Highlights
-
•
Combination of thidiazuron and naphthalene acetic acid induced callus growth in Withania somnifera.
-
•
Red light improved callus growth with lower antioxidant enzymes activities.
-
•
Violet light enhanced the total phenolic and flavonoid content in callus culture of W. somnifera.
-
•
Withaferin A and chlorogenic acid were detected in callus cultures.
Keywords: Withania, Light, Phenolic, Withanolides, Callus, Anti-oxidant
Abstract
Withania somnifera L. is an endangered medicinal plant of higher market value. The in vitro callus cultures were established on Murashige and Skoog (MS) media augmented with different plant growth regulators. The MS medium containing 0.5 mg∙L−1 of each TDZ and NAA was found to be optimal for callus formation and growth. Further, callus cultures were raised in different light wavelengths to find the right wavelength carrying the photons for the ideal cell growth of W. somnifera. Among the different wavelengths, red light was best for maximum biomass accumulation in callus culture. However, violet light condition was proven to be favouring the phenols and flavonoids synthesis in the callus cultures. Compared to other wavelengths, red light grown callus extract showed significantly higher content of chlorogenic acid, and withaferin A. This study concludes that red light treatment was optimum for maximum biomass accumulation and anti-oxidant activity in calli of W. somnifera.
1. Introduction
Withania (W.) somnifera is an important medicinal plant commonly known as winter cherry, Indian ginseng or poison gooseberry, belongs to Solanaceae or nightshade family. Traditionally, it is used as medicine for more than 3000 years against various ailments, and it is an essential constituent of over 200 traditional medicinal formulations [1]. Some of the important therapeutic properties of W. somnifera are immunoregulatory, anti-cancer, anti-arthritic and recovery from neurodegenerative disorders [[2], [3], [4]]. The higher market demand for W. somnifera has caused a tremendous burden on its natural reservoirs during the last decades. Due to habitat destruction, over exploitation and illegal collection of this plant, it is at the verge of eminent danger of extinction in Pakistan. Considering its paramount medicinal significance novel means and ways should be implemented on the conservation and sustainable utilization of this important plant [5]. In comparison to conventional cultivation procedures, plant callus cultures have emerged as a promising platform for the biosynthesis of valuable metabolites in limited time and space [5,6].
The successful establishment of callus culture is crucial for commercial scale production and it needs the proper balance of plant growth regulators (PGRs) and controlled environmental condition [7]. Generally, auxin and cytokinin either alone or in combination results callus formation from the explants. However, there are different auxins and cytokinin compounds to whom plant cells respond differentially and makes it hard to find the right PGR for the callus initiation [8]. So we designed this study to look for the exact PGRs combinations for the callus formation from leaf explant of W. somnifera L.
Other than PGRs, light also play vital role in plant growth and development as it is the source of photons to drive the photosynthesis reaction. Different plant species responds to light differently due to genetic and biochemical variations among plant species. Also it is the fact that sunlight caries different energy packets (photons) in the form of light wavelengths and plants are specialised to utilise certain range of photons and dissipate the rest as heat. In the open field plants are specialised to cope with the heat produced by the dissipated light. However, in vitro tissue culture conditions a high amount of energy is consumed to maintain the growth room conditions for optimal plant cell, tissues and organ growths. For this, LED technology have been proven to be promising which enabled the tissue culturist to reduce the cost. Plant responds differentially to the respective wavelengths or colours of light through photoreceptors called phytochromes, cryptochromes and phytotropin [9]. As a number of in-vitro studies have mentioned light having significant effects on callus growth and morphogenesis, inhibition of axillary shoot proliferation and induction of specific enzyme activity which are linked with the production of important secondary products [10,11]. By now three kind of photoreceptors known as phytochrome (red and far red detector), cryptochrome (blue and UV-B detector) and phototropin (blue and UV-A detector) are thought to be involved in plant development [12]. There are some reports on the involvement of light on phytohormone synthesis, for example it has been shown that CK biosynthesis is stimulated to occur in red light, but is prevented when far red is used [13]. It is presumed that monochromatic light regimes may help to optimize and control plant cell growth by evoking the photo-oxidative changes that may lead to the increased content of phytochemicals [14].
However, information regarding the role of monochromatic light conditions on plant cell growth and secondary metabolites content of W. somnifera is lacking. This is the first report to study the effect of different light wavelengths on W. somnifera callus growth and medicinal compounds production to achieve sustainability. It is well understood that secondary metabolites producing pathways are significantly affected by environmental factors, and light quality/quantity have proven to be an effective elicitor for enhanced secondary metabolite content [14]. Therefore, we performed this study to determine the impacts of different wavelengths of light on callus growth and secondary metabolites accumulation of W. somnifera. Moreover, the biochemical markers like total protein content, protease activity, per-oxidase activity, superoxide dismutase activity and malondialdehyde content along with HPLC based quantification of withanolides were studied to determine the plant secondary metabolism during callus formation in response to different wavelengths of light.
2. Materials and methods
2.1. Leaf explant preparation and callus culture initiation
Explants (leaf sections∼2.5 cm) were excised from intact leaves of W. somnifera L. plant, grown in pots in greenhouse. During sterilization, the explants were thoroughly rinsed with ethanol (70%) for 5 min followed by rinsing for 3 min in 0.1% (w/v) mercuric chloride solution (HgCl2). Finally, the explants were washed three times with sterile distilled water and were dried on sterile blotting paper. The sterilized explants were cultured for callus initiation on Murashige and Skoog (MS) [15] medium containing 3% sucrose and 0.8% agar in 100 ml conical flasks supplemented with combined or individual concentration of thidiazuron : TDZ (0.1, 0.5 and 1 mg∙L−1), benzyladenine : BAP (0.5 and 0.1 mg∙L−1) and α-naphthalene acetic acid : NAA (0.1, 0.5, 1.0 and 1.5 mg∙L−1). The media pH was adjusted to 5.8 ± 0.2 prior to autoclave at 121 °C for 30 min. Three replicates of each treatment were maintained at 16h photoperiod under cool-white light (∼50 μmol/m2/sec) and 25 ± 2 °C. Data on the growth patterns during callus organogenesis was recorded after 5 weeks of culture as percent callus formation (PCF; %) and callus biomass formation (fresh weight and dry weight). A 100% callus formation means all the cultured leaf explant were responsive to a certain PGRs treatment.
2.2. Effects of monochromatic lights on callus growth
For different monochromatic light treatments, the protocol of Tariq et al. [16] was used. Briefly, for violet (350–400 nm), blue (380–560 nm), green (480–670 nm), yellow (530–780 nm) and red (610–715 nm) illuminations tubes of Philips (32 W), Keliang (220 V; 50 Hz), Litex (40 W), Philips (36 W) and Binxiang (25 W) private limited were used, respectively. The MS media having 0.5 mg∙L−1 of both TDZ and NAA, placed under the cool white fluorescent light (16:8 h light and dark) was used as control and all experiments were performed in triplicates. After five weeks of treatments PCF, callus fresh weight (g explant−1) and dry weight (g explant−1) were recorded as mean ± standard error.
2.3. Estimation of total phenolics and flavonoids content
Each callus culture was washed with sterile distilled water. Excess water was removed by gently pressing the samples on filter paper and finally oven dried at 60 °C for 24 h. Dry biomass (DBM) was taken by weighing these dried calluses and were subjected for metabolite extraction as per the method of Khan et al. [17]. Briefly, 100 mg of each finely ground dried powder of the samples was soaked in 10 ml methanol (80%; v/v) and sonicated 3 times for ten min with an interval of 30 min. It was followed by a centrifugation of 10 min at 8 × 103 rpm. The supernatants were decanted into sterile storage tubes and were immediately used or stored at 4 °C for further analysis.
For total phenolics content (TPC) estimation the reported method of Adil et al. [11] was used and expressed as mg Gallic acid equivalents (GAE) g−1 DW. Total flavonoid content (TFC) was calorimetrically determined according to the method of Khan et al. [17] and was expressed as mg quercetin equivalents (QAE) g−1 DW.
2.4. Anti-oxidant potential
The 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity (DFRSA), total antioxidant capacity (TAC) and total reducing power (TRP) were used for estimation of the anti-oxidant potential of W. somnifera callus. For DPPH free radical scavenging activity, the described protocol of Abbasi et al. [18] was used. The method of Jafri et al. [19] was used for estimation of TAC and TRP. Briefly, 0.1 ml of callus extract was mixed with 1 ml of reaction mixture containing sulfuric acid (0.6 M), sodium phosphate (28 mM) and ammonium molybdate (4 mM) and incubated for 90 min in a water bath at 95 °C. After cooling (to room temperature) the antioxidant potential of the sample was measured at 697 nm using UV/VIS-DAD spectrophotometer (Agilent 8453) and was expressed as mg ascorbic acid equivalent. While, 0.1 ml of methanol plus reagent solution was used as blank. Whereas, for TRP assay 0.2 ml of callus extract was mixed with 0.5 ml phosphate buffer (2 mM; pH = 6.6) followed by addition of 0.5 ml potassium fericynide [K3Fe (CN)6] and incubated for 20 min in water bath at 50 °C. After cooling, 10% of tri-chloroacetic acid was added and centrifuged at 3000 rpm for 10 min to isolate 0.5 ml of upper the layer. The isolated upper layer was mixed with an equal volume of distilled water followed by addition of 100 μl of 0.1% of ferric chloride (FeCl3). The absorbance of reaction mixture was measured at 700 nm where 200 μl of methanol was used as control and reducing power was expressed as mg ascorbic acid equivalent.
2.5. Plant cell protecting enzymes activities
Fresh callus was harvested after 35 days from each of the tested light treatments and processed for protein extraction using the method of Nayyar and Gupta [20]. For total protein content (TPC) estimation the method of Lowry et al. [21] was used. Bovine serum albumin (BSA) was used as standard and total protein content was expressed as mg BSA equivalent. The super oxide dismutase (SOD) activity was estimated according to the protocol of Adil et al [11]. Where the reaction mixture was prepared by sequentially mixing of 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 mM p-nitro blue tetrazolium chloride (NBT), 2 mM riboflavin, 0.1 mM EDTA and 60 μl of enzyme extract. The previously reported protocol of Chisari et al. [22] was followed for peroxidase activity (POD). Briefly, a reaction mixture of 27.5 mM H2O2, 100 mM Guaiacol, 50 mM K-phosphate buffer of pH 7 and 20 μl of samples was prepared.
2.6. Protease activity and MDA content in callus cultures
Callus protease activity was determined by the Lowry-Folin method as described by McDonald and Chen [23]. Briefly, 100 mg of powdered callus was incubated in 4 ml of 1% casein in citrate phosphate buffer; pH 7.0 for one hour at 30 °C, followed by addition of 5 ml trichloroacetic acid (5%) to precipitate the residual protein and then centrifuged at 13,000 g for 15 min. The obtained 5 ml supernatant was mixed with 5 ml of reaction mixture (2% sodium carbonate, 2.7% sodium potassium tartrate and 1% copper sulphate) followed by addition of NaOH (1 N) and incubated for 10 min. Finally FC reagent was added to the reaction mixture and absorbance was measured at 660 nm after 60 min of incubation. The previously ascribed thiobarbituric acid (TBA) based method of Bailly et al. [24] was used for Malondialdehyde (MDA) content estimation and MDA content was expressed as μM/g FW.
2.7. HPLC based secondary metabolite analysis
HPLC analysis for withanolides was performed with some modification to the method of Mundkinajeddu et al. [25]. The Jasco HPLC system (LC-Net II), consisting of a quaternary pump (PU-2089), an autosampler (AS-2059 Plus) and a PDA detector (MDA-2018) was used for putative detection of withanolides. The compounds separation were achieved using 5 μm Luna C18 250 × 4.6 column and Solvents (A = Water Ultra-Pure + 0.05% TFA (pH. 2.6), B = MeOH HPLC grade). Mobile phase was run using gradient elution at 0, 15, 20 and 23 min of 30, 80, 80 and 30% of solvent-B respectively. The flow rate was 0.7 ml/min and injection volume was 20 μl. The eluent was detected and analysed at 230 nm. To get a clearer picture of the variation in root samples we integrated the peaks area region of 15–26 min which were assigned as global withanolides area (data not shown). For background correction standards of chlorogenic acid (100 mg L−1), and withaferin A (146 μM) were passed through HPLC to assign respective peaks.
2.8. Statistical
Mean values of three replicates from the treatments were subjected to analysis of variance (ANOVA) and significant difference were separated using one-way ANOVA with Tukey's test using Statistix software (8.1 versions). For graphical presentation Origin lab (8.5) was used and error bars represent standard error (SE).
3. Results and discussion
3.1. Plant growth regulators effect callus formation and biomass
Plant growth regulators plays an important role in plant cells growth and development. The combination of auxin and cytokinin, and their concentration are crucial for callus formation. However a generalization for a common combination PGRs and concentration to induce callus formation is not possible [26]. Thus it needs to study each and every plant species for successful callus formation. The success of callus formation is crucial for studying plant cell responses to environmental cues in a controlled environment. Also a callus culture can be used for biotransformation studies to modify a certain compound for novel biological functions [27].
In this experiments leaf explant were cultured on MS media with varying PGR`s concentration and combinations. These growth regulators differentially effected W. somnifera L. callus growth in term of percent callus formation, callus fresh and dry weights (Table 1). The cytokinin, BA failed to induce callus and the leaf explant turned brown after one week of culture in growth room. While in auxin, NAA containing medium callus initiation was observed but its growth was overwhelmed by adventitious root formation. To balance this, its combination with BA and TDZ were tested and maximum (78%) callus formation was reached on MS medium containing 0.5 mg·L−1 of each TDZ and NAA (Table 1). At this media higher callus fresh weight (3.5 g per explant) and dry weight (0.29 g per explant) was harvested and the obtained callus was compact in texture (Table 1). However, Rani et al. [28] reported maximum callus induction from cotyledon explant of W. somnifera on MS medium containing 2,4-D and Kin. In agreement with current findings, Ali and Abbasi [29] reported callus induction in leaf explant of Artemisia absinthium on MS medium containing TDZ and NAA. In ours finding BA failed to induce callus while previously it has been declared as potent PGR for variety of plant tissue culture experiments [30]. The inhibitory action of BA in this study might be due to the assumption that cytokinin in cultured tissues induce ethylene biosynthesis [31]. Where ethylene induce leaf senescence during the plant life course which defines the season and plant growth.
Table 1.
The implication of different plant growth regulators (PGRs) for W. somnifera callus induction and growth. Data values are means ± standard errors of three replicates.
| PGRs (mg·L−1) |
|||||
|---|---|---|---|---|---|
| TDZ | BAP | NAA | CIF (%) | FW (g explant−1) | DW (g explant−1) |
| 1 | 0 | 0 | 49.3 ± 2.84EF | 0.46 ± 0.61CDE | 0.05 ± 0.007H |
| 0 | 0 | 0.5 | 38.4 ± 1.2H | 0.16 ± 0.13DE | 0.04 ± 0.005I |
| 0 | 0.5 | 0 | NDI | NDE | NDj |
| 0.1 | 0 | 0.1 | 55.6 ± 1.76D | 1.68 ± 0.21B | 0.11 ± 0.003E |
| 0.1 | 0 | 0.5 | 62.67 ± 1.45BC | 1.6 ± 0.26B | 0.18 ± 0.0015C |
| 0.1 | 0 | 1.0 | 66.3 ± 2.60BC | 1.8 ± 0.23B | 0.17 ± 0.002C |
| 0.1 | 0 | 1.5 | 50.3 ± 1.76EF | 0.78 ± 0.28C | 0.08 ± 0.008F |
| 0.5 | 0 | 0.5 | 78.3 ± 1.35A* | 3.5 ± 1.02A* | 0.29 ± 0.0019A* |
| 0 | 0.1 | 0.5 | 43.67 ± 1.9GH | 0.97 ± 0.09C | 0.105 ± 0.007E |
| 0 | 0.1 | 1.5 | 61.3 ± 0.89C | 0.83 ± 0.046C | 0.062 ± 0.0037G |
| 0 | 0.1 | 2.0 | 46.68 ± 2.85FG | 0.68 ± 0.078CD | 0.14 ± 0.005D |
| 0 | 0.5 | 0.5 | 67.89 ± 1.22B | 0.94 ± 0.05C | 0.21 ± 0.001B |
Different alphabets represent significant difference in columns at P < 0.05.
3.2. Light triggers plant cell morphogenetic and secondary metabolites patterns
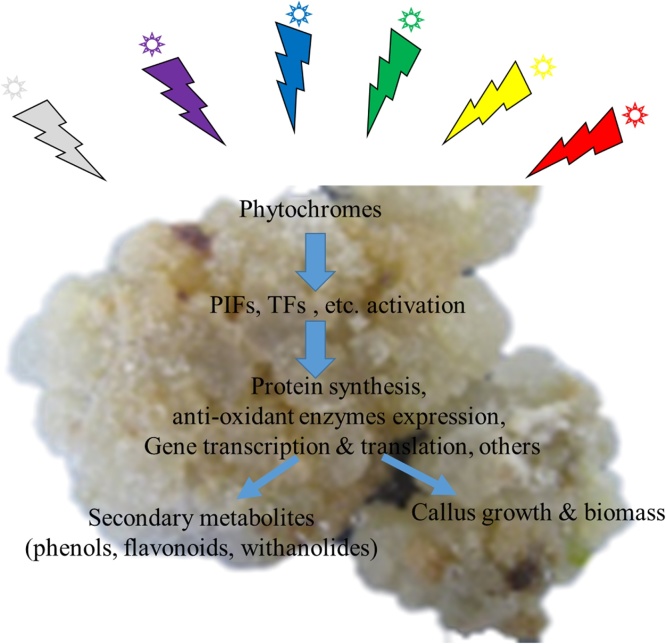
Light is the major source of energy for plants and it is harnessed by plant photosynthetic machinery to synthesise energy rich compounds to thrive. The light received from sun is mainly composed of UV (<400 nm), visible (400 to 700 nm), and far-red (>700 nm) wavelengths. Where plant absorb mainly red and blue lights which plays important roles in plant morphogenesis and regulation of certain biosynthetic pathways in plant cells (Fig. 1). Conventionally, in plant tissue culture it is mainly sourced from white fluoresce tubes but with recent technological intervention the availability of different light colours have made it plausible to study the individual light wavelength effecting plant growth. Hence, different plant species responds differently to individual light colours this study highlights for the first time the role of different lights in the growth of W. somnifera L. callus cultures.
Fig. 1.
Different light colours are sensed by phytochromes in callus and in-turn activates photochromes interacting factors (PIFs) and transcription factors (TFs) leading to arrays of events to regulate callus growth and secondary metabolism.
3.2.1. Light wavelength effect percent callus formation and biomass accumulation
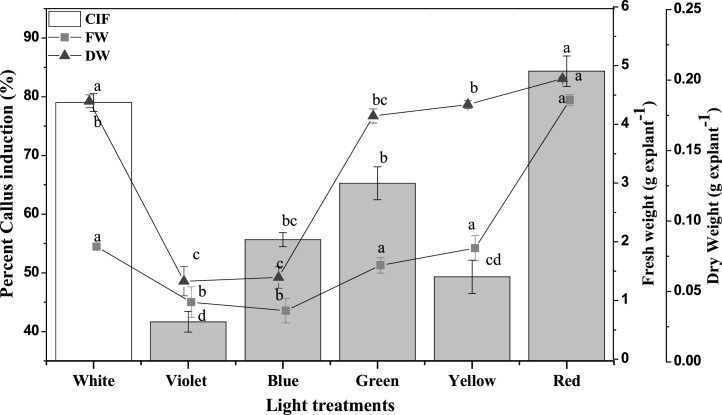
Light signalling pathways regulate the growth, differentiation and metabolism in callus cultures (Fig. 1). It is believed that different light wavelengths, deferentially evokes different responses. Such as green light at highest PPFD increases shoot elongation in lettuce plant [32]. The results in this study indicate that red light of wavelength ∼610-715 nm was effective and resulted maximum (84%) callus formation from leaf explant of W. somnifera. This was followed by white fluorescence and lower (41%) callus formation was observed in violet light (Fig. 2). Red light been reported to enhance the transport and biosynthesis of PGRs in plants which may have led to higher percent callus formation in leaf explants of W. somnifera [32]. This study suggests the involvement of red light receptors in increasing the callus formation from leaf explant. While, Morini et al. [33] suggested the blue light receptors based enhanced callusing in leaf explant of Cydonia oblonga Mill.
Fig. 2.
Effect of different light wavelengths on percent callus formation (%) and callus growth in leaf explant of W. somnifera. Data columns and points with error bars are mean of three replicates and different alphabets indicate significance at P < 0.05.
The callus growth in term of callus fresh and dry weight were evoked in red light condition. Compared to other lights, red light wavelength produced significantly higher callus fresh (4.4 g) and dry weight (0.2 g) (Fig. 2). Compared to the callus obtained under red and control (white) light, the callus under violet light was of minimum fresh and dry weight. Similarly, previous studies have reported increased biomass formation in plants under red light illumination [11,31]. While, Johkan et al. [32] reported green light to enhance biomass formation in lettuce plant. The higher nutrients accumulation in callus culture under red light condition could be the possible reason for the higher biomass formation [34]. Also, light wavelengths effect sugar metabolism in plant cells that leads to variations in biomass formation [35]. Additional to sugar metabolism, the higher nitrate reductase activity in red light irradiance increases nitrogen metabolism and might also be the possible explanation for increase biomass formation in callus culture [36]. Arias et al. [35], reported substrate consumption in cell suspension culture of Thevetia pervuviana was varyingly effected by light treatment.
3.2.2. Callus total soluble protein and MDA content, and enzymes activities
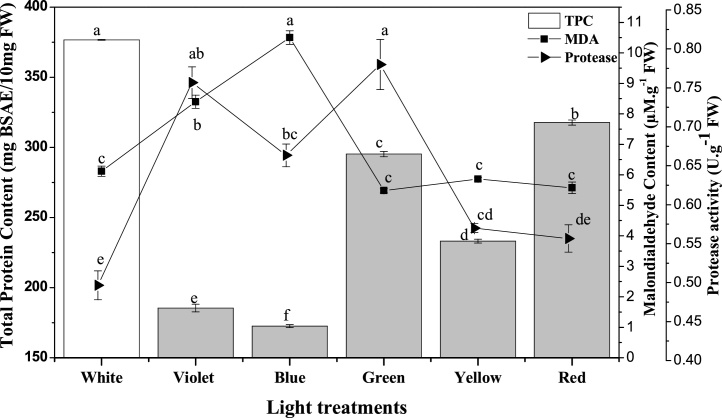
The soluble protein content may act as osmotic-regulator and serves as the reservoir for various plant enzymes to modulated plant cell growth and metabolism [11]. While protease activity is crucial for plant innate immunity and helps to process signals [37]. The effect of different light spectra on callus total protein content, protease activity and MDA content after 7 weeks of culture is shown in Fig. 3. MDA, routinely used as an indicator of lipid peroxidation and indicates the ROS burst in cells in certain unfavourable conditions. Maximum total protein content of 376.6 μg BSAE/10 mg FW was observed in callus treated with white fluorescence light while at the same treatment the protease activity (6.1 U·g−1 FW) and MDA (0.47 μM·g−1 FW) content were lowest then blue and violet light treated callus (Fig. 3). Red light stands second in total protein content and protease activity, while minimum total protein (172.6 μg BSAE/10 mg FW) content was observed in callus treated with blue light. The variation in total protein content represents the active state of plant cell at certain light treatment. Higher the total protein content means higher expression and transcription of genes to run and process the signal perceived by cell. In literature the soluble protein content varies with the type of plant species, and certain generalization hard to make that certain light will lead to higher protein and MDA level [11,[37], [38], [39]].
Fig. 3.
Total protein content, MDA and proteases activities are influenced by different light wavelengths in callus cultures of W. somnifera.
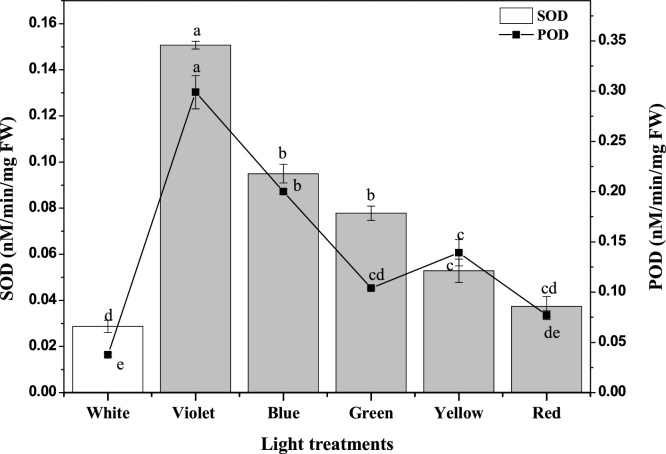
Among antioxidant enzymes activities, SOD and POD activities were study in callus cultures. These enzymes are important to eliminate ROS from plant cells which are produced during the plant metabolism. The maximum SOD (0.15 nM/min/mg FW) and POD (0.29 nM/min/mg FW) activity in violet-light treated callus that was followed by blue light (0.09 and 0.2 nM/min/mg FW; respectively) (Fig. 4). The decline in the SOD and POD level were observed in calli treated with red and white lights, and might be due to the lesser degree of oxidative damage induced by these wavelengths (lights) (Fig. 4). As the first line of defence SOD catalyse the conversion of superoxide anion radical (produced in cell due to high light energy stress) into H2O2 and superoxide anion radical [40]. Furthermore, the H2O2 is decomposed by POD by oxidation of co-substrate such as phenols or other antioxidant compounds [41]. Contrarily to ours finding, Shohael et al. [42] reported higher SOD activity in red light treated somatic embryos of E. senticosus.
Fig. 4.
Activity of anti-oxidative enzymes, Peroxidase (POD) and superoxide dismutase (SOD) in callus cultures were varying effected by different light treatments. Values are mean ± SE of three replicates and alphabets shows significance at P value < 0.05.
3.2.3. Callus secondary metabolites profile in response to different lights
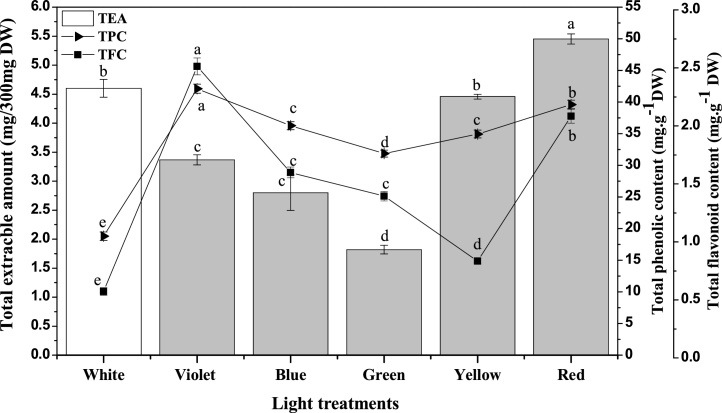
Secondary metabolites are important compounds produced by plants at dispense of environmental and plant growth factors. Their concentration in plants grown in wild varies and sometime found in lower concentrations which compromises the efficacy of medicinal formulations. While the callus culture being in control environment offers the opportunity to produce them consistently in higher amount [43]. But it need to be optimised and light plays important role in obtaining the higher yield of metabolites [44]. These secondary metabolites can be extracted using organic solvents and its amount may correspond to the level of different compound produced in plant cells. Maximum extract amount of 5.4 mg/300 mg DW was observed in calli of W. somnifera L., cultured under red-light for 7 weeks, this was followed by the values 4.6 mg (white) and 4.45 mg (yellow) (Fig. 5). While minimum amount of extract was noticed for callus obtained under green-light treatment. Compared to other light treatments, violet-light grown callus contained higher amount of total phenol and flavonoids (42 and 2.5 mg·g−1 DW, respectively). The red-light treated callus stands second with values of 39.6 mg·g−1 DW and 2.08 mg·g−1 DW for phenolic and flavonoid, respectively. Significantly, lower phenolic (18.8 mg·g1 DW) and flavonoid (0.57 mg·g−1DW) were observed in callus treated with white-light. These compounds play an important role in plant stress alleviation and the variation in their content corresponds to the degree of stress caused by different monochromatic lights.
Fig. 5.
The light wavelengths effect callus extractable amount, total phenolic content (TPC) and total flavonoid content (TFC) in callus cultures of W. somnifera L.
Phenolic and flavonoid compounds are of higher importance in human nutraceuticals. The results of this study suggests the ability of different light wavelengths to alter or modify both the concentration and profile of phenolic and flavonoids in callus culture of W. somnifera L. It was observed that violet-light favoured the phenolic and flavonoid formation while white-light failed to do so. Contrarily, Samuoliene et al. [45] higher phenolic content in lettuce plant when grown in red LED light for 16 h photoperiod. However, in accord to ours finding, Wang et al. [46] reported enhanced secondary metabolites production in red light treated hairy roots of Artemisia annua.
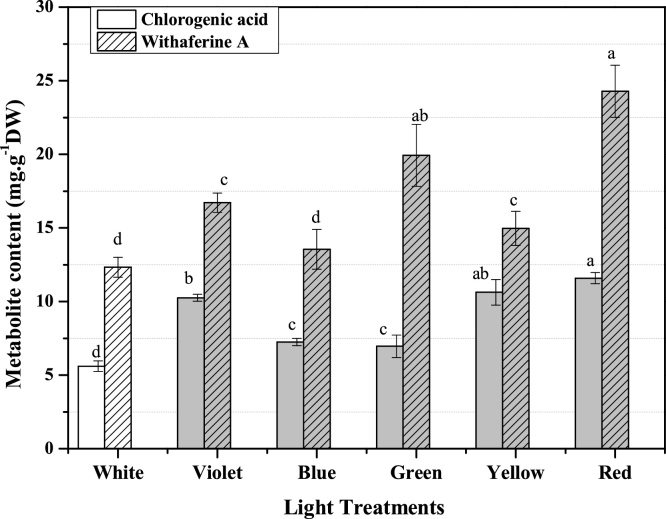
Furthermore, the withanolides content in calluses of W. somnifera showed significant variation due to the varying light colours (wavelengths) used in the experiment. On HPLC chromatogram the chlorogenic acid (11.6 ± 0.4), and withaferin A (24.3 ± 1.8) were peaked to maximum level in extract of red-light treated calli of W. somnifera (Fig. 6.). In withaferin A content callus grown in green light condition stood second while lower (12.3 ± 0.7) amount was detected in white light grown callus. Since withanolides biosynthesis involves the conversion of phenylalanine to cinnamic acid by phenyl ammonia lyase (PAL) enzyme [47]. Therefore it might be hypothesized that PAL enzyme has been triggered during callus growth under red light for signalling the phenylpropanaoid pathway to circumvent the in-vitro oxidative stress by production of higher level of withanolides for acquiring callus organogenesis in leaf explants. In literature there is no available report to study the effect of light wavelength on callus growth and withaferin A synthesis in W. somnifera.
Fig. 6.
The synthesis of chlorogenic acid and withaferine A in callus culture of W. somnifera were effected by different light treatments.
3.2.4. Antioxidant activity in callus cultures
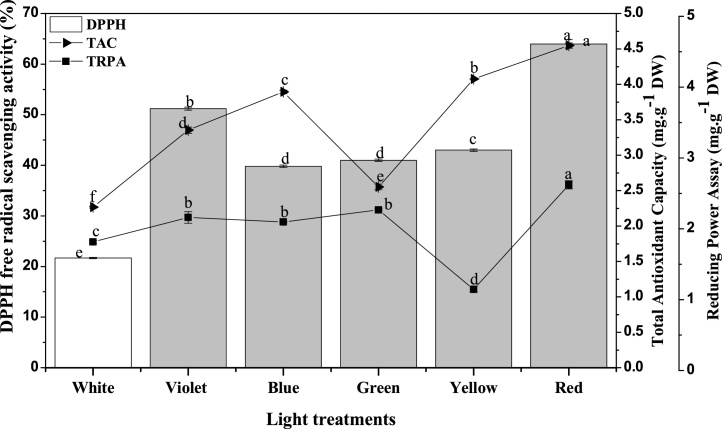
W. somnifera L. is a medicinal plant and its callus culture could be used as an alternative source to the wild grown whole plant. The antioxidant activity through different methods (i.e. DPPH, total antioxidant activity and reducing power assays) of its callus extract explains and evaluate the potential medicinal value. It was observed that callus cultures grown in different light conditions showed significant variation in DPPH free radical scavenging activity, TAC and RPA. The antioxidant activity, measured through DPPH robust method was in the range of lower ∼ 20% and higher ∼70% (Fig. 7). Compared to other lights, red light-grown callus cultures showed higher antioxidant activities (DPPH, TAC, and RPA) and this could be aligned with the higher secondary metabolites. The TAC of callus cultures were measured in the range of 4.5 – 2.6 mg AAE·g−1 DW while the RPA based antioxidant activity was in range of 2.6 – 1.8 mg AAE·g−1 DW. Contrarily, Kapoor et al. [48] observed higher antioxidant activity (DPPH and TAC) in blue-light grown callus culture of Rhodiola imbricate and they believed the increase was because of higher secondary metabolites accumulation at the respective light condition.
Fig. 7.
Light induced changes in DPPH free radical scavenging activity, total antioxidant capacity and reducing power assay in callus cultures of W. somnifera L.
4. Conclusion
In conclusion, the morphogenetic and biochemical signatures of W. somnifera callus cultures were differentially regulated by the different wavelengths of light. W. somnifera is a medicinal plant of high industrial importance and considered as the rich source of withanolides and other secondary metabolites. It was concluded that red-light condition is supportive for maximum biomass accumulation. While violet light condition stimulated the maximum phenolic, and flavonoid synthesis in callus cultures. The lower SOD and POD activities, and MDA content in callus, grown in red light condition was anticipated for higher biomass formation. From production point of red light condition is empirical for both biomass and withaferin A synthesis, and its utilization in future experiments will be the best strategy W. somnifera callus cultivation in vitro for withanolides production. The antioxidant activity of callus extracts further confirmed its potential medicinal value and could be used as an alternative source of antioxidant compounds. This strategy is advantageous over other biotechnological strategies as it is easy to implement in the in vitro conditions.
Author’s contribution
MA did the research work, data analyses and manuscript write-up. BHA conceived the idea and helped in experiment design. IH assisted in biochemical analysis and critically reviewed the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors highly acknowledge the support of higher education commission (HEC) of Pakistan.
References
- 1.Kaileh M., Berghe W.V., Heyerick A., Horion J., Piette J., Libert C., De Keukeleire D., Essawi T., Haegeman G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 2.Mirjalili M.H., Moyano E., Bonfill M., Cusido R.M., Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh N., Bhalla M., de Jager P., Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011;8 doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bano A., Sharma N., Dhaliwal H.S., Sharma V. A systematic and comprehensive review on Withania somnifera (L.) dunal-an indian ginseng. Br. J. Pharm. Res. 2015;7:63–75. [Google Scholar]
- 5.Adil M., Jeong B.R. In vitro cultivation of Panax ginseng CA Meyer. Ind. Crops Prod. 2018;122:239–251. [Google Scholar]
- 6.Kazmi A., Khan M., Ali H. Biotechnological approaches for production of bioactive secondary metabolites in Nigella sativa: an up-to-date review. Int. J. Second. Metab. 2019;6(2):172–195. [Google Scholar]
- 7.Ali A., Mohammad S., Khan M.A., Raja N.I., Arif M., Kamil A., Mashwani Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019;47(1):715–724. doi: 10.1080/21691401.2019.1577884. [DOI] [PubMed] [Google Scholar]
- 8.Reema Y., Khan M.A., Ullah N., Khan I., Hayat O., Shehzad M.A., Khan I., Taj F., Din N., Khan A., Naeem I., Ali H. Biosynthesis of anti-leishmanial natural products in callus cultures of Artemisia scoparia, Artificial Cells. Nanomed. Biotechnol. 2019;47(1):1122–1131. doi: 10.1080/21691401.2019.1593856. [DOI] [PubMed] [Google Scholar]
- 9.Mohammad S., Khan M.A., Ali A., Khan L., Khan M.S. Feasible production of biomass and natural antioxidants through callus cultures in response to varying light intensities in olive (Olea europaea. L) cult. Arbosana. J. Photochem. Photobiol. B Biol. 2019;193:140–147. doi: 10.1016/j.jphotobiol.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Afshari R.T., Angoshtari R., Kalantari S. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics. 2011;4(2):60. [Google Scholar]
- 11.Adil M., Ren X., Jeong B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019;196 doi: 10.1016/j.jphotobiol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Casal J.J. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. J. Photochem. Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Ascencio-Cabral A. Plant regeneration of Carica papaya L. through somatic embryogenesis in response to light quality, gelling agent and phloridzin. Sci. Hortic. 2008;118(2):155–160. [Google Scholar]
- 14.Ali H., Khan M.A., Kayani W.K., Dilshad E., Rani R., Khan R.S. Production of biomass and medicinal metabolites through adventitious roots in Ajuga bracteosa under different spectral lights. J. Photochem. Photobiol. B Biol. 2019;193:109–117. doi: 10.1016/j.jphotobiol.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 16.Tariq U., Ali M., Abbasi B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B: Biol. 2014;130:264–271. doi: 10.1016/j.jphotobiol.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Khan M.A., Abbasi B.H., Ahmed N., Ali H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind. Crops Prod. 2013;46:105–110. [Google Scholar]
- 18.Abbasi B.H., Ali J., Ali M., Zia M., Bokhari S.A., Khan M.A. Free radical scavenging activity in in vitro-derived tissues of Eruca sativa. Toxicol. Ind. Health. 2016;32:98–105. doi: 10.1177/0748233713498450. [DOI] [PubMed] [Google Scholar]
- 19.Jafri L., Saleem S., Kondrytuk T.P., Haq Iu., Ullah N., Pezzuto J.M., Mirza B. Hedera nepalensis K. Koch: a novel source of natural Cancer Chemopreventive and anticancerous compounds. Phytother. Res. 2016;30:447–453. doi: 10.1002/ptr.5546. [DOI] [PubMed] [Google Scholar]
- 20.Nayyar H., Gupta D. Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006;58:106–113. [Google Scholar]
- 21.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Chisari M., Barbagallo R.N., Spagna G. Characterization and role of polyphenol oxidase and peroxidase in browning of fresh-cut melon. J. Agric. Food Chem. 2007;56:132–138. doi: 10.1021/jf0721491. [DOI] [PubMed] [Google Scholar]
- 23.Mcdonald C., Chen L. The lowry modification of the folin reagent for determination of proteinase activity. Anal. Biochem. 1965;10:175. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- 24.Bailly C., Benamar A., Corbineau F., Come D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 1996;97:104–110. [Google Scholar]
- 25.Mundkinajeddu D., Sawant L.P., Koshy R., Akunuri P., Singh V.K., Mayachari A., Sharaf M.H., Balasubramanian M., Agarwal A. Development and validation of high performance liquid chromatography method for simultaneous estimation of flavonoid glycosides in Withania somnifera aerial parts. ISRN Anal. Chem. 2014;2014 [Google Scholar]
- 26.George E.F., Hall M.A., De Klerk G.-J. Plant Propagation by Tissue Culture. Springer; 2008. Effects of the physical environment; pp. 423–464. [Google Scholar]
- 27.Nasib A., Musharraf S.G., Hussain S., Khan S., Anjum S., Ali S., Atta-ur-Rahman, Choudhary M.I. Biotransformation of (−)-ambrox by cell suspension cultures of Actinidia deliciosa. J. Nat. Prod. 2006;69(6):957–959. doi: 10.1021/np050221o. [DOI] [PubMed] [Google Scholar]
- 28.Rani G., Virk G., Nagpal A. Callus induction and plantlet regeneration in Withania somnifera (L.) Dunal. In Vitro Cell. Dev. Biol. 2003;39:468–474. [Google Scholar]
- 29.Ali M., Abbasi B.H. Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in Callus Cultures of Artemisia absinthium L. Appl. Biochem. Biotechnol. 2014;172:2363–2376. doi: 10.1007/s12010-013-0663-7. [DOI] [PubMed] [Google Scholar]
- 30.Kaviani B., Kazemi D. Callus induction and organogenesis capacity from lamina explant of Petunia× hybrida F1 induced by BA and NAA. J. Ornamental Plants. 2017;7(3):157–162. [Google Scholar]
- 31.Gaspar T., Kevers C., Penel C., Greppin H., Reid D.M., Thorpe T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol. Plant. 1996;32(4):272–289. [Google Scholar]
- 32.Johkan M., Shoji K., Goto F., Hahida S.N., Yoshihara T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012;75:128–133. [Google Scholar]
- 33.Morini S., D’Onofrio C., Bellocchi G., Fisichella M. Effect of 2, 4-D and light quality on callus production and differentiation from in vitro cultured quince leaves. Plant Cell Tissue Organ Cult. 2000;63:47–55. [Google Scholar]
- 34.Manivannan A., Soundararajan P., Park Y.G., Wei H., Kim S.H., Jeong B.R. Blue and red light-emitting diodes improve the growth and physiology of in vitro-grown carnations ‘Green Beauty’and ‘Purple Beauty’. Hortic. Environ. Biotechnol. 2017;58(1):12–20. [Google Scholar]
- 35.Arias J.P., Zapata K., Rojano B., Arias M. Effect of light wavelength on cell growth, content of phenolic compounds and antioxidant activity in cell suspension cultures of Thevetia peruviana. J. Photochem. Photobiol. B Biol. 2016;163:87–91. doi: 10.1016/j.jphotobiol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Lillo C., Appenroth K.J. Light regulation of nitrate reductase in higher plants: which photoreceptors are involved? Plant Biol. 2001;3:455–465. [Google Scholar]
- 37.Balakireva A.V., Zamyantnin A.A. Indispensable role of proteases in plant innate immunity. Int. J. Mol. Sci. 2018;19:629. doi: 10.3390/ijms19020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong B.R., Sivanesan I. Impact of light quality and sucrose on adventitious shoot regeneration and bioactive compound accumulation in Ajuga multiflora Bunge. Sci. Hortic. 2018;236:222–228. [Google Scholar]
- 39.Verma S.K., Gantait S., Jeong B.R., Hwang S.J. Enhanced growth and cardenolides production in Digitalis purpurea under the influence of different LED exposures in the plant factory. Sci. Rep. 2018:8. doi: 10.1038/s41598-018-36113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewir Y., Chakrabarty D., Ali M., Hahn E., Paek K. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006;58:93–99. [Google Scholar]
- 41.Meloni D.A., Oliva M.A., Martinez C.A., Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003;49:69–76. [Google Scholar]
- 42.Shohael A., Ali M., Yu K., Hahn E., Islam R., Paek K. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006;41:1179–1185. [Google Scholar]
- 43.Yue W., Ming Q., Lin M., Rahman K., Zheng C., Han T., Qin L. Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016;36:215–232. doi: 10.3109/07388551.2014.923986. [DOI] [PubMed] [Google Scholar]
- 44.Guo B., Liu Y.-G., Yan Q., Liu C.-Z. Spectral composition of irradiation regulates the cell growth and flavonoids biosynthesis in callus cultures of Saussurea medusa Maxim. Plant Growth Regul. 2007;52:259–263. [Google Scholar]
- 45.Samuolienė G., Sirtautas R., Brazaitytė A., Duchovskis P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012;134(3):1494–1499. doi: 10.1016/j.foodchem.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Zhang H., Zhao B., Yuan X. Improved growth of Artemisia annua L hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 2001;23:1971. [Google Scholar]
- 47.Khan M.A., Abbasi B.H., Ali H., Ali M., Adil M., Hussain I. Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organ Cult. (PCTOC) 2015;120(1):127–139. [Google Scholar]
- 48.Kapoor S., Raghuvanshi R., Bhardwaj P., Sood H., Saxena S., Chaurasia O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018;183:258–265. doi: 10.1016/j.jphotobiol.2018.04.018. [DOI] [PubMed] [Google Scholar]