Abstract
Background
The clinicopathological continuity between amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) is well known. Although ALS demonstrates language symptoms similar to FTLD, including semantic dementia, word reading impairments in ALS have not been well studied. “Jukujikun” are Kanji-written words with irregular pronunciation comparable to “exception words” and useful for detecting semantic deficits in Japan. We conducted a cross-sectional study to investigate Jukujikun reading impairments and related network changes in ALS.
Methods
We enrolled 71 ALS patients and 69 healthy controls (HCs). Age-, sex-, and education matched HCs were recruited from another cohort study concurrently with patient registration. We examined neuropsychological factors including low frequency Jukujikun reading. We performed resting-state functional magnetic resonance imaging with voxel-based graph analysis on a subset of participants who agreed.
Findings
Low frequency Jukujikun score was decreased in ALS (15·0[11·0–19·0](median[25–75 percentile])) compared with HCs (19·0[17·3–20·0]) (p < 0·001, effect size = 0·43). Fifty-two percent of ALS (N = 37) with low frequency Jukujikun score ≤ 5th percentile of HCs was classified as ALS with positive Jukujikun deficit (ALS-JD+). Compared with HCs, ALS-JD+ showed decreased degree centrality in the right lingual/fusiform gyrus, where connectivities with regions associated with word perception, semantic processing, or speech production were decreased. They also showed increased degree centrality in the left inferior/middle temporal gyrus, associated with increased connectivities involving semantic processing.
Interpretation
Dysfunction of the “hub” in the right lingual/fusiform gyrus can affect semantic deficit in ALS. Considering neuropsychological symptoms as network impairments is vital for understanding various diseases.
Fund
MHLW and MEXT, Japan.
Keywords: Amyotrophic lateral sclerosis, Semantic deficits, Language impairments, Resting-state functional MRI, Voxel-based graph theoretical analysis
Research in context.
Evidence before this study
Approximately 15% of amyotrophic lateral sclerosis (ALS) patients have clinical features similar to frontotemporal lobar degeneration (FTLD). Moreover, 9·1–40% of ALS patients can show language impairment, such as exception word reading and production difficulties similar to semantic dementia (SD) and progressive non-fluent aphasia (PNFA). “Jukujikun” is a category of words with irregular pronunciation and is often used to detect semantic impairments in patients with SD in Japan. However, it is not known whether Jukujikun reading impairments exist in ALS patients and what brain networks would be associated with the impairment.
Added value of this study
Fifty-two percent of ALS patients showed Jukujikun reading impairments. Voxel-based graph theoretical analysis revealed the right fusiform/lingual gyrus as a hub region associated with the impairment. Subsequent seed-based analysis showed decreased connectivity of the right fusiform/lingual gyrus with regions associated with visual word perception, semantic processing, and speech beyond hypothesis-driven study based on local pathology. Voxel-based graph theoretical hub analysis may uncover the network-based impairments of specific cognitive tasks.
Implications of all the available evidence
Jukujikun reading promises to be a sensitive tool for the detection of early semantic impairment in ALS patients. Based on findings of brain atrophy in pathological and conventional imaging studies, the left temporal pole has been considered a causal lesion of Jukujikun reading impairments. However, our voxel-based graph theoretical analysis indicated that a hub dysfunction in the right lingual/fusiform gyrus, associated with decreased connectivity with other regions relevant to reading Jukujikun, could be a major causal network alteration leading to semantic impairments assessed by Jukujikun reading. Our results suggest that clinical symptoms are derived from network impairments including multiple relevant areas rather than from one single lesion. In addition, this perspective is important for understanding the clinical symptoms in various diseases.
Alt-text: Unlabelled Box
1. Introduction
Amyotrophic lateral sclerosis (ALS) is considered a neurodegenerative disease that shows selective involvement of motor systems. However, approximately 15% of ALS patients have clinical features of frontotemporal lobar degeneration (FTLD) during the course of the illness [1]. TAR DNA binding protein of 43 kDa (TDP-43) is a common causal protein of ALS and FTLD [2]. Although 9·1–40% of patients with ALS also show language domain impairment [3] similar to semantic dementia (SD) and progressive non-fluent aphasia (PNFA) [4], the neural and network basis of language impairments in ALS remains unclear.
In English-speaking countries, regular words (words with regular spelling-to-sound correspondence, such as “apple”) and exception words (words with irregular pronunciation to the corresponding spelling, such as “yacht” or “sew”, etc.) are generally used to evaluate the ability of word reading [5]. Patients with SD show characteristic impairments called surface dyslexia in reading exception words related to activated semantic processing [5].
The written Japanese language has two orthographies: Kana is a phonemic symbol, and Kanji is a nonphonemic morphogram. Approximately 2000 Kanji characters are commonly used [6], and most Kanji characters have two or more readings. Depending on the reading consistency of the component characters, Kanji words can be classified into three categories: consistent words, inconsistent-typical words, and inconsistent-atypical words [7]. Consistent words and inconsistent-typical words can be correctly pronounced due to the regular correspondence of letters and sounds. Inconsistent-atypical words including “Jukujikun” cannot be pronounced by the regular readings for the characters. Thus, although Kanji words cannot be simply compared with words in other languages in terms of the differences of their writing and reading systems, consistent words and inconsistent-typical words are analogous to “regular words” in English-speaking countries, and inconsistent-atypical words including “Jukujikun” are comparable to “exception words” [8].
“Jukujikun” is a category of words for which the entire word has a unique reading that does not correspond to the standard reading of any of the constituent characters. For example, when the characters “雪” (=snow) read “YUKI” or “SETSU” and “崩” (=collapse) read “HOH” are combined to make the word “雪崩” (=avalanche), the reading becomes “NADARE”, while this word is never read “YUKI-HOH” or “SETSU-HOH.” The sounds “NA”, “DA”, or “RE” are never used for the characters “雪” or “崩” in other words.
In Japan, “Jukujikun” is often used for evaluating semantic impairments in patients with SD [9]. However, whether Jukujikun reading ability is affected in patients with ALS is unknown. Notably, although lesions to the left temporal pole are considered associated with exception word reading impairment [5], no reports have addressed the relationship between Jukujikun impairments and resting-state network (RSN) abnormalities. Recently, extensive functional connectivity alterations across the temporal, frontal, parietal, and occipital lobes beyond the core atrophic regions in the anterior temporal lobes and related language networks were reported in SD [10], and widespread connectivity alterations were also reported in ALS [11,12], suggesting that impairment of Jukujikun in ALS may be associated with the more widespread and unknown regions beyond the left anterior temporal lobe involvement.
In this study, we investigated Jukujikun reading and RSN findings in patients with non-familial ALS. To investigate alterations in RSN, we used Intrinsic Connectivity Contrast(ICC) [13]. ICC is a voxel-based graph theoretical analysis approach that computes degree centrality, a network measure reflecting the number of connections a given voxel has to the rest of the brain. ICC requires no prior information or assumptions of relevant regions-of-interest since it computes degree centrality at every voxel within the brain making it very useful in investigating brain network changes. However, ICC could not reveal the specific connections among regions to the observed changes. As such, we additionally performed seed-to-voxel analysis using the regions extracted from ICC results as seeds to further elucidate the relevant connectivity changes associated with ICC findings.
2. Methods
2.1. Study design and participants
A total of 140 participants, non-familial ALS patients (n = 71) and age-, sex-, and education-matched healthy controls (HCs) (n = 69), were included in this cross-sectional study. ALS patients were recruited from referrals to the Department of Neurology at Nagoya University from April 2016 to December 2017. We excluded patients who had a medical history of stroke or brain injury, structural lesions on MRI, and a family history of ALS or FTLD. All ALS patients fulfilled the criteria for probable laboratory-supported, probable, or definite ALS based on the El Escorial criteria [14]. Concurrently with patient registration, we continuously recruited age-, sex-, and education-matched HCs from an ongoing cohort study [15] conducted by the Brain and Mind Research Centre (BMRC) of Nagoya University from April 2016 to December 2017. The number of HCs was approximately equal to that of ALS. The exclusion criteria were: a score of 88 or less on Addenbrooke's Cognitive Examination-revised (ACE-R) score, history of neurological disease, structural lesions on MRI, or moderate or higher degree of atrophy or white matter (WM) degeneration on MRI. We also excluded one healthy participant whose scores were outliers on the word reading test and picture naming. Finally, 68 HCs and 71 ALS patients were included for clinical analysis. Out of the 71 ALS patients, we performed an imaging study in 34 patients who agreed to participate. The average time period between neuropsychological exams and MRI acquisition was 10·2[1·5, 11·5] days (median [IQR]). Additionally, 34 age-, sex- and education-matched HCs were included for MRI analysis. The study conformed to the Ethical Guidelines for Medical and Health Research Involving Human Subjects endorsed by the Japanese government. The study protocol was approved by the Ethics Review Committee of the Nagoya University Graduate School of Medicine, and written informed consent was obtained from all participants.
2.2. Neuropsychological and language assessments
For all participants, general cognitive function was assessed using the Mini-Mental State Examination (MMSE) and ACE-R. Nonverbal intellectual ability was assessed using Raven's Coloured Progressive Matrices (RCPM). Frontal lobe cognitive function was examined by the Frontal Assessment Battery (FAB). Executive function, working memory and inhibitory control function were estimated by a one-minute phonemic verbal fluency test (Ka), a digit span test (both forward and backward) and the Stroop test. Language ability was assessed by categorical verbal fluency (animal naming), picture naming in the Sophia Analysis of Language in Aphasia (SALA), noun similarity judgement in the SALA and noun picture matching in the Test of Lexical Processing in Aphasia (TLPA). All participants completed a self-reporting instrument concerning anxiety and depression (Beck Depression Inventory II (BDI II)).
2.3. Word reading test
Twenty consistent words based on a previous report [7] were included. We uniquely selected 20 sets of Jukujikun so that the word characteristics of familiarity, frequency, and imageability were matched to those of the consistent words. In order to detect slight impairment, 20 sets of lower frequency Jukujikun were also prepared (Table 1). Between the two Jukujikun groups with different frequencies, imageability is only matched. This is because, among the three word characteristics, familiarity and frequency are correlated [16]. Below, we refer to the three categories as “consistent words”, “high frequency Jukujikun”, and “low frequency Jukujikun.”
Table 1.
Word characteristics and the word list used in the study.
| Word category | Familiarity | Frequency | Imageability |
|---|---|---|---|
| Consistent word | 5·36 (0·58) | 2·96 (0·64) | 4·08 (0·56) |
| Jukujikun, High frequency | 5·26 (0·64) | 2·72 (0·57) | 4·38 (0·77) |
| Jukujikun, Low frequency | 4·62 (0·71)⁎ | 1·77 (0·39)⁎ | 4·17 (0·54) |
| Example of error reactions on representative five words |
List of the other 15 words in each category | ||||||
|---|---|---|---|---|---|---|---|
| Words | Meaning | Reading | Overregularisation | ||||
| Consistent words (20 words) | 集計 | aggregate | Shukei | – | 永続”Eizoku” | 返信”Henshin” | 急送Kyusou” |
| 区別 | distinction | Kubetsu | – | 任務”Ninmu” | 銀貨”Ginka” | 理学”Rigaku” | |
| 信任 | confidence | Shinnin | – | 製剤”Seizai” | 動脈”Doumyaku” | 満開”Mankai” | |
| 予告 | notice | Yokoku | – | 軍備”Gunbi” | 試練”Shiren” | 低温”Teion” | |
| 観察 | observation | Kansatsu | – | 持続”Jizoku” | 医局”Ikyoku” | 改選”Kaisen” | |
| Jukujikun high frequency (20 words) | 雪崩 | avalanche | Nadare | Setsuhou/Yukikuzure | 師走”Shiwasu” | 名残”Nagori” | 素人”Shirouto” |
| 玄人 | professional | Kurouto | Gennjinn | 笑顔”Egao” | 牡丹”Botan” | 寄席”Yose” | |
| 田舎 | countryside | Inaka | Densha | 早苗”Sanae” | 蚊帳”Kaya” | 神楽”Kagura” | |
| 果物 | fruit | Kudamono | Kabutsu | 硫黄”Iou” | 乙女”Otome” | 今宵”Koyoi” | |
| 為替 | money order | Kawase | Tamekae | 吹雪”Fubuki” | 息吹”Ibuki” | 若人”Wakoudo” | |
| Jukujikun low frequency (20 words) | 足袋 | Japanese socks | Tabi | Sokutai/Ashibukuro | 真似”Mane” | 海原”Unabara” | 屏風”Byoubu” |
| 太刀 | sward | Tachi | Taitou/Futoha | 稲荷”Inari” | 暖簾”Noren” | 巫女”Miko” | |
| 海女 | woman shell diver | Ama | Kaijo | 蕎麦”Soba” | 野良”Nora” | 洒落”Share” | |
| 雑魚 | small fish | Zako | Zatsugyo | 日和”Hiyori” | 稚児”Chigo” | 桟敷”Sajiki” | |
| 黄昏 | twilight | Tasogare | Koukon | 胡瓜”Kyuuri” | 草履”Zouri” | 土筆”Tsukushi” | |
The word characteristics are equivalent between Consistent word and High-frequency Jukujikun.
Examples of errors by reading exception words with regular reading are shown as overregularization.
“Overregularisation” is an error pattern made by reading exception words as they are spelled. Typical overregularization errors obtained in the study are shown.
, compared with high frequency Jukujikun by two-sample t-test, p < 0·05.
Word characteristics were obtained from the NTT psycholinguistic database [[16], [17], [18]]. This major massive database in Japan contains word and character information, such as familiarity, frequency, accent, orthography, imageability, and other important psycholinguistic properties. In this database, the word frequency section includes approximately 340,000 words, and the other sections include approximately 70,000 words each. The number of recorded words is comparable to the worldwide database [19,20].
We used a display monitor to show exam words one by one. Participants were asked to read aloud the displayed words. The number of correct answers was used as the score, giving 20 as the full score for each category. In order to highlight the characteristics of patients who are impaired reading Jukujikun, we further subdivided the ALS patients into two groups, ALS with positive Jukujikun deficit group (ALS-JD+) and ALS with negative Jukujikun deficit group (ALS-JD-). The cut-off was set to the fifth percentile of the HCs' score in the low-frequency Jukujikun.
2.4. MRI acquisition
All MRI scans were performed using a Siemens Magnetom Verio (Siemens, Erlangen, Germany) 3·0-T scanner with a 32-channel head coil at BMRC. High-resolution T1-weighted images (TI-WI) were acquired using the following parameters: repetition time (TR) = 2·5 s, echo time (TE) = 2·48 ms, 192 sagittal slices with 1-mm thickness, field of view (FOV) = 256 mm, 256 × 256 matrix size, and an in-plane voxel resolution of 1 × 1 mm2. Eight-minute eyes-closed resting-state functional images were obtained with echo-planar imaging (EPI) using the following parameters: TR = 2·5 s, TE = 30 ms, 39 transversal slices with a 0·5-mm interslice interval and a 3-mm thickness, FOV = 192 mm, 64 × 64 matrix dimension, flip angle = 80 degrees, and 198 volumes.
2.5. Voxel-based morphometry
All T1-WI were preprocessed using the SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fl.ion.ucl.ac.uk/spm/software/spm12/) running in MATLAB (R2016a; MathWorks, Natick, MA, USA). The T1-WI images were segmented into grey matter (GM), WM, cerebrospinal fluid (CSF), and other nonbrain tissues and normalised to the standard Montreal Neurological Institute (MNI) space using the Diffeomorphic Anatomical Registration through Exponentiated Lie (DARTEL) method [21], and spatially smoothed by an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. Then, preprocessed GM images were analysed using two-sample t-tests for group comparisons. Age, sex, education, and total brain volume were included as covariates. The significance level for the results was set at p < 0·05 after correcting for multiple comparisons using cluster-level familywise error correction (FWEc) with a cluster-forming threshold of p = 0·001.
2.6. Resting-state fMRI analysis
2.6.1. Preprocessing
Functional MRI data were preprocessed using the default pipeline as the standard procedure implemented in the CONN Toolbox [22] running on MATLAB. The first five volumes of each subject's data were discarded. Then, the images were realigned, unwarped to remove dynamic EPI distortions [23], slice-time corrected, coregistered to the bias corrected T1-WI, segmented, normalised to the MNI space, resampled to an isotropic voxel resolution of 2 × 2 × 2 mm3 and smoothed using an 8-mm FWHM Gaussian filter. Moreover, blood-oxygenation level-dependent (BOLD) signal noise was reduced with the CompCor approach. Bandpass filtering was performed within the frequency window of 0·01–0·1 Hz. ART-based outlier detection and scrubbing were also performed to further eliminate the effects of head motion.
2.6.2. Intrinsic connectivity contrast
Intrinsic connectivity contrast (ICC) [13] is a whole-brain voxel-based hypothesis-free analysis based on graph theory. Specifically, we used the ICC-power (ICC-p) metric, which represents the average of the square of the connectivity values of a given voxel to all other voxels within the brain. This metric can be expressed as
where x and y represent the spatial locations of two arbitrary voxels, r(x,y) is the connectivity value between locations x and y, and Ω represents the set of all brain voxels. ICC-p can be interpreted as a “weighted” degree centrality, a network measure representing the number of connections between a given voxel and the rest of the brain, obtained with the connectivity threshold set to 0. The advantages of this approach are as follows. First, there is no need for defining regions of interest (ROIs), and second, the ICC map can be computed without applying arbitrary thresholds. Therefore, no prior information or assumptions are required.
We calculated this ICC-p value for each subject and performed group comparisons that included ALS vs. HC, ALS-JD+ vs. HC, ALS-JD- vs. HC, and ALS-JD+ vs. ALS-JD-. The analysis was performed using the CONN toolbox considering age, sex, and education as nuisance covariates. Statistical significance was set using a height threshold of p < 0·001 and a false discovery rate (FDR)-corrected cluster-size threshold of p < 0·05. We also calculated the mean ICC-p values of the clusters with significantly different ICC-p values between groups.
We also evaluated the correlation between mean ICC-p values and Jukujikun reading ability or ACE-R scores. The partial correlation coefficient and p value were calculated using Spearman's partial correlation, accounting for age, sex, and education as covariates, and the significance of the obtained p values was assessed with FDR adjustment using MATLAB.
2.6.3. Seed-based analysis
We subsequently performed a seed-to-voxel analysis to identify the origin of the degree alterations. First, all the regions with significantly different ICC-p values were extracted as ROIs and used as seed regions. The average filtered BOLD signal in each seed region was evaluated, and its bivariate correlation with the BOLD signal in all other voxels in the brain was computed. Then, we performed group comparisons based on Jukujikun reading results. We applied two-sample t-tests with age, sex, and education as nuisance covariates. Statistical significance was set at a height threshold of p < 0·001 and an FDR-corrected cluster-size threshold of p < 0·05.
2.7. Statistical analyses
Clinical data were evaluated with the Shapiro–Wilk test for normality. To compare ALS and HCs, we performed the Mann–Whitney U test, Chi-square test, Fisher's exact-test, or two-sample t-test. Kruskal–Wallis test was performed for three group comparison and post hoc Mann-Whitney U test with Bonferroni correction along with The Hodges-Lehman method was also conducted. Effect size (r) was calculated using the formula: (Z: standardized value for the U-value, N: sample size). Spearman's rank correlation test was performed to obtain the correlation coefficients between low frequency Jukujikun score and other examinations for semantic evaluation. P values <0·05 were considered statistically significant. Statistical analyses were conducted with the Statistical Package for the Social Sciences (SPSS) version 24·0 (IBM Corp., Armonk, NY, USA) and statistical program R.
3. Results
3.1. Subject demographics and clinical characteristics
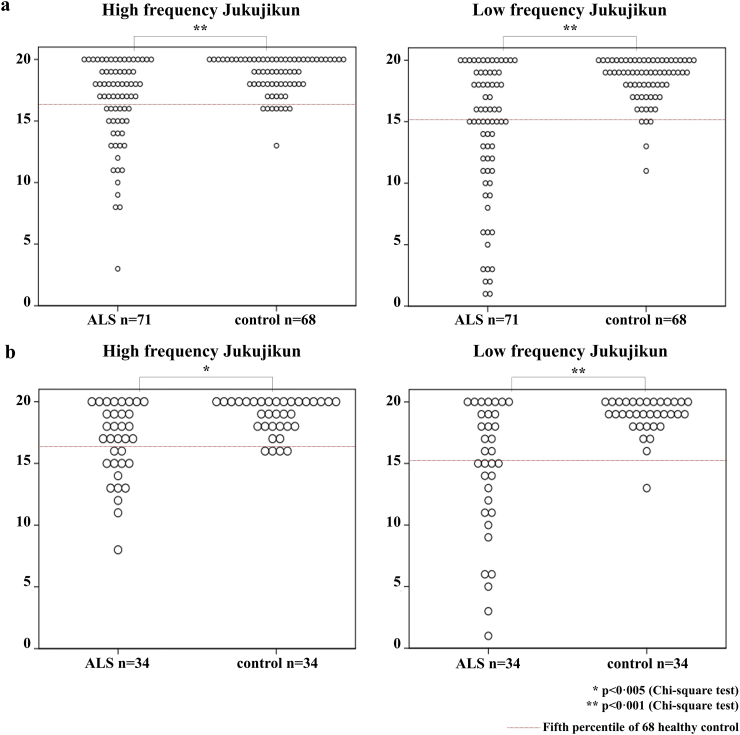
Participant demographics and clinical findings are described in Table 2, and participants who later underwent MRI scanning are shown in Table 3. ALS patients showed a significant reduction in various cognitive function test scores: MMSE(28·0 [26·0–29·0] for ALS vs. 30·0 [29·0–30·0] for HCs), ACE-R(94·0 [86·0–98·0] vs. 98·0 [95·0–99·0]), FAB(15·0 [13·0–17·0] vs. 16·0 [15·0–17·0]), phonemic fluency (10·0 [8·0–13·0] vs. 12·0 [9·0–15·0]), picture naming (61·0 [58·0–62·0] vs. 63·0 [61·3–64·0]), and noun similarity judgement (47·0 [46·0–48·0] vs. 48·0 [47·0–48·0]) (Table 2). However, the score variation in low frequency Jukujikun was the most remarkable (15·0 [11·0–19·0] (vs. 19·0 [17·3–20·0]; p < 0·001, r = 0·43) (Table 2, Fig. 1). Low frequency Jukujikun score showed significant correlations with semantic examinations such as picture naming (Spearman's rho = 0·657, p < 0·001) and noun similarity judgement (rho = 0·608, p < 0·001) (Supplemental Table 1). No patients fulfilled the criteria of SD or PNFA. Based on the fifth percentile value of the 68 HCs, we subdivided the patients into two groups: those who had scores of low frequency Jukujikun 15 or less were assigned to the ALS-JD+ group, and the others were assigned to the ALS-JD- group. Among the 71 patients, 37 were classified as ALS-JD+, and the remaining 34 were classified as ALS-JD- (Fig. 1a). Similarly, 17 out of 34 patients who underwent MRI were classified as ALS-JD+ (Fig. 1b). The ALS-JD+ group was significantly less educated than the ALS-JD- and HC groups: median differences between groups for education years evaluated by using the Hodges-Lehmann nonparametric estimator were −2·0(95% CI: −3·0, 0·0) for ALS-JD+ vs. ALS-JD-, −1·0(−3·0, 0·0) for ALS-JD+ vs. HC, and 0·0(0·0, 0·0) for ALS-JD- vs. HC (Table 2, Supplemental Table 2). However, the other demographics were not significantly different. Cognitive examinations showed significant reductions in various domains in the ALS-JD+ group compared with those in the ALS-JD- and HC groups, whereas the ALS-JD- and HC groups showed no significant difference: MMSE (Kruskal–Wallis H(2) = 11·56, p = 0·003; Hodges-Lehmann median estimate = −1·0(95% CI: −2·0, 0·0) for ALS-JD+ vs. ALS-JD- and −2·0(−3·0, −1·0) for ALS-JD+ vs. HC), ACE-R (H(2) = 19·69, p < 0·001; −7·0(−13·0, −2·0) and −9·0(−14·0, −4·0)), FAB(H(2) = 9·95, p = 0·007; −2·0(−3·0, 0·0) and − 2·0(−3·0,-1·0)), Picture naming(H(2) = 17·16, p < 0·001; −3·0(−5·0, −2·0) and −4·0(−5·0, −2·0)). The difference of low frequency Jukujikun score was the most remarkable: H(2) = 35·95, p < 0·001; −7·0(−10·0, −6·0) and −7·0(−9·0, −6·0)) (Table 2, Supplemental Table 2). The backgrounds between participants who underwent MRI and those who did not were not significantly different, and the trend of less education and extensive cognitive deficits in the ALS-JD+ group was similar in both groups (Table 3). Similar to other cognitive examinations, Jukujikun reading scores could be influenced by educational background. Thus, we subdivided all participants into two groups based on years of education: high educational background group (≥14 years, n = 51 (ALS/HC = 25/26)) and low educational background group (≤12 years, n = 88 (ALS/HC = 46/42)). In each educational subgroup, we compared low frequency Jukujikun scores between ALS and HCs. Then, in both subgroups nearly half of patients had scores of the fifth percentile of HC or less (high educational background group: 40·0% (n = 10), low educational background group: 45·7% (n = 21)) (Supplemental Table 3, Supplemental Fig. 1).
Table 2.
Demographics and clinical characteristics of the participants.
| ALS |
HC |
p value | |||
|---|---|---|---|---|---|
| Total |
ALS-JD+ |
ALS-JD- |
|||
| n = 71 | n = 37 | n = 34 | n = 68 | ||
| Demographic | |||||
| Age (years) | 66·0 [62·0–70·0] | 68·0 [61·5–71·5] | 66·0 [62·8–69·3] | 69·0 [62·0–72·0] | 0·325† |
| Sex (m/f) | 46/25 | 26/11 | 20/14 | 34/34 | 0·131‡ |
| Education(years) | 12·0 [12·0–16·0] | 12·0 [9·0–12·0]b, d | 13·0 [12·0–16·0] | 12·0 [12·0–16·0] | 0·003† |
| Handedness(r/l) | 71/0 | 37/0 | 34/0 | 68/0 | – |
| Years from first symptom | 1·4 [0·9–2·7] | 1·2 [0·8–2·5] | 1·6 [0·9–2·8] | N/A | 0·637§ |
| ALSFRS-R(48) | 41·0 [37·0–43·0] | 40·0 [37·0–43·0] | 41·0 [39·0–43·0] | N/A | 0·384§ |
| General cognition | |||||
| MMSE(30) | 28·0 [26·0–29·0]⁎⁎ | 27·0 [25·5–29·0]b | 29·0 [27·8–30·0] | 30·0 [29·0–30·0] | <0·001† |
| ACE-R(100) | 94·0 [86·0–98·0]⁎⁎ | 88·0 [77·5–96·0]b, d | 96·5 [93·0–98·0] | 98·0 [95·0–99·0] | <0·001† |
| RCPM(36) | 32·0 [27·0–34·0] | 30·0 [25·5–34·0] | 33·5 [30·8–34·3] | 31·5 [30·0–34·0] | 0·058† |
| Frontal and executive | |||||
| FAB(18) | 15·0 [13·0–17·0]⁎⁎ | 14·0 [12·0–16·0]b, c | 16·0 [15·0–17·0] | 16·0 [15·0–17·0] | 0·001† |
| Phonemic fluency | 10·0 [8·0–13·0]⁎⁎ | 9·0 [5·0–12·5]b, c | 11·0 [9·0–14·0] | 12·0 [9·0–15·0] | 0·002† |
| Forward digit span | 6·0 [5·0–7·0] | 6·0 [5·0–6·0]a | 6·0 [6·0–7·0] | 6·0 [5·0–6·0] | 0·016† |
| Backward digit span | 4·0 [4·0–5·0] | 4·0 [3·0–5·0]a | 5·0 [4·0–5·0] | 4·0 [4·0–5·0] | 0·041† |
| Stroop test(part2-pat1) | 10·4 [6·7–17·8] | 11·1 [6·0–21·1] | 10·0 [6·8–15·0] | 9·6 [6·7–14·5] | 0·576† |
| Language production | |||||
| Semantic fluency | 16·0 [12·0–20·0] | 13·0 [10·0–19·0]b, c | 17·0 [14·0–21·0] | 18·0 [15·0–20·0] | 0·007† |
| Picture naming(SALA, 64) | 61·0 [58·0–62·0]⁎⁎ | 59·0 [54·0–61·5]b, d | 62·0 [61·0–63·0] | 63·0 [61·3–64·0] | <0·001† |
| Language comprehension | |||||
| Noun picture matching(TLPA, 40) | 40·0 [40·0–40·0]⁎ | 40·0 [39·0–40·0]b, c | 40·0 [40·0–40·0] | 40·0 [40·0–40·0] | 0·007† |
| Noun similarity judgement(SALA, 48) | 47·0 [46·0–48·0]⁎⁎ | 46·0 [44·5–48·0]b, d | 48·0 [47·0–48·0] | 48·0 [47·0–48·0] | <0·001† |
| Word reading | |||||
| Consistent word(20) | 20·0 [20·0–20·0]⁎⁎ | 20·0 [19·0–20·0]b, c | 20·0 [20·0–20·0] | 20·0 [20·0–20·0] | <0·001† |
| High frequency Jukujikun(20) | 17·0 [15·0–19·0]⁎⁎ | 15·0 [12·5–17·0]b, d | 19·0 [18·0–20·0] | 19·0 [18·0–20·0] | <0·001† |
| Low frequency Jukujikun (20) | 15·0 [11·0–19·0]⁎⁎ | 11·0 [6·0–14·5]b, d | 19·0 [17·8–20·0] | 19·0 [17·3–20·0] | <0·001† |
| Mood | |||||
| BDI II | 11·0 [4·0–15·8]⁎⁎ | 8·5 [2·8–16·8]b, d | 11·5 [5·0–15·0] | 3·0 [1·0–6·0] | <0·001† |
Values are shown as the median [25–75 percentile].
Described p values represent significance in the three-group comparison among ALS-JD+, ALS-JD- and HCs except for Years from first symptom and ALSFRS-R, where p values represent the significance between ALS-JD+ vs. ALS-JD-.
ALSFRS-R: Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; MMSE: Mini-Mental State Examination; ACE-R: Addenbrooke's Cognitive Examination-Revised; RCPM: Raven's Coloured Progressive Matrices; FAB: Frontal Assessment Battery; SALA: Sophia Analysis of Language in Aphasia; TLPA: Test of Lexical Processing in Aphasia; BDI II: Beck Depression Inventory II; ALS: amyotrophic lateral sclerosis; HC: healthy control.
Compared with HCs by Mann–Whitney test, p < 0·05.
Compared with HCs by Mann–Whitney test, p < 0·01.
Kruskal–Wallis test followed by Bonferroni adjustment.
Chi-square test.
Mann–Whitney test.
Compared with HCs, p < 0·05.
Compared with HCs, p < 0·01.
Compared with ALS-JD-, p < 0·05.
Compared with ALS-JD-, p < 0·01.
Table 3.
Demographics and clinical characteristics of the participants who were included in the MRI analysis.
| ALS |
HC |
p value | |||
|---|---|---|---|---|---|
| Total |
ALS-JD+ |
ALS-JD- |
|||
| n = 34 | n = 17 | n = 17 | n = 34 | ||
| Demographic | |||||
| Age (years) | 67·0 [62·0–70·0] | 66·0 [61·5–70·5] | 68·0 [64·0–69·5] | 64·5 [61·0–71·0] | 0·922† |
| Sex (m/f) | 24/10 | 13/4 | 11/6 | 24/10 | 0·753‡ |
| Education (years) | 12·0 [12·0–16·0] | 12·0 [9·0–13·0]a | 14·0 [12·0–16·0] | 12·0 [12·0–16·0] | 0·017† |
| Handedness (r/l) | 34/0 | 34/0 | 34/0 | 34/0 | – |
| Years from first symptom | 1·3 [0·9–2·6] | 1·0 [0·8–2·1] | 2·0 [1·0–3·4] | N/A | 0·092§ |
| ALSFRS-R(48) | 42·0 [39·0–45·0] | 43·0 [39·5–45·0] | 42·0 [39·0–45·0] | N/A | 0·267§ |
| General cognition | |||||
| MMSE(30) | 29·0 [27·0–29·0]⁎⁎ | 28·0 [26·5–29·5]b, c | 29·0 [27·5–29·0] | 30·0 [29·0–30·0] | 0·003† |
| ACE-R(100) | 95·0 [90·5–98·0]⁎⁎ | 91·0 [84·5–96·0]b | 96·0 [94·0–98·0] | 99·0 [95·0–99·0] | <0·001† |
| RCPM(36) | 33·0 [29·8–34·3] | 31·0 [27·5–34·0] | 34·0 [32·0–35·0] | 32·5 [30·8–34·0] | 0·159† |
| Frontal and executive | |||||
| FAB(18) | 15·0 [14·0–16·3]⁎ | 14·0 [12·5–16·5]b | 16·0 [15·0–16·5] | 16·0 [15·8–17·0] | 0·007† |
| Phonemic fluency | 11·0 [9·0–13·0] | 10·0 [5·5–14·5] | 12·0 [9·5–13·0] | 12·0 [9·0–15·0] | 0·217† |
| Forward digit span | 6·0 [5·0–7·0] | 6·0 [5·0–6·0]a | 7·0 [6·0–7·5] | 6·0 [5·0–6·3] | 0·023† |
| Backward digit span | 4·5 [3·8–5·0] | 4·0 [3·0–5·0] | 5·0 [4·0–5·0] | 4·0 [4·0–5·0] | 0·204† |
| Stroop test(part2-pat1) | 11·0 [7·3–17·2] | 11·1 [6·9–23·1] | 10·9 [7·2–16·1] | 9·1 [7·0–12·0] | 0·525† |
| Language production | |||||
| Semantic fluency | 17·0 [10·0–21·3] | 14·0 [10·0–21·0] | 17·0 [15·0–22·0] | 18·0 [14·0–21·0] | 0·367† |
| Picture naming(SALA, 64) | 61·5 [59·8–62·3]⁎⁎ | 60·0 [57·0–62·0]b, c | 62·0 [61·0–63·0] | 63·0 [62·0–64·0] | <0·001† |
| Language comprehension | |||||
| Noun picture matching(TLPA, 40) | 40·0 [40·0–40·0] | 40·0 [40·0–40·0] | 40·0 [40·0–40·0] | 40·0 [40·0–40·0] | 0·727† |
| Noun similarity judgement(SALA, 48) | 47·0 [45·8–48·0]⁎ | 46·0 [44·5–48·0]a | 47·0 [47·0–48·0] | 48·0 [47·0–48·0] | 0·022† |
| Word reading | |||||
| Consistent word(20) | 20·0 [19·8–20·0]⁎ | 20·0 [19·0–20·0]b, c | 20·0 [20·0–20·0] | 20·0 [20·0–20·0] | 0·002† |
| High frequency Jukujikun(20) | 17·0 [15·0–19·3]⁎⁎ | 15·0 [13·0–17·0]b, d | 19·0 [17·5–20·0] | 19·5 [18·0–20·0] | <0·001† |
| Low frequency Jukujikun (20) | 15·5 [11·0–19·0]⁎⁎ | 11·0 [6·0–14·5]b, d | 19·0 [17·0–20·0] | 19·0 [18·0–20·0] | <0·001† |
| Mood | |||||
| BDI II | 10·0 [3·5–15·5]⁎ | 5·0 [1·0–15·0]a | 11·0 [5·0–17·3] | 4·0 [1·5–7·5] | 0·034† |
Values are shown as the median [25–75 percentile].
Described p values represent significance in the three-group comparison among ALS-JD+, ALS-JD- and HCs.
ALSFRS-R: Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; MMSE: Mini-Mental State Examination; ACE-R: Addenbrooke's Cognitive Examination-Revised; RCPM: Raven's Coloured Progressive Matrices; FAB: Frontal Assessment Battery; SALA: Sophia Analysis of Language in Aphasia; TLPA: Test of Lexical Processing in Aphasia; BDI II: Beck Depression Inventory II; ALS: amyotrophic lateral sclerosis; HC: healthy control.
Compared with HCs by Mann–Whitney test, p < 0·05.
Compared with HCs by Mann–Whitney test p < 0·01.
Kruskal–Wallis test followed by Bonferroni adjustment.
Chi-square test.
Mann–Whitney test.
Compared with HCs, p < 0·05.
Compared with HCs, p < 0·01.
Compared with ALS-JD-, p < 0·05.
Compared with ALS-JD-, p < 0·01.
Fig. 1.
Distribution map of Jukujikun score.
There were some patients who showed significant score reduction in reading Jukujikun, and this trend was more remarkable in low frequency Jukujikun: based on the fifth percentile value of the HCs' Jukujikun score, we compared the number of participants with Jukujikun reading deficit between ALS and HCs. Significantly more participants showed lower scores in the ALS group than HCs (p < 0·005, Chi-square test). We defined patients who had scores of low frequency Jukujikun 15 or less, which corresponds to the fifth percentile of the 68 HCs, as the “ALS with positive Jukujikun deficit group (ALS-JD+)” and the other patients as the “ALS with negative Jukujikun deficit group (ALS-JD-).” The data of all the participants are shown in a, the data of the participants enrolled in MRI study are shown in b; both data sets showed similar tendency.
3.2. Voxel-based morphometry does not correlate with the involvement of Jukujikun
Compared with HCs, ALS exhibited a significant reduction in GM volume (GMV) near the right parahippocampal gyrus, and the ALS-JD+ showed GMV reduction in the anterior part of the left temporal lobe (Fig. 2, Supplemental Table 4). There were no significant differences in GMV between the ALS-JD- and HC groups or between the ALS-JD+ and ALS-JD- groups. Furthermore, low frequency Jukujikun scores did not show any significant correlation with GMV in the ALS group.
Fig. 2.
Voxel-based morphometry findings.
The comparison of ALS vs. HCs showed GM atrophy in the right hippocampus in ALS. ALS-JD+ vs. HCs showed GM atrophy in the anterior medial part of the left temporal lobe. ALS-JD- vs. HCs and ALS-JD+ vs. ALS-JD- showed no significant region. The significance level was set at p < 0·05 after correcting for multiple comparisons using a cluster-level family-wise error correction (FWEc) with a cluster-forming threshold of p = 0·001. The detailed coordinates are shown in Supplemental Table 4.
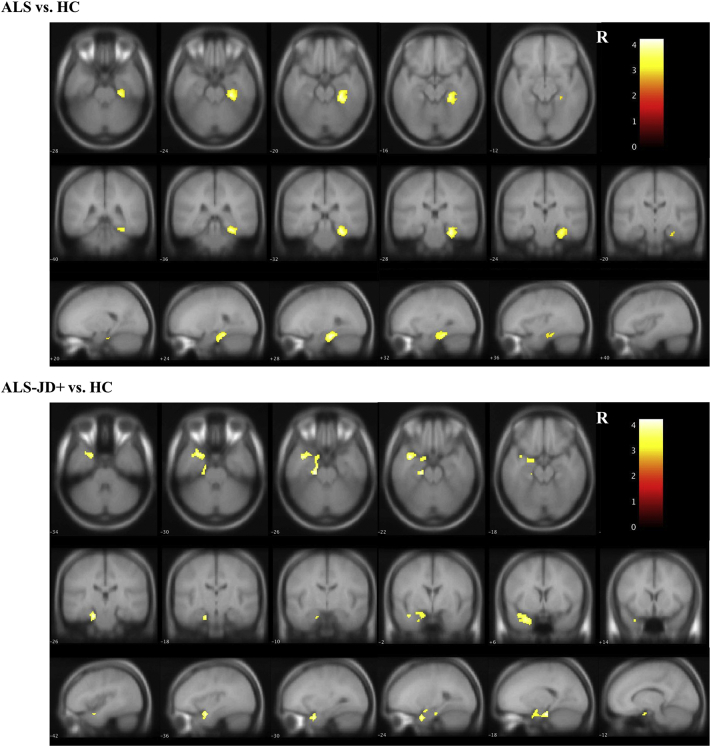
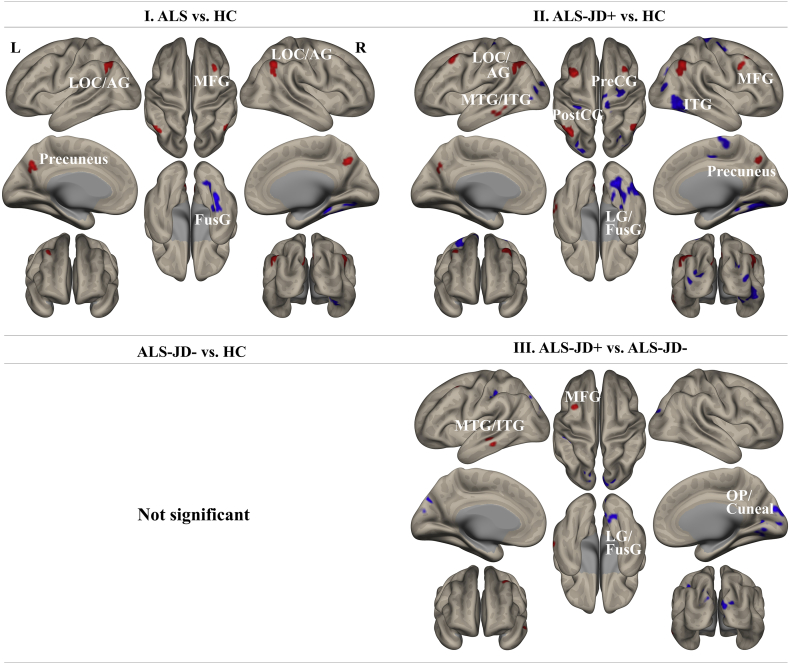
3.3. Degree centrality alterations in ALS and ALS-JD+
Fig. 3 and Supplemental Table 5 show the regions with degree centrality alterations in ICC analysis. ALS vs. HCs revealed significantly reduced ICC-p value (degree centrality) in seven regions, and ALS-JD+ vs. HCs and ALS-JD+ vs. ALS-JD- revealed twelve and six regions with decreased degree centrality, respectively.
Fig. 3.
Analysis of intrinsic connectivity contrast.
The comparison of ALS vs. HCs showed decreased ICC-p values in the posterior right fusiform and the lingual gyrus. ALS-JD+ vs. HC and ALS-JD+ vs. ALS-JD- revealed similar regions where the ICC-p value was decreased.
The left inferior/middle temporal gyrus and the left middle frontal gyrus showed increased ICC-p values in comparisons of both ALS-JD+ vs. HCs and ALS-JD+ vs. ALS-JD-. Comparison of ALS-JD- vs. HCs showed no significant area. Statistical significance was set at a threshold of p < 0·001 and an FDR-corrected cluster-size threshold of p < 0·05. The major locations are described below, and the detailed coordinates are shown in Supplemental Table 5.
AG: angular gyrus; FusG: fusiform gyrus; ICC: intracalcarine cortex; ITG: inferior temporal gyrus; LG: lingual gyrus; LOC: lateral occipital cortex; MFG: middle frontal gyrus; MTG: middle temporal gyrus; OP: occipital pole; PostCG: postcentral gyrus; PreCG: precentral gyrus.
Decreased degree centrality was most remarkable in the right fusiform/lingual gyrus, followed by the posterior part of the bilateral lateral occipital cortex and postcentral gyrus in ALS-JD+ compared with that in HCs. The reduced degree centrality in the right fusiform/lingual gyrus was also observed in ALS compared with that in HCs. Additionally, compared with ALS-JD-, ALS-JD+ showed decreased degree centrality in the right fusiform/lingual gyrus, followed by the right occipital pole/cuneus and left posterior central gyrus (Fig. 3, Supplemental Table 4).
The degree centrality in the left inferior/middle temporal gyrus and left middle frontal gyrus was significantly increased in ALS-JD+ compared with that in HCs and ALS-JD-. There was no significant difference in degree centrality between ALS-JD- and HC.
3.4. Correlation analysis of degree centrality and Jukujikun score
We investigated the correlation between ICC-p values and low frequency Jukujikun scores in 12 regions, which showed significant degree centrality alterations in ALS-JD+ compared with those in HCs. Six of the 12 regions, including the right fusiform/lingual gyrus, right precentral gyrus, bilateral lateral occipital gyrus, right precentral/postcentral gyrus, and left postcentral gyrus, showed significant correlations. Similarly, the comparison of ALS-JD+ vs. ALS-JD- showed a significant correlation between ICC-p values and low frequency Jukujikun scores in the following regions: the right fusiform/lingual gyrus, bilateral cuneus, left middle and inferior temporal gyri, left middle frontal gyrus, and left postcentral gyrus. However, there was no significant correlation between ICC-p values and ACE-R scores, which represent general cognition, in the clusters where the correlation between ICC-p values and low frequency Jukujikun scores was significant (Table 4).
Table 4.
Partial correlation analysis between ICC-p values and scores of low frequency Jukujikun or ACE-R.
| Cluster | Peak location |
Major location | Jukujikun |
ACE-R |
|||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Side | rho | p-value | rho | p-value | ||
| II-1 | 40 | −56 | −2 | R | FusG, LOC, ITG, LG, PaHC | 0·403 | 0·024⁎ | 0·328 | 0·071 |
| II-2 | −40 | −64 | 50 | L | LOC, AG | −0·204 | 0·270 | −0·322 | 0·077 |
| II-3 | 50 | −60 | 40 | R | LOC, AG | −0·264 | 0·151 | −0·292 | 0·112 |
| II-4 | 30 | −12 | 64 | R | PreCG, SFG, SMA | 0·502 | 0·004⁎ | 0·157 | 0·399 |
| II-5 | −32 | −78 | 16 | L | LOC, OP | 0·475 | 0·007⁎ | 0·373 | 0·039 |
| II-6 | −32 | 14 | 50 | L | MidFG | −0·370 | 0·040 | −0·274 | 0·135 |
| II-7 | 28 | −72 | 8 | R | LOC, LOC | 0·496 | 0·005⁎ | 0·228 | 0·218 |
| II-8 | 8 | −26 | 70 | R | PreCG, PostCG | 0·462 | 0·009⁎ | 0·355 | 0·050 |
| II-9 | 8 | −64 | 40 | – | Precuneus | −0·037 | 0·843 | −0·290 | 0·113 |
| II-10 | 38 | 10 | 36 | R | MidFG | −0·230 | 0·214 | −0·145 | 0·435 |
| II-11 | −52 | −40 | −14 | L | ITG, MTG | −0·281 | 0·126 | −0·199 | 0·284 |
| II-12 | −30 | −28 | 68 | L | PostCG | 0·457 | 0·010⁎ | 0·352 | 0·052 |
| III-1 | 26 | −74 | 8 | R | LG, ICC, FusG | 0·611 | <0·001⁎ | 0·295 | 0·107 |
| III-2 | 4 | −84 | 22 | R | OP, Cuneal, ICC | 0·617 | <0·001⁎ | 0·260 | 0·158 |
| III-3 | −18 | −82 | 16 | L | Cuneal | 0·567 | 0·001⁎ | 0·290 | 0·113 |
| III-4 | −58 | −28 | −14 | L | MTG | −0·458 | 0·010⁎ | −0·410 | 0·022 |
| III-5 | −34 | 12 | 56 | L | MidFG | −0·427 | 0·016⁎ | −0·327 | 0·073 |
| III-6 | −42 | −30 | 30 | L | PostCG | 0·655 | <0·001⁎ | 0·171 | 0·357 |
Partial correlation coefficients (=rho) and p-values were calculated using Spearman's partial correlation, accounting for age, sex and education as covariates.
The statistical significance threshold was set at p < 0·05 using FDR-adjusted p-value (shown with *).
AG: angular gyrus; FDR: false discovery rate; FusG: fusiform gyrus; ICC: intracalcarine cortex; ITG: inferior temporal gyrus; LG: lingual gyrus; LOC: lateral occipital cortex; Mid FG: middle frontal gyrus; MTG: middle temporal gyrus; OP: occipital pole; PaHC: parahippocampal gyrus; PostCG: postcentral gyrus; PreCG: precentral gyrus; SFG: superior frontal gyrus; SMA: supplementary motor cortex.
3.5. Seed-based analysis
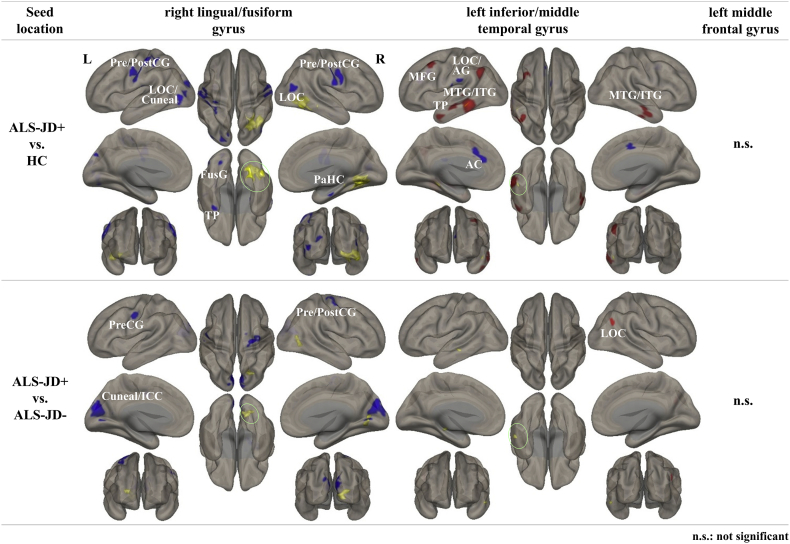
Decreased degree centrality in the right lingual/fusiform gyrus and increased degree centrality in the left inferior/middle temporal gyrus and left middle frontal gyrus were commonly observed in both the ALS-JD+ vs. HC and ALS-JD+ vs. ALS-JD- comparisons. Thus, we performed seed-based analyses using these three regions as seed ROIs.
Seed-based analysis from the ROI in the right lingual/fusiform gyrus revealed decreased functional connectivity with the bilateral peri-central sulcus, bilateral lateral occipital cortex, left temporal pole, and right hippocampus in ALS-JD+ compared with that in HCs (Fig. 4, Supplemental Table 6). There was significantly increased functional connectivity between the ROIs in the left inferior/middle temporal gyrus and bilateral inferior and middle temporal gyri, left angular gyrus, left temporal pole, left peri-sylvian sulcus, or right cerebellum in ALS-JD+ compared with that in HCs.
Fig. 4.
Seed-based analysis.
The three areas where degree centrality (ICC-p) alternation was commonly detected in comparisons of both ALS-JD+ vs. HCs and ALS-JD+ vs. ALS-JD- were used as ROIs. The ROIs are shown with the regions coloured yellow in the green circle. Statistical significance was set at p < 0·001 and an FDR-corrected cluster-size threshold of p < 0·05. The major locations are described below, and the detailed coordinates are shown in Supplemental Table 6.
AC: anterior cingulate cortex; AG: angular gyrus; SMG: supramarginal gyrus; FusG: fusiform gyrus; ICC: intracalcarine cortex; ITG: inferior temporal gyrus; LG: lingual gyrus; LOC: lateral occipital cortex; MFG: middle frontal gyrus; MTG: middle temporal gyrus; OP: occipital pole; PaCiG: paracingulate gyrus; PaHC: parahippocampal gyrus; PO: parietal operculum cortex; PreCG: precentral gyrus; PostCG: postcentral gyrus; SFG: superior frontal gyrus; TP: temporal pole. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Compared with ALS-JD-, ALS-JD+ showed decreased connectivity values between the ROIs in the right lingual/fusiform gyrus and bilateral visual-related area or bilateral peri-central sulcus. ALS-JD+ also showed increased connectivity between the ROIs in the left inferior/middle temporal gyrus and right lateral occipital cortex.
No significant connectivity change from the seed ROI in the left middle frontal gyrus was observed.
4. Discussion
In this study, compared with HCs, approximately half of ALS patients had a low frequency Jukujikun score of the fifth percentile or less, suggesting that Jukujikun could be an early marker for language impairment in Japanese ALS patients. Decreased degree centrality in the right fusiform/lingual gyrus associated with decreased functional connectivity values between the right fusiform/lingual gyrus and the regions involved in semantic processing appears to be related to Jukujikun impairment in ALS. Thus, brain network analysis using voxel-based graph analysis could provide novel insights into the involvement of information processing systems in ALS related to Jukujikun impairment.
4.1. Jukujikun reading in ALS
Few reports have mentioned impairments in low frequency Jukujikun reading in ALS patients [24]. Nonetheless, Jukujikun reading impairment is a characteristic symptom of SD, similar to impairments in reading exception words by SD patients in English-speaking countries [9]. This phenomenon known as surface dyslexia is not specific to patients with SD, but it has been reported to reflect a more general loss of semantic knowledge especially for less frequent items [25,26]. Our results showed a correlation between low frequency Jukujikun and other semantic examinations, and it was suggested that low frequency Jukujikun reflected semantic deficits. In addition, although it is supposed that surface dyslexia in Japanese arises from a reading process similar to that in English [26], there is a difference in writing system between Kanji written words and alphabetic words. Typically, words written in Kanji elicit images of the constituent characters because Kanji themselves have meanings. Because Jukujikun are exception words written in Kanji, semantic processing is more closely related to Jukujikun reading, especially reading of low frequency words.
In this study, ALS-JD+ had significantly fewer years of education than that of ALS-JD- and HCs. Nevertheless, regardless of educational background, nearly half of patients had scores of the fifth percentile of HC or less. This suggests that reduction of Jukujikun reading scores could not be fully explained by educational background alone. However, more detailed consideration using education matched participants or prospective assessment may be needed for more accurate evaluation.
Based on our results, Jukujikun reading impairments and semantic impairments are commonly involved in ALS and support the idea that FTLD and ALS are continuous diseases. Besides, ALS-JD+ group were more impaired than other ALS patients in various cognitive tasks including executive functions. This is also consistent with the previous report that pure syndromes of SD and PNFA are less common than mixed language and executive syndromes in ALS [27]. In this study, all the patients were non-familial ALS, but genetic investigations for the diagnosis of sporadic ALS were not thoroughly performed. Although Japanese ALS patients rarely show C9orf72 expansion [28], which has been reported to be more common to cause cognitive impairments, it cannot be affirmed that there are no genetic mutations in this study. For a better characterisation of semantic deficits in sporadic ALS, genetic investigations will be crucial.
4.2. Decreased degree centrality in the right lingual/fusiform gyrus and their network disconnection is associated with Jukujikun impairment in ALS
Decreased degree centrality in the posterior region of the right fusiform gyrus and lingual gyrus indicates deterioration of its function as a hub in ALS patients. In addition, this region showed significantly decreased degree centrality in ALS-JD+, correlating with the score of low frequency Jukujikun.
The right fusiform gyrus (as well as the left fusiform gyrus) is reportedly involved in language processing and Kanji recognition [6] and is related to better performance [29,30]. The right side is particularly employed to a greater extent when language tasks become more difficult in older people [31]. Moreover, the right lingual gyrus is associated with global shape processing of words or objects [32]. These findings are consistent with the fact that Jukujikun are Kanji-written words, not easy to read, and require global attention to the entire word because the exceptional readings are unique to their entire character strings. Although the right hemisphere involvement and global attention requirement to the whole word are not limited to Kanji words, further analysis using other languages and different writing systems are desired for more generalisation.
Furthermore, the hub deterioration in the right fusiform/lingual gyrus was derived from the disconnection with areas related to a semantic process in ALS-JD+, including the lateral occipital cortex for visual perception, processing, and memory of shape [33]; the left anterior temporal lobe, right hippocampus and parahippocampus for storage and construction of semantic memory [34]; and the peri-central gyrus for speech production. Network problems among these areas cause difficulties in recognising words, accessing semantic memory, recalling specific readings, and reading aloud correctly.
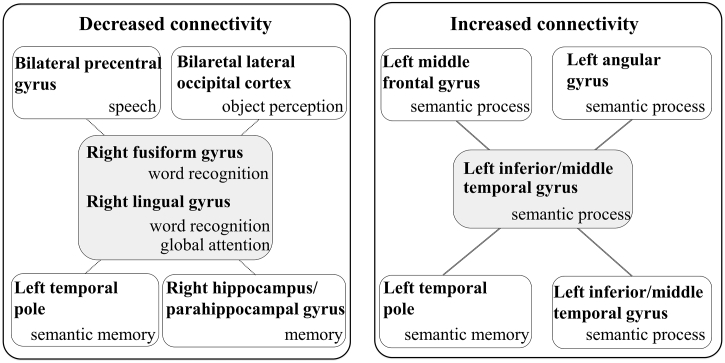
Based on these findings, we consider that the hub dysfunction in the right fusiform/lingual gyrus and the entangled reduced functional connectivity with several regions could cause impairments in low frequency Jukujikun in ALS patients (Fig. 5).
Fig. 5.
Schema of network alternations related to low frequency Jukujikun reading in ALS.
It has been suggested that impairment of Jukujikun reading in ALS is related to decreased connectivity centred at the right lingual/fusiform gyrus and increased connectivity centred at the left inferior/middle temporal gyrus. The network alternation indicated hub dysfunction at the right lingual/fusiform gyrus, its reduced connectivity with some regions associated with the Jukujikun reading process, and increased connectivity at the left inferior/middle temporal gyrus related to semantic processing.
The network impairments we found in this study could include multifocal factors other than semantic processing. Therefore, in order to specifically extract semantic processing or to discuss general inferences about semantic processing, further analyses would be needed such as rsfMRI combined with other sematic test batteries or task-based fMRI.
4.3. Increased degree centrality in the left inferior/middle temporal gyrus corresponding to impairment in low frequency Jukujikun
The left inferior/middle temporal gyrus showed increased degree centrality in ALS-JD+ compared with that in HCs, and this alteration was considered to be derived from a strengthened network with areas associated with semantic processing, including the bilateral inferior and middle temporal gyri [35], left angular gyrus [36], left middle frontal gyrus [6], and left anterior temporal lobe (Fig. 5).
The left inferior/middle temporal gyrus is reportedly involved in lexico-semantic processing [37]. For the anterior temporal lobe, the region that showed increased connectivity with the left inferior/middle temporal gyrus was located more laterally than the region that showed decreased connectivity with the right fusiform/lingual gyrus. Based on previous findings from patients with SD, more lateral and posterior regions of the anterior temporal lobe are associated with semantic memory [5]. In addition, the left intraparietal sulcus, which plays a role in orthographical-to-phonological reading processes without semantic process involvement, is activated during reading low frequency exception words in SD patients but not in HCs [5]. The left angular gyrus detected in our results overlaps with this area. Thus, the left inferior/middle temporal gyri may strengthen their connectivities with areas related to semantic processes and those related to other reading processes without semantic process involvement. Collectively, increased degree centrality in the left inferior/middle temporal gyrus and their connected regions mentioned above may play important roles in the compensatory process against semantic impairment in ALS.
5. Conclusion
Approximately half of ALS patients showed low frequency Jukujikun reading impairment. “Hub” dysfunction in the right lingual/fusiform gyrus could be crucial for its network basis. The ICC approach can elucidate the unsolved networks associated with specific cognitive function in neurodegenerative diseases. Our results suggest that clinical symptoms are not solely derived from one lesion but from network impairment that could include many relevant areas connected to hubs. This perspective is important for understanding the symptoms of various diseases.
6. Limitation
We evaluated semantic deficits exclusively with language in particular Jukujikun reading ability. In order to draw general inferences about semantic processing, additional semantic evaluation would be needed. Besides, although we performed cognitive examinations in this study, it is also necessary to investigate social cognition, emotions and behavioural features for the better understanding of cognitive characteristics in ALS patients. Further, we enrolled non-familial ALS patients, but not all the patients were genetically confirmed as sporadic ALS. Genetic investigation will be necessary for a better characterisation of sporadic ALS. We consecutively registered patients with ALS in Nagoya University Hospital. As for the controls, we enrolled participants from a cohort study, in which healthy volunteers whose age was over 20 from Nagoya City and neighbouring areas through public invitations. We believe that there is no restriction or selection bias to participate in this cohort. However, we did not investigate whether this cohort completely corresponds to the general population or not.
Acknowledgements/Funding
This work was supported by Grants-in-Aid from the Research Committee of Central Nervous System Degenerative Diseases by the Ministry of Health, Labour, and Welfare and by the Integrated Research on Neuropsychiatric Disorders, a project carried out under the Strategic Research for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This work was also supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (grant number 80569781) and a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Ageing and Dementia Control) (26117002) from MEXT. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Declaration of competing interests
The authors declare no competing interests.
Author contributions
AO, RO, MM, KK, and YK designed the study, coordinated its execution and data analysis, and contributed to data collection. AO, HW, MK, and GS wrote the manuscript, contributed to data interpretation and revised the manuscript. YR, RN, and NA contributed to data collection and revised the manuscript. RO, YT, MM, TK, KI, TY, and KH contributed to data collection. Statistical analysis was conducted by AO, KK, MN, and EB. All authors read and approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.022.
Appendix A. Supplementary data
Supplementary material
References
- 1.Goldstein L.H., Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M., Sampathu D.M., Kwong L.K. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Leslie F.V.C., Hsieh S., Caga J. Semantic deficits in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener. 2015;16:46–53. doi: 10.3109/21678421.2014.987301. [DOI] [PubMed] [Google Scholar]
- 4.Burrell J.R., Halliday G.M., Kril J.J. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388:919–931. doi: 10.1016/S0140-6736(16)00737-6. [DOI] [PubMed] [Google Scholar]
- 5.Wilson S.M., Brambati S.M., Henry R.G. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132:71–86. doi: 10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ino T., Nakai R., Azuma T., Kimura T., Fukuyama H. Recognition and reading aloud of kana and kanji word: an fMRI study. Brain Res Bull. 2009;78:232–239. doi: 10.1016/j.brainresbull.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Ijuin M., Patterson K. Consistency, frequency, and lexicality effects in naming Japanese Kanji. Artic J Exp Psychol Hum Percept Perform. 1999;25:382–407. [Google Scholar]
- 8.Wydell T.N., Leong C.K., Tamaoka K. What matters in Kanji word naming: consistency, regularity, or On/Kun-reading difference? An Interdiscip J. 1998;10:359–373. [Google Scholar]
- 9.Sasanuma S., Patterson K. Non-semantic reading in Kanji and English: Universal and language-specific features. In: de Gelder B., Morais J., editors. Speech and Reading: A Comparative Approach. Taylor & Francis; Oxford: 1995. pp. 207–225. [Google Scholar]
- 10.Landin-Romero R., Tan R., Hodges J.R., Kumfor F. An update on semantic dementia: genetics, imaging, and pathology. Alzheimer's Res Ther. 2016;8:1–9. doi: 10.1186/s13195-016-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loewe K., Machts J., Kaufmann J. Widespread temporo-occipital lobe dysfunction in amyotrophic lateral sclerosis. Sci Rep. 2017;7 doi: 10.1038/srep40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C., Hu X., Hu J. Altered brain network in amyotrophic lateral sclerosis: a resting graph theory-based network study at voxel-wise level. Front Neurosci. 2016;10:204. doi: 10.3389/fnins.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martuzzi R., Ramani R., Qiu M., Shen X., Papademetris X., Constable R.T. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage. 2011;58:1044–1050. doi: 10.1016/j.neuroimage.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 15.Bagarinao E., Watanabe H., Maesawa S. An unbiased data-driven age-related structural brain parcellation for the identification of intrinsic brain volume changes over the adult lifespan. Neuroimage. 2018;169:134–144. doi: 10.1016/j.neuroimage.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Amano S., Kondo K. Vol. 7. Sanseido; Tokyo: 2000. Nihongo-no Goi-tokusei [Lexical properties of Japanese] [Google Scholar]
- 17.Amano S., Kondo K. Vol. 1. Sanseido; Tokyo: 1999. Nihongo-no Goi-tokusei [Lexical properties of Japanese] [Google Scholar]
- 18.Amano S., Kondo K. Vol. 8. Sanseido; Tokyo: 2005. Nihongo-no Goi-tokusei [Lexical properties of Japanese] [Google Scholar]
- 19.Coltheart M. The MRC psycholinguistic database. Q. J. Exp. Psychol. Sec. 1981;33:497–505. [Google Scholar]
- 20.Burnage G. Centre for Lexical Information; Nijmegen: 1990. CELEX: A Guide for Users. [Google Scholar]
- 21.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 23.Andersson J.L.R., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 24.Iroi A., Okuma Y., Fukae J., Fujisima K., Goto K., Mizuno Y. Amyotrophic lateral sclerosis presented with Alexia of Kanji and word meaning aphasia [in Japanese] Sink Kenkyu No Shinpo (Brain Nerve) 2002;54:903–907. [PubMed] [Google Scholar]
- 25.Woollams A.M., Ralph M.A.L., Plaut D.C., Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114:316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- 26.Fushimi T., Komori K., Ikeda M., Lambon Ralph M.A., Patterson K. The association between semantic dementia and surface dyslexia in Japanese. Neuropsychologia. 2009;47:1061–1068. doi: 10.1016/j.neuropsychologia.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Saxon J.A., Harris J.M., Thompson J.C. Semantic dementia, progressive non-fluent aphasia and their association with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:711–712. doi: 10.1136/jnnp-2016-314912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura R., Sone J., Atsuta N. Next-generation sequencing of 28 ALS-related genes in a Japanese ALS cohort. Neurobiol Aging. 2016;39:219.e1–219.e8. doi: 10.1016/j.neurobiolaging.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 29.van Ettinger-Veenstra H.M., Hällgren M., Karlsson T. Right-hemispheric brain activation correlates to language performance. Neuroimage. 2009;49:3481–3488. doi: 10.1016/j.neuroimage.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly K.M., Allendorfer J.B., Szaflarski J.P. Right hemispheric participation in semantic decision improves performance. Brain Res. 2011;1419:105–116. doi: 10.1016/j.brainres.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman P., Morcom A.M. Age-related changes in the neural networks supporting semantic cognition: a meta-analysis of 47 functional neuroimaging studies. Neurosci Biobehav Rev. 2018;84:134–150. doi: 10.1016/j.neubiorev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Fink G.R., Halligan P.W., Marshall J.C., Frith C.D., Frackowiak R.S.J., Dolan R.J. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- 33.Karanian J.M., Slotnick S.D. Memory for shape reactivates the lateral occipital complex. Brain Res. 2015;1603:124–132. doi: 10.1016/j.brainres.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Hoenig K., Scheef L. Mediotemporal contributions to semantic processing: fMRI evidence from ambiguity processing during semantic context verification. Hippocampus. 2005;15:597–609. doi: 10.1002/hipo.20080. [DOI] [PubMed] [Google Scholar]
- 35.Graves W.W., Desai R., Humphries C., Seidenberg M.S., Binder J.R. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex. 2010;20:1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder J.R., McKiernan K.A., Parsons M.E. Neural correlates of lexical access during visual word recognition. J Cogn Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- 37.Ramsay S., Cardebat D., Nespoulous J., Wise R., Rascol A., Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material