beneficial or detrimental. Additionally, ADCC targeting other epitopes, including the V1 V2 epitope which was a correlate of protection in the RV144 trial [6,72,89], non-gp120 epitopes, novel epitopes yet to be defined, and polyclonal ADCC responses should also be explored as potential correlates of protection to inform vaccine and therapeutic design.
Funding sources
This study was funded by NIH grant R01AI076105 and NIH fellowship F30AI136636. Funding sources were not involved in the study design, data collection or analysis, or preparation of the manuscript.
Author contributions
J.O., N.N., J.S., G.J.S and R.N. participated in the design of the study. N.N. conducted the experiments. N.N., J.S. and B.R. participated in analysis of the data. N.N. and J.O. wrote the manuscript with input from all authors.
Declaration of Competing Interest
Dr. Naiman has nothing to disclose.
Dr. Slyker has nothing to disclose.
Dr. Richardson reports personal fees from Gilead, personal fees from PATH, personal fees from Theratechnologies, outside the submitted work.
Dr. John-Stewart reports grants from NIH, during the conduct of the study; grants from NIH, grants from CDC, grants from Thrasher, grants from IMPAACT, personal fees from UpToDate, personal fees from UW, outside the submitted work.
Dr. Nduati has nothing to disclose.
Dr. Overbaugh has nothing to disclose.
Acknowledgements
We would like to thank Caelan Radford for making the 17b Fab fragments, Jesse Bloom for providing the FI6v3-producing cells, and Michel Nussenzweig for providing the IgG1 expression vectors. We would like to thank all of the participants and investigators of the Nairobi Breastfeeding Clinical Trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.072.
Appendix A. Supplementary data
Supplementary material
References
- 1.Lynch R.M., Yamamoto T., McDermott A.B. HIV vaccine research and discovery in the nonhuman primates model: a unified theory in acquisition prevention and control of SIV infection. Curr. Opin. HIV AIDS. 2013;8:288–294. doi: 10.1097/COH.0b013e328361cfe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Excler J.L., Robb M.L., Kim J.H. Prospects for a globally effective HIV-1 vaccine. Am. J. Prev. Med. 2015;49:S307–S318. doi: 10.1016/j.amepre.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Escolano A., Dosenovic P., Nussenzweig M.C. Progress toward active or passive HIV-1 vaccination. J. Exp. Med. 2017;214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomaras G.D., Plotkin S.A. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol. Rev. 2017;275:245–261. doi: 10.1111/imr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 6.Haynes B.F., Gilbert P.B., McElrath M.J. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montefiori D.C., Karnasuta C., Huang Y. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan C., Slyker J.A., Overbaugh J. Chapter two - the role of immune responses in HIV mother-to-child transmission. In: Kielian M., Mettenleiter T.C., Roossinck M.J., editors. Advances in Virus Research. vol. 100. Academic Press; 2018. pp. 19–40. [DOI] [PubMed] [Google Scholar]
- 9.Milligan C., Richardson B.A., John-Stewart G., Nduati R., Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch J.B., Nduati R., Blish C.A. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J. Virol. 2011;85:5252–5261. doi: 10.1128/JVI.02216-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh B.S., Shaffer N., Coughlin R. Dynamics of maternal IgG antibody decay and HIV-specific antibody synthesis in infants born to seropositive mothers. The NYC perinatal HIV transmission study group. AIDS Res. Hum. Retrovir. 1993;9:907–912. doi: 10.1089/aid.1993.9.907. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez M., Ludwig D.A., Khan S.S. Has highly active antiretroviral therapy increased the time to seroreversion in HIV exposed but uninfected children? Clin. Infect. Dis. 2012;55:1255–1261. doi: 10.1093/cid/cis662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mother-to-child transmission of HIV infection. The European Collaborative StudyLancet. 1988;2:1039–1043. [PubMed] [Google Scholar]
- 14.Simpson B.J., Andiman W.A. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention. Pediatrics. 1994;93:840–842. [PubMed] [Google Scholar]
- 15.Sohn A.H., Thanh T.C., Thinh le Q. Failure of human immunodeficiency virus enzyme immunoassay to rule out infection among polymerase chain reaction-negative Vietnamese infants at 12 months of age. Pediatr. Infect. Dis. J. 2009;28:273–276. doi: 10.1097/INF.0b013e31818e03b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chantry C.J., Cooper E.R., Pelton S.I., Zorilla C., Hillyer G.V., Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr. Infect. Dis. J. 1995;14:382–387. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Van de Perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21:3374–3376. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 18.Mabuka J., Nduati R., Odem-Davis K., Peterson D., Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tranchat C., Van de Perre P., Simonon-Sorel A. Maternal humoral factors associated with perinatal human immunodeficiency virus type-1 transmission in a cohort from Kigali, Rwanda, 1988–1994. J Infect. 1999;39:213–220. doi: 10.1016/s0163-4453(99)90052-x. [DOI] [PubMed] [Google Scholar]
- 20.Ljunggren K., Moschese V., Broliden P.A. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J. Infect. Dis. 1990;161:198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- 21.Broliden K., Sievers E., Tovo P.A. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin. Exp. Immunol. 1993;93:56–64. doi: 10.1111/j.1365-2249.1993.tb06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard J., Prevost J., Alsahafi N., Ding S., Finzi A. Impact of HIV-1 envelope conformation on ADCC responses. Trends Microbiol. 2018;26:253–265. doi: 10.1016/j.tim.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Tolbert W.D., Sherburn R.T., Van V., Pazgier M. Structural basis for epitopes in the gp120 cluster a region that invokes potent effector cell activity. Viruses. 2019;11 doi: 10.3390/v11010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeVico A.L. CD4-induced epitopes in the HIV envelope glycoprotein, gp120. Curr. HIV Res. 2007;5:561–571. doi: 10.2174/157016207782418560. [DOI] [PubMed] [Google Scholar]
- 25.Wilen C.B., Tilton J.C., Doms R.W. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan Y., Pazgier M., Sajadi M.M. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari G., Pollara J., Kozink D. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 2011;85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams K.L., Cortez V., Dingens A.S. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine. 2015;2:1464–1477. doi: 10.1016/j.ebiom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonsignori M., Pollara J., Moody M.A. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand S.P., Prevost J., Baril S. Two families of Env antibodies efficiently engage fc-gamma receptors and eliminate HIV-1-infected cells. J. Virol. 2019;93 doi: 10.1128/JVI.01823-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding S., Veillette M., Coutu M. A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J. Virol. 2015;90:2127–2134. doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard J., Prevost J., Baxter A.E. Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses. MBio. 2018;9 doi: 10.1128/mBio.00358-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W.S., Prevost J., Richard J. CD4- and time-dependent susceptibility of HIV-1-infected cells to antibody-dependent cellular cytotoxicity. J. Virol. 2019;93 doi: 10.1128/JVI.01901-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gohain N., Tolbert W.D., Acharya P. Cocrystal structures of antibody N60-i3 and antibody JR4 in complex with gp120 define more cluster A epitopes involved in effective antibody-dependent effector function against HIV-1. J. Virol. 2015;89:8840–8854. doi: 10.1128/JVI.01232-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Bredow B., Arias J.F., Heyer L.N. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J. Virol. 2016;90:6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevost J., Zoubchenok D., Richard J. Influence of the envelope gp120 Phe 43 cavity on HIV-1 sensitivity to antibody-dependent cell-mediated cytotoxicity responses. J. Virol. 2017;91 doi: 10.1128/JVI.02452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham T.N., Lukhele S., Hajjar F., Routy J.P., Cohen E.A. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology. 2014;11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veillette M., Desormeaux A., Medjahed H. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J. Virol. 2014;88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya P., Tolbert W.D., Gohain N. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J. Virol. 2014;88:12895–12906. doi: 10.1128/JVI.02194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolbert W.D., Gohain N., Alsahafi N. Targeting the late stage of HIV-1 entry for antibody-dependent cellular cytotoxicity: structural basis for Env epitopes in the C11 region. Structure. 2017;25:1719–31 e4. doi: 10.1016/j.str.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolbert W.D., Gohain N., Veillette M. Paring down HIV Env: design and crystal structure of a stabilized inner domain of HIV-1 gp120 displaying a major ADCC target of the A32 region. Structure. 2016;24:697–709. doi: 10.1016/j.str.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollara J., Bonsignori M., Moody M.A. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 2014;88:7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alsahafi N., Bakouche N., Kazemi M. An asymmetric opening of HIV-1 envelope mediates antibody-dependent cellular cytotoxicity. Cell Host Microbe. 2019;25:578–87 e5. doi: 10.1016/j.chom.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomaras G.D., Ferrari G., Shen X. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeVico A., Fouts T., Lewis G.K. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17477–17482. doi: 10.1073/pnas.0707399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fouts T.R., Bagley K., Prado I.J. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E992–E999. doi: 10.1073/pnas.1423669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santra S., Tomaras G.D., Warrier R. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nduati R., John G., Mbori-Ngacha D. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 49.Panteleeff D.D., John G., Nduati R. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J. Clin. Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emery S., Bodrug S., Richardson B.A. Evaluation of performance of the gen-probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hezareh M., Hessell A.J., Jensen R.C., van de Winkel J.G., Parren P.W. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 2001;75:12161–12168. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wines B.D., Powell M.S., Parren P.W., Barnes N., Hogarth P.M. The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors Fc gamma RI and Fc gamma RIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J. Immunol (Baltimore Md : 1950) 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 53.Williams K.L., Stumpf M., Naiman N.E. Identification of HIV gp41-specific antibodies that mediate killing of infected cells. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Roman V.R., Florese R.H., Patterson L.J. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Wu X., Parast A.B., Richardson B.A. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J. Virol. 2006;80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baum L.L., Cassutt K.J., Knigge K. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 57.Madhavi V., Wines B.D., Amin J. HIV-1 Env- and Vpu-specific antibody-dependent cellular cytotoxicity responses associated with elite control of HIV. J. Virol. 2017;91 doi: 10.1128/JVI.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madhavi V., Wren L.H., Center R.J. Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design. AIDS. 2014;28:1859–1870. doi: 10.1097/QAD.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 59.Lambotte O., Ferrari G., Moog C. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. Aids. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kulkarni A., Kurle S., Shete A. Indian long-term non-Progressors show broad ADCC responses with preferential recognition of V3 region of envelope and a region from tat protein. Front. Immunol. 2017;8:5. doi: 10.3389/fimmu.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhande J., Angadi M., Murugavel K.G. Brief report: the anti-HIV-1 ADCC-mediating antibodies from Cervicovaginal secretions of HIV-infected women have an ability to mediate lysing of autologous CD4+ HIV-infected cells. J. Acquir. Immune Defic. Syndr. 2018;79:277–282. doi: 10.1097/QAI.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 62.Sawyer L.A., Katzenstein D.A., Hendry R.M. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 1990;6:341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 63.Forthal D.N., Landucci G., Haubrich R. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad R., Sindhu S.T., Toma E. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 2001;21:227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 65.Chung A.W., Navis M., Isitman G. Activation of NK cells by ADCC antibodies and HIV disease progression. J. Acquir. Immune Defic. Syndr. 2011;58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia M., Li D., He X. Impaired natural killer cell-induced antibody-dependent cell-mediated cytotoxicity is associated with human immunodeficiency virus-1 disease progression. Clin. Exp. Immunol. 2013;171:107–116. doi: 10.1111/j.1365-2249.2012.04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson S.E., Rollman E., Chung A.W. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol. 2011;24:359–368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz M.J., Salido J., Abusamra L. Evaluation of different parameters of humoral and cellular immune responses in HIV Serodiscordant heterosexual couples: humoral response potentially implicated in modulating transmission rates. EBioMedicine. 2017;26:25–37. doi: 10.1016/j.ebiom.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wren L.H., Chung A.W., Isitman G. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richard J., Pacheco B., Gohain N. Co-receptor binding site antibodies enable CD4-mimetics to expose conserved anti-cluster a ADCC epitopes on HIV-1 envelope glycoproteins. EBioMedicine. 2016;12:208–218. doi: 10.1016/j.ebiom.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson A.D., Hoffmann M.M., Parks C.A., Dasari S., Schrum A.G., Gil D. IgG Fab fragments forming bivalent complexes by a conformational mechanism that is reversible by osmolytes. J. Biol. Chem. 2012;287:42936–42950. doi: 10.1074/jbc.M112.410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao H.X., Bonsignori M., Alam S.M. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alsahafi N., Richard J., Prevost J. Impaired downregulation of NKG2D ligands by Nef proteins from elite controllers sensitizes HIV-1-infected cells to antibody-dependent cellular cytotoxicity. J. Virol. 2017;91 doi: 10.1128/JVI.00109-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard J., Prevost J., von Bredow B. BST-2 expression modulates small CD4-mimetic sensitization of HIV-1-infected cells to antibody-dependent cellular cytotoxicity. J. Virol. 2017;91 doi: 10.1128/JVI.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veillette M., Coutu M., Richard J. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J. Virol. 2015;89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruel T., Guivel-Benhassine F., Lorin V. Lack of ADCC breadth of human nonneutralizing anti-HIV-1 antibodies. J. Virol. 2017;91 doi: 10.1128/JVI.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prevost J., Richard J., Medjahed H. Incomplete downregulation of CD4 expression affects HIV-1 Env conformation and antibody-dependent cellular cytotoxicity responses. J. Virol. 2018;92 doi: 10.1128/JVI.00484-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alsahafi N., Ding S., Richard J. Nef proteins from HIV-1 elite controllers are inefficient at preventing antibody-dependent cellular cytotoxicity. J. Virol. 2015;90:2993–3002. doi: 10.1128/JVI.02973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez R.A., Hamlin R.E., Monroe A. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J. Virol. 2014;88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arias J.F., Heyer L.N., von Bredow B. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Bredow B., Arias J.F., Heyer L.N. Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity. J. Virol. 2015;89:10648–10655. doi: 10.1128/JVI.01911-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neil S.J., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 83.Van Damme N., Goff D., Katsura C. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richard J., Veillette M., Ding S. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine. 2016;3:122–134. doi: 10.1016/j.ebiom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez D.R., Fong Y., Li S.H. Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell. 2019;178:190–201 e11. doi: 10.1016/j.cell.2019.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kramski M., Schorcht A., Johnston A.P. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. J. Immunol. Methods. 2012;384:51–61. doi: 10.1016/j.jim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Kramski M., Parsons M.S., Stratov I., Kent S.J. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr. HIV Res. 2013;11:388–406. doi: 10.2174/1570162x113116660061. [DOI] [PubMed] [Google Scholar]
- 88.Alrubayyi A., Schuetz A., Lal K.G. A flow cytometry based assay that simultaneously measures cytotoxicity and monocyte mediated antibody dependent effector activity. J. Immunol. Methods. 2018;462:74–82. doi: 10.1016/j.jim.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Zolla-Pazner S., deCamp A., Gilbert P.B. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material