Abstract
Background
This study aims to evaluate the quality of preclinical data, determine the effect sizes, and identify experimental measures that inform efficacy using mesenchymal stromal (or stem) cells (MSC) therapy in animal models of rheumatoid arthritis (RA).
Methods
Literature searches were performed on MSC preclinical studies to treat RA. MSC treatment effect sizes were determined by the most commonly used outcome measures, including paw thickness, clinical score, and histological score.
Findings
A total of 48 studies and 94 treatment arms were included, among which 42 studies and 79 treatment arms reported that MSC improved outcomes. The effect sizes of RA treatments using MSC, when compared to the controls, were: paw thickness was ameliorated by 53.6% (95% confidence interval (CI): 26.7% −80.4%), histological score was decreased by 44.9% (95% CI: 33.3% −56.6%), and clinical score was decreased by 29.9% (95% CI: 16.7% −43.0%). Specifically, our results indicated that human umbilical cord derived MSC led to large improvements of the clinical score (−42.1%) and histological score (−51.4%).
Interpretation
To the best of our knowledge, this meta-analysis is to quantitatively answer whether MSC represent a robust RA treatment in animal models. It suggests that in preclinical studies, MSC have consistently exhibited therapeutic benefits. The findings demonstrate a need for considering variations in different animal models and treatment protocols in future studies using MSC to treat RA in humans to maximise the therapeutic gains in the era of precision medicine.
Funds
NIH [1DP2CA195763], Baylx Inc.: BI-206512, NINDS/NIH Training Grant [Award# NS082174].
Keywords: Mesenchymal stromal (or stem) cells, MSC, Rheumatoid arthritis, Pre-clinical study, Clinical trials, Meta-analysis
Abbreviations: AA, adjuvant-induced arthritis; AD, adipose tissues; AIA, adjuvant-induced arthritis; AM, amniotic membrane; BM, bone marrow; CAIA, collagen antibody-induced arthritis; CarrIA, carrageenan-induced arthritis; CI, confidence interval; CIA, collagen-induced arthritis; DMARDs, non-biologic disease-modifying anti-rheumatic drugs; hPG, proteoglycan-induced arthritis; ED, exfoliated deciduous teeth; ESC, embryonic stem cells; GI, gingival tissue; hUC-MSC, MSC derived from human umbilical cords; IA, intra-articular injection; IBM, intra-bone marrow injection; IDO, indoleamine 2, 3-Dioxygenase; IL-6, interleukin-6; IL, intralymphatic injection; IM, intramuscular injection; IP, intraperitoneal injection; IS, intrasplenic injection; IV, intravenous injection; LRT, likelihood ratio test; MD, mean difference; MOA, mechanism of action; MSC, mesenchymal stromal cells; NSAIDs, non-steroidal anti-inflammatory drugs; OE, olfactory ecto; OIA, ovalbumin-induced arthritis; PBS, phosphate buffered saline; RA, rheumatoid arthritis; REML, restricted maximum-likelihood estimator; RoB, risk of bias; SC, subcutaneous injection; SF, synovial fluid; SM, synovial membrane; SMD, Standardised mean difference; STA, K/BxN serum-transfer arthritis; SYRCLE, SYstematic Review Centre for Laboratory Animal Experimentation; TGF, transforming growth factors; TNF, tumor necrosis factor; Treg, regulatory T cells; UC, umbilical cord; UCB, umbilical cord blood
Research in context.
Evidence before this study
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily affects the joints. Stem cell therapy, especially mesenchymal stromal (or stem) cell (MSC) therapy, is emerging as a potential medical treatment for RA. From animal models to clinical trials, MSC have shown promise in the treatment of many immune disorder diseases and have been widely investigated for their beneficial therapeutic effects in rheumatic diseases including RA. Several preclinical studies have verified the efficacy of MSC in the treatment of RA, and positive results in clinical trials have also been reported. We conducted a comprehensive literature search for articles evaluating the therapeutic potential of MSC in RA from databases PubMed and Web of Science by June 2019. The search terms used were (mesenchymal OR mesenchymal stem cell OR mesenchymal stromal cell OR MSC) AND (rheumatoid arthritis OR rheumatoid OR arthritis OR RA), which were sufficiently broad to capture the majority of the published MSC in RA animal model data. All studies included in this meta-analysis were done in animal models of RA treated using non-genetically modified or “native” MSC. Studies with a high risk of any bias were excluded from the meta-analysis using the SYRCLE's risk of bias tool. In addition, studies that failed to present sample sizes, standard deviations, or lacked numerical/graphical results required for evaluating the effect sizes objectively, were also excluded in the parametric meta-analysis.
Added value of this study
This meta-analysis is a comprehensive and quantitative analysis of published pre-clinical studies using MSC to treat RA. Many studies of MSC treatment of RA animal models have shown case specific MSC treatment efficacy, but a broader analysis of the field was necessary to identify which experimental parameters produced the largest treatment outcomes. We found that there were considerable efficacy variations, across donor and recipient species, routes of administration, MSC tissue of origin, timing of MSC introduction, transplant types, dosage of MSC administration and number of injections. These variables account for significant variation in treatment outcomes between studies, and should be considered carefully when designing future pre-clinical and human clinical trials of MSC to treat RA.
Implications of all the available evidence
This meta-analysis supports the hypothesis that MSC provide therapeutic benefit with large effect sizes in the RA treatment. This work is the first meta-analysis article to attempt exploration of studying heterogeneity from different MSC study designs, including different donor species, tissue of origin, injection routes and dosages of administration. Our analysis could also be used to inform guidelines (e.g., experimental design, power calculation, etc.) for the future clinical translation of MSC to the bedside.
Alt-text: Unlabelled Box
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease and systemic disorder that primarily affects the joints [1]. In RA, the immune system attacks the synovial membrane, causing chronic inflammation, disintegration of bone and cartilage, and potential damage to other organs [2,3]. In the United States, it is estimated that about 1.5 million adults are affected by RA [4]. The conventional treatment regimen is a progression through corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), non-biologic disease-modifying anti-rheumatic drugs (DMARDs), and biologic DMARDs (e.g., anti-tumour necrosis factor (anti-TNF) [5,6]. Unfortunately, 15–40% of RA patients become resistant to long-term treatments and can become non-responsive to all existing clinical therapies [7,8]. Therefore, there is an exigent need for novel RA therapies [9,10].
Stem cell therapies are emerging as potential medical treatments for RA. In particular, mesenchymal stem or stromal cells (MSC) are a type of multipotent adult stem cells, which are currently used in many clinical trials. Cells meeting the MSC minimal criteria (per International Society for Cell Therapy guidelines) [11] have been isolated from bone marrow (BM) [12,13], adipose tissues (AD) [[14], [15], [16]], umbilical cord (UC) [[17], [18], [19]] and gingival tissue (G) [20,21]. They can be quickly expanded in vitro and used as an “off-the-shelf” allogeneic cell therapy due to their immune-evasive properties [22]. From animal models to clinical trials, MSC have shown promise in the treatment of many diseases including tissue injuries and immune disorders [[22], [23], [24], [25], [26]]. In particular, MSC have been widely investigated for their beneficial therapeutic effects in rheumatic diseases [27,28] including RA, in both preclinical studies [29] and clinical studies [[30], [31], [32], [33]]. Possible mechanisms of MSC combating RA include MSC-immune cell contact, induced death of effector lymphocytes and/or induction of regulatory T (Treg) cells, and production of soluble mediators, including anti-inflammatory cytokines such as Transforming growth factors (TGFs) and Indoleamine 2, 3-Dioxygenase (IDO) [[34], [35], [36]]. Preliminary insight of mechanism of action (MOA) based on the use of MSC derived from human umbilical cords (hUC-MSC) in RA treatment in human clinical trials revealed increased levels of CD4+ CD25+ Foxp3+ Treg cells and decreased levels of pro-inflammatory factors including IL-6 and TNF-alpha in circulation [30,33].
The purpose of this meta-analysis is to review and analyse preclinical studies of MSC in the treatment of RA. Several variables were compared, including donor species, tissues of origin, routes of administration, transplant types (i.e., autologous, allogeneic and xenogeneic). This meta-analysis also aims to identify optimal treatment variables and conditions by meta-regression and subgroup analysis, which can inform future experimental and trial designs. This analysis began by reviewing each study's quality using an Risk of bias (RoB) tool for animal intervention studies, presented by the SYstematic Review Centre for Laboratory Animal Experimentation (SYRCLE): SYRCLE's RoB tool [37]. Next, the effect size of MSC treatment was determined for the clinical score, paw thickness and histological scores. Finally, the MSC efficacy was examined across several variables of interest including dosage, number of injections, tissue source of MSC, MSC donor species, and other parameters listed in Table 1, Table 2.
Table 1.
Pre-clinical studies using MSC to treat RA included in this study.
| Author (year) | Arm | P < 0.05 | MSC favour? | Origin | Donor | Control | Transplant type | Treatment protocol | Rcpt | Age | n |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. (2011)S1 | 1 | Y | Y | AD | Human | PBS/Other | Xenogenic | CIA, no booster, IV, Multiple | Mouse | 8w | 10 |
| #Garimella, et al. (2015)S2 |
1 | Y | Y | AD | Murine | PBS | Autologous | CIA, with booster, IP, Single | Mouse | 8-10w | 6–7 |
| Chen et al. (2013)S3 | 1 | Y | Y | Other (G) | Human | Nil | Xenogenic | CIA, IV, Single | Mouse | 8-10w | 6 |
| 2 | Y | Y | Other (G) | Human | Nil | Xenogenic | CIA, IV, Single | Mouse | 8-10w | 6 | |
| 3 | Y | Y | Other (G) | Human | Nil | Xenogenic | CIA, IV, Single | Mouse | 8-10w | 6 | |
| Lee et al. (2015)S4 | 1 | Y | Y | BM | Other | PBS | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 7-9w | 5 |
| 2 | Y | Y | Other (SF) |
Other | PBS | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 7-9w | 5 | |
| Augello et al. (2007)S5 | 1 | N | Y | BM | Murine | PBS | Allogeneic | CIA, with booster, IP, Multiple | Mouse | 6w | 6 |
| #Chen et al. (2009)S6 | 1 | Y | N | BM | Murine | Nil | Autologous | CIA, with booster, IV, Single | Mouse | 8-10w | N/A |
| Lopez-Santalla,et al. (2015)S7 | 1 | Y | Y | AD | Human | Other | Xenogenic | CIA, with booster, IV, Single | Mouse | 8w | 64 |
| #Greish et al. (2012)S8 |
1 | Y | Y | UC | Human | PBS/Other | Xenogenic | AIA, no booster, IA, Single | Rat | N/A | 8 |
| Gonzalo-Gil, et al. (2016)S9 |
1 | Y | Y | Other (ESC) | Human | PBS | Xenogenic | CIA, IP, Single | Mouse | 10w | 7 |
| 2 | Y | Y | Other (ESC) | Human | PBS | Xenogenic | CIA, IP, Multiple | Mouse | 10w | 4 | |
| 3 | Y | Y | Other (ESC) | Human | PBS | Xenogenic | CIA, IP, Multiple | Mouse | 10w | 20 | |
| Mao et al. (2010)S10 | 1 | Y | Y | N/A | Rat | PBS | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 6 |
| #Swart et al. (2016)S11 | 1 | Y | Y | BM | Murine | PBS | Autologous | hPG, IP, Multiple | Mouse | N/A | 10 |
| 2 | Y | Y | BM | Murine | PBS | Autologous | hPG, IA, Multiple | Mouse | N/A | 10 | |
| #Papado-poulou et al. (2012)S12 | 1 | N | N | BM | Rat | PBS/Other | Allogeneic | AIA, IV. Single | Rat | 7w | 4–5 |
| 2 | N | N | BM | Rat | PBS/Other | Allogeneic | AIA, IV. Multiple | Rat | 7w | 4–5 | |
| 3 | N | N | BM | Rat | PBS/Other | Allogeneic | AIA, IP, Multiple | Rat | 7w | 4–5 | |
| 4 | N | N | BM | Rat | PBS/Other | Allogeneic | AIA, IBM, Multiple | Rat | 7w | 4–5 | |
| 5 | N | N | BM | Rat | PBS/Other | Allogeneic | AIA, IS, Multiple | Rat | 7w | 4–5 | |
| 6 | Y | Y | BM | Murine | PBS/Other | Allogeneic | STA, IP, Multiple | Mouse | 7w | 4–5 | |
| Rui et al. (2016)S13 | 1 | Y | Y | BM | Murine | PBS | Allogeneic | CIA, with booster, IV, Multiple | Mouse | 8-10w | 6 |
| 2 | Y | Y | Other (OE) |
Murine | PBS | Allogeneic | CIA, with booster, IV, Multiple | Mouse | 8-10w | 6 | |
| #Djouad et al. (2005)S14 |
1 | N | Y | N/A | Murine | N/A | Allogeneic | CIA, with booster, IV, Single | Mouse | 8-10w | 5–11 |
| 2 | N | N | N/A | Murine | N/A | Allogeneic | CIA, with booster, IV, Single | Mouse | 8-10w | 5–11 | |
| 3 | N | Y | N/A | Murine | N/A | Allogeneic | CIA, with booster, IP, Single | Mouse | 8-10w | 5–11 | |
| 4 | N | Y | N/A | Murine | N/A | Allogeneic | CIA, with booster, IM, Single | Mouse | 8-10w | 5–11 | |
| 5 | N | Y | N/A | Murine | N/A | Allogeneic | CIA, with booster, IA, Single | Mouse | 8-10w | 5–11 | |
| Santos et al. (2013)S15 | 1 | N | Y | UC | Human | PBS | Xenogenic | AIA, no booster, IA, Multiple | Rat | 16w | 8 |
| 2 | N | Y | UC | Human | PBS | Xenogenic | AIA, no booster, IA, Multiple | Rat | 16w | 8 | |
| 3 | Y | Y | UC | Human | PBS | Xenogenic | AIA, no booster, IP, Multiple | Rat | 16w | 8 | |
| Wu et al. (2012)S16 | 1 | N | Y | UC | Human | PBS | Xenogenic | CIA, with booster, IA, Single | Mouse | 7-8w | 6 |
| Kim et al. (2014)S17 | 1 | N | Y | AD | Human | PBS | Xenogenic | Curdlan, IP, Multiple | Mouse | 10-12w | 6 |
| Liu et al. (2010)S18 |
1 | Y | Y | UC | Human | PBS/Other | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 6-8w | 10 |
| Shu et al. (2015)S19 | 1 | Y | Y | Other (AM) | Human | Nil/PBS | Xenogenic | CIA, with booster, IP, Single | Rat | N/A | 6 |
| Park et al. (2016)S20 | 1 | Y | Y | UC | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 |
| 2 | Y | Y | BM | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 3 | Y | Y | AD | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 4 | Y | Y | UC | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 5 | Y | Y | BM | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 6 | Y | Y | AD | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 7 | Y | Y | BM | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 8 | Y | Y | BM | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| 9 | Y | Y | BM | Human | Other | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 5 | |
| #Gonzalez et al. (2009)S21 | 1 | Y | Y | AD | Human | PBS/Other | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 7-10w | 8–11 |
| 2 | Y | Y | AD | Murine | PBS/Other | Allogeneic | CIA, with booster, IP, Multiple | Mouse | 7-10w | 8–10 | |
| 3 | Y | Y | AD | Murine | PBS/Other | Autologous | CIA, with booster, IP, Multiple | Mouse | 7-10w | 8–10 | |
| 4 | Y | Y | AD | Human | PBS/Other | Xenogenic | CIA, with booster, IA, Single | Mouse | 7-10w | 8–11 | |
| #Zhao et al. (2015)S22 | 1 | Y | Y | UC | Human | PBS/Other | Xenogenic | CIA, with booster, IV, Single | Rat | 8w | N/A |
| #Bouffi et al. (2010)S23 | 1 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A |
| 2 | Y | Y | BM | Murine | N/A | Allogeneic | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| 3 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| 4 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| 5 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| 6 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| 7 | Y | Y | BM | Murine | N/A | Autologous | CIA, with booster, IV, Multiple | Mouse | 9-10w | N/A | |
| Sullivan et al. (2012)S24 | 1 | Y | N | BM | Murine | PBS | Autologous | CIA, with booster, IV, Single | Mouse | 7-9w | 12 |
| 2 | Y | N | BM | Murine | PBS | Allogeneic | CIA, with booster, IV, Single | Mouse | 7-9w | 12 | |
| 3 | Y | N | BM | Murine | PBS | Allogeneic | CIA, with booster, IV, Single | Mouse | 7-9w | 12 | |
| Schurgers et al. (2010)S25 | 1 | N | N | BM | Murine | PBS | Autologous | CIA, no booster, IV, Single | Mouse | 8-12w | 9 |
| Liu et al. (2015)S26 |
1 | Y | Y | UC | Human | PBS | Xenogenic | CIA, with booster, IV, Single | Mouse | 6-8w | 5 |
| Choi et al. (2008)S27 | 1 | N | N | BM | Murine | PBS | Autologous | CIA, with booster, IV, Multiple | Mouse | 8-12w | 10 |
| Park et al. (2011)S28 |
1 | N | N | BM | Murine | PBS | Autologous | CIA, with booster, IP, Single | Mouse | N/A | 6 |
| Parolini et al. (2014)S29 | 1 | Y | Y | Other (AM) | Human | PBS | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 8w | 8–10 |
| #2 | Y | Y | Other (AM) | Murine | PBS | Allogeneic | CIA, with booster, IP, Multiple | Mouse | 8w | 8–10 | |
| Sullivan et al. (2013)S30 | 1 | N | N | BM | Murine | PBS | Allogeneic | CIA, with booster, IV, Single | Mouse | 7-9w | 10 |
| El-Denshary, et al. (2013)S31 |
1 | Y | Y | BM | Murine | PBS | Allogeneic | CIA, with booster, IV, Single | Mouse | 6w | 10 |
| Choi et al. (2016)S32 | 1 | Y | Y | AD | Human | PBS | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 12 |
| #Luz-Crawford, et al. (2015)S33 |
1 | Y | Y | BM | Murine | PBS/Other | Allogeneic | CIA, with booster, IV, Multiple | Mouse | 9-10w | 16 |
| Kehoe et al. (2014)S34 | 1 | Y | Y | BM | Murine | PBS | Autologous | AIA, IA, Single | Mouse | 7-8w | 6 |
| Gu et al. (2016)S35 |
1 | Y | Y | Other (G) | Murine | PBS | Allogeneic | CIA, with booster, IV, Single | Mouse | 6-8w | 6 |
| Luo et al. (2019)S36 | 1 | Y | Y | Other (G) | Human | Nil | Xenogenic | CIA, IV, Single | Mouse | 8-10w | 6 |
| 2 | Y | Y | Other (G) | Human | Nil | Xenogenic | CIA, IV, Single | Mouse | 8-10w | 4 | |
| Nam et al. (2018)S37 | 1 | Y | Y | BM | Human | Nil | Xenogenic | CAIA, IP, Multiple | Mouse | 6w | 10 |
| Park et al. (2017)S38 | 1 | Y | Y | BM | Human | Nil | Xenogenic | CIA, IP, Multiple | Mouse | 6w | 5 |
| Shin et al. (2016)S39 | 1 | Y | Y | Other (UCB) | Human | Nil | Xenogenic | CIA, with booster, IP, Multiple | Mouse | 6-8w | 5 |
| 2 | Y | Y | Other (UCB) | Human | Nil | Xenogenic | CIA, with booster, IV, Single | Mouse | 6-8w | 7 | |
| Feng et al. (2018)S40 | 1 | Y | Y | UC | Human | PBS | Xenogenic | CIA, with booster, IV, Single | Mouse | N/A | 5 |
| Zhang et al. (2019)S41 | 1 | Y | Y | BM | Human | PBS | Xenogenic | CIA, with booster, IV, Single | Mouse | 8w | 6 |
| 2 | Y | Y | UC | Human | PBS | Xenogenic | CIA, with booster, IV, Single | Mouse | 8w | 6 | |
| 3 | Y | Y | Other (ED) | Human | PBS | Xenogenic | CIA, with booster, IV, Single | Mouse | 8w | 6 | |
| #Tian et al. (2019)S42 | 1 | Y | Y | BM | Rat | PBS | Autologous | CIA, with booster, IV, Single | Rat | 3-4w | N/A |
| Abd-Elhalem et al. (2018)S43 | 1 | Y | Y | BM | Rat | Nil | Autologous | AIA, with booster, Transplant | Rat | 6w | 6 |
| Mancheño-Corvo et al. (2017)S44 | 1 | Y | Y | AD | Human | Ringer | Xenogenic | CIA, with booster, IL, Multiple | Mouse | 8w | 34 |
| 2 | Y | Y | AD | Human | Ringer | Xenogenic | CIA, with booster, IL, Multiple | Mouse | 8w | 34 | |
| 3 | N | N | AD | Human | Ringer | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 8w | 40 | |
| Li et al. (2017)S45 |
1 | Y | Y | BM | Murine | Nil | Autologous | CIA, with booster, IV, Multiple | Mouse | 7w | 5 |
| Yan et al. (2017)S46 | 1 | Y | Y | SM | Human | PBS | Xenogenic | CIA, with booster, IA, Multiple | Mouse | 7-9w | 8 |
| Sun et al. (2017)S47 | 1 | N | Y | UC | Human | PBS | Xenogenic | CIA, with booster, IP, Single | Mouse | 6-8w | 5 |
| Yu et al. (2018)S48 | 1 | Y | Y | Other (UCB) | Human | PBS | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 6w | 5 |
| 2 | Y | Y | Other (UCB) | Human | PBS | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 6w | 5 | |
| 3 | Y | Y | Other (UCB) | Human | PBS | Xenogenic | CIA, with booster, IV, Multiple | Mouse | 6w | 5 |
Abbreviations: Age: age of induction, AA: adjuvant-induced arthritis, AD: adipose tissue, AIA: adjuvant-induced arthritis, AM: amniotic membrane, BM: bone marrow, CAIA: collagen antibody-induced arthritis, CarrIA: carrageenan-induced arthritis, CIA: collagen-induced arthritis, ED: Exfoliated deciduous teeth, ESC: Embryonic stem cells, GI: gingival tissue, hPG: proteoglycan-induced arthritis, IA: intra-articular injection, IBM: intra-bone marrow injection, IL: intralymphatic injection, IM: intramuscular injection, IP: intraperitoneal injection, IS: intrasplenic injection, IV: intravenous injection, Multiple: multiple injections, n: sample size, N/A: not reported, OE: Olfactory ecto, OIA: ovalbumin-induced arthritis, Origin: tissue of origin, PBS: phosphate-buffered saline, Rcpt: recipient specie, Ringer: Ringer's Lactate, SC: subcutaneous injection, SF: Synovial fluid, Single: single injection, SM: Synovial Membrane, STA: K/BxN serum-transfer arthritis, UC: umbilical cord tissue, UCB: umbilical cord blood, w: weeks.
#: studies that have been excluded from the parametric meta-analysis due to missing values or high risk of biases.
Table 2.
Study arms categorised by experimental variables of interest and their qualitative effect size.
| Experimental variables | Total no. of arms | Favour MSC | Favour control |
|---|---|---|---|
| 94 | 79 | 15 | |
| Donor species | |||
| Human | 49 | 48 | 1 |
| Mouse | 35 | 26 | 9 |
| Rat | 8 | 3 | 5 |
| Others | 2 | 2 | 0 |
| Recipient species | |||
| Mouse | 81 | 71 | 10 |
| Rat | 13 | 8 | 5 |
| MSC transplant types | |||
| Autologous | 19 | 14 | 5 |
| Allogeneic | 23 | 14 | 9 |
| Xenogenic | 52 | 51 | 1 |
| MSC tissue of origin | |||
| Umbilical cord | 13 | 13 | 0 |
| Bone marrow | 40 | 27 | 13 |
| Adipocyte | 14 | 13 | 1 |
| Others/Unknown | 27 | 26 | 1 |
| Route of administration | |||
| IV | 54 | 43 | 11 |
| IP | 25 | 23 | 2 |
| IA | 9 | 9 | 0 |
| Others | 6 | 4 | 2 |
| RA model | |||
| CIA | 78 | 68 | 10 |
| AIA | 11 | 6 | 5 |
| Others | 5 | 5 | 0 |
2. Materials and methods
Literature search and inclusion criteria. (See Supplementary materials.)
Data extraction. (See Supplementary materials.)
Evaluating the risk of bias. (See Supplementary materials.)
2.1. Effect size estimation
The effect sizes of MSC therapy were analysed based on the three different indicators most frequently used in preclinical MSC in RA animal studies: [1] clinical score (a semi-quantitatively summative macroscopic measure of animal anatomical conditions), [2] histological score (a pathological microscopic measure of joint conditions), and [3] paw thickness (a measurement of paw swelling to determine arthritis severity) [38]. Due to methodical variations in histology scoring, we separated histological data into a “general” score if the paper combined several scoring parameters into an overall score. In papers that listed individual histology assessment parameters (bone erosion, cartilage damage, and inflammation) we reported them individually. We averaged (presented as mean) both general and individual histological scores of each treatment arm. The changes in mean clinical scores and paw thickness were evaluated with the measurements before and after MSC treatments. If the changes in clinical score and paw thickness were not provided directly from the included literature, these indicators were calculated by subtracting the mean endpoint measurement by the baseline (standard deviations were estimated by assuming all the measurements were independent). All treatment arms included in the quantitative meta-analysis used PBS as the control group, which is the most commonly used control, although some high quality studies included multiple control groups (e.g. fibroblast and methotrexate). All study design differences were normalised before comparing the PBS treated RA group and MSC treatment RA group. Normalisation of data and effect sizes were determined by the method outlined in Vesterinen et al. 2013. In brief, the effect sizes were normalised to the sham control group (i.e. healthy animals) [39]. If there was no difference between the MSC treated RA group and the sham control group, it was scored 0. The directionality was positive if indicating more pathological conditions, therefore a higher score means worse disease conditions. Thus, positive efficacy from MSC treated RA group shows a negative normalised value. The effect sizes were estimated by subtracting the normalised values of the MSC treated RA group by the PBS treated RA group. All the effect sizes are unitless due to normalisation (Eq. 1). Some studies included multiple treatment arms, so to avoid overestimating treatment effects and unit-of-analysis error, we split the PBS treated RA group so that it could be compared to each treatment arm separately, without being counted more than once. If there were not enough PBS-treated RA mice to split, the MSC treated groups were combined. Standardised mean difference (SMD) using exact Hedge's G effect sizes was also performed as sensitivity analysis.
| (1) |
2.2. Statistical analysis, subgroup analysis and meta-regression
The random-effects model method in the R package “meta” was used to calculate the mean effect size, 95% CIs, forest plots, and significance. Heterogeneity was calculated/analysed in the restricted maximum-likelihood estimator (REML) with the Ι2 and τ2 values, which are the ratio of true heterogeneity to total observed variation and the variance between studies respectively. A subgroup analysis was conducted using routes of administration, donor species, transplant types, or tissue of origin of MSC as categorical variables assuming there is a common τ2 among the groups. A mixed-effects model of regression was performed in the “metafor” package in R to address heterogeneity. Variations in MSC tissues of origin, MSC donor species, routes of administration, dosage of administration, number of injections and transplant types were tested as covariate separately. After fitting moderators, residual heterogeneity was assessed by the “adjusted R2 value”, which can be used to test variation in the effect size. Potential interactions between the moderators were also tested. An omnibus test, as well as all pairwise comparisons between the factor levels, were used for the statistical test of the moderator, and a likelihood ratio test (LRT) was used to test the interaction. The confidence intervals were adjusted with the Knapp-Hartung method. The p-value threshold was set to 0.1 for heterogeneity, to increase the power of the test, and 0.05 for other tests. Funnel plots were drawn with the meta package to assess the publication bias, if there were at least nine included studies in that indicator. Trim and fill correction were done where there was significant asymmetry of the plot.
3. Results
3.1. Study characteristics
The article selection process is summarised in Fig. S1 (See Supplementary Materials). Literature searches retrieved 4745 articles from PubMed and the Institute for Scientific Information Web of Science, in which 661 were duplicates. A total of 3912 articles were excluded by title alone. Overall, articles were screened by title and abstract, however, a further 111 did not meet the inclusion criteria. The remaining 61 full text articles were assessed for eligibility resulting in 48 articles meeting the criteria to be included in the meta-analysis review (Table 1).
A total of 48 studies and 94 treatment arms were identified (Table 1), which were further categorised based on experimental variables of interest listed in Table 2. In this meta-analysis, 86.2% of the treatment arms used mouse models, and 13.8% of the treatment arms used rat models. The RA models included mainly collagen-induced arthritis (CIA) induction (83.0%) and adjuvant-induced arthritis (AIA) induction (11.7%). Among 94 treatment arms, 72.3% used an immunisation booster for model induction. Human derived MSC were used in 52.1% of the 94 treatment arms, 37.2% used murine MSC, and 8.5% used rat derived MSC. The tissue of origin of MSC varied by study, such that 13.8% of 94 treatment arms used umbilical cord, 42.6% bone marrow, and 14.9% adipose. Other MSC tissue of origin including gingiva was used in 28.7% of the treatment arms. Transplant types were also compared, such that 55.3% of the 94 treatment arms were xenogeneic, 24.5% allogeneic, and 20.2% autologous. The routes of administration were intravenous (IV) injection in 57.4% of 94 treatment arms, 26.6% intraperitoneal (IP) injection and 9.6% intra-articular (IA) injection (Table 1, Table 2).
3.2. Quality of included studies
The quality of studies was assessed (Fig. S2, Supplementary materials). Most studies avoided selection/reporting bias, and all reported the baseline characteristics (Q2). Selective outcome (Q9) and other sources of bias (Q10) appeared to be low in these reports. Nonetheless, few studies attempted to report the strategies to mitigate potential performance bias, detection bias and attrition bias. Therefore, there was uncertainty regarding the actual risk of bias. Notably, some of the studies included blindness on evaluation protocol (Q7: 35.42% of “yes”) in their study.
3.3. Effect size
Three indicators were used to study effect sizes in this meta-analysis review: [1] clinical score, [2] histological score, and [3] paw thickness. Qualitative analysis was done to show the effect size of MSC administration in preclinical studies of RA (Table 2). MSC improved outcomes (i.e., at least one indicator was improved) in 87.5% of the 48 studies and in 84.0% of the 94 treatment arms (Table 1, Table 2). Clinical score difference was used to illustrate effect size for MSC administration in 73.4% of the 94 treatment arms, 38.3% included histological scoring and 39.4% included paw thickness difference. In terms of clinical score, 87.0% of 69 treatment arms favoured MSC treatment, while 13.0% favoured the PBS control treatment. Histological scoring showed 91.7% of the 36 total treatment arms favoured MSC treatment, vs 8.3% for PBS control. Finally, paw thickness measurements showed 94.6% of the 37 treatment arms favoured MSC treatment, vs 5.4% for PBS control. Interestingly, 60.0% of the treatment arms that favoured PBS control treatment used MSC derived from mice; however, 98.0% of human derived MSC improved RA pathophysiology (Table 2).
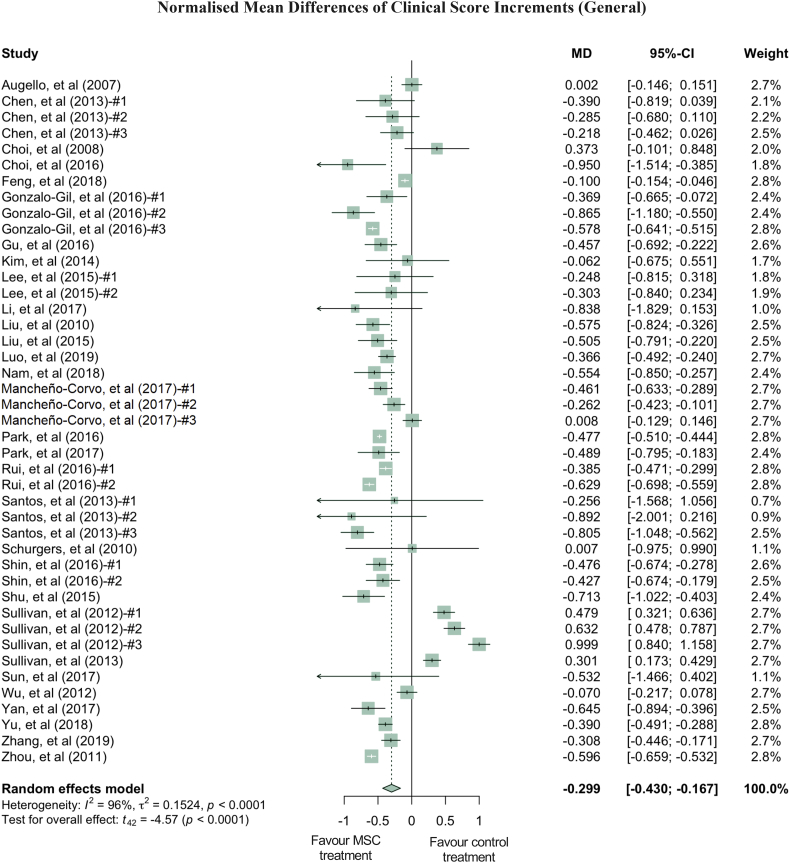
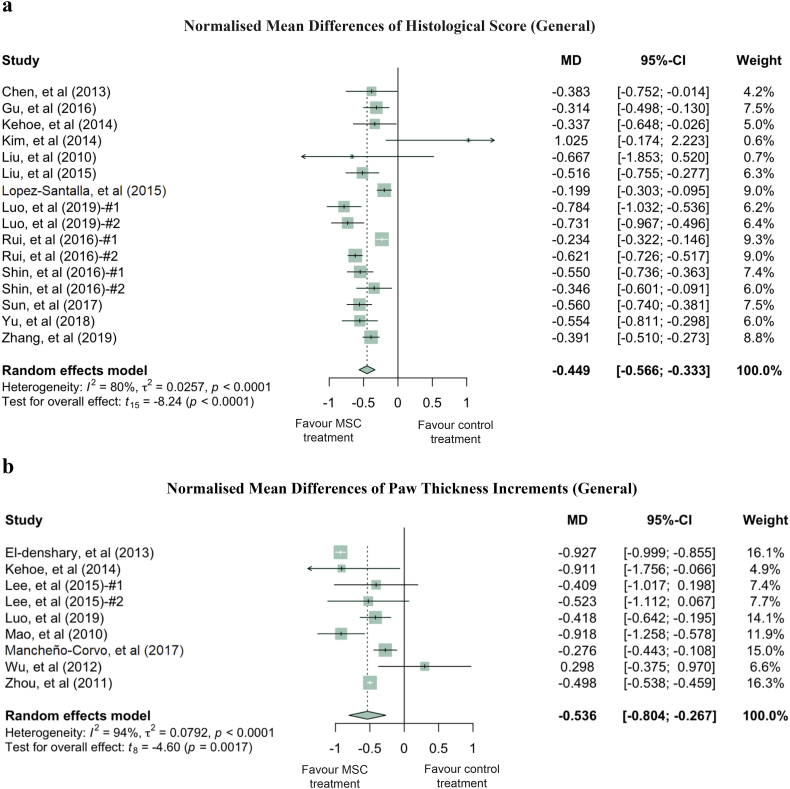
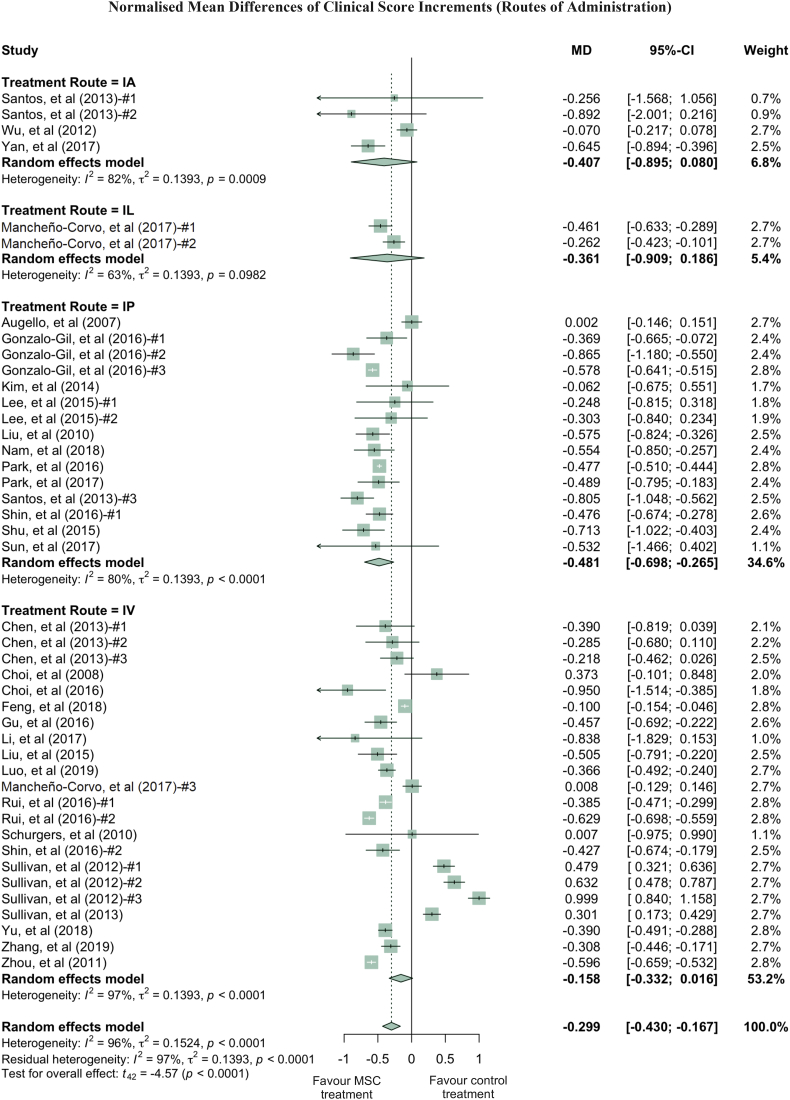
Quantitatively, clinical scores of the MSC treated RA groups were reduced by an average of 29.9% compared to the PBS treated RA groups (Fig. 1). While this reduction was statistically significant (p-value <0.0001), it contained both total and sampling variabilities. The estimated total heterogeneity (τ2) was 0.1524, and Ι2, which is defined as a ratio of total heterogeneity to total variability, was 96% (p-value <0.0001) [39]. Second, there was a 44.9% decrease in histological scores (p-value <0.0001) in MSC treated groups compared to PBS groups in 8 studies (with 9 treatment arms) which used a general histological score obtained by summarising the pathological condition of the joint tissues (Fig. 2a). Despite the heterogeneity of this indicator being statistically significant (p-value <0.0001), it is much lower than the clinical score heterogeneity. Some of the studies grouped histology measurements into categories, such as bone erosion, cartilage damage and inflammation. For example, 4 studies (5 treatment arms) reported the histological score for bone erosion, and the normalised mean difference was −0.538 (p-value = 0.0210) when comparing the MSC treated groups to the PBS control groups (Fig. S3a, Supplementary Materials). MSC treatment significantly decreased cartilage damage by 51.6% and reduced inflammation by 42.3% (Fig. S3b and S3c, respectively, Supplementary Materials). Third, paw thickness in MSC treatment groups was significantly reduced, on average, by 53.6% (p-value = 0.0017). Variations between these studies, using paw thickness as an indicator, were relatively small (τ2 = 0.0792) (Fig. 2b). As a sensitivity analysis, similar conclusions can be drawn for clinical score and histological score when using the standardised mean difference (SMD) method (Fig. S4a and S4b, Supplementary Materials). Notably, 88.9% of the 9 included treatment arms showed results favouring MSC treatment in terms of paw thickness. The wide confidence interval of the SMD estimate of paw thickness could be due to the high inter-study variability (Fig. S4c, Supplementary Materials).
Fig. 1.
Forest plots showing the normalised mean difference (MD) and 95% CI of the clinical score for each study included in the meta-analysis. The graph was generated using the meta package in R. All results have been normalised with sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2.
Fig. 2.
Forest plots showing the normalised mean difference (MD) and 95% CI of (a) histological score, (b) paw thickness for each study included in the meta-analysis. The graphs were generated using the meta package in R. All results have been normalised with sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2.
More than one method was used in our study to estimate effect sizes. Systematic results showed that MSC produced a significantly beneficial treatment effect in 42 of the 48 studies and 79 of the 94 treatment arms, based on the three indicators (paw thickness, clinical score and histological score). Notably, majority of the meta-analysis results remain robust in the sensitivity analysis. Over all studies combined (by SMD), there was a drop (1.383, p-value = 0.0008) in clinical score, a decrease (1.931, p-value <0.0001) of histological score, and a reduction (2.814, p-value = 0.0639) of paw thickness (inflammation) (Fig. S4, Supplementary Materials). The non-statistically significant decrease of paw thickness by SMD might be due to high inter-study heterogeneity, which is a result of the presence of high standard deviation in some of the included studies [40].
3.4. Subgroup analysis
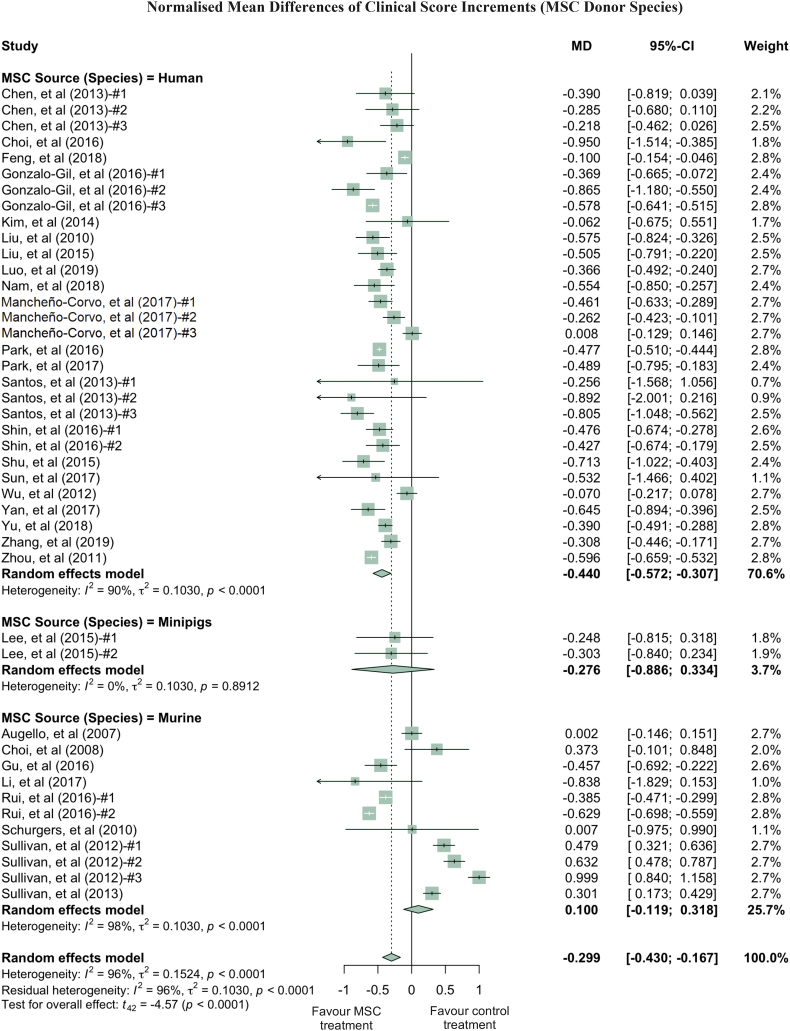
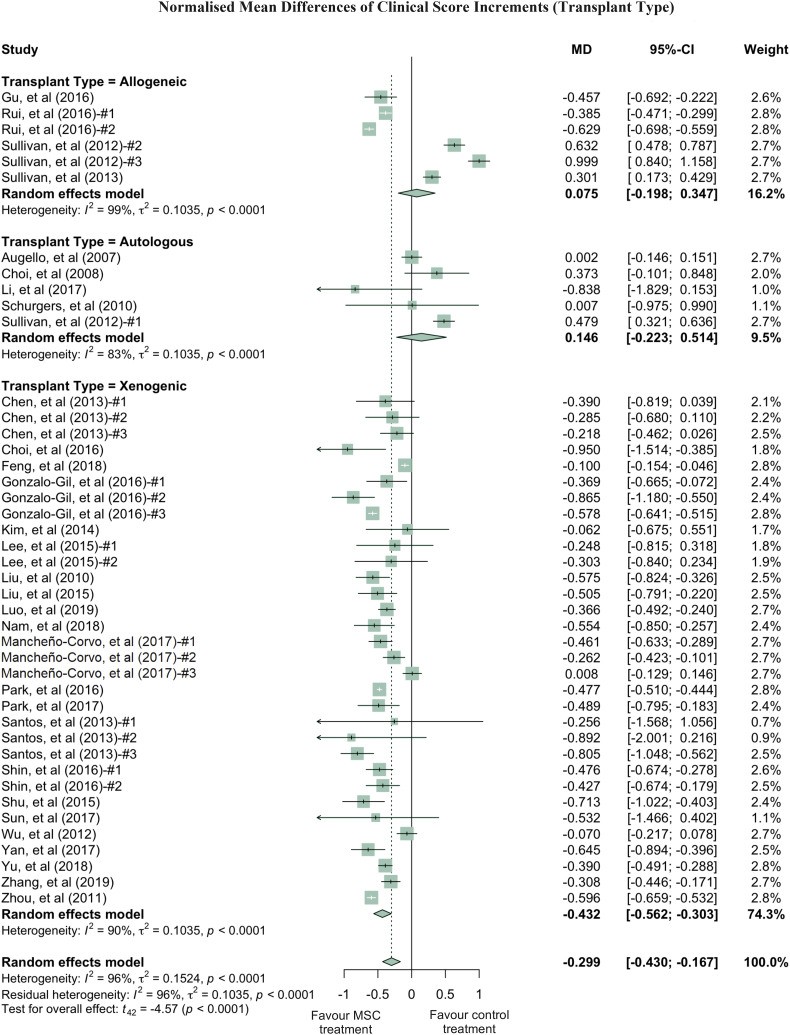
Clinical scores varied dramatically among the studies, with a τ2 value of 0.1542. Thus, we performed further analysis by grouping studies according to the donor species, tissue of origin, transplant types and administration routes of MSC used in treatment (Fig. 3, Fig. 4, Fig. 5, Fig. 6). Notably, human derived MSC demonstrated more consistent and effective clinical results, with a further 14.1% reduction in the clinical score compared to the overall effect size (Fig. 3). The superior treatment effect of human derived MSC is statistically significant compared to mouse derived counterparts, which is indicated by the non-overlapping confidence intervals between human and mouse MSC groups. Interestingly, xenogeneic MSC give significantly better treatment efficacy in terms of clinical score than those from other transplant types (Fig. 4). MSC from different tissue of origin including umbilical cord, gingiva and adipose showed consistent improvement of clinical score, whereas bone marrow derived MSC showed a trend of favouring PBS control treatment (Fig. 5). By comparing clinical scores for the donor species and MSC tissue of origin of the MSC simultaneously, we discovered that human MSC, especially MSC derived from adipose tissue and umbilical cord tissue, provided better therapeutic effects, supported by at least four independent studies (Fig. S5, Supplementary Materials). Notably, UC-MSC and gingival tissue derived MSC (G-MSC) showed consistent and robust efficacy as well as the beneficial effect in the subgroup analysis of general histological score (Fig. S6, Supplementary materials). When comparing the studies by different administration routes, IP injection gave a more significant reduction in clinical score. The decreases in clinical scores with other administration routes, such as IV injection, were not significant (Fig. 6). Nonetheless, this result might be subject to other factors. For example, most of studies conducting IV injections used bone marrow MSC (BM-MSC), with which inconsistent results were reported among different studies (Fig. 6, Table 1). Specifically, in the studies from Sullivan et al., which accounts for most of the IV injection treatment arms favouring PBS control treatment, the MSC used have inconsistent cell quality (e.g. low CD73 expression), isolation protocols and low dosage (1 × 105 cells).
Fig. 3.
Forest plots showing normalised mean difference (MD) of clinical score changes and 95% CI for the subgroup of MSC donor species. The graph was generated using the meta package in R. All results were normalised with the sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2.
Fig. 4.
Forest plots showing normalised mean difference (MD) of clinical score changes and 95% CI for the subgroup of transplant types. The graph was generated using the meta package in R. All results were normalised with the sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2.
Fig. 5.
Forest plots showing normalised mean difference (MD) of clinical score changes and 95% CI for the subgroup MSC tissue of origin. The graph was generated using the meta package in R. All results were normalised with the sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2. Mixed* indicates the treatment arm contains more than one type of MSC tissue of origin.
Fig. 6.
Forest plots showing normalised mean difference (MD) of clinical score changes and 95% CI for the subgroup of routes of administration. The graph was generated using the meta package in R. All results were normalised with the sham control group as described in the methods. For all the plots, the vertical line indicates no effect, left hand side indicates favouring MSC treatment while right side indicates favouring PBS control treatment. The size of the box indicates the weighting of each study, and the thin horizontal whisker indicates the 95% CI. Random-effects model was used to summarise the effect sizes. Heterogeneity is denoted by the Ι2 and τ2.
3.5. Meta-regression of effect size
To address the heterogeneity from different variables and investigate the correlation between experimental parameters and effect size, meta-regression was performed on clinical scores. Predefined potential moderators were tested. We found that either routes of MSC administration or dosage of MSC contributed little heterogeneity, when analysed separately (Table S2, Supplementary materials). On the other hand, consistent with the subgrouping results, variation in MSC donor species accounted for 32.43% (p-value = 0.0006) of the heterogeneity, which was the highest source of variability among tested single moderators (Fig. 3 and Table S2, Supplementary materials). Regression on transplant types of MSC resulted in a 32.11% reduction in heterogeneity (p-value = 0.0006) (Table S2, Supplementary materials). Moreover, adjusting the tissue of origin of the MSC could reduce the total heterogeneity by 27.58% (p-value = 0.0315). The interaction between donor species and MSC tissue of origin was significant (p-value = 0.0413), accounting for 43.94% of the heterogeneity (Table S2, Supplementary materials).
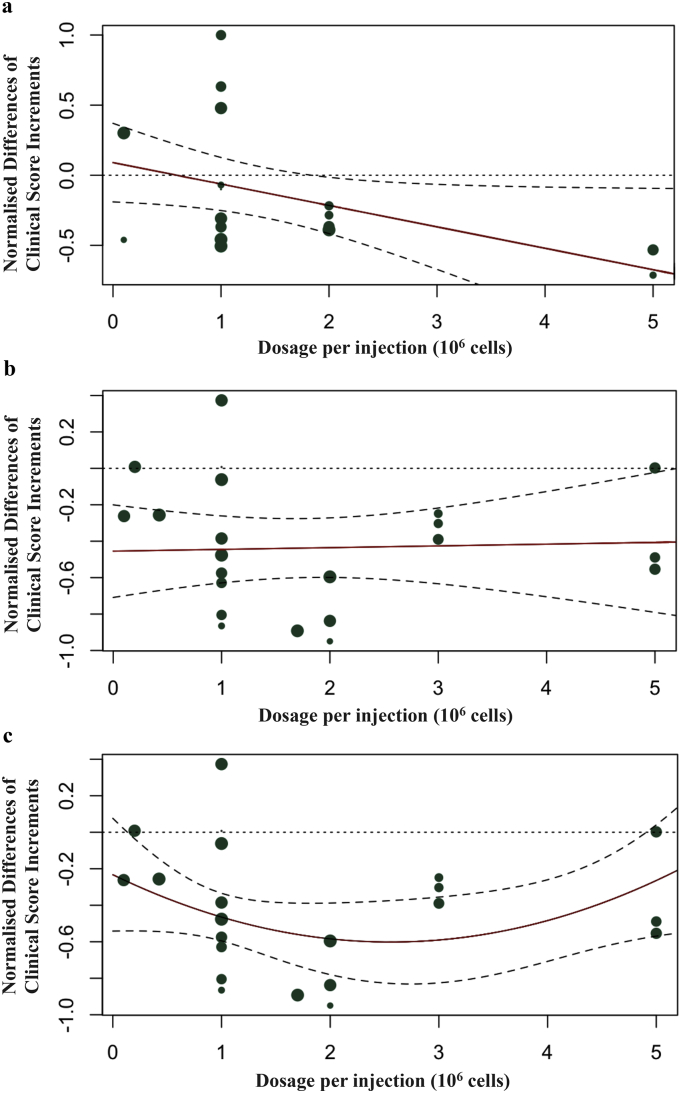
The number of injections administered is another parameter that should be considered. When the number of injection (categorised into single or multiple injections) was adjusted separately, the reduction in heterogeneity was significant (p-value = 0.0147) (Table S2, Supplementary materials). Furthermore, there might be interactions between dosage of MSC and number of injection in the treatment protocol. When the interaction between dosage and number of injection was tested, the heterogeneity was decreased by 19.91% (moderator p-value = 0.0205, interaction p-value = 0.0722) (Table S2, Supplementary materials). A single injection with ≥2 × 106 cell dosage correlated with better clinical outcomes (Fig. 7a). However, multiple injections treatment arms did not show any correlation between cell dosage and outcomes in clinical score, when a linear regression was used (Fig. 7b), but if a quadratic regression was used, there was a better outcome for 2 to 3 × 106 cell dosage (p-value = 0.0391) (Fig. 7c and Table S3, Supplementary materials). By combining the results above, a final model was built with MSC donor species, MSC tissue of origin, transplant types, treatment dosage, and number of injections adjusted. This final model explained 58.04% of the heterogeneity (p-value = 0.0017) (Table S2, Supplementary materials).
Fig. 7.
Regression model with regression line is shown in (a) single MSC injection treatment, (b) multiple MSC injections treatment (linear regression), and (c) multiple MSC injections treatment (quadratic regression). The size of the dot is proportional to number of injections. The dashed lines in (a, b, and c) represent the 95% CIs. The red line represents the trend of interactions between dosage of MSC and normalised difference in clinical score changes. The size of the dot is proportional to the weighting given to the study and the detailed weighting of each study could be found in Fig. 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
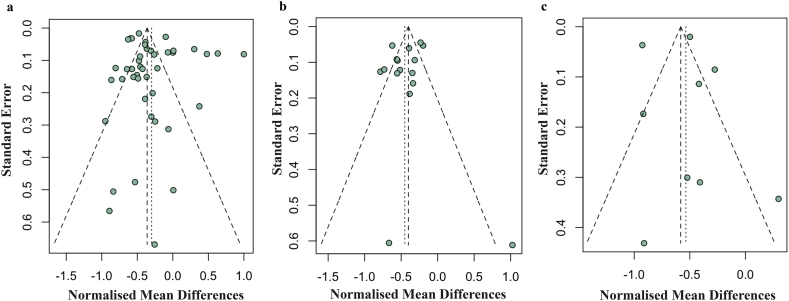
3.6. Evaluation of publication bias
Funnel plots for clinical score, histological score, and paw thickness were used to assess the publication bias. There is no significant asymmetry for all the indicators included in this study in which the p-values of the funnel plot asymmetry for clinical score, histological score and paw thickness were 0.2060, 0.4007 and 0.9719 respectively (Fig. 8). Thus, there is no observable publication bias in this meta-analysis. Multiple studies that reported positive results were out of the ±1.96 standard error boundary in the funnel plot for histological score (Fig. 8b), so we performed a correction using the trim-and-fill method. After correction, the symmetry centre shifted to around +0.0545 (Fig. S7b, Supplementary materials). No obvious change could be also found in the funnel plot for clinical score and paw thickness, after trim-and-fill (Fig. S7a and S7c, Supplementary materials).
Fig. 8.
Funnel plot for (a) clinical score, (b) histological score, and (c) paw thickness. The funnel plots used either a model without regression (horizontal axis is the normalised mean difference), or a model after regression (horizontal axis is the residual value). Each dot in the figure represents a study, with the y-axis signifying study quality and the x-axis showing the study results.
4. Discussion
This meta-analysis examined preclinical studies of MSC used to treat RA and supports the hypothesis that MSC provide therapeutic benefit with large effect sizes. This work is the meta-analysis article to attempt exploration of study heterogeneity from different MSC study designs, including different donor species, tissues of origin, injection routes and dosages of administration [41]. We found that the major contributor of heterogeneity is donor species of MSC, which accounts for 32.43% of the variations (Table S2, Supplementary materials). Number of injections, MSC tissue of origin and transplant types also account for substantial amount of heterogeneity. Notably, when considering MSC treatment dosage and number of injections together, the variation decreased by 19.91% (Table S2, Supplementary materials).
This analysis could be used to inform guidelines (e.g., experimental design, power calculation, etc.) for the future clinical translation of MSC to the bedside. Even though benefits were significant across species, delivery routes, time of administration in RA treatment, this study has provided evidence that human derived MSC are promising candidates for RA treatment in terms of the efficacy. Our results support further translational studies of human derived MSC in the treatment of RA in humans.
The quality of studies and risk of bias were reviewed to investigate their translational potentials. Since most of the published papers related to MSC-based RA treatment did not include double-blinded studies, the overall quality of the studies was lowered with increased risk of bias. Even though most of the studies tried to avoid selection bias and reporting bias, few studies attempted to report their strategies to mitigate the potential performance bias, detection bias or attrition bias. Therefore, the results from this meta-analysis should be interpreted with caution. Instead of excluding potentially biased studies from this review, we tried to increase the sample size, and therefore the statistical power, by including more relevant publications. To improve the overall quality of the studies, we recommend that future studies related to MSC-based RA treatment should include double-blinded studies in their protocols.
Intriguingly, from the meta-regression, a single high dosage (≥2 × 106 cells) is associated with a higher effect size in clinical score (Fig. 7a). Our results also suggest that multiple injections within a range of 2 × 106 to 3 × 106 cell dosage per injection lead to an optimal treatment outcome (Fig. 7c). In clinical studies, it is reported that even one injection of MSC would be sufficient for therapeutic benefits, suggesting a single moderate dose of MSC treatment may lead to RA remission, without the need for frequent, routine administrations [30,31]. The results of our meta-analysis agree with the observations from previous clinical studies [[30], [31], [32], [33]]. For further studies, especially for early phase clinical trials, exploration of the optimal dosage range (i.e., the range of dosage that could exert pharmacological effects without significant toxicity) of MSC treatments is needed because few studies have been done on the dose-response relationship of MSC therapy in RA.
Human derived MSC were used in 49 of the total 94 treatment arms, and almost all of them showed therapeutic benefits (Fig. 3, Fig. 4, Fig. 5, Fig. 6, and Table 2). Mouse derived MSC showed therapeutic benefits in 74.3% of the 35 treatment arms, and majority of the studies that favoured PBS control treatment were from mouse derived MSC (9 effects in 35 treatment arms), which suggests that human derived MSC may present better therapeutic effects compared to those from mice, in animal RA models. This observation could be related to the fact that human derived MSC were of xenogeneic implantation in the animal models of RA. These findings also implicate that autologous or donor recipient HLA matching MSC may not be necessary for more effective therapeutic outcomes in human RA treatment. In fact, this finding suggests that an appropriate level of immune incompatibility between donor cells and the host may be beneficial for cell therapy in some context.
We also investigated the effects of different MSC tissue of origin (Table 2 and Fig. 5) and found that UC-MSC (13 out of 13 reported positive effects; all human derived), AD-MSC (13 out of 14 reported positive effects) and G-MSC (5 out of 5 reported positive effects) have shown better RA treatment, compared to other tissue of origin, such as BM-MSC (27 out of 40 reported positive effects, whereas 13 treatment arms reporting negative therapeutic effects). There were no statistically significant differences between UC-MSC, AD-MSC and G-MSC clinical scores found in this study. However, UC-MSC and G-MSC showed better treatment outcomes in regard to histological scores. Additionally, autoantibody levels are considered as an important parameter regarding MSC treatments in RA [21,42]. Our results showed that MSC significantly decreased the serum levels of anti-collagen II (IgG) antibodies (Figs. S8, S9 and Table S1, Supplementary materials).
Future studies of autoimmune disease animal models treated with stem cells should take into account the inherent problems with having heterogeneity between studies. It would improve the reliability of data coming from these studies if they attempted to minimise variation of comparable data, such as the animal models being used, the methods for evaluating disease levels, the number of experimental groups evaluated in a single study, and the methods used to prepare cells. Other variables such as the stem cell's species and tissue origin, timing of treatments relative to disease, cell dosage, and delivery routes should be optimised to the parameters that have been previously proven most efficacious, unless further data is provided as to why these optimal methods were not used. In certain cases, one of these parameters may be better than others, but until more studies try to minimise heterogeneity of other parameters, the relative merits of each study will not be clear. The data in this analysis could be used to choose optimum parameters and minimise heterogeneity in future studies. Finally, when the field further progresses in human RA clinical trials of MSC, patient/disease heterogeneity and disease stages are among the most critical variables that can affect MSC's therapeutic efficacy. Note that the consequences and implications of these analyses may be restricted to animal models of arthritis and could not be directly extrapolated to rheumatoid arthritis in humans. The differences in the models versus human diseases and the fact that most studies are xenogeneic make the relevance to human disease debatable. In the era of precision medicine, therefore, correlations between patient/disease characteristics (e.g. based on genetic analyses and biomarkers), clinical outcomes, MSC characteristics as well as preclinical animal data will be critical to define MSC mechanism of actions and identify MSC products for given patient subpopulations.
RA is a debilitating chronic disorder that affects 1.3 million Americans. The current treatments, including NSAIDs, conventional synthetic DMARDs, and biologic DMARDs [43], do not restore immune balance and are associated with side effects. Importantly, 30–40% patients are refractory to current treatments [9,44]. Accumulating clinical evidence (Table S4, Supplementary Materials) has demonstrated that MSC (including AD, BM, UC, or UC blood derived) are potent in modulating the immune system and improving RA symptoms through production of trophic and anti-inflammatory factors, and the induction of self-tolerance [[30], [31], [32], [33],[45], [46], [47]]. For example, pioneering studies testing hUC-MSC products have been conducted, in several indications including RA. Results of two clinical studies, one in adult refractory RA patients (n = 172) and the other in refractory juvenile idiopathic arthritis (JIA) patients (n = 10), have demonstrated the safety and preliminary efficacy of hUC-MSC products in RA treatment [30,33].
Both animal and human clinical studies have demonstrated that MSC could modulate the immune system and decrease RA symptoms [30,34,35]. These studies indicate that the alleviation of disease symptoms are probably caused by the innate ability of MSC to dampen the immune response and induce tolerance [34]. For instance, transplantation of hUC-MSC into RA patients led to a significant increase in CD4+ CD25+ Foxp3+ Treg cells, a decrease in IL-6 and TNF-alpha, and induction of disease remission [30]. Further analysis on variables, including Treg cells levels, and pro−/anti-inflammatory factors, will be performed to evaluate the treatment effects from MSC on RA. Several factors from preclinical and clinical findings support the conclusion that MSC therapy can be a safe and effective treatment for patients who are refractory to current RA therapies [31,32]. The role and influence of MSC on promoting or inhibiting existing tumour development are controversial [48]. Due to mixed results of MSC on tumour relapse [49,50], toxicology studies including tumorigenicity would be recommended before MSC administration to patients and caution should be taken for patients with existing malignancy due to the unknown effect of MSC on tumours. MSC have an overall positive safety record in hundreds of clinical trials across many different disease indications [51]. All in all, this review suggests MSC therapy have a high potential to become a valuable treatment option for human RA disease.
5. Conclusions
To the best of our knowledge, this meta-analysis is to quantitatively answer whether MSC represent a robust RA treatment option in animal models. It suggests that in preclinical studies in RA animal models, MSC have consistently exhibited therapeutic benefits based on clinical scores, histological scores and paw thickness. These findings were also robust after correction of publication bias. We also found that there were considerable efficacy variations, across donor and recipient species, routes of administration, MSC tissue of origin, timing of MSC introduction, transplant types, dosage of MSC administration and number of injections. These findings demonstrate the need for considering variations in different animal models and treatment protocols in future studies using MSC to treat RA in humans, in order to maximise the therapeutic gains in the era of precision medicine.
Declarations
Availability of data and supporting materials section. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This work is supported by NIH [1DP2CA195763] and Baylx Inc.: BI-206512. H.P.F is in part supported by a pre-doctoral fellowship from the National Institute of Neurological Disorders and Stroke (NINDS/NIH) Training Grant [Award# NS082174].
Authors' contributions
L.L. conducted study conception and design, performed data acquisition and interpretation, and drafted the manuscript. C.W.W. participated in study design, performed analysis and interpretation of data, and revised the manuscript. M.H. carried out data acquisition and interpretation, revised the manuscript and coordinated the project. H.P.F. revised the manuscript and participated in data interpretation. G.L. edited the manuscript. Y.L. participated in study design and conception, and edited the manuscript. W.L. conducted study conception and design, and revised the manuscript. W.Z. was responsible for conception and design of the study, revised the manuscript and supervised overall project. L.L., C.W.W., W.L. and W.Z. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version to be published.
Declaration of Competing Interest
Dr. Weian Zhao reports grants from NIH, grant from Baylx Inc., during the conduct of the study. Dr. Zhao was a co-founder of and received personal fees from Baylx Inc. Dr. Zhao received grants and personal fees from Velox Biosystems Inc., as well as grants and personal fees from Amberstone Biosciences, outside the submitted work. Dr. Wenbin Liao is a co-founder and employee of Baylx Inc. aims to use UC-MSC to treat disease indications including RA. Henry P. Farhoodi reports fellowship from NINDS/NIH. Dr. Linan Liu, Chi W. Wong, Menglu Han and Henry P. Farhoodi report grant from Baylx Inc., during the conduct of the study. Dr. Guangyang Liu and Dr. Yongjun Liu have financial relationships with Beijng Beilai Biotechnology Corporation Ltd. that develops MSC-based products.
Acknowledgements
We thank Dr. Steven C. Cramer for discussion and comments. We are grateful to Shengyu Gao for the help in this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.073.
Contributor Information
Wenbin Liao, Email: wliao@baylxinc.com.
Weian Zhao, Email: weianz@uci.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hunt L., Emery P. Defining populations at risk of rheumatoid arthritis: the first steps to prevention. Nat Rev Rheumatol. 2014;10(9):521–530. doi: 10.1038/nrrheum.2014.82. [DOI] [PubMed] [Google Scholar]
- 2.Smolen J.S., Aletaha D., Redlich K. The pathogenesis of rheumatoid arthritis: new insights from old clinical data? Nat Rev Rheumatol. 2012;8(4):235–243. doi: 10.1038/nrrheum.2012.23. [DOI] [PubMed] [Google Scholar]
- 3.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl. 5):v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 4.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 5.Smolen J.S., Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 6.Choy E.H., Kavanaugh A.F., Jones S.A. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9(3):154–163. doi: 10.1038/nrrheum.2013.8. [DOI] [PubMed] [Google Scholar]
- 7.Weinblatt M.E., Keystone E.C., Furst D.E., Moreland L.W., Weisman M.H., Birbara C.A. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 8.Lipsky P.E., Van Der Heijde D.M., St. Clair E.W., Furst D.E., Breedveld F.C., Kalden J.R. Infliximab and methotrexate in the treatment of rheumatoid arthritis. New Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 9.Vander Cruyssen B., Van Looy S., Wyns B., Westhovens R., Durez P., Van den Bosch F. Four-year follow-up of infliximab therapy in rheumatoid arthritis patients with long-standing refractory disease: attrition and long-term evolution of disease activity. Arthritis Res Ther. 2006;8(4):R112. doi: 10.1186/ar2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis J.R., Singh J.A. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Nam Y., Jung S.M., Rim Y.A., Jung H., Lee K., Park N. Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park N., Rim Y.A., Jung H., Kim J., Yi H., Kim Y. Etanercept-synthesising Mesenchymal stem cells efficiently ameliorate collagen-induced arthritis. Sci Rep. 2017;7 doi: 10.1038/srep39593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B., Yuan J., Zhou Y., Ghawji M., Jr., Deng Y.P., Lee A.J. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011;141(3):328–337. doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Santalla M., Mancheno-Corvo P., Menta R., Lopez-Belmonte J., DelaRosa O., Bueren J.A. Human adipose-derived Mesenchymal stem cells modulate experimental autoimmune arthritis by modifying early adaptive T cell responses. Stem Cells. 2015;33(12):3493–3503. doi: 10.1002/stem.2113. [DOI] [PubMed] [Google Scholar]
- 16.Choi E.W., Shin I.S., Song J.W., Lee M., Yun T.W., Yang J. Effects of transplantation of CTLA4Ig-overexpressing adipose tissue-derived Mesenchymal stem cells in mice with sustained severe rheumatoid arthritis. Cell Transplant. 2016;25(2):243–259. doi: 10.3727/096368915X688470. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Mu R., Wang S., Long L., Liu X., Li R. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12(6):R210. doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R., Li X., Zhang Z.Y., Zhou M., Sun Y., Su D.L. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci Rep. 2015;5 doi: 10.1038/srep12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Z., Zhai Y., Zheng Z., Yang L., Luo X., Dong X. Loss of A20 in BM-MSCs regulates the Th17/Treg balance in Rheumatoid arthritis. Sci Rep. 2018;8(1):427. doi: 10.1038/s41598-017-18693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M., Su W., Lin X., Guo Z., Wang J., Zhang Q. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65(5):1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Y., Wu W., Gu J., Zhang X., Dang J., Wang J. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine. 2019;43:620–631. doi: 10.1016/j.ebiom.2019.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uccelli A., Prockop D.J. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol. 2010;22(6):768–774. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Parekkadan B., Milwid J.M. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X.-M., Liu H., Ding J., Wang J., Wang Y., Yang M. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of Thermogel-encapsulated bone marrow Mesenchymal stem cells. Plos One. 2015;10(3) doi: 10.1371/journal.pone.0120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyndall A. Mesenchymal stem cell treatments in rheumatology: a glass half full? Nat Rev Rheumatol. 2014;10(2):117–124. doi: 10.1038/nrrheum.2013.166. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey E., Gonzalez M.A., Varela N., O'Valle F., Hernandez-Cortes P., Rico L. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Wang L., Cong X., Liu G., Zhou J., Bai B. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev. 2013;22(24):3192–3202. doi: 10.1089/scd.2013.0023. [DOI] [PubMed] [Google Scholar]
- 31.Álvaro-Gracia J.M., Jover J.A., García-Vicuña R., Carreño L., Alonso A., Marsal S. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 32.Liang J., Li X., Zhang H., Wang D., Feng X., Wang H. Allogeneic mesenchymal stem cells transplantation in patients with refractory RA. Clin Rheumatol. 2012;31(1):157–161. doi: 10.1007/s10067-011-1816-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Zhang Y., Li H., Hong J., Chen X., Li M. Clinical observation of employment of umbilical cord derived Mesenchymal stem cell for juvenile idiopathic arthritis therapy. Stem Cells Int. 2016;2016:9165267. doi: 10.1155/2016/9165267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H.K., Lim S.H., Chung I.S., Park Y., Park M.J., Kim J.Y. Preclinical efficacy and mechanisms of mesenchymal stem cells in animal models of autoimmune diseases. Immune Netw. 2014;14(2):81–88. doi: 10.4110/in.2014.14.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pers Y.M., Jorgensen C. Mesenchymal stromal cells: updates and therapeutic outlook in rheumatic diseases. J Clin Med. 2013;2(4):201–213. doi: 10.3390/jcm2040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Jawhari J.J., El-Sherbiny Y.M., Jones E.A., McGonagle D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM. 2014;107(7):505–514. doi: 10.1093/qjmed/hcu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1) doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosloniec E.F., Cremer M., Kang A.H., Myers L.K., Brand D.D. Collagen-induced arthritis. Curr Protoc Immunol. 2010;15(5):1–25. doi: 10.1002/0471142735.im1505s89. Chapter 15:Unit. [DOI] [PubMed] [Google Scholar]
- 39.Vesterinen H., Sena E., Egan K., Hirst T., Churolov L., Currie G. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92–102. doi: 10.1016/j.jneumeth.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Takeshima N., Sozu T., Tajika A., Ogawa Y., Hayasaka Y., Furukawa T.A. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14(1):30. doi: 10.1186/1471-2288-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hynes K., Bright R., Proudman S., Haynes D., Gronthos S., Bartold M. Immunomodulatory properties of mesenchymal stem cell in experimental arthritis in rat and mouse models: a systematic review. Semin Arthritis Rheum. 2016;46(1):1–19. doi: 10.1016/j.semarthrit.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Song Y.W., Kang E.H. Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM. 2010;103(3):139–146. doi: 10.1093/qjmed/hcp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh J.A., Saag K.G., Bridges S.L., Jr., Akl E.A., Bannuru R.R., Sullivan M.C. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 44.Rubbert-Roth A., Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl. 1):S1. doi: 10.1186/ar2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghoryani M., Shariati-Sarabi Z., Tavakkol-Afshari J., Ghasemi A., Poursamimi J., Mohammadi M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: a successful clinical trial in Iran. Biomed Pharmacother. 2019;109:1834–1840. doi: 10.1016/j.biopha.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 46.Park E.H., Hs Lim, Lee S., Roh K., Seo K.W., Kang K.S. Intravenous infusion of umbilical cord blood-derived Mesenchymal stem cells in rheumatoid arthritis: a phase Ia clinical trial. Stem Cells Transl Med. 2018;7(9):636–642. doi: 10.1002/sctm.18-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shadmanfar S., Labibzadeh N., Emadedin M., Jaroughi N., Azimian V., Mardpour S. Intra-articular knee implantation of autologous bone marrow–derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(4):499–506. doi: 10.1016/j.jcyt.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Droujinine I.A., Eckert M.A., Zhao W. To grab the stroma by the horns: from biology to cancer therapy with mesenchymal stem cells. Oncotarget. 2013;4(5):651. doi: 10.18632/oncotarget.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L., Chen S., Yang P., Cao H., Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10(1):182. doi: 10.1186/s13287-019-1287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 51.Kim N., Cho S.-G. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28(4):387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material