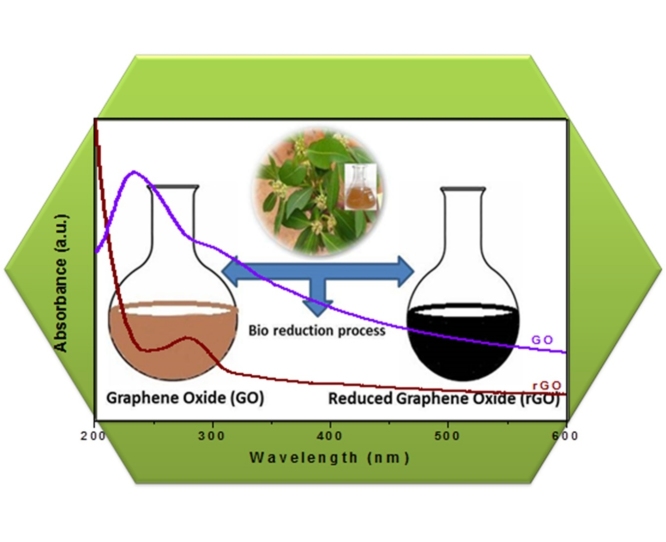
Graphical abstract
Keywords: Euphorbia heterophylla (L.), Reduced graphene oxide (rGO), X-ray diffraction, Cytotoxicity
Highlights
-
•
A simple and facile biocompatible method for the reduction of graphene oxide (GO).
-
•
Reduction of graphene oxide (rGO) from leaves extract of Euphorbia heterophylla (L.).
-
•
The rGO shows significant cytotoxicity on A549- Human Lung Cancer cell line and HepG2-Human Hepatocarcinomatous Cell lines.
-
•
Green synthesis of rGO is very easy, in expensive, cheap, non-toxic, biocompatibility and ecofriendly.
Abstract
Facile and biocompatible synthesis of reduced graphene oxide from graphene oxide as a precursor and aqueous leaves extract of Euphorbia heterophylla (L.), act as a reducing /capping /stabilizing agent by green chemistry approaches. The obtained product was analyzed by Ultraviolet-Visible spectroscopy (UV–vis), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy and Scanning electron Microscopy (SEM). In addition to this, the significant cytotoxicity of rGO studied against cancerous cell lines such as A549- Human Lung cancer cell line and HepG2-Human Hepatocarcinoma Cell lines in-vitro. These results indicate that the biocompatible synthesis of rGO is straightforward, inexpensive and environmentally friendly for promising large-scale production of industrial purpose and then finding further biomedical applications.
1. Introduction
Graphene is a single layer of sp2 hybridization of carbon atoms bonded in a hexagonal honeycomb two-dimensional crystal lattice structure which exhibits the large surface area, exceptional electronic, mechanical and biological properties. A wide range of applications have been explored using graphene-based materials [[1], [2], [3]]. Various methods have been reported for the preparation of graphene materials such as micromechanical cleavage of graphite [4], chemical vapour deposition [5], and chemical reduction [6] of graphene oxide. These methods are highly expensive, hazardous and not environmentally friendly [7,8]. However, some of the investigators proposed the biocompatible synthesis of reduced graphene oxide (rGO) is a simple, cost-effective and eco-friendly [9]. Biomolecules assisted synthesis of rGO were reported from different bioactive compounds such as gelatin, L-ascorbic acid, green tea polyphenol, chitosan, and bovine serum albumin [[10], [11], [12], [13], [14]]. However, some these biomolecules especially L-ascorbic acid, have been employed for the preparation of reduced graphene oxide possessing certain defects [15].
Great medicinal values and also eco-friendly nature of the phytochemicals makes an attractive and alternatives to the conventional toxic reducing agents. The plant derivate rGO is highly advantages because of the presence of numerous bioactive molecules which act as a reducing agent for the stabilization of product which are useful in several applications. Euphorbia heterophylla (Linn.) is one of the traditional medicines that is being used for the curing of various diseases [[16], [17], [18], [19], [20], [21], [22]]. Here, we report, the biocompatible conversion of reduced graphene oxide (rGO) from Euphorbia heterophylla (L.) in a simple process [23]. The obtained the product was studied using different characterization techniques such as UV–vis, XRD, FT-IR, Raman and SEM. Then after, the rGO was tested for cytotoxicity effects against human cancer cell such as A549 and HepG2 in-vitro study.
2. Experimental
2.1. Chemicals
The chemicals procured from Sigma Aldrich, Merk, SD-Fine chemicals, and Himedia Pvt. Ltd and used without any further purifications. All the reagents were prepared from double deionized water.
2.2. Preparation of extract and preliminary phytochemical analysis from Euphorbia heterophylla (L.) leaves
The preparation of plant extracts from the leaves of Euphorbia heterophylla (L.) using double distilled water by soxhlet extraction apparatus [24]. After completion of the process, the materials were stored at 4 °C until further use. The aqueous leaves extract of Euphorbia heterophylla was qualitatively examined for various phytochemicals using the standard test as described [25].
2.3. Preparation of reduced graphene oxide (rGO)
The graphene oxide (GO) was prepared by slight modification of Hummers method [26]. For the synthesis of rGO, 10 mg of Euphorbia heterophylla leaves extracts were mixed with the 40 mg of GO in 100 ml of distilled water which was sonicated for 30–40 min. After the sonication process, the reaction mixture was transferred to a reaction flask, thoroughly shook, mixed using magnetic stirrer and kept in oil bath by maintaining 95 °C for 12 h. After the completion of the reaction process, the deoxygenation of GO was observed by a visual color changing from brown to black. The obtained, stable black dispersion product was centrifuged (Centrifuge 5430AR Eppendorf, Hamburg, Germany) at 10,000 rpm for 10 min. The supernatant liquid was discarded, the suspension was washed with deionized water several times and then dried, stored in vials for further studies.
2.4. Characterization
The optical studies of samples were recorded by Ultraviolet-visible spectrophotometer (Perkin Elmer Lamda-35). The structural analysis of samples was carried out using Shimadzu X-ray diffractometer (PXRD-7000) using Cu Ka radiation (k =1.541 Å). The determination of functional groups for the samples using the Perkin Elmer Spectrum BX FT-IR system. The modes of vibrations of samples were analyzed by using HORIBA Lab Ram HR800 spectrometer with recorded at several different spots in backscattering geometry using 514.5 nm Ar + laser. The morphological features of samples were carried out on using Scanning Electron (SEM- Hitachi-3000 model).
2.5. In vitro cytotoxicity activity of reduced graphene oxide (rGO)
The in-vitro analysis of cytotoxicity was carried out using rGO obtained from E.heterophylla leaves extracts against human cancer cell lines such as Lung cancer cell line (A549) and Hepatocarcinoma cell line (HepG2) (National Centre for Cell Science, Pune, India) [27,28]. The cancer cell lines were grown in Dulbecco's Modified Eagle's Medium (DMEM) with supplemented fetal bovine serum with antibiotics. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. The cells were seeded in 96 well plates at a density 25 × 103 cells/well. The cells were further evaluated for cytotoxicity of various concentration rGO (25, 50, 100, 200 and 400 μg/mL) against cancer cell lines in the presence of MTT assay. MTT is a yellow dye which is reduced into purple colour formazon crystals in the presence of activity shows in mitochondrial succinate dehydrogenase enzyme in viable cells. The statistical analysis of cytotoxicity of rGO against cancer cell lines in the presence of MTT to the viability of cell was evaluated the IC50 value was determined by using linear regression equation, i.e. Y = Mx + C. Here, Y = 50, M and C values were derived.
3. Result and discussion
Generally, the plant materials contain various bioactive compounds. Here, the plant extract of Euphorbia heterophylla has bioactive phytochemicals such as alkaloids, flavonoids, tannins, saponin etc., as shown in the Table 1. We have prepared the reduced graphene oxide (rGO) using graphene oxide (GO) as a precursor and leaves extract of Euphorbia heterophylla as a reducing/capping/stabilizing agent. In reaction process, the phytochemicals of Euphorbia heterophylla undergo a free radical mechanism by donating hydrogen radical for the reduction of GO to rGO [[29], [30], [31], [32], [33]]. Obtained rGO has been characterized and used for the evaluation of biological activities.
Table 1.
The phytochemical investigation of Aqueous Leaves extract of E.heterophylla.
| Plant Name | Test | Results |
|---|---|---|
| E.heterophylla | Alkaloids | Present |
| Flavonoids | Present | |
| Tannins | Present | |
| Glycosides | Present | |
| Terpenoids | Present | |
| Sterols | Present | |
| Saponins | Present | |
| Carbohydrates | Present |
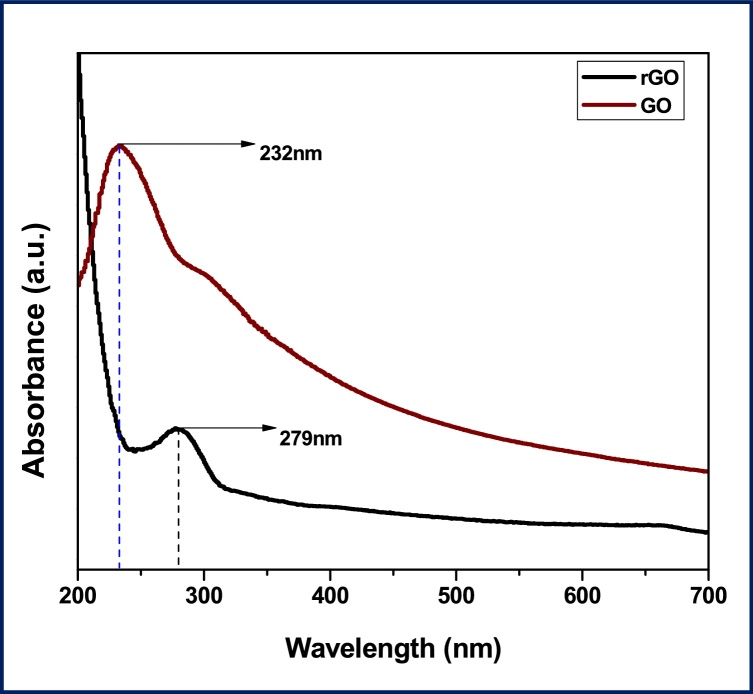
The UV–vis absorption spectra of GO and rGO exhibiting the surface plasma resonance (SPR) is shown in Fig. 1. The absorption of GO observed at 232 nm, which attributes to the π-π bond interaction of transition with aromatic C—C interaction, whereas the absorption peak at 295 nm is attributed to the n–π transition of C O bond interaction [34]. The absorption peak at 279 nm is the characteristic peak of rGO indicates that the aromatic C—C bond was observed. Similarly, this type of UV–vis absorption spectra was found for the reduction of graphene oxide (GO) using reducing agents such as L-ascorbic acid, L-glutathione and L-cysteine in the earlier reports [[35], [36], [37]]. Earlier reports shows that different bio-masses had been used for the reduction of graphene oxide (GO) using E.coli [38] Shewanella species etc. [39].
Fig. 1.
shows the UV–vis spectra of graphene oxide (GO) and reduced graphene oxide (rGO).
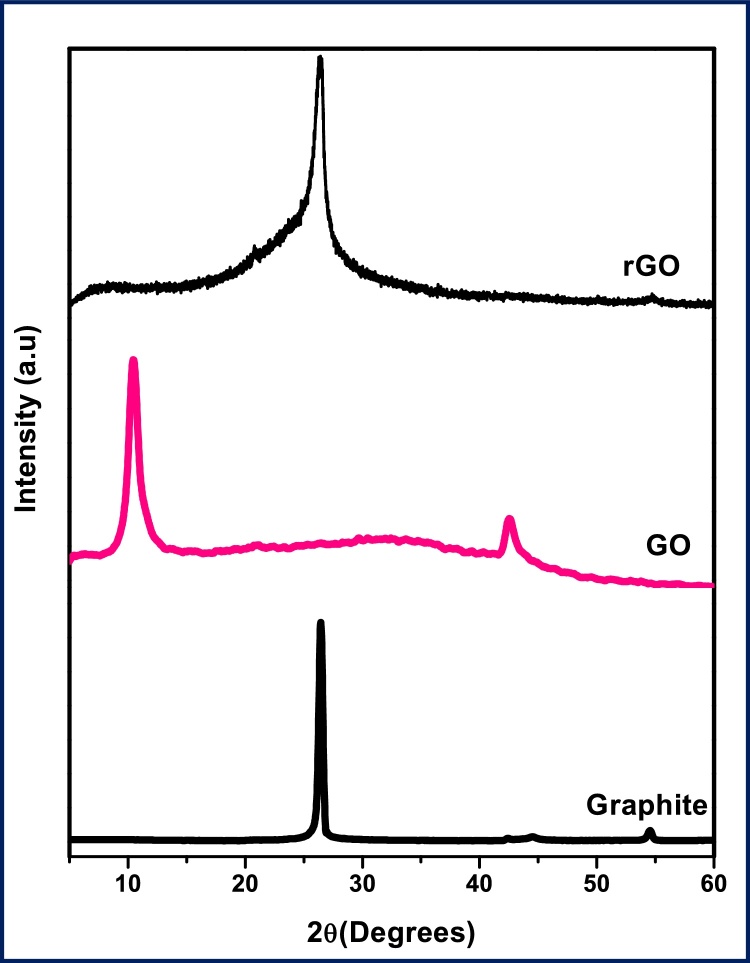
The XRD patterns of graphene oxide (GO) and reduced graphene oxide (rGO) as shown in the Fig. 2. The diffraction peak of carbon (001) of bulk GO sheets observed at the reflection 2θ = 10.5°, which is corresponded to d-space or interlayer distance of 0.84 nm [40]. The higher value of d-space GO sheets indicates that, intercalation of water molecules and the formation of oxygen-containing functional groups between the layers of graphite. After the reduction, the characteristic peak of GO sheets (001) at 10.5° disappeared, and a new peak reflection (002) of reduced graphene oxide was observed at 2θ = 26.6˚ which corresponds to the d-space of about 0.34 nm. The obtained inter-layer distance value of rGO is in good agreement with the reported value for graphene [41]. Decreasing the interlayer spacing in rGO indicates that exfoliation has been occurred at a larger extent by the removal of oxygen and water contents from the interlayers. Commercial graphite and exfoliated graphene sheets show reflection peak at 2θ = 26.4°. The reflection peak of graphite (002) has high intense, sharp and strong diffraction but exfoliated graphene (002) has broad and less intense reflection due to the reduction of thickness because of a breakdown of layers in bulk graphite. Except for (002) reflection of rGO, no other reflections were observed, which indicates that the absence of oxygen or other functional groups in graphene sheets [42].
Fig. 2.
shows the XRD pattern of graphite, graphene oxide(GO) and reduced graphene oxide (rGO).
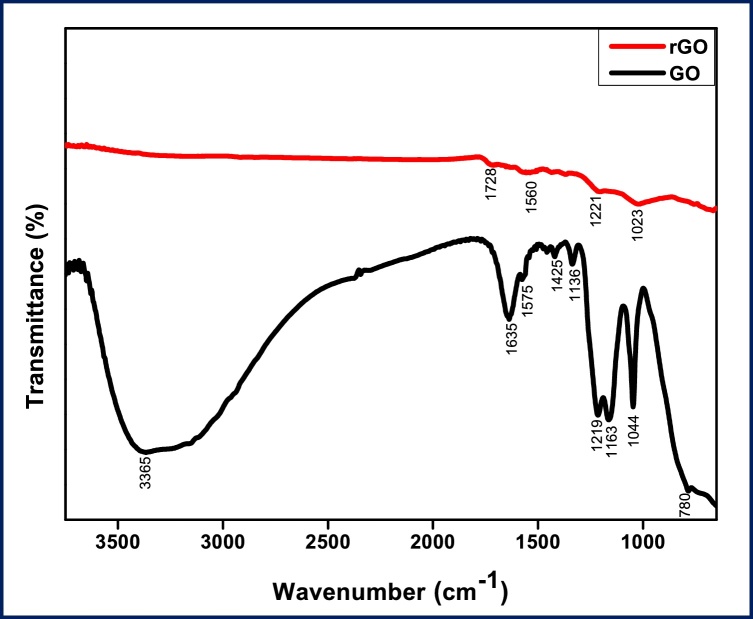
The FTIR studies of GO and rGO have been shown in Fig. 3. The presence of different oxygen functional groups confirms the oxidation of graphite to GO. The broadbands of GO observed at 3365 cm−1 and 1635 cm−1 are attributed to the −OH stretching vibrations. The characteristic band at 1575 cm−1 is due to the C O stretching vibration of carboxylic groups. Peaks at 1425 cm-1, 1336 cm-1 and 1219 cm-1 are assigned to C–H vibration and C–O-stretching frequencies. In the case of rGO, Intensities of the characteristic peaks at 1728 cm-1 of C–O stretching and carboxylic −OH stretching vibrations (3179 cm−1 and 1620 cm-1) are highly reduced [43,44]. In rGO, low intense absorption peaks at 1560, 1121 and 1023 cm-1 are assigned to the C O stretching and C C bending vibrations with the presence of bioactive compounds of plants extract.
Fig. 3.
shows the FT-IR spectra of graphene oxide(GO) and reduced graphene oxide (rGO).
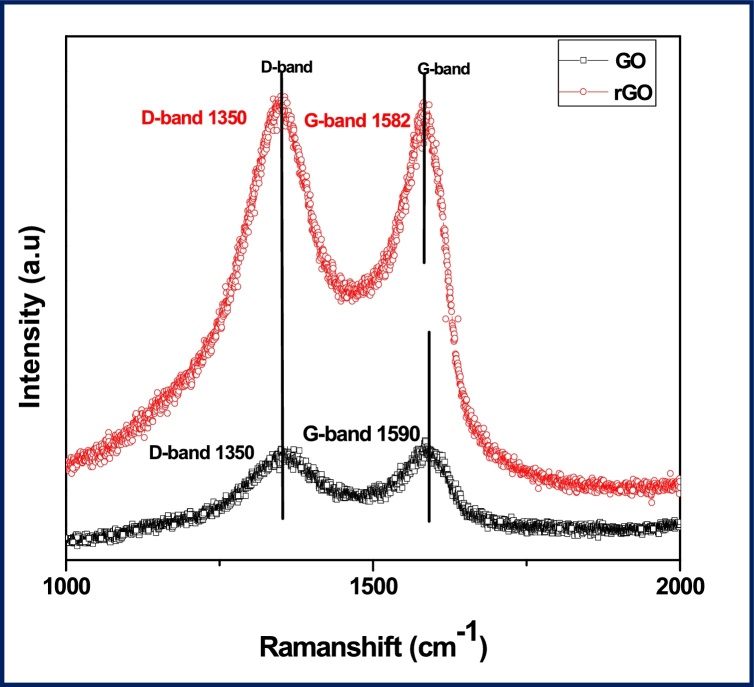
The Raman spectra of GO and rGO have been shown in Fig. 4. The spectra of GO exhibiting the D and G bands are broadened and shifted to 1350 cm−1 and 1590 cm−1. In case of rGO, D and G bands are appeared at 1350 cm−1 and 1582 cm−1 respectively. It is known that D band originates from the edges can be attributed to the symmetry and the G band corresponds to the first-order scattering of the E2g modulation of sp2 domain of graphite. Moreover, the relative strength of the D band compared to the G band depends strongly on the amount of disorder in the graphitic materials [45]. The D/G intensity ratio of rGO (ID/IG) is 1.01, slightly smaller than that of GO (ID/IG = 0.95). Thus, it could be deduced that the extensive oxidation and reduction had induced a certain amount of decrease in the size of in-plane sp2 domains, an increase of the edge planes, as well as the expansion of the disorder in the prepared reduced graphene oxide [46].
Fig. 4.
shows the Raman spectra of graphene oxide(GO) and reduced graphene oxide (rGO).
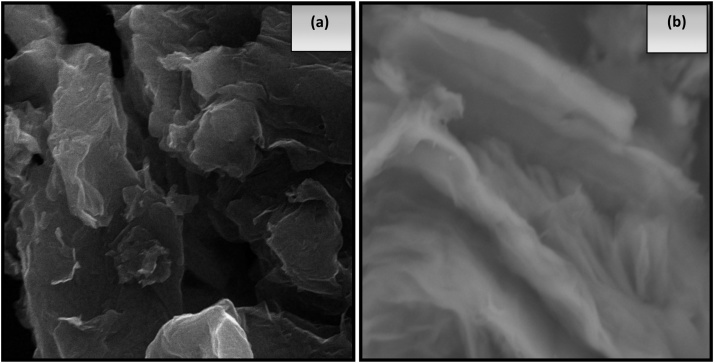
The morphological features of GO and rGO have been shown in Fig. 5. The SEM micrograph of GO Fig. 5a shows that large number of layers have been stacked to each other and formed into thick and bulk sheet clusters which are opaque in nature. But, Euphorbia heterophylla assisted rGO (Fig. 5b) are creased, transparent in nature, has smooth surface, overlapped with each other showing uniform sheets which indicates the exfoliation of few layered rGO which is also supported by x-ray diffraction pattern.
Fig. 5.
shows the SEM Micrograph of (a) Graphene oxide(GO) and (b) Reduced graphene oxide (rGO).
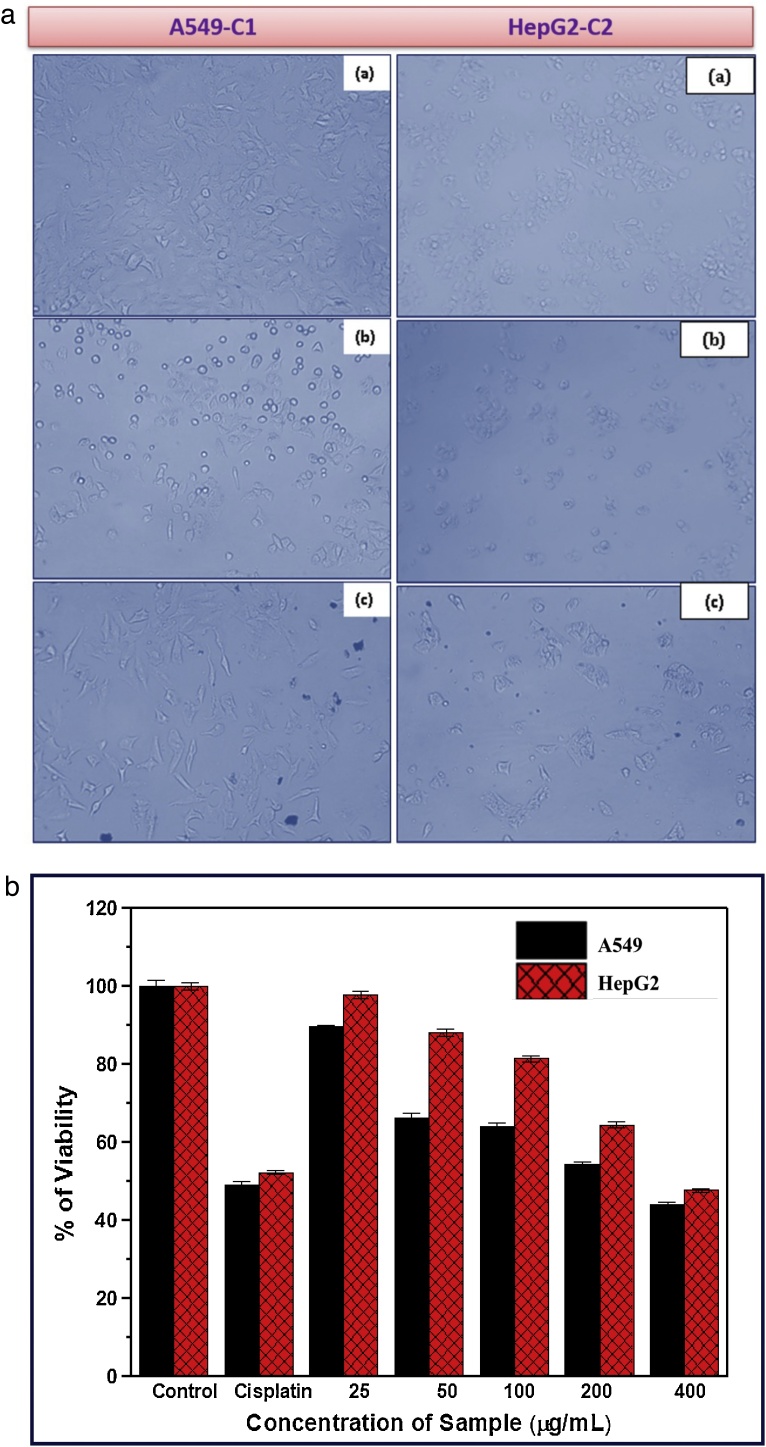
The cytotoxicity of rGO has been evaluated against cancer cell lines such as A549 and HepG2 by measuring the cellular reduction in the presence of MTT. The results indicating the cytotoxicity of rGO against A549 and HepG2 cell line has been shown in Fig. 6(a) & (b), and then the summarized results are shown in Table 2. The cytotoxicity mechanism of rGO against cancer cell lines may the exterior membrane of a cell, they interact with the plasma membrane or extra-cellular matrix and enter the cell, mainly through diffusion, endocytosis and/or binding to receptors [[47], [48], [49]]. The potential toxic effects of graphene mainly depend on its physicochemical characteristics, the nature of its interaction with cells and accumulation in specific organs. Up on interaction with light, graphene can generate reactive oxygen species, which in turn can cause oxidative stress, loss in cell functionality, pro-inflammatory responses and mitochondrial damage. Uptake of graphene into the nucleus may cause DNA-strand breaks and induction of gene expression via the activation of transcription factors, cell death and genotoxicity. [50,51].
Fig. 6.
(a). Photo images of cytotoxicity activity against A549- C1 and HepG2-C2 cancer cell lines with (a) Untreated with cell (b) cell treated with standard drug (c) cell treated with rGO. (b). Shows the graphical representation of reduced graphene oxide (rGO) with different Concentration against A549 Human Lung Cancer Cell line and HepG2-Human Hepatocarcinoma Cell Line.
Table 2.
Cytotoxicity of reduced graphene oxide (rGO) against Cancer cell lines.
| Cell lines | Standard Cisplatin | Concentration of rGO (μg/mL) |
IC50(μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| (25μM) | Control | 25 | 50 | 100 | 200 | 400 | ||
| A549 | 49.09 | 100 | 89.76 | 66.29 | 64.05 | 54.25 | 43.85 | 297.81 |
| HepG2 | 52.23 | 100 | 99.79 | 88.03 | 81.44 | 64.50 | 47.66 | 357.53 |
4. Conclusion
The reduced graphene oxide (rGO) nanosheets have been successfully exfoliated by a simple and biocompatible method using Euphorbia heterophylla [L.] leaves extract as a reducing agent in an aqueous medium. The obtained product was well characterized with the help of analytical tools such as UV–vis, XRD, FT-IR, Raman spectra and SEM techniques. The reduced graphene oxide from E. heterophylla showed significant cytotoxic activity against cancer cell lines such as A549 and HepG2. The cytotoxicity results of rGO against cancer cell lines show irregular morphological analysis of treated with standard drug cisplatin and rGO from E. heterophylla, whereas, regular morphology was observed in controlled cells. However, these results concluded that plant-derived reduced graphene oxide is very useful in biomedical as well as environmental applications.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors greatly acknowledge Tumkur University for providing the research and lab facilities to carry out this work.
References
- 1.Huang Y., Dong X., Shi Y., Li C.M., Li L.J., Chen P. Nano electronic biosensors based on CVD grown graphene. Nanoscale. 2010;2:1485–1488. doi: 10.1039/c0nr00142b. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Li P., Chen Y., He J., Zhang W., Schmidt O.G., Li Y. Pure thiophene–sulfur doped reduced graphene oxide: synthesis, structure, and electrical properties. Nanoscale. 2014;6:7281–7287. doi: 10.1039/c3nr05061k. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Li Z.H., Wang J., Li J.H., Lin Y.H. Graphene and graphene oxide: bio functionalization and applications in biotechnology. Trends Biotechnol. 2011;29:205–212. doi: 10.1016/j.tibtech.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 5.Wang G., Shen X., Wang B., Yao J., Park J. Synthesis and characterization of hydrophilic and organophilic graphene nano sheets. Carbon. 2009;47:1359–1364. [Google Scholar]
- 6.Stankovich S., Dikin D.A., Dommett G.H., Kohlhaas K.M., Zimney E.J., Stach E.A., Piner R.D., Nguyen S.T., Ruoff R.S. Graphene-based composite materials. Nature. 2006;422:282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 7.Bai H., Xu Y., Zhao L., Li C., Shi G. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. 2009;13:1667–1669. doi: 10.1039/b821805f. [DOI] [PubMed] [Google Scholar]
- 8.Shen J., Hu Y., Li C., Qin C., Shi M., Ye M. Layer-by-layer self-assembly of graphene nanoplatelets. Langmuir. 2009;25:6122–6128. doi: 10.1021/la900126g. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Li P., Chen Y., He J., Zheng B., Liu J., Qi F. The green synthesis of reduced graphene oxide by the ethanol-thermal reaction and its electrical properties. Mater. Lett. 2014;116:416–419. [Google Scholar]
- 10.Liu K., Zhang J., Cheng F., Zheng T., Wang C., Zhu J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011;21:12034–12040. [Google Scholar]
- 11.Merino M.J.F., Guardia L., Paredes J.I., Rodil S.V., Fernandez P.S., Alonos A.M., Tascon J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C. 2010;114:6426–6432. [Google Scholar]
- 12.Wang Y., Shi Z., Yin J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its bio composites. ACS Appl. Mater. Interfaces. 2011;3:1127–1133. doi: 10.1021/am1012613. [DOI] [PubMed] [Google Scholar]
- 13.Fang M., Long J., Zhao W., Wang L., Chen G. pH-responsive chitosan-mediated graphene dispersions. Langmuir. 2010;26:16771–16774. doi: 10.1021/la102703b. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Fu S., Yuan B., Li Y., Deng Z. Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J. Am. Chem. Soc. 2010;132:7279–7281. doi: 10.1021/ja100938r. [DOI] [PubMed] [Google Scholar]
- 15.Gao J., Liu F., Liu Y., Ma N., Wang Z., Zhang X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010;22:2213–2218. [Google Scholar]
- 16.Falodun A., Agbakwuru E.O.P. Phyto-chemical analysis and laxative activity of the leaf extracts of Euphorbia heterophylla L. (Euphorbiaceae) Pak. J. Sci. Ind. Res. 2004;47:345–348. [Google Scholar]
- 17.Uduak A., Essiett Kola, Ajibesin K. Antimicrobial activities of some Euphorbiaceae plants used in the traditional medicine of akwa ibom state of Nigeria. Ethnobot. Leaflet. 2010;14:654–664. [Google Scholar]
- 18.Kesavan K., Deepa A., Shobana G., Jothi G., Sridharan G. Preliminary phytochemical screening and in vitro antioxidant potential of Euphorbia heterophylla(L.) Int. J. Pharm. Pharm. Sci. 2014;6:549–553. [Google Scholar]
- 19.Falodun A., Okunrobo L.O., Uzoamaka N. Phytochemical screening and anti-inflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae) Afr. J. Biotechnol. 2006;5:529–531. [Google Scholar]
- 20.Sundaram M.M., Karthikeyan K., Sudarsanam D., Brindha P. Antimicrobial and anticancer studies on Euphorbia heterophylla. J. Pharm. Res. 2010;3:2332–2333. [Google Scholar]
- 21.Annapurna A., Hatware K. Effect of aqueous extract of Euphorbia heterophylla on blood glucose levels of alloxan induced diabetic rats. Int. J. Res. Pharm. Chem. 2014;4:669–672. [Google Scholar]
- 22.James O., Emmanuel T. Phytochemical composition, bioactivity and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. Int. J. Pharm. Biomed. Res. 2010;1:54–63. [Google Scholar]
- 23.Hou D., Liu Q., Cheng H., Zhang H., Wang S. Green reduction of graphene oxide via Lycium barbarum extract. J. Solid State Chem. 2017;246:351–356. [Google Scholar]
- 24.Raja Naika H., Krishna V. Antimicrobial activity of extracts from the leaves of Clematis gouriana roxb. Int. J. Biomed. Pharma. Sci. 2006;1:69–72. [Google Scholar]
- 25.Kokate C.K., Purohith A.P., Gokhale S.B. Text Book of Pharmacognosy. 23rdedn. Niraliprakashan; Pune: 2003. pp. 25–27. [Google Scholar]
- 26.Montree S., Pattarachai S., Poramane C., Tanas S., Patcharaporn S. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon. 2012;50:5156–5161. [Google Scholar]
- 27.Lingaraju K., Raja Naika H., Nagabhushana H., Nagaraju G. Euphorbia heterophylla (L.) mediated fabrication of ZnO NPs: characterization and evaluation of antibacterial and anticancer properties. Biocatal. Agric. Biotechnol. 2019;18:100894. [Google Scholar]
- 28.Chang Y., Yang S.T., Liu J.H., Dong E., Wang Y., Cao A., Liu Y., Wang H. Invitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011;200:201–210. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Shi Z., Yin J J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its bio composites. ACS Appl. Mater. Interfaces. 2011;3:1127–1133. doi: 10.1021/am1012613. [DOI] [PubMed] [Google Scholar]
- 30.Hou D., Liu Q., Cheng H., Li K., Wang D., Zhang H. Chrysanthemum extract assisted green reduction of graphene oxide. Mater. Chem. Phys. 2016;183:76–82. [Google Scholar]
- 31.Udayabhanu S., Muralikrishna, Kishore B., Nagabhushana H., Suresh D., Sharma S.C., Nagaraju G. One pot green synthesis of MnCO3–rGO composite hybrid superstructure: application to lithium ion battery and biosensor. New J. Chem. 2017;41:12854–12865. [Google Scholar]
- 32.Hui K.S., Hui K.N., Dinh D.A., Tsang C.H., Cho Y.R., Zhou W., Hong X., Chun H.H. Green synthesis of dimension-controlled silver nanoparticle–graphene oxide with insitu ultrasonication. Acta Mater. 2014;64:326–332. [Google Scholar]
- 33.Adyani S.H., Soleimani E. Green synthesis of Ag/Fe3O4/RGO nanocomposites by Punica granatum peel extract: catalytic activity for reduction of organic pollutants. Int. J. Hydro Energy. 2019;44(5):2711–2730. [Google Scholar]
- 34.Stankovich S., Dikin D.A., Piner R.D., Kohlhaas K.A., Kleinhammes A., Jia Y., Wu Y., Nguyen S.T., Ruoff R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45:1558–1565. [Google Scholar]
- 35.Zhang J.L., Yang H.J., Shen G.X., Cheng P., Zhang J.Y., Guo S.W. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010;46:1112–1114. doi: 10.1039/b917705a. [DOI] [PubMed] [Google Scholar]
- 36.Pham T.A., Kim J.S., Kim J.S., Jeong Y.T. One-step reduction of graphene oxide with L-glutathione. Colloids Surf. A: Physicochem. Eng. Asp. 2011;384:543–548. [Google Scholar]
- 37.Chen S., Cai W., Piner R.D., Suk J.W., Wu Y., Ren Y., Kang Y., Ruoff R.S. Synthesis and characterization of large-area graphene and graphite films on commercial Cu-Ni alloy foils. Nano Lett. 2011;9:3519–3525. doi: 10.1021/nl201699j. [DOI] [PubMed] [Google Scholar]
- 38.Gurunathan S., Han J.W., Eppakayala V., Kim J.H. Microbial reduction of graphene oxide by Escherichia coli: a green chemistry approach. Colloids Surf. B: Bio Interfaces. 2013;102:772–777. doi: 10.1016/j.colsurfb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Wang G., Qian E., Saltikov C.W., Jiao Y., Li Y Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011;4:563–570. [Google Scholar]
- 40.Marcano D.C., Kosynkin D.V., Berlin J.M., Sinitskii A., Sun Z., Slesarev A., Alemany L.B., Lu W., Tour J.M. Improved synthesis of graphene oxide. ACS Nano. 2010;4:4806–4814. doi: 10.1021/nn1006368. [DOI] [PubMed] [Google Scholar]
- 41.Moon I.K., Lee J., Ruoff R.S., Lee H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010;1:73. doi: 10.1038/ncomms1067. [DOI] [PubMed] [Google Scholar]
- 42.Saiful Badri M.A., Salleh M.M., Noor N.F., Rahman M.Y.A., Umar A.A. Green synthesis of few-layered graphene from aqueous processed graphite exfoliation for graphene thin film preparation. Mater. Chem. Phys. 2017;193:212–219. [Google Scholar]
- 43.He Y., Cui H. Synthesis of highly chemiluminescent graphene oxide/silver nanoparticle nano-composites and their analytical applications. J. Mater. Chem. 2012;22:9086. [Google Scholar]
- 44.Polavarapu L., Mourdikoudis S., Santos I.P., Juste J.P. Nanocrystal engineering of noble metals and metal chalcogenides: controlling the morphology, composition and crystallinity. Cryst. Eng. Commun. 2015;17:3727–3762. [Google Scholar]
- 45.Kudin K.N., Ozbas B., Schniepp H.C., Prud’homme R.K., Aksay I.A., Ca R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008;8(1):36–41. doi: 10.1021/nl071822y. [DOI] [PubMed] [Google Scholar]
- 46.Navarro C.G., Weitz R.T., Bittner A.M., Scolari M., Mews A., Burghard M., Kern K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007:3499–34503. doi: 10.1021/nl072090c. [DOI] [PubMed] [Google Scholar]
- 47.Tabish T.A., Pranjol M.Z.I., Hayat H., Rahat A.A., Abdullah T.M., Whatmore J.L., Zhang S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology. 2017;28(50) doi: 10.1088/1361-6528/aa95a8. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y., Shen H., Tu X., Zhang Z. Assessing in vivo toxicity of graphene materials: current methods and future outlook. Nanomedicine. 2014;9(10):1565–1580. doi: 10.2217/nnm.14.68. [DOI] [PubMed] [Google Scholar]
- 49.Wilding L. Jennifer, Bodmer F. Walter. Cancer cell lines for drug discovery and development. Cancer Res. 2014;74(9):2377–2384. doi: 10.1158/0008-5472.CAN-13-2971. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X., Dorn M., Vogt J., Spemann D., Yu W., Mao Z., Estrela-Lopis I., Donath E., Gao C. A quantitative study of the intracellular concentration of graphene/noble metal nanoparticle composites and their cytotoxicity. Nanoscale. 2014;6:8535–8542. doi: 10.1039/c4nr01763c. [DOI] [PubMed] [Google Scholar]
- 51.Gurunathan S., Han J.W., Park J.H., Kim E., Choi Y.J., Kwon D.N., Kim J.H. Reduced graphene oxide–silver nanoparticle nanocomposite: a potential anticancer nano therapy. Int. J. Nanomed. Nanosurg. 2015;10:6257–6276. doi: 10.2147/IJN.S92449. [DOI] [PMC free article] [PubMed] [Google Scholar]