Abstract
Background
Long term low-dose benzene exposure leads to the inhibition of haematopoiesis. However, the underlying mechanisms remained poorly defined, especially mediated by early effector molecules.
Methods
Here, we first found in mRNA microarray that pyroptotic classic genes (Casp1, 4, 5, and IL1β) were up-regulated and represented dose-dependent differential expression in controls, low-dose benzene-exposed and chronic benzene-poisoned workers, and the expression of Casp1 and IL1β were confirmed in low-dose benzene-exposed workers and was accompanied with elevated potent proinflammatory IL1β. In vitro studies showed that benzene metabolites induced AHH-1 cell pyroptosis through activating Aim2/Casp1 pathway with the increased expression of GSDMD. Meanwhile, TET2 overexpression was elevated in vivo and in vitro and it was positively correlated with IL1β. Further, we verified that pyroptosis caused by 1,4-BQ could be ameliorated in vitro by RNAi or pretreatment with Dimethyloxalylglycine (DMOG), the inhibitor of TET2.
Findings
Exposure to benzene can trigger pyroptosis via TET2 directly regulating the Aim2/Casp1 signaling pathway to cause haematotoxicity.
Interpretation
Benzene metabolites induced pyroptotic cell death through activation of the Aim2/Casp1 pathway which can be regulated by Tet2 overexpression. Tet2 may be a potential risk factor and is implicated in the development of benzene-related diseases.
Fund
National Natural Science Foundation of China; the Support Project of High–level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan; Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education.
Keywords: Benzene, TET2, Pyroptosis, Inflammatory, Hematotoxicity
Research in context.
Evidence before this study
Benzene absorbed into the human body can form active metabolites and induce haematotoxicity. However, the detailed mechanism is still unclear. Research found that chronic inflammation is likely to play a significant role in benzene-induced disease initiation and progression. Under these circumstances, cells pyroptosis, a highly inflammatory form of programmed cell death, was reported, and inhibiting cell pyroptosis increased survival. Meanwhile, Tet2, a DNA methylcytosine dioxygenase, was identified that can regulate inflammasome-associated genes to induce cells pyroptosis. We hypothesized that TET2 might be involved in benzene-induced pyroptotic cell death.
Added value of this study
In the present study, we found benzene exposure induced the specific expression of pyroptotic-related genes and inflammatory response in human. In vitro, we confirmed that benzene metabolites induced pyroptotic cell death through activating the Aim2/Casp1 pathway which can be regulated by Tet2 overexpression. Tet2 may be a potential risk factor and is implicated in the development of benzene-related diseases. This is the first time that a direct effect of benzene exposure on pyroptosis has been reported, it may provide a new perspective for early prevention and intervention of benzene-induced disease.
Implications of all the available evidence
Our study demonstrates that exposure to benzene was able to induce pyroptosis cell death and inflammatory response via up-regulation of TET2. TET2 may be a potential early biological effect biomarker and preventive target for benzene toxicity.
Alt-text: Unlabelled Box
1. Introduction
Benzene, as an important organic chemical, is widely existed in the workplace and in the general environment [14]. People exposed to short-term high-dose benzene will arise anaesthesia of the central nervous system and organ failure while long-term low-dose exposure to benzene causes decrease in the peripheral blood cell count, chronic benzene poisoning, even hematologic malignancies such as myelodys plastic syndromes (MDS) and leukemia [12,14]. Up to present, the metabolic pathway of benzene in human body and the hazard outcome are well known [25], but the mechanism of benzene toxicity is unclear. Exploring the microscopic mechanism will greatly promote the screening of early, special and sensitive biomarkers, so there still no early, special and sensitive biomarkers were found for benzene-induced diseases. However, it is distressing to see that the production and consumption of pure benzene still increasing gradually in developing countries. Researchers concluded that the workforce in China still have high airborne concentrations of benzene. According to the National report on Occupational Diseases issued by National Health Commission of the People's Republic of China from 2011 to 2017 (Fig. S1), the number and the incidence of poisoning and leukemia cases caused by benzene have remained stubbornly high in China. Therefore, it is necessary to study the detailed toxic mechanisms of benzene, which helps with their health care and risk screening.
Pyroptosis is a proinflammatory form of regulated cell death which were verified rely on the active precursor of caspases family including the classical Casp1 active precursor or non-classical Casp4, Casp5, and Casp11 active precursor [17]. These inflammatory Caspases precursor one side cleave the pro-inflammatory cytokines IL1β and IL18 to produce active forms [17], for another, cleave the pyroptotic pore-forming protein gasdermin D(GSDMD), generating an N-terminal cleavage product transferring to cell membrane that triggers cell membrane perforation promoting IL1β and IL18 active forms release [24]. Among them, IL1β, as potent proinflammatory cytokine, induce secondary cytokine production (e.g., IL6, IL8, and CSF) through recruiting and activating other immune cells such as lymphocytec, leading to the occurrence of severe inflammatory response [23]. Limit studies have shown that pyroptosis may be involved in multiple types of haematological diseases such as MDS and acute myelogenous leukemia (AML). For example, researcher found that pyroptotic and other cellular inflammatory mediators promoted progression of MDS [1]. Rodriguez-Cimadevilla JC, et al., has reported that IL1β can stimulate AML growth [21]. Those works offered a new insight into the occurrence and development of haematological diseases associated with cell pyroptosis death.
Plenty of research have been reported that generation of caspases precursors depend on a multi-molecular platform usually comprised of a cytosolic sensor, an adaptor of ASC (apoptotic speck-likeprotein containing CARD) and an effector of pro-Caspase-1 [17]. Inflammasomes, as pattern recognition receptors, play important roles in potential disease development and cancer promotion. Research has found that carbon nanotubes and crystalline silica [11], thiopyran derivative [6], and cadmium [5] all can activate inflammasomes, trigger cell pyroptosis death, finally lead diseases or cancer. Of these inflammasomes, melanoma 2 (AIM2)-like receptor was reported that it can activate Casp1 in response to molecular signals from pathogens and other dangerous stimuli, simultaneously, have a role in the reduction of cell proliferation by cell cycle arrest, and were found to be highly expression in leukemia without TET2 mutations [19,29]. AIM2 is part of the inflammasome and can drive pyroptosis and proteolytic cleavage of the proinflammatory cytokines pro-IL1β and pro-IL18 [16]. However, whether benzene and its metabolite can induce pyroptotic cell death by activating Aim2, and the role and precise mechanisms of pyroptosis in benzene induced hematotoxicity remains unclear.
TET2 (Ten-Eleven Translocation 2) gene, a member of the TET family of enzymes located on chromosome 4q24, and its protein product TET2 can modify DNA through catalyzing the conversion of 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) [7]. Accumulating evidence shows that TET2 is critical for the expression of Aim2. For example, Aim2 were found hypermethylated with low expression level in chronic myelomonocytic leukemia(CMML) patients with mutant TET2 [29] while another research found that increasing the expression of TET2 will up-regulate the expression of Aim2 by inhibiting global changes in 5-hmC levels in human leukemic cells [26]. In patients with cryopyrin associated periodic syndromes, Aim2 showed demethylation accompanied by subsequent activation of gene expression, however the mRNA levels of Aim2 showed decreased upregulation when inhibit the expression of TET2 [27]. Those results reveal that inflammasome-associated genes Aim2 activation depend on TET2. Recently, research found that redox-active quinones induces the conversion of 5mC to 5hmC depend on the existence of TET2 [31] and TET2 is a sensitive indicator responding for quinine derivatives in cells. Based on the above research, we hypothesis that TET2 might be involved in benzene-induced pyroptotic cell death through activating Aim2.
In this study, we first attempt to identify whether benzene and its metabolite can induce pyroptotic cell death and the underlying mechanism in both in vitro and in vivo. The results reveal that benzene metabolite promote TET2 expression activating Aim2/Casp1pathway to trigger cell pyroptosis death and inflammatory overactivation which induced hematotoxicity. These observations are of great significance for the early health screening and prevention of benzene exposure.
2. Materials and methods
2.1. Characteristics of subjects
140 subjects including 69 low-level benzene-exposed workers and 71 references were recruited with gender, BMI, medical history, smoking and drinking matched listed in Table S2. Among them, the peripheral blood cells of 28 male controls and 28 male low-level benzene-exposed workers matched confounding factors such as age, height, weight and lifestyle included in smoking and drinking were collected for RNA extraction. Benzene-exposed workers who were engaged in spraying lacquer were selected from a factory in Beijing, China and the average concentration of benzene in the air was 1.82 ± 1.16 mg/m3 while the highest concentration of benzene was 3.5 mg/m3 which lower than National occupational exposure limit for benzene concentration(6 mg/m3) in China. Controls employed in the same factory were not exposed to benzene or any other known occupational carcinogen. A detailed questionnaire including gender, age, BMI, smoking history, alcohol consumption, and family and personal medical history were interviewed and subjects with autoimmune disorder, inflammatory disease and angiocardiopathy were excluded. This study was approved by the Institutional Review Board of Capital Medical University, and informed consent was obtained from all subjects prior to their recruitment into the study.
2.2. Routine blood detection and sample preparation
Blood samples were collected from subjects for hematology analysis immediately. Medical parameters, including total white blood cell counts (WBC), neutrophil (NEUT), lymphocyte (LYMPH), red blood cell counts (RBC), hemoglobin (HGB), blood platelet (PLT), alanine aminotransferase (ALT) were detected using an automated blood analyzer (Brand). Then the blood samples were centrifuged at 3000 rpm for 10 min to isolate the serum fraction. Blood samples and serum fraction were stored at −80 °C prior to other analysis.
2.3. mRNA microarray
Study subjects in mRNA microarray are mainly engaged in painting and control group is the office staff of the same unit. Chronic benzene poisoning was diagnosed according to diagnostic criteria and principles of occupational benzene poisoning (GBZ 68–2008). To control the impact of confounding factors, age, gender, medical history, the history of occupation and lifestyle such as smoking and drinking were matched showed in Table S1. And the detailed information was expounded as previously described [8]. mRNA microarray was performed as previously described [8]. Total RNA was extracted from human peripheral blood cells using TRIzol reagent (Invitrogen, USA), then purified RNA was amplified and transcribed into cRNA. cDNA was labeled and hybridized to the GeneChip Human Gene 2.0 ST Array (Affymetrix). The experimentations were scrutinized by GeneChip® Command Console® Software (AGCC), meanwhile, the acquired array images were analyzed by Affymetrix GeneChip Operating Software. Affymetrix® Expression Console™ Software were used to control QC analysis of Gene 2.0 ST Array data.
2.4. Reagents and antibodies
1,4-Benzoquinone (1,4-BQ), Thiazolyl Blue Tetrazolium Bromideand (MTT), and Dimethyloxalylglycine (DMOG, TET2 inhibitor), Ac-YVAD-cmk (Casp1 inhibitor) were purchased from Sigma-Aldrich (USA). FAM-FLICA® Caspase-1 Assay Kit was purchased from ImmunoChemistry Technologies (USA)., Irreversible caspase-1inhibitor (ab141388) was purchased from Abcam (UK). RevertAid First Strand cDNA Synthesis Kit was obtained from ThermoFisher Scientific (USA) while KAPA SYBR® Fast Mastermix were acquired from KAPABiosystems(USA). Human IL1β ELISA Kit, Human IL6 ELISA Kit, Human IL8 ELISA Kit, Human IL10 ELISA Kit, and Goat Anti-Rabbit IgG H&L (FITC), were acquired from Abcam (UK). TET2 antibodies, Aim2, CASP1, IL1β, β-actin and anti-mouse/rabbit IgG antibody were purchased from Cell Signaling Technologie (USA) while GSDMD was acquired from Proeintech.
2.5. Cell culture and treatment
The AHH-1 cells were human B lymphocytes immortalized by Epstein-Barr virus and obtained from the National Institute for Radiological Protection, China CDC (Chinese Center for Medical Response to Radiation Emergency) in 2016. The cell lines were maintained in RPMI-1640 contained 10% donor equine serum (HyClone, USA) and 1% antibiotic (100 U/ml penicillin and 100 mg/ml streptomycin) cultured in a 37 °C humidified incubator with 5% CO2. Culture media was replaced every 3 days. AHH-1 cells used for cell vitality detection were grown in 96-well plates whereas those used for qRT-PCR, immunoblotting, LDH assay was plated at 6-well plates or 25 cm2-cell culture bottles. Culture media was replaced every 3 days. Cells were plated at a density of 1.0 × 106 cells per milliliter (ml).
2.6. Cell viability
Cell viability of AHH-1 cell lines was determined using MTT assay. Briefly, total 1.0 × 105 cells per hole were seeded into 96-well plates for 12 h or 24 h treated with different doses of 1, 4-BQ (0, 5, 10, 20, 40 μM) and cultured for 12 h and 24 h, respectively, then 10 μl MTT solution with the density of 5 mg/ml was added and incubated for 4 h at 37 °C, 12 h and 24 h after cultured, 150 μL Dimethyl sulfoxide (DMSO) was added and shaking gently about 10 min. The absorbance was measured at 492 nm.
2.7. Flow cytometry
Pyroptosis analysis was performed by flow cytometry according to the manufacturer's instruction. Briefly, AHH-1 cells were treated with 1,4-BQ for the indicated time, harvested, washed, and stained with Casp1 fluorescent inhibitor probe (FAM-YVAD-FMK) at room temperature for 1 h. Remove media, wash cells 3 times, and resuspend cells using propidium Iodide (PI). Resuspended cells were then analyzed by flow cytometry (ACEA NovoCyte®, ACEA Biosciences Inc., Hangzhou, Zhejiang, China), and the data were analyzed by ACEA NovoExpress® software.
2.8. ELISA
The serum fraction was isolated from blood samples with centrifuging at 3000 rpm for 10 min and were stored at −80 °C prior to analysis. AHH-1 cells were treated with 1,4-BQ for 24 h at 37 °C. Cell culture supernatants were collected, and cells was lysed for immediate use. Inflammatory molecules (IL1β, IL8, IL6, and IL10) in serum of human and in cell lysis solution and were analyzed using ELISA kit following the manufacturer's instructions. Negative controls were samples containing no cell lysate or no substrate were included.
2.9. Immunofluorescence
AHH-1 Cells were treated with 1,4-BQ for 24 h, then harvested to a centrifugal tube with the volume of 1.5 ml. Then, 100 μL PBS solution was added and gently blew evenly. Absorbing 50 μl suspension dropped to a cover slide, dried by airing. Adding 200 μL 4% paraformaldehyde fixed for 20 min at room temperature. After washing three times with PBS, the plates were incubated for 20 min with 200 μL 0.3% Triton X-100 at room temperature. Then cells were stained with antibody and diluted in blocking solution overnight at 4 °C. The next day, cells were incubated with secondary antibody at room temperature for 90 min before being mounted in the photobleach resistant mounting media containing DAPI Vectashield. Cells were then imaged using a Laser confocal microscope (TCS SP5, Leica, USA).
2.10. RNA-seq analysis
Total RNA was isolated from 5 × 106 cells and used for RNA-seq analysis. cDNA library construction was performed by Shanghai Jiaying Biotechnology using using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA). The products were purified using the AMPure XP system and sequencing were performed on the Agilent Bioanalyzer 2100 system. According the manufacturer's recommendations, carrying out clustering of the index-coded samples on a cBot Cluster Generation System using a HiSeq 2500 PE Cluster Kit (Illumia, USA), then, the prepared libraries were sequenced on an Illumina Hiseq 2500 platform and reads were produced.
2.11. Cell transfection or inhibitor
Lentiviral particles of TET2 interference were purchased from GeneChem (Shanghai, China). 1 × 105 cells AHH-1 cells were transfected with 5 × 106 transducing units of lentivirus in combination with HitransG A transfection enhancement reagent following manufacturer's instructions. After72 h, observing the situation of green fluorescent protein marker detected infection efficiency by STED ultra-high resolution confocal microscope (TCS SP8 STED, Leica, USA). In addition, the concentration of TET2 inhibitor (DMOG) was set at 500 μM and AHH-1 cells were treated with this chemical for 2 h before exposure to 1,4-BQ. The caspase-1 inhibitor Ac-YVAD-CMK was dissolved in DMSO and used at a concentration of 50 μM for 2 h before exposure to 1,4-BQ.
2.12. RNA isolation and qRT-PCR assay
Total RNA was extracted from human peripheral blood cells or AHH-1 cells using Column Blood RNAOUT (TIANDZ, China) according to the manufacturer's protocol. qRT-PCR analysis was performed using RevertAid First strand cDNA (Thermo Fisher Scientific, USA) and KAPA SYBR® Fast Mastermix (KAPABiosystems, USA) according to the manufacturer's protocol. Data were normalized to β-actin expression. qRT-PCR was performed on Bio-Rad (CFX96™ optics module). Each sample was repeated three times. The primers for mRNA were designed and synthesized by Sangon Biotech (Shanghai, China) and are listed in Table S6.
2.13. Western blot
AHH-1 cells treated with 1,4-BQ for 24 h were harvested in cold lysis buffer which every 1 ml lysis buffer contained 10 μl phosphatase inhibitor, 1 μl protease inhibitor, and 5 μl PMSF. Incubated with ice for 30 min followed by brief sonication and centrifugation to remove cell debris. Protein concentration of cell lysates was determined using a BCA Protein Assay Kit (Dingguo Changsheng biotechnology, Beijing, China). The equal amounts of total protein added bromophenol blue indicator were loaded onto an 8%, 10%, or 15% SDS–PAGE gel. The Samples were transferred to a PVDF membrane in different time before blocked with 5% BSA in TBST for 2 h, and then probed overnight with shaking at 4 °C using primary antibodies. The next day, washed with TBST for three times and incubated with secondary antibody at room temperature for 90 min. ECL reagent (Thermo Fisher Scientific, USA) were used detected with the blots.
2.14. Statistical analysis
Statistical analyses were performed by SPSS version 20.0 software. The correlation between either two variables was investigated using pearson analysis. Statistical differences were calculated using one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test or Student's t-test to compare the differences among groups. Data were expressed as the mean ± S.D. of independent experiments, p < .05 was statistically significant.
3. Results
3.1. Benzene exposure-induced pyroptotic promoting inflammatory response result in leucocyte decrease in benzene-exposed workers
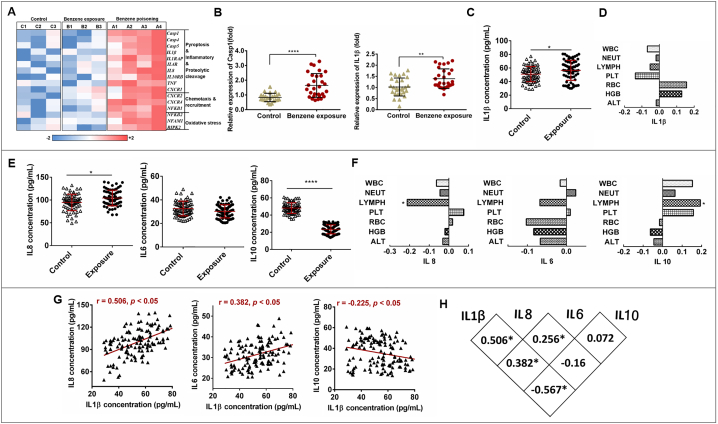
To identify differentially expressed of mRNA associated with benzene hematotoxicity, mRNA microarray was conducted in three health subjects, three benzene-exposed workers, and four benzene poisoning patients matched in age, gender and other confounding factors. Especially, several pyroptotic classic genes such as Casp1, 4, 5, and IL1β were up-regulated and represented dose-dependent differential expression (Fig. 1A). Then, to further validate the above results and explore the effect of benzene exposure, we recruited total 140 subjects including 69 workers with benzene exposure and 71 healthy controls as references. The WBC, RBC, and PLT show significant decreased in female subjects exposed to benzene (Table S1). Thereinto, 28 controls and 28 low-level benzene exposed workers were matched to extract the total RNA of human blood. qRT-PCR were used to detect the expression of Casp1 and IL1β which is crucial to mediate pyroptosis. As a result, the expressions of Casp1 and IL1β were all up-regulated in benzene-exposed workers (Fig. 1B). ELISA were used to detect the level of pyroptosis classical proinflammatory factor in all 140 subjects. The results in Fig. 1C show that benzene exposure increased IL1β release and IL1β showed a negative correlation trend with leucocyte including WBC, NEUT, LYMPH, and PLT although there is no significant difference (Fig. 1D). Dysregulation of the inflammatory response is a key driver of many types of leukemia and pyroptosis cell death are important drivers of this inflammatory response. To investigate the effect of benzene exposure on inflammation, IL8, IL6 and IL10 were detected in all subjects. The results in Fig. 1E show that the level of IL8 were significantly increased in benzene-exposed groups compared to that of controls, meanwhile, anti-inflammatory factor of IL-10 were quite lower than controls, and, proinflammatory IL8 showed significantly negative correlated with LYMPH while IL-10 showed positive correlated with LYMPH(Fig. 1F), which may represent inflammatory overactivation occurred in benzene exposure workers. Moreover, increased level of IL8 showed strongly positive with IL1β (r = 0.506, p < .05), whereas IL10 was negative correlated with IL1β (r = 0.225, p = .05). In addition, although IL6 showed no significant difference in two group, it was found to be positive correlated with IL1β (r = 0.382, p = .05) (Fig. 1G and H). Taken together, these results indicate that benzene exposure induce the specific expression of pyroptotic-related genes and inflammatory response in human, pyroptotic may participation in leucocyte decrease.
Fig. 1.
Effect of benzene exposure on pyroptosis-associated genes and inflammatory factors. (A) Heat map of the log2-transformed expression level of mRNAs associated with benzene hematotoxicity. The expressed mRNAs among three groups including benzene poisoning group (n = 4), benzene-exposed group (n = 3), and health control group without benzene exposure (n = 3). (B) qRT-PCR analysis of Casp1 and IL1β expression in benzene-exposed group (n = 28) and health control group (n = 28). The peripheral blood cells of 28 male controls and 28 male low-level benzene-exposed workers matched confounding factors such as age, BMI and lifestyle included in smoking and drinking were collected for qRT-PCR. (C) Serum pyroptosis classical pro-inflammatory factors IL1β. (D) The correlation analysis between IL1β and basic blood test in all subjects (n = 140). (E) Serum inflammatory factors IL8, IL6, and IL10 level in benzene-exposed group (n = 71) and health control group (n = 69). (F) The correlation analysis between inflammatory factors and basic blood test in all subjects (n = 140) (G) The correlation analysis between IL1β with IL8, IL6, and IL10 in all subjects (n = 140). Data are shown as mean ± SD. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, and ⁎⁎⁎⁎p < .0001.
3.2. 1,4-BQ decreased cell viability and induced pyroptotic cell death
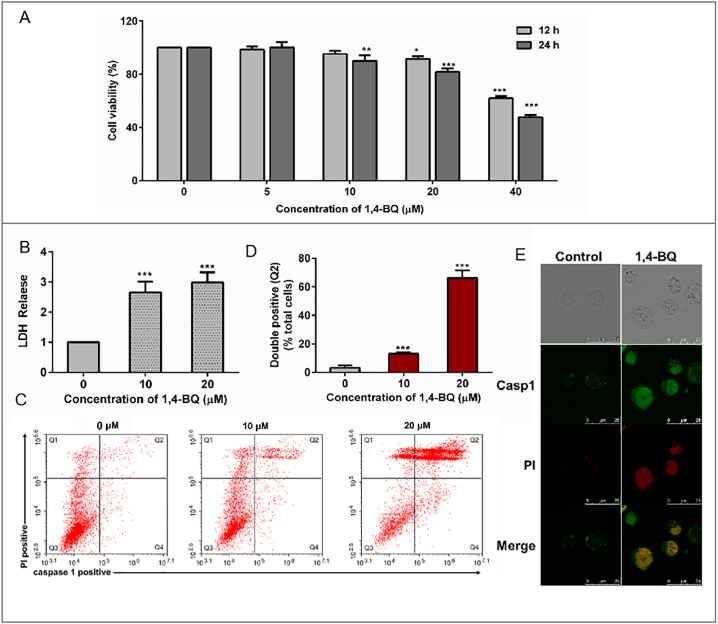
To verify whether benzene exposure induced the occurring of pyroptosis cell death, we cultured normal B lymphocyte cells (AHH-1 cells) which were recognized as the essential component of the body's immunological function. B-cell is sensitive to external stimuli and is bridge of environmental factors and immune disorders, such as autoimmune diseases, lymphoma, and leukemia(Bao and Cao [2]). To evaluate the cytotoxicity of the 1,4-BQ, AHH-1 cells were treated with different doses of 1,4-BQ (0, 5, 10, 20, and 40 μM) for 12 h and 24 h, the data showed that 1,4-BQ decreased the cell viability in a dose- and time-dependent manner (Fig. 2A). Briley, the viability of AHH-1 cells induced by 1, 4-BQ for 24 h in was significantly lower than that of control. The viability of 10 μM group was 90.17% while 20 μM group was 81.69%, but in 40 μM group was decreased to 47.75% compared to control. To study the effect of low-dose benzene metabolism exposure on AHH-1 cells, we guarantee the vitality of the cells at >80% compared to control. Pyroptotic cell death was assessed by measuring the release of lactate dehydrogenase (LDH) and double-positive staining of activated CASP 1 and PI. We found that 1,4-BQ increased the release of LDH dose-dependently in AHH-1 cells (Fig. 2B). To determine whether the death of AHH-1 induced by 1,4-BQ is a result of pyroptosis, we exposed AHH-1 cells to 1,4-BQ (10 and 20 μM) and detected activated Caspase-1 and PI using flow cytometry after 24 h. As shown in Fig. 2C and D, the proportion of double-positive (activated Caspase-1 and PI) AHH-1 (Q2) significantly increased in 10 μM (12.57%) and 20 μM (69.25%) 1,4-BQ groups (p < .001 versus 0 μM). The immunofluorescence assay combined with LSCM was performed to observe the expression of Casp1 and PI uptake. As shown in Fig. 2E, CASP 1 is not expressed in the untreated control cells, whereas it is accumulated in the 1,4-BQ-treated cells accompanied with PI uptake increasing.
Fig. 2.
1,4-BQ-induced cytotoxicity and pyroptosis in AHH-1 cells. (A) Cell viability was measured by MTT assay. AHH-1 cells were treated in a dose-dependent manner with 1,4-BQ for 12 or 24 h. (B) Cytotoxicity was analyzed by LDH assay. (C) Detected double positivity of Casp1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI (Q2) using flow cytometry after 24 h. The proportions of Q2 were then presented with histogram. (D) Representative flow cytometry scatter plots. (E) LSCM images of the AHH-1 cells with Casp1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI after treatment with 1,4-BQ for 24 h. Scale bar: 25 μm. Data are shown as mean ± SD of at least three independent experiments. ⁎p < .05, ⁎⁎p < .01, and ⁎⁎⁎p < .001.
To confirm the effects of 1,4-BQ on cell pyroptosis in AHH-1cells, TEM, LSCM, and ELISA assays were performed. As shown in Fig. 3A, the cellular morphology changed with cell swelling, cell membrane protruding and cell nucleus atrophy after treating with 1,4-BQ. It was reported that GSDMD cleavage will drive pyroptosis and release interleukin IL1β to extracellular space(Shi et al. [24]). Our studies have shown that the expression of GSDMD increased and accumulated as specific form throughout the cytomembrane in the 1,4-treated cells (Fig. 3B). subsequently, ELISA results in Fig. 3C showed that the effect of 1,4-BQ on proinflammatory and anti-inflammatory cytokines level. Briefly, the expression of IL1β, and IL8 was markedly elevated in AHH-1 cell treated with 1,4-BQ, however, IL10, as an anti-inflammatory molecular, decreased in 1,4-BQ group. Therefore, 1,4-BQ decreased cell viability and induced cell pyroptotic death accompanied by inflammation response. Previous study found that Necrosulfonamide (NSA) can directly bind to GSDMD inhibiting p30-GSDMD oligomerization and block interleukin-1β (IL-1β) release [20]. In the present study, the results in Fig. 3D show that NSA rescued the expression of IL1β, meanwhile, the expression of IL6 and IL8 was effectively suppressed which means pyroptotic cell death contributed to inflammation induced by benzene metabolic.
Fig. 3.
1,4-BQ changes cellular morphology and enhance the pyroptotic pore-forming protein GSDMD and inflammatory factors accumulation in the AHH-1 cells. (A) TEM images show that the cellular morphology changed with cell swelling, cell membrane protruding and cell nucleus atrophy after treating with 1,4-BQ (20 μM) for 24 h. (B) LSCM images of indirect immunofluorescence analysis show the GSDMD distribution patterns in the control and 1,4-BQ-treated cells. GSDMD (red) and DAPI staining of nuclei (blue) is shown. Scale bar: 25 μm. The punctate of GSDMD in cell in the untreated control and 1,4-BQ-treated cells is shown. (C) Effects of 1,4-BQ on inflammatory factors IL1β, IL8, IL6, and IL10 were detected using ELISA. (D) The effect of NAS on inflammatory in AHH-1 cells. The AHH-1 cells were pretreated with NSA at a concentration of 8 μM for 4 h, then the cells were treated with 1,4-BQ for another 24 h. Data are expressed as mean ± standard deviation from three independent experiments. ⁎p < .05, ⁎⁎p < .01, and ⁎⁎⁎p < .001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. 1,4-BQ induce pyroptotic cell death via activating the Aim2/Casp1 pathways
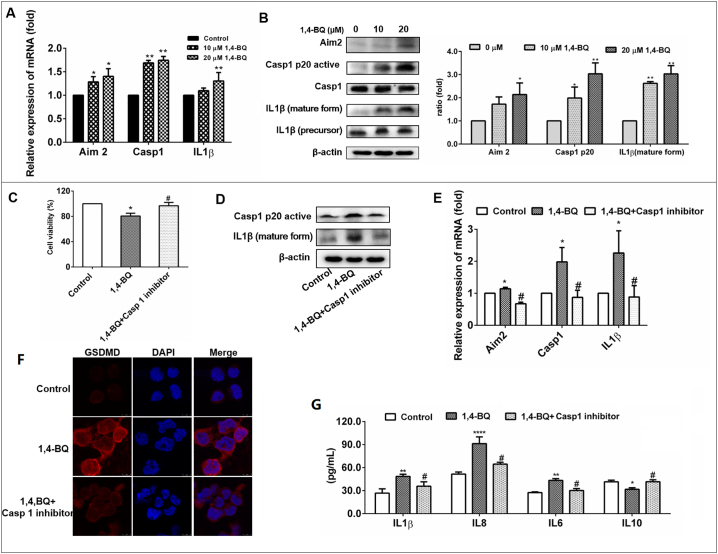
Aim 2, as an inflammasome, can activate Casp 1 in response to molecular signals from pathogens and other dangerous stimuli [13]. Previous study reported that Aim 2 showed highly expression in leukemia [29]. Then, we evaluated whether the 1,4-BQ induced cell pyroptotic death via the Aim2/Casp1 pathways. qRT-PCR assays were conducted to determine whether the Aim2/CASP1 pathway was activated. The mRNA level of Aim2 was increased in AHH-1cells after exposure to 1,4-BQ (10 and 20 μM) for 24 h. 1,4-BQ also upregulated the gene expression of CASP1, IL1β in a dose-dependent manner, which was consistent with the results from population (Fig. 4A). Subsequently, immunoblotting results showed that, in AHH-1cells, 1,4-BQ treatment resulted in a significant dose - dependent increase in the protein expression of Aim2, Casp1 p20 and mature IL1β (Fig. 4B), which suggested the Aim2 inflammasome and CASP1 pathway was activated. These results indicated that 1,4-BQ activated Aim2/Casp1 pathways triggering pyroptotic cell death.
Fig. 4.
1,4-BQ activate Aim2/Casp1 signal pathways in AHH-1 cells.
(A) 1,4-BQ up-regulated pro-pyroptosis associated genes including Aim2, casp1, and IL1β in AHH-1 cells. qRT-PCR analysis of Aim2, casp1, and IL1β expression in AHH-1cells followed by 1,4-BQ treatments for 24 h. (B) 1,4-BQ up-regulated inflammasome-related protein Aim2, Casp1 and IL1β expression in AHH-1 treated with 1,4-BQ for 24 h. WB analysis of Aim2, activated Casp1 p20, Casp1, Activated IL1β, and IL1β in AHH-1 cells followed by 1,4-BQ treatment for 24 h. β-actin was used as a control. (C) Casp1 inhibitor (Ac-YVAD-cmk) rescues 1,4-BQ-induced the decrease of AHH-1 cell vitality. (D) Casp1 inhibitor inhibits 1,4-BQ-induced Casp1 and IL1β activation. (E) Casp1 inhibitor rescues 1,4-BQ-induced the mRNA expression of Aim2, Casp1, and IL1β. (F) Casp1 inhibitor inhibits 1,4-BQ-induced GSDMD increase and accumulation. (G) Casp1 inhibitor inhibits 1,4-BQ-induced IL1β, IL8, IL6, and IL10 expression. Data are expressed as mean ± standard deviation from three independent experiments. ⁎p < .05, ⁎⁎p < .01, and ⁎⁎⁎p < .001.
To verify whether 1,4-BQ induced cell pyroptotic through Casp1 pathway, we speculated that the inhibition of Casp1 would suppress 1,4-BQ-induced cell pyroptotic. First, the Casp1 inhibitor (Ac-YVAD-cmk) was used to detect the 1,4-BQ-induced cell vitality change. The results in Fig. 4C show that the Casp1 inhibitor treatment rescued AHH-1 cell vitality in the 1,4-BQ-treated and Casp1 inhibitor-treated groups, meanwhile, Casp1 inhibitor treatment decreased the expression of active Casp1and IL1β in the 1,4-BQ-treated and Casp1 inhibitor-treated groups (Fig. 4D and E). In addition, Casp1 inhibitor treatment also inhibited the upregulation and accumulation of GSDMD induced by 1,4-BQ, indicating that the cell pyroptotic induction was suppressed (Fig. 4F). Furthermore, the expression of IL1β, IL8, IL6 and IL10 analysis revealed that Casp1 inhibitor treatment decreased inflammatory reaction in the 1,4-BQ-treated cells and Casp1 inhibitor treated cells (Fig. 4G). Thus, the inhibition of Casp1 by Casp1 inhibitor treatment suppressed 1,4-BQ-induced cell pyroptotic, suggesting that 1,4-BQ induce cell pyroptotic via activating Casp1 pathway.
3.4. Upregulated of TET2 in benzene-exposed workers and in AHH-1 cells treated with 1,4-BQ, and was associated with pyroptotic and inflammatory factors
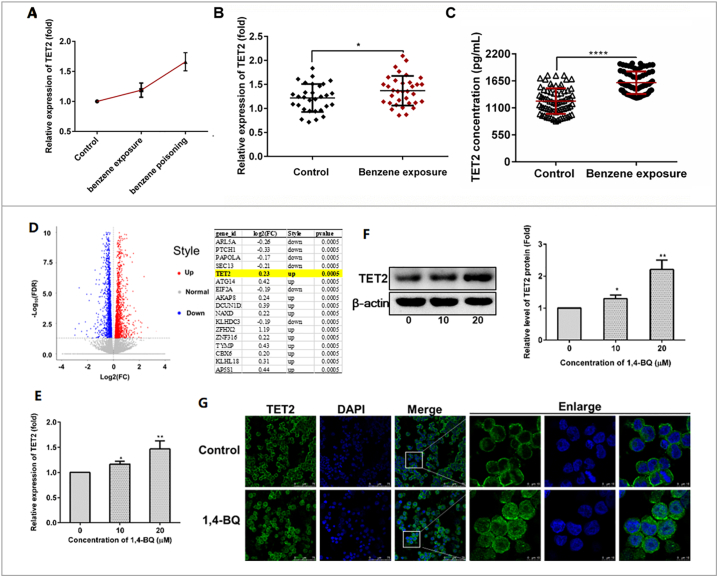
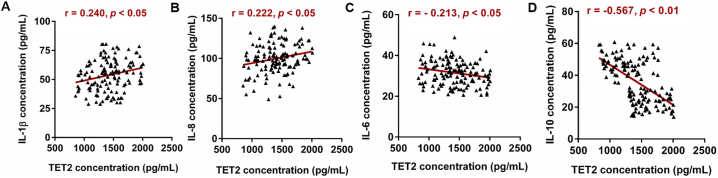
The gene TET2, which was found not only have a hand in myeloid malignancies but play an important role in inflammatory response, drew our attention. Extraordinary, TET2 was found to be strongly associated the expression of Aim2. In the present study, mRNA microarray result in Fig. 5A showed that TET2 were up-regulated and represented dose-dependent differential expression in three health subjects, three benzene-exposed workers, and four benzene poisoning patients. Then, in low dose benzene-exposed worker, the expression of TET2 was significantly increased (Fig. 5B). Concentrations of TET2 protein concentrations in serum showed the same trend with gene in two group (Fig. 5C). Meanwhile, increased expression of TET2 showed a negative correlation trend with WBC, NEUT, LYMPH, and PLT (Table S3). This revealed that both TET2 gene and protein increased in workers exposed to benzene and Tet2 took part in the toxicity of benzene. Then, to elucidate the molecular mechanisms by which benzene induced Aim2/Casp1 activation, we performed RNA-seq to compare gene expression between 1,4-BQ-exposed AHH-1 cells and control cells. The results illustrated that some genes were dysregulated. Our screening showed that TET2 were significantly higher in 1,4-BQ treated AHH-1 cells as compared to that in control cells which was consistent with the results from population (Fig. 5D). qRT-PCR result showed that the expression of Tet2 was also increased in AHH-1 cells in a dose-dependent manner (Fig. 5E) which was consistent with the results from Western blot (Fig. 5F). Meanwhile, Immunofluorescence assay combined with LSCM was performed to observe the pattern of TET2 distribution. As shown in Fig. 5G, TET2 is mainly assembled in the cytoplasm in the untreated control cells, whereas it is increased and partly entered the location of the nucleus in the 1,4-BQ-treated cells. TET2 may participate in blood toxicity of benzene. Then, our works has found that expose to benzene markedly changes inflammatory-related genes expression. Previous Research has also discovered that Tet2 can selectively mediate repression of interleukin-6 transcription during inflammation resolution in innate myeloid cells [30]. So, we performed the correlation analysis to explore whether TET2 was associated with the level of inflammation factor in all 140 subjects. Results showed that TET2 was positively correlated with IL1β (p < .05, r = 0.240) and IL8 (p < .05, r = 0.222). While, the TET2 expression was negative correlated with IL6 (p < .05, r = −0.213) and IL10(p < .05, r = −0.567) (Fig. 6). Taken together, these data suggest that TET2 is involved in benzene toxicity and plays a potent role in benzene exposure-mediated inflammation. Next the detailed mechanism was required.
Fig. 5.
Benzene and its metabolite promote TET2 expression in vivo and in vitro.
(A) mRNA microarray showed that only the expressions of TET2 were significantly up regulated in benzene-contacted subjects [benzene poisoning group (n = 4), benzene-exposed group (n = 3), and health control group without benzene exposure (n = 3)]. (B and C) The results of qPCR and ELISA confirmed that the level of TET2 was significantly increased in benzene-exposed workers. (D) RNA-Seq results showed that the expressions of TET2 were significantly upregulated in AHH-1 cells treated with 1,4-BQ for 24 h. (E, F, and G) qRT-PCR, Immunoblotting, and Immunofluorescence analysis of TET2 expression in AHH-1cells followed by 1,4-BQ treatments for 24 h. All results were replicated at least three times independently. The data are presented as means ± SD. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, and ⁎⁎⁎⁎p < .0001 versus the control group.
Fig. 6.
The correlation analysis between TET2 expression and inflammatory factors. n = 140. *Statistically significant correlation between two variables, p < .05.
3.5. Inhibition of TET2 could attenuate pyroptosis cell death and proinflammatory cytokine release from exposure to 1,4-BQ
In the present study, we have observed that exposure to 1,4-BQ significantly increased the expression of TET2 in AHH-1 cells compared with control cells in a concentration-dependent manner and the expression of TET2 was significantly higher in benzene exposure workers than that in controls. Simultaneously, TET2 was associated with inflammatory factors. However, whether TET2 might be involved in benzene toxicity by regulating cell pyroptosis and inflammatory, and the detailed mechanism need to be verified. In order to carry out the functional study of TET2, AHH-1 cells were transfected by lentivirus vectors with TET2 or empty lentiviral vectors for 72 h. When the transfection efficiency was up to 80% which identified by green fluorescent protein, the transfected cells could be successful used for the following experiments. As shown in Fig. 7A, the ratio of green fluorescent protein expression was up from 80% to 90%. And qRT-PCR showed that the expression of TET2 in Si-TET2 was lower than that in Si-NC suggesting that the expression of TET2 was successful down-regulated in Si-TET2 group. Meanwhile, in Si-NC group, 1,4-BQ treatment increased the TET2 expression as untransfected cells which indicating that lentivirus transfection had no effect on TET2 expression in AHH-1 cells. All above results proved that the lentiviral interference model was constructed successfully. Next, we investigated the consequence of TET2 knockdown on the LDH release and the Aim2/Casp1 pathways. As shown in Fig. 7B, TET2 knockdown decreased LDH release in the 1,4-BQ-treated group. Meanwhile, we studied the effects of TET2 knockdown on 1,4-BQ-induced pyroptosis, western blot assay revealed that the knockdown of TET2 reversed 1,4-BQ-induced augmentation of Aim2 inflammasome (Fig. 7C). Quantitative analysis of pyroptosis classical pathway genes further revealed that the knockdown of TET2 decreased the expression of Aim2, Casp1, and IL1β in the 1,4-BQ-treated group (Fig. 7D).
Fig. 7.
Effects of TET2 on the regulation of cell pyroptosis and inflammatory. (A) Expression of TET2 in AHH-1 cells transfected with lentivirus vectors of TET2(Si-TET2) or empty lentiviral vectors (Si-NC) following 1,4-BQ treated for 24 h. (B) LDH assay were used to evaluate cytotoxicity in AHH-1 cells with down-regulation of TET2 following treating with 1,4-BQ for 24 h. (C) Western blot analysis of Aim2 expression in AHH-1 cells with down-regulation of TET2 followed by 1,4-BQ treatments for 24 h. (D) qRT-PCR analysis of Aim2 signal pathways related genes in AHH-1 cells with down-regulation of TET2 following treating with 1,4-BQ for 24 h. (E) The AHH-1 cells were pretreated with 500 μM DMOG for 2 h, then the cells were treated with 20 μM of 1,4-BQ for another 24 h. Thereafter, (E) cells were harvested and the expressions of TET2 were analyzed by western blot (F) LSCM images of the AHH-1 cells with Casp1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI with DMOG treatment before 1,4 -BQ induced. (E) qRT-PCR analysis of Aim2 signal pathways related genes. (H and I) ELISA analysis of Intracellular (H) and Extracellular (I) inflammatory factors in including IL1β, IL8, and IL6 in AHH-1 cells with DMOG following treating with 1,4-BQ for 24 h. All results were replicated at least three times independently. (G) A scheme illustrating the mechanism by which benzene metabolic induces pyroptosis and pro-inflammatory release of AHH-cells. The data are presented as means ± SD. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, and ⁎⁎⁎⁎p < .0001 versus the control group.
In recent years, small molecules have been widely used in scientific research, especially, some has entered clinical application. Previous study has found that the catalytic activity of the TETs is strongly dependent on the homeostasis of iron(II) (Fe2+) and/or α-ketoglutarate (α-KG). Fortunately, the scholars have verified that DMOG is a cell permeable competitor of α-KG, and were used to inhibit the 5hmC formation in TCBQ-treated MRC-5 cells which depended on the absence of Tet proteins [31]. In this study, MTT assay found that DMOG at a concentration of 500 μM treated AHH-1 for 24 h was no effect on cell vitality (Fig. S2), meanwhile, the expression of TET2 can be successful inhibited in AHH-1 cells treated with DMOG at a concentration of 500 μM for 2 h before exposure to 1,4-BQ (Fig. 7E). Then, to confirm the cell pyroptosis effects after inhibiting the expression of TET2 with DMOG in AHH-1cells, we observed the expression of Casp1 and PI uptake. As shown in (Fig. 7F), the accumulation of Casp1 and PI decreased in AHH-1 cells after inhibiting TET2 expression. Simultaneously, qRT-PCR results show that the expression of pyroptosis classical genes were suppressed (Fig. 7G). Finally, we performed Luminex assays to measure the levels of inflammatory cytokine IL1β, IL6, and IL8 in the intracellular and released into the medium in AHH-1 cells after TET2 downregulation. Our results showed that the synthesize and secrete of IL1β and IL8 all decreased. Although other measured cytokines showed different behavior (secretion decreased production after TET2 inhibition, and no changes of synthesize were detected). These results suggested that inhibiting up-regulation of TET2 induced by 1,4-BQ reduced cell pyroptosis death, thereby decreasing the release of proinflammatory cytokine.
Overall, our results demonstrate that benzene metabolite promote TET2 expression activating inflammasome to trigger cell pyroptosis and inflammatory induced hematotoxicity (Fig. 7G).
4. Discussion
Identifying the consensus mechanism of benzene-induced hematotoxicity and any additional biomarkers which specificity predicted future incidence of benzene-induced disease has been one of most challenging issues in the field of health care and risk screening of benzene-induced disease initiation and progression [9]. In this study, we performed mRNA microarray in three health subjects, three benzene-exposed workers, and four benzene poisoning patients to search for specificity response gene and pathway that are involved in long- low dose benzene exposure. We successfully identified that pyroptosis and inflammation related gene expression gradually increased with the process of benzene exposure. The most important finding of this work is Tet2 overexpression induced by benzene metabolite mediated cell pyroptosis death promoted inflammation overactivation by directly regulating Aim2/Caspase1 pathway, opening a new avenue for study of the pathogenesis of xenobiotic pollutants exposure and provides novel proof for risk assessment of benzene.
Cell pyroptosis death is found to be a potential toxic effect of xenobiotic pollutants that results in over-activation of inflammation, tissue damage, even malignant tumors [1,6,27]. It was reported pyroptosis is a highly inflammatory form of programmed cell death drove by caspases activation accompanied with release of pro-inflammatory cytokines IL1β [18]. Release of IL1β attract other immune cells to release of secondary inflammatory and expand inflammatory response [23]. A large epidemiology study conducted by the Shanghai Health Study (SHS) from 2001 to 2009 provided evidences that chronic inflammation is likely to play a more significant role in benzene-induced disease initiation and progression [9]. Therefore, we propose that benzene may trigger cell pyroptosis death to promote and maintain the overactivation of inflammation. Here, we identified Aim2 inflammasome as a cytosolic sensor can total be activated by benzene metabolic which is critical for controlling cell proliferation and resulting in cell pyroptosis death through induced Casp1 activation. Activated casp1 led the expression of GSDMD increased and accumulated as specific form throughout the cytomembrane changed cellular morphology and increased IL1β production in AHH-1 cell treated with benzene metabolic. Casp1 and IL1β were reported to be classical signaling pathway gene of pyroptosis [3]. Meanwhile, the expression of Casp1 and IL1β show significant difference between two groups and markedly upregulated in low dose benzene-exposed workers accompanied with serum IL1β release increased which confirmed the occurrence of pyroptosis. Others living animal experiment showed benzene metabolic exposure induced rats secreted higher levels of proinflammatory cytokines IL1β which support us results [10]. Furthermore, inflammation factors included in this study showed that IL8 was significantly higher while IL-10 show significantly lower levels in low dose benzene-exposed workers than controls. IL8 was cited as a proinflammatory mediator and high levels of IL8 have been shown association with multiple types of diseases such as obesity, lung injury, and cancer [23]. IL10, an anti-inflammatory cytokine. Its high expression enhances B cell survival and proliferation while a decrease of IL10 cannot effectively inhibit release of pro-inflammatory cytokines to maintain inflammatory balance. Lower level of IL10 have been observed associated with the risk of developing childhood acute lymphoblastic leukemia [4]). As for IL6, acts as both a pro- inflammatory cytokine and an anti-inflammatory myokine in human, is an important mediator of the acute phase response involved in many biological functions [22]. However, inhibitor of pyroptotic rescued inflammation overactivation induced by benzene metabolite. It pointed out that cell pyroptosis death may be one of important potential toxic effect of benzene that results in chronic inflammation of the body, leading peripheral leukocytes reduction. Therefore, we confirmed that benzene induced cell pyroptotic death through activating Aim2/ Casp1 pathway was one of important chronic toxic effects mechanisms of benzene related diseases.
To further elucidate the direct molecular mechanisms of 1,4-BQ induced cell pyroptosis death, TET2, which were reported to be essential for its myeloid malignancy-suppressive function in hematopoietic stem progenitor cells [32] and, simultaneously, play an important role in inflammatory response in an enzymatic activity independent way [30], drew our attention. In this study, we found the expression of TET2 significantly increased in benzene exposed workers and Tet2 expression was positively correlated with classical pyroptosis proinflammatory IL1β. Interestingly, TET2 were significantly associated with most of inflammatory factor only among the benzene-exposed subjects, indicating the possible association between TET2 and the systemic inflammation in this benzene exposure group (Table S4 and S5). In Vitro experiments results showed that inhibition of TET2 using lentivirus interference test or small molecule inhibitor could attenuate pyroptosis cell death through depressing the expression of Aim2 in AHH-1 cells exposure to 1,4-BQ which strengthened our crowd research. Interestingly, Aim2 was reported that showed a hypermethylation in MDS, especially in CMML patients with TET2 mutations [28,29] and Pier Paolo Pandolfi, et al., reported that the expression of Aim2 rendered same direction change with Tet2 expression levels [26] indicted inflammasome Aim2 expression depend on the absence of TET2 which supported our results. Redox-active quinones such as TCBQ or TCHQ led to the genome-wide DNA methylation changes by influencing the 5mc to 5hmc conversion through induced reactive oxygen production to increase the catalytic activity of TET dioxygenases [31] may be one possible explanation that the up-regulation of TET2 in benzene exposure workers. Thus, we discover an important role of TET2 participated in benzene-triggered cell pyroptosis via directly mediating the expression of Aim2. Previous study found that Tet2 play important role in in resolving inflammatory in an enzymatic activity independent way [30]. Such conflicting data compared with before might be attributed to diversity functions of TET2. For example, upregulated of TET2 induced inflammasome-related genes Aim2 demethylation associated with increased gene expression [27] while Tet2 repressed transcription of IL-6 and inflammatory via histone deacetylation [30]. However, it's been surprising to us, in our study, TET2 showed negative correlated with IL-6 although no different level in benzene exposure workers and controls which was consistent with previous data [30]. One possible explanation is that previous research all based on TET2 mutation or TET2 knock-out model to study the damage to cells or population. Few data focused the harm due to the increasing expression of TET2 in body. Recently study suggested that TET2 activity induced genetic instability and mutagenesis [15] which was a very dangerous process in disease development. In our study, all results indicated that Tet2 overexpression induced by benzene and its metabolite promoted cell pyroptosis death and inflammatory. Tet2 overexpression maybe a potential risk factors and is implicated in the development of multifarious illnesses. To the best of our knowledge, this is the first time that a direct effect of benzene exposure on cell pyroptosis death has been reported. It may provide a new perspective for early prevent and intervention of benzene related diseases.
However, hematotoxicity refers to the continuous cytotoxicity change and decline count of peripheral blood cell in people which mainly caused by the injury of hematopoietic stem cells, hematopoietic stem cells maybe the most suitable study model of benzene hematotoxicity. Therefore, further studies such as those of hematopoietic stem cells-based mechanism research, are warranted to explore the role of benzene exposure in benzene-induced disease initiation and progression, to uncover the mechanisms involved, and to accumulate further evidence regarding the biomarkers of environmental pollutants and the etiology and pathogenesis of benzene hematotoxicity.
5. Conclusion
In conclusion, we have clarified the direct effect of benzene exposure, specifically in the aspect of cell pyroptosis death. Our results illustrated that exposure to benzene was able to induce cell pyroptosis death and inflammatory response via the up-regulation of TET2. TET2 may be a potential early biological effect biomarker and preventive target for benzene toxicity.
Acknowledgments
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81773397, 81472957), the Support Project of High–level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (CIT&TCD 20170323), Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201810025032). We would like to thank the participants for taking part in this study.
Author contributions
Xiaoli Guo designed and conducted experiments, analyzed data, and wrote the manuscript. Wen Zhong conducted some experiments. Yujiao Chen provided advice and established techniques of flow cytometry. Wei Zhang and Jing Ren helped to collect blood samples and information from patients. Ai GAO designed the study, supervised the research, interpreted the data, and revised the manuscript.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.056.
Appendix A. Supplementary data
Supplementary material
References
- 1.Banerjee T., Calvi L.M., Becker M.W., Liesveld J.L. Flaming and fanning: the spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019;36:57–69. doi: 10.1016/j.blre.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao Y., Cao X. Epigenetic control of B cell development and B-cell-related immune disorders. Clin Rev Allergy Immunol. 2016;50:301–311. doi: 10.1007/s12016-015-8494-7. [DOI] [PubMed] [Google Scholar]
- 3.Bolivar B.E., Vogel T.P., Bouchier-Hayes L. Inflammatory caspase regulation: maintaining balance between inflammation and cell death in health and disease. FEBS J. 2019;286:2628–2644. doi: 10.1111/febs.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J.S., Zhou M., Buffler P.A., Chokkalingam A.P., Metayer C., Wiemels J.L. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2011;20:1736–1740. doi: 10.1158/1055-9965.EPI-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Lu Y., Cao Z. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16. doi: 10.1016/j.toxlet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Weng B., Li H. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-kappaB mediated apoptosis-to-pyroptosis switch. Apoptosis. 2019;24:74–82. doi: 10.1007/s10495-018-1499-y. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y., Li X., Cassady K., Zou Z., Zhang X. TET2 function in hematopoietic malignancies, immune regulation, and DNA repair. Front Oncol. 2019;9:210. doi: 10.3389/fonc.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao A., Yang J., Yang G., Niu P., Tian L. Differential gene expression profiling analysis in workers occupationally exposed to benzene. Sci Total Environ. 2014;472:872–879. doi: 10.1016/j.scitotenv.2013.11.089. [DOI] [PubMed] [Google Scholar]
- 9.Gross S.A., Paustenbach D.J. Shanghai Health Study (2001–2009): What was learned about benzene health effects? Crit Rev Toxicol. 2018;48:217–251. doi: 10.1080/10408444.2017.1401581. [DOI] [PubMed] [Google Scholar]
- 10.Heluany C.S., Kupa L.V.K., Viana M.N., Fernandes C.M., Farsky S.H.P. Hydroquinone exposure worsens the symptomatology of rheumatoid arthritis. Chem Biol Interact. 2018;291:120–127. doi: 10.1016/j.cbi.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Hindman B., Ma Q. Carbon nanotubes and crystalline silica stimulate robust ROS production, inflammasome activation, and IL-1beta secretion in macrophages to induce myofibroblast transformation. Arch Toxicol. 2019;93:887–907. doi: 10.1007/s00204-019-02411-y. [DOI] [PubMed] [Google Scholar]
- 12.Janitz A.E., Campbell J.E., Magzamen S., Pate A., Stoner J.A., Peck J.D. Benzene and childhood acute leukemia in Oklahoma. Environ Res. 2017;158:167–173. doi: 10.1016/j.envres.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesavardhana S., Kanneganti T.D. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29:201–210. doi: 10.1093/intimm/dxx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loomis D., Guyton K.Z., Grosse Y. Carcinogenicity of benzene. Lancet Oncol. 2017;18:1574–1575. doi: 10.1016/S1470-2045(17)30832-X. [DOI] [PubMed] [Google Scholar]
- 15.Mahfoudhi E., Talhaoui I., Cabagnols X. TET2-mediated 5-hydroxymethylcytosine induces genetic instability and mutagenesis. DNA Repair (Amst) 2016;43:78–88. doi: 10.1016/j.dnarep.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Man S.M., Karki R., Kanneganti T.D. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269–280. doi: 10.1002/eji.201545839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patsos G., Germann A., Gebert J., Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer. 2010;126:1838–1849. doi: 10.1002/ijc.24905. [DOI] [PubMed] [Google Scholar]
- 20.Rathkey J.K., Zhao J., Liu Z., Chen Y. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3(26) doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Cimadevilla J.C., Beauchemin V., Villeneuve L., Letendre F., Shaw A., Hoang T. Coordinate secretion of interleukin-1 beta and granulocyte-macrophage colony-stimulating factor by the blast cells of acute myeloblastic leukemia: role of interleukin-1 as an endogenous inducer. Blood. 1990;76:1481–1489. [PubMed] [Google Scholar]
- 22.Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology (Oxford) 2018;57:ii43–ii50. doi: 10.1093/rheumatology/kex513. [DOI] [PubMed] [Google Scholar]
- 23.Schett G., Dayer J.M., Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 24.Shi J., Zhao Y., Wang K. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 25.Smith M.T., Zhang L., McHale C.M., Skibola C.F., Rappaport S.M. Benzene, the exposome and future investigations of leukemia etiology. Chem Biol Interact. 2011;192:155–159. doi: 10.1016/j.cbi.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song S.J., Ito K., Ala U. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vento-Tormo R., Alvarez-Errico D., Garcia-Gomez A. DNA demethylation of inflammasome-associated genes is enhanced in patients with cryopyrin-associated periodic syndromes. J Allergy Clin Immunol. 2017;139:202–211. doi: 10.1016/j.jaci.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki J., Jelinek J., Lu Y. TET2 mutations affect non-CpG Island DNA methylation at enhancers and transcription factor-binding sites in chronic Myelomonocytic leukemia. Cancer Res. 2015;75:2833–2843. doi: 10.1158/0008-5472.CAN-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki J., Taby R., Vasanthakumar A. Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics. 2012;7:201–207. doi: 10.4161/epi.7.2.19015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q., Zhao K., Shen Q. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B., Yang Y., Wang X. Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic Acids Res. 2014;42:1593–1605. doi: 10.1093/nar/gkt1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z., Chen S., Zhu X. The catalytic activity of TET2 is essential for its myeloid malignancy-suppressive function in hematopoietic stem/progenitor cells. Leukemia. 2016;30:1784–1788. doi: 10.1038/leu.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material