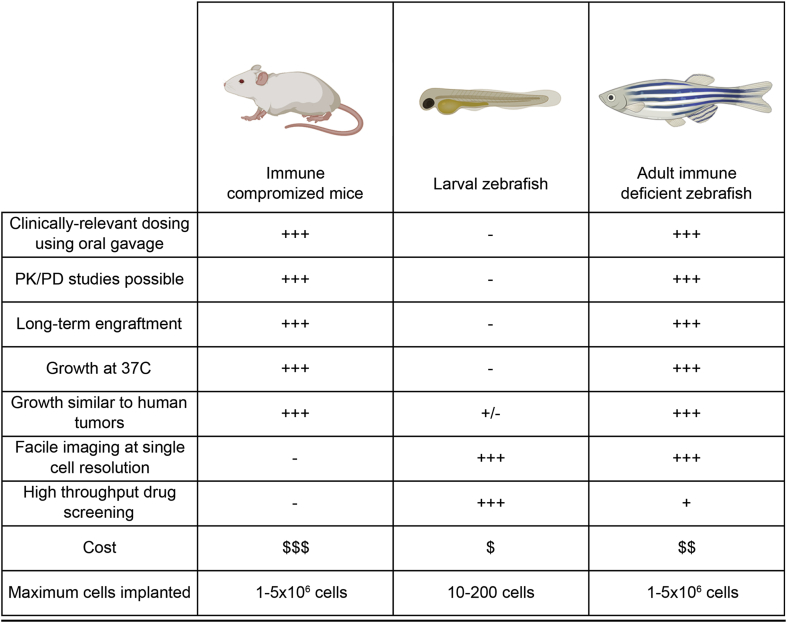
Immune compromised mice are an invaluable model for xenograft cell transplantation studies. To date, engraftment into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice has become the gold standard for accessing cell growth, differentiation, pluripotency of stem cell populations, and therapy responses in human cancers [1] (Fig. 1). However, these murine models are expensive and single cell imaging of engrafted cells requires complex imaging techniques including surgical window implantation into engrafted mice [1]. By contrast, zebrafish have many attributes that make it an ideal cell transplantation model including high fecundity, low cost and optical clarity (Fig. 1). Current zebrafish xenotransplantation studies have predominantly been carried out using larval recipients, as the adaptive immune system only becomes fully functional by three-weeks post fertilization [2]. Despite many important findings coming from cancer xenograft studies using larval zebrafish including elegant pre-clinical modeling and assessing patient responses to therapy [2], they are necessarily limited by the numbers of cells that can be engrafted and a short experimental time window that is usually confined to 5–7 days (Fig. 1). In addition, larval zebrafish engraftment studies are carried out at 34 °C–35 °C; yet, many human tumor cells do not grow well at these non-physiological temperatures and most do not form tumor masses akin to those found in xenografted mice or primary human tumors. Finally, because of their small body size and ability to only deliver drugs using submersion therapy, achieving accurate drug dosing and assessing pharmacokinetics is impossible using larval xenografts.
Fig. 1.
Comparison of mouse and zebrafish xenograft cell transplant models.
To address the limitations of short-term larval engraftment studies, the community has created an array of partially immune compromised strains of zebrafish that robustly engraft allogeneic tissues [[3], [4], [5], [6]]. Building on these successes, an optically-clear, homozygous compound mutant prkdcD3612fs, il2rgaY91fs (prkdc−/−, il2rga−/−) casper-strain zebrafish has been developed that lack T, B and natural killer (NK) cells [7] (Fig. 1). These immune deficient animals can survive at 37 °C and robustly engraft a variety of fluorescently-labeled human cancers for in excess of 28 days. Remarkably, the growth kinetics, histology, cell proliferation and apoptotic rates are largely similar when compared to the same tumor engrafted into NSG mice. Yan et al. went on to identify a drug combination, olaparib PARP-inhibitor and the DNA damaging agent temozolomide, that curbed growth of pediatric rhabdomyosarcoma muscle cancers. Moreover, optical clarity of the prkdc−/−, il2rga−/− casper-strain zebrafish allowed dynamic visualization of therapy responses in vivo using clinically relevant dosing using oral gavage and the four-color FUCCI4-cell cycle reporter. Importantly, this work also confirmed that this same drug combination elicited potent anti-tumor responses in mouse xenografts and had the similiar pharmacokinetics as observed in mouse and human [7]. Based on these results, olaparib and temozolomide combination is soon to be tested in a phase II clinical trial, providing the first example of clinical translation for cancer therapy originating from zebrafish xenograft studies.
Achieving robust, long-term engraftment of human cancer cells into adult immune compromised zebrafish is an important step forward in preclinical animal modeling. The zebrafish offers many complementing traits to their murine counterparts. First, the optical clarity of casper-strain immune compromised zebrafish is ideal for high-resolution imaging of different cancer cell processes. For example, by using conventional confocal microscopy and a one-step anesthesia procedure involving submerging the animal in tricaine-infused water, we were able to carry out photoconversion cell lineage-tracing of single engrafted tumor cells with an imaging depth of up to 300 μm, in an average of 5 min per transplant animal. Secondly, engrafting patient-derived cancer xenografts (PDXs) into the prkdc−/−, il2rga−/− model is particularly exciting [7]. The eventual hope is that engrafting a patient's tumor into large cohorts of animals will permit testing of a wide array of clinically available drugs, pairing responses in zebrafish avatars with clinical decision-making that stratifies patients into the most suitable treatment for their tumor. Finally, the adult immunocompromised zebrafish model also has the potential to transform pre-clinical animal modeling and drug discovery by administering drugs orally in a clinically achievable manner, increasing the throughput of in vivo screening, and providing faster imaging endpoints that capitalize on the ability to assess drug affects in real-time and at single cell resolution (Fig. 1).
Despite the recent successes in engrafting human cancers into immune-deficient zebrafish, the current prkdc−/−, il2rga−/− model is far from perfect. Engraftment efficiency of most human cancer types ranged from 50% to 90%, with some tumor types never engrafting into the model [7]. Rejection in a subset of recipient animals is likely due to several reasons. First, like immune compromised scid mice, the prkdc mutant zebrafish develop “leakiness” over time, resulting in the retention of small populations of residual B cells [3,8]. Because, a subset of teleost B cells have phagocytic activity [9], it is likely that B cell retention may directly impact tumor cell killing. Second, zebrafish have two distinct populations of NK cells [8]. Importantly, the NK-lysin-expressing subset are retained in prkdc−/−, il2rga−/− zebrafish and likely have the ability to produce cytotoxic, anti-microbial peptides that might account for rejection of transplanted cells [8]. Finally, current engraftment studies into the prkdc−/−, il2rga−/− zebrafish require pre-treating fish with clodronate liposomes to deplete macrophages [7]. It is possible that rejection occurs only in those animals with incomplete clearing of macrophages. Clearly, new immune compromised zebrafish models with mutations that fully deplete T, B, macrophages, and all NK cell subsets will likely provide superior engraftment models in the future.
With the established prkdc−/−, il2rga−/− zebrafish and the further development of new immunodeficient zebrafish models, we envision the widespread application of immune compromised zebrafish for the study of cancer biology, stem cell and regenerative biology, and assessing therapy responses in vivo. Future studies will likely see the engraftment of human embryonic stem cells and induced pluripotent stem cells (iPSCs). When paired with the large-scale drug discovery platforms available in the zebrafish model, it is also likely that high throughput screens will identify novel factors that influence self-renewal and lineage specification directly within live animals. New transgenic approaches will also likely be complexed with available immune-deficient zebrafish, providing the ability to express human cytokines that support the growth of human CD34+ cord blood cells and peripheral mononuclear blood cells (PMBCs). These humanized models would provide powerful tools to visualize human blood cell development and witness stem cell self-renewal divisions in vivo, akin to elegant studies already available to the allogeneic zebrafish model [10]. Moreover, creating “humanized” zebrafish would also be extremely useful for cancer immunotherapy studies, allowing visualization of immune cell/cancer cell interactions, quantifying cytotoxic responses of the modified immune cells, and assessing specificity of on target killing.
In conclusion, developing adult immune compromised zebrafish models has provided a much needed step in the evolution of transplantation biology. Engraftment studies using adult immune compromised zebrafish will surely provide unique insights into cancer biology, stem cell and regenerative medicine, and drug discovery, especially in the context of performing large-scale drug studies using oral gavage and visualizing cell responses at single cell resolution in vivo.
Disclosure
Dr. Langenau has a patent pending on the immune-compromised zebrafish models. Other authors declare no conflicts of interest.
Acknowledgements
This work is supported by NIH grant R24OD016761, R01CA154923, R01CA215118, R01CA211734, the Liddy Shriver Sarcoma Initiative, the MGH Research Scholars Program, the Millett-O'Neill Sommelier Foundation, the Tosteson & Fund for Medical Discovery Fellowship from MGH (Y.C.), and the Alex's Lemonade Stand Foundation Young Investigator Award (Y.C.).
References
- 1.Day C.P., Merlino G., Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163(1):39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fior R., Povoa V., Mendes R.V., Carvalho T., Gomes A., Figueiredo N. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc Natl Acad Sci U S A. 2017;114(39):8234–8243. doi: 10.1073/pnas.1618389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J.C., Tang Q., Yordan N.T., Moore F.E., Garcia E.G., Lobbardi R. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J Exp Med. 2016;213(12):2575–2589. doi: 10.1084/jem.20160378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Q., Abdelfattah N.S., Blackburn J.S., Moore J.C., Martinez S.A., Moore F.E. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat Methods. 2014;11(8):821–824. doi: 10.1038/nmeth.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess I., Iwanami N., Schorpp M., Boehm T. Zebrafish model for allogeneic hematopoietic cell transplantation not requiring preconditioning. Proc Natl Acad Sci U S A. 2013;110(11):4327–4332. doi: 10.1073/pnas.1219847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wienholds E., Schulte-Merker S., Walderich B., Plasterk R.H. Target-selected inactivation of the zebrafish rag1 gene. Science (New York, NY) 2002;297(5578):99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 7.Yan C., Brunson D.C., Tang Q., Do D., Iftimia N.A., Moore J.C. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019;177(7):1903–1914. doi: 10.1016/j.cell.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q., Iyer S., Lobbardi R., Moore J.C., Chen H., Lareau C. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J Exp Med. 2017;214(10):2875–2887. doi: 10.1084/jem.20170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Barreda D.R., Zhang Y.A., Boshra H., Gelman A.E., Lapatra S. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7(10):1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 10.Tamplin O.J., Durand E.M., Carr L.A., Childs S.J., Hagedorn E.J., Li P. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160(1–2):241–252. doi: 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]