Abstract
Epithelioid hemangioma of bone is a rare, locally aggressive but benign vascular tumor that is now recognized as a distinct entity from other vascular neoplasms. It is often difficult to distinguish on imaging studies from other vascular tumors, including epithelioid hemangioendothelioma, which can lead to misdiagnosis and inappropriate treatment. We present the characteristic features and multimodality imaging findings in the case of a 24-year-old female with multifocal epithelioid hemangioma of the first and second metacarpal bones with extension into the surrounding soft tissue.
Keywords: Vascular tumor, Musculoskeletal, Epithelioid hemangioma, Metacarpal
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare intermediate-grade vascular tumor that displays a highly variable clinical course [1], [2]. It was first described as a tumor of soft tissues with a distinct epithelioid or histiocytoid endothelial cell histologic makeup that had an intermediate clinical course between that of benign hemangioma and a malignant angiosarcoma [3]. EHE can involve single or multiple organs and can be uni- or multifocal, which has been shown to correlate with overall outcomes and survival in several studies [4], [5], [6]. In both single and multiorgan involvement, EHE is most commonly found in the liver (21%), lung (12%), liver plus lung (18%), and bone (14%), although many other sites have been reported [2], [4].
Clinical symptoms vary widely based on location of the tumor. If associated with soft tissue of the extremities, it can present as a poorly-circumscribed mass with pain, local swelling, and pathologic fracture, but many patients are asymptomatic [2], [7], [8]. When associated with the lung, patients can present with chest or pleuritic pain, but again, are usually asymptomatic. Concurrently, when found in the liver, patients can describe nonspecific symptoms such as weight loss, right upper quadrant pain, or symptoms associated with hepatic failure [1].
Radiographically, EHE of bone appears as a unicentric or a multicentric clustering of lytic lesions at the epiphyses of bone and is commonly associated with periosteal sclerosis, cortical disruption, and expansion [7], [9]. It has been reported that in up to 40% of cases, there may be soft tissue components [7]. It can be challenging to identify on imaging and could be easily mistaken for carcinoma due to its epithelial appearance. Thus, understanding its presentation and characteristic imaging is paramount in early identification and treatment. In this report, we present a case of EHE occurring in the first and second metacarpal bones with involvement of surrounding soft tissue discovered in radiologic studies and verified by pathology as literature is limited regarding EHE in the bones of the hand.
Case
A 24-year-old female with no significant past medical history presented with a 7-month history of left thumb and wrist pain associated with intermittent weakness and swelling that did not improve with NSAID use. She had initially been diagnosed with left wrist tendonitis at an outpatient clinic 3 months prior and was prescribed wrist exercises, a soft wrist brace, and topical pain relief agents, which provided no relief. No imaging was performed at this time given the lack of any known injury to the area, and the patient's age. She reported that with lifting or holding heavy objects, or with prolonged use, she experienced stiffness and swelling of her left first digit and wrist that was occasionally accompanied by first and second digit paresthesias. She could also feel 2 small bumps on her left wrist that seemed to be growing larger, but were not erythematous and did not demonstrate overlying skin changes. On physical exam, there was tenderness at the base of the left first and second digits with mild swelling of the wrist. No bruising, erythema, or warmth was noted, and the patient was able to demonstrate full range of motion of her wrist with some discomfort.
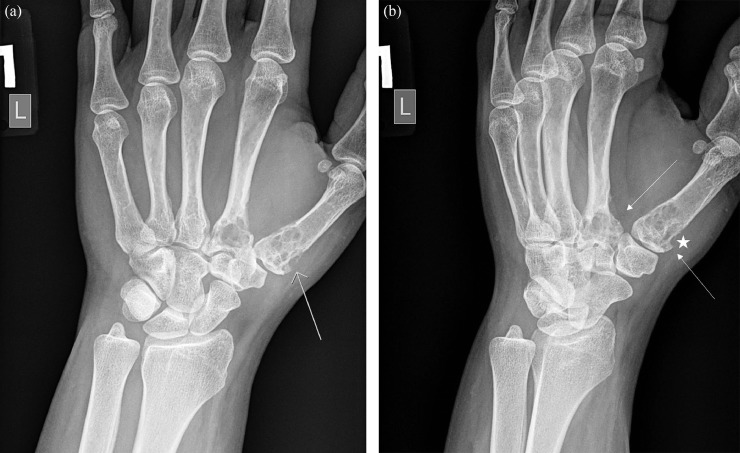
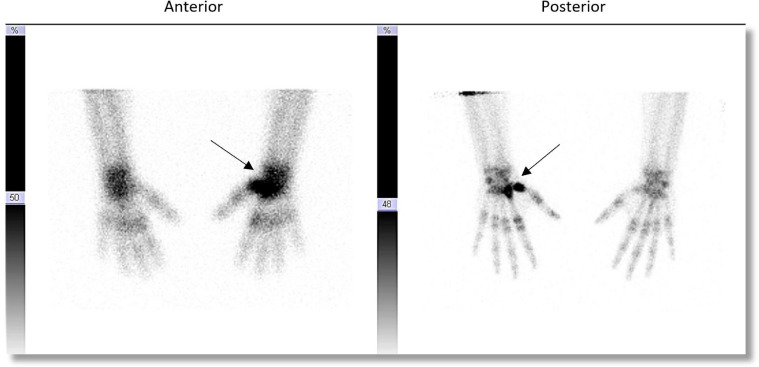
Initial evaluation included a left wrist radiograph that revealed multiple lytic lesions with smooth, scalloped margins throughout the proximal first and second metacarpal bones, with thin sclerotic margin (Fig. 1). A bone scan was then performed to evaluate for the possibility of additional sites as well as a potential site for biopsy. A whole-body bone scan showed no abnormal osseous tracer uptake separate from the lytic lesions of the left first and second metacarpals (Fig. 2).
Fig. 1.
(a) Left wrist AP (a) and oblique (b) radiographs showing multiple lytic areas involving the proximal first and second metacarpal bones (white arrows). At least one of these lesions demonstrates adjacent cortical disruption (white star). (b) Left wrist AP (a) and oblique (b) radiographs showing multiple lytic areas involving the proximal first and second metacarpal bones (white arrows). At least one of these lesions demonstrates adjacent cortical disruption (white star).
Fig. 2.
Bone scan of the hands in anterior and posterior projections demonstrates focally increased radiotracer activity at the left proximal metacarpal and carpal bones (arrow).
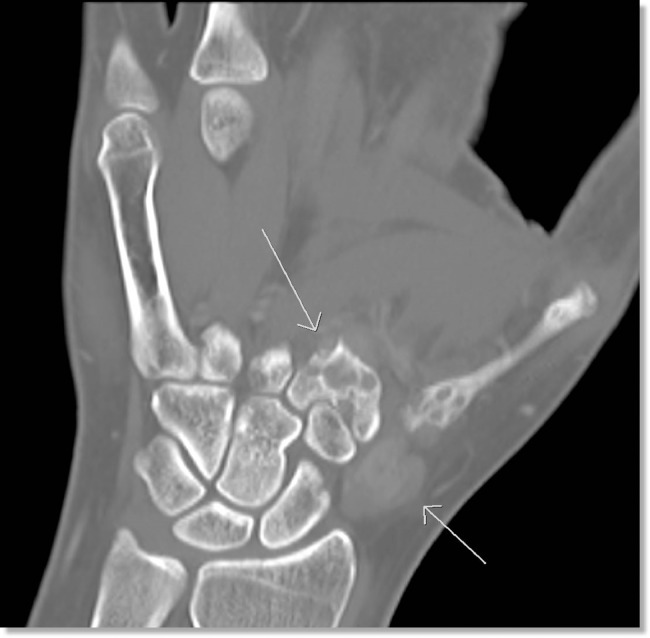
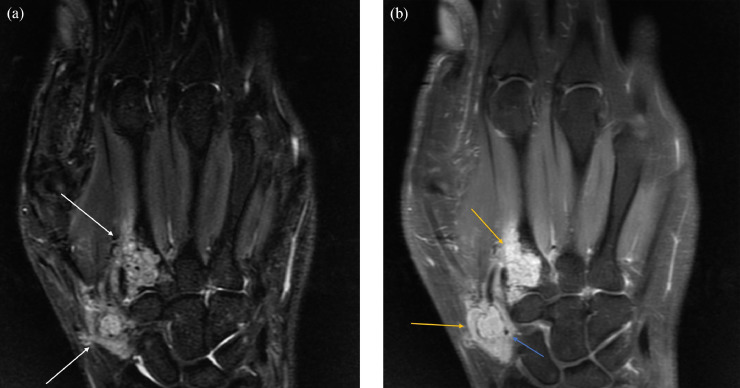
This was followed by magnetic resonance imaging (MRI) of the left upper extremity with and without contrast, demonstrating a locally aggressive neoplastic process with scattered foci of signal void that could have represented chondroid matrix or vascular flow voids. There was associated scalloping and cortical disruption, as well as extensive extraosseous enhancing soft tissue components (Fig. 3). Further characterization of the bony component with computed tomography of the left upper extremity with and without contrast demonstrated an avidly enhancing soft tissue process with involvement of the first and second metacarpal bases as well as adjacent soft tissue (Fig. 4). This soft tissue mass lesion did not have computed tomography evidence of internal matrix, effectively excluding chondroid lesion from the differential, making a benign, locally aggressive vascular soft tissue neoplasm the most likely imaging diagnosis. Additional potential differential considerations such as atypical infection, plasmacytoma, and metastasis were considered much less likely give the patient's constellation of clinical symptoms, absence of systemic symptoms, and age. She was then referred to orthopedic oncology, where a biopsy of the mass was obtained. This mass was subsequently surgically excised.
Fig. 3.
Noncontrast enhanced CT of the left hand redemonstrates multiple lytic lesions in the proximal first and second metacarpals in addition to adjacent soft tissue mass (white arrows).
Fig. 4.
(a) MRI left hand with contrast. (a) STIR. (b) T1-weighted postcontrast. Multiple lytic lesions in the proximal first and second metacarpals which demonstrate heterogeneous high T2/STIR signal (white arrows) and avid homogeneous contrast enhancement (yellow arrows) with intratumoral flow voids (blue arrow). (b) MRI left hand with contrast. (a) STIR. (b) T1-weighted postcontrast. Multiple lytic lesions in the proximal first and second metacarpals which demonstrate heterogeneous high T2/STIR signal (white arrows) and avid homogeneous contrast enhancement (yellow arrows) with intratumoral flow voids (blue arrow).
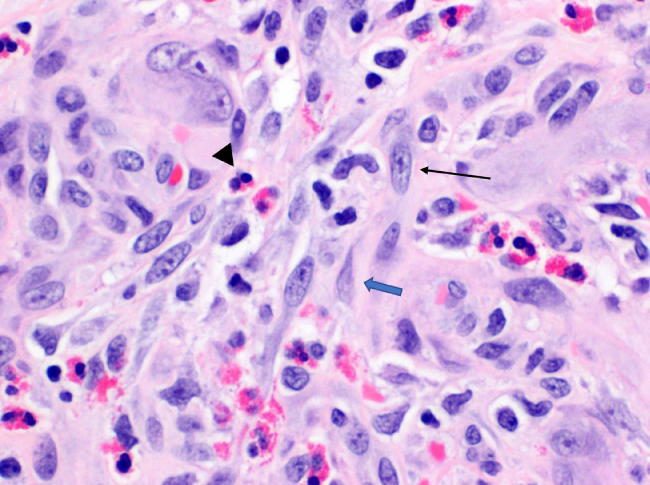
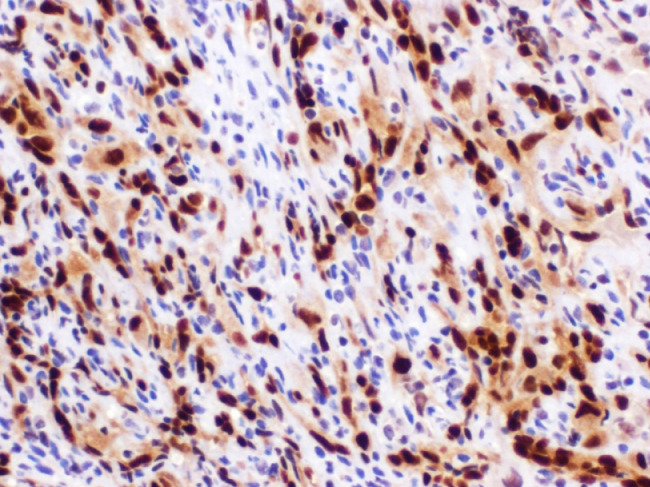
Hematoxylin and eosin stain revealed proliferation of blood vessels with epithelioid endothelial cells without atypia (Fig. 5). Pathology then performed a nuclear stain, ERG, to confirm the endothelial nature of the cells (Fig. 6). To further prove the benign nature, an additional smooth muscle actin stain was performed, which demonstrated pericytes surrounding the capillaries, a finding not seen in angiosarcoma. Given these pathologic findings, the final diagnosis was EH.
Fig. 5.
Hemotoxylin and eosin stain of the specimen magnified 400× reveals plump endothelioid cells (black arrow) and admixed eosinophils (black arrowhead). Additional myopericyte is noted, cells which wrap around endothelial cells and line the small vasculature of the body such as capillaries (blue arrow).
Fig. 6.
High power image of an ERG nuclear stain, which confirms the proliferating endothelial cells of the tumor (brown stained cells).
Discussion
Radiographically, EH is described as a well-defined lytic lesion commonly associated with cortical expansion. However, occasional cortical disruption with soft tissue extension has been reported [2], [3], [4]. Because of the overlapping features, it can be challenging to differentiate EH from other vascular or epithelial lesions such as EHE or carcinoma. In this report, we present a case of EH of the first and second metacarpal bones in a 24-year-old female with involvement of surrounding soft tissue discovered in radiologic studies and verified by pathology as literature is limited regarding EH in the bones of the hand. Correct identification of EH is essential, as treatment and prognosis differ from that of higher-grade vascular tumors.
Epithelioid hemangioma (EH) is a rare, benign, yet locally aggressive, vascular tumor that can be difficult to distinguish from the spectrum of vascular abnormalities, ranging from hemangioma to angiosarcoma, by imaging. First described as angiolymphoid hyperplasia with eosinophilia, epithelioid hemangioma is a now a well-recognized distinct clinicopathologic entity, separate from other vascular tumors of bone [2], [3], [7]. While most consider EH a benign neoplasm, there have been reports of associated lymph node involvement and instances of local recurrence [2], [4], [6]. In fact, the most recent 2013 World Health Organization Classification designated it as intermediate grade, “locally aggressive, rarely metastasizing” elevating it along the spectrum, although to a lesser degree than malignant EHE and angiosarcoma [9]. While increasing the grade to intermediate level, there have been no reported deaths associated with this disease process [2], [4], [7]. The pathogenesis of EH is largely unknown, although it may represent an unusual reactive process after local trauma, infections, arteriovenous shunting, or hyperestrogenemia [1], [10]. This tumor usually appears from the third to sixth decades of life (average age of 35) and affects males and females equally [2]. Most reported cases of EH arise in the skin and soft tissues, particularly of head and neck. It can also occur as an osseous tumor, and rarely as a purely intravascular lesion [1]. In a study of 50 cases, osseous EH involved long tubular bones (40%), distal lower extremities (18%), flat bones (18%), vertebrae (16%), and small bones of the hand (8%) [2]. EHs are typically solitary lesions, but up to 25% display multifocal disease. Nielsen et al, reported up to 18% of lesions involving more than 1 bone in his series of 50 cases and some had been described to have lesions involving bone as well as soft tissue or parenchymal organs [2]. These findings are thought to represent multicentricity rather than metastatic disease. EH typically presents with a local painful mass unlike conventional hemangiomas, however, many are found incidentally on imaging and are asymptomatic [3]. As in our case, the patient may present with soft tissue components associated with swelling. Our patient seemed to also experience symptoms of medial nerve impingement/constriction, possibly due to swelling that seemed to correlate with increased usage.
Radiographically, these lesions are challenging to distinguish from other lytic lesions of bone, although the thin, well-defined geographic type 1B margins favor a benign process. In our case, multiple enchondromatosis, including Ollier disease and Maffucci Syndrome, sarcoid, as well as metastatic carcinoma, were initially included in the differential. With MRI imaging, our differential expanded to include chondrosarcoma, enchondroma protuberans, or other chondroid-matrix lesions due to the multifocal cortical erosions and enhanced soft tissue components, suggesting a locally aggressive process. Low signal foci were seen on T2-weighted sequences that could represent small vascular flow voids in the setting of vascular malformations which expanded the differential to include tumors of vascular origin such as hemangioma, soft tissue venous malformations, and multifocal EH and EHE. EH have been described on radiographs as a well-defined solitary lytic lesion supporting this diagnosis. In up to 25% of cases, EH can show mixed lytic and sclerotic patterns of bone disruption with septations. Although not common, EH can also have associated cortical disruption with a thick reactive periosteal new bone formation [2], [3], [7]. On MRI, EH appears isointense or slightly hyperintense to skeletal muscle on T1-weighted images, and heterogeneous but predominately hyperintense on T2-weighted images [4], [6]. These findings overlap with those of other vascular bone tumors, particularly EHE, and can be impossible to distinguish between without histologic correlation [1], [3], [6], [8], [11].
Morphologically, EH has lobulated or multinodular growth with borders pushing into the medullary cavity or soft tissues. They show variable vasoformative properties and form mature vessels with open lumens containing erythrocytes [6]. Microscopically, the lobules commonly show a hypocellular periphery and gradually transition to a hypercellular center containing well-formed compact vessels lined with plump epithelioid endothelial cells which can protrude into the lumen creating a “tombstone-like” appearance [5]. These epithelioid endothelial cells contain variable nuclei (round-oval to cleaved) with evenly dispersed chromatin and have abundant eosinophilic cytoplasm. The mitotic activity is usually low supporting its benign nature [5]. A loose connective tissue stroma with inflammatory infiltrate such as eosinophils, lymphocytes, and plasma cells is usually present [5]. EH can be easily mistaken for other vascular tumors, particularly when comparing it to EHE; however, there are distinct histologic differences. EHE is composed of cords and strands with solid aggregates of cells with round, oval, and cuboidal shaped epithelioid endothelial cells with abundant eosinophilic cytoplasm embedded in a myxohyaline stroma [12], [13]. EH does not show the extracellular hyalinized matrix typical of EHE. Furthermore, EHE has a more primitive corded arrangement compared to the mature vessels of EH and lacks its typical inflammatory infiltrate. EHE has also been related to a t(1;3)(p36;q25) translocation gene with WWTRi-CAMTA-1 fusion gene that can be identified with FISH and RT-PCR [14]. EH can be distinguished from malignant lesions like angiosarcoma and metastatic carcinoma by the absence of nuclear pleomorphism, anaplasia, and cytologic atypia [3].
Distinguishing between these neoplasms is of the utmost importance due to differences in prognosis and treatment strategy. The preferred treatment of EH is intralesional curettage or, when appropriate, marginal en-bloc excision. In a few cases, patients were treated conservatively after biopsy with tumor resolution [2]. In contrast, EHE should be treated with wide surgical excision of the lesion with clear margins with possible adjuvant radiation due to the risk for metastasis increased mortality [2], [15]. EH has approximately a 10% risk of local recurrence after treatment [5]. As demonstrated in this case, multimodality imaging is essential to narrow the wide differential from benign vascular tumors such as EH, from malignant tumors such as EHE as treatment and prognosis differ from that of higher-grade vascular tumors.
References
- 1.Ko J.S., Billings S.D. Diagnostically challenging epithelioid vascular tumors. Surg Pathol Clin. 2015;8(3):331–351. doi: 10.1016/j.path.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen G.P., Srivastava A., Kattapuram S., Deshpande V., Oconnell J.X., Mangham C.D. Epithelioid hemangioma of bone revisited: a study of 50 cases. Am J Surg Pathol. 2009;33(2):270–277. doi: 10.1097/PAS.0b013e31817f6d51. [DOI] [PubMed] [Google Scholar]
- 3.Wenger D.E., Wold L.E. Benign vascular lesions of bone: radiologic and pathologic features. Skelet Radiol. 2000;29(2):63–74. doi: 10.1007/s002560050012. [DOI] [PubMed] [Google Scholar]
- 4.Schenker K., Blumer S., Jaramillo D., Treece A.L., Bhatia A. Epithelioid hemangioma of bone: radiologic and magnetic resonance imaging characteristics with histopathological correlation. Pediatr Radiol. 2017;47(12):1631–1637. doi: 10.1007/s00247-017-3922-x. [DOI] [PubMed] [Google Scholar]
- 5.Hart J.L., Edgar M.A., Gardner J.M. Vascular tumors of bone. Semin Diagn Pathol. 2014;31(1):30–38. doi: 10.1053/j.semdp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Errani C., Zhang L., Panicek D.M., Healey J.H., Antonescu C.R. Epithelioid hemangioma of bone and soft tissue: a reappraisal of a controversial entity. Clin Orthop Relat Res. 2012;470(5):1498–1506. doi: 10.1007/s11999-011-2070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Errani C., Vanal D., Gambarotti M., Alberghini M., Picci P., Faldini C. Vascular bone tumors: a proposal of a classification based on clinicopathological, radiographic and genetic features. Skelet Radiol. 2012;41(12):1495–1507. doi: 10.1007/s00256-012-1510-6. [DOI] [PubMed] [Google Scholar]
- 8.Bregman J.A., Jordanov M.I. Epithelioid hemangioma occurring in the radial styloid of a 17-year-old boy-an unusual presentation of an uncommon neoplasm. Clin Imaging. 2014;38(6):899–902. doi: 10.1016/j.clinimag.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CDM, WHO classification of tumours of soft tissue and bone 2013.
- 10.Fetsch J.F., Weiss S.W. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia) Mod Pathol. 1991;4(4):449–455. [PubMed] [Google Scholar]
- 11.Duncan S.F., Krochmal D.J., Craft R.O., Merritt M.V., Smith A.A. Epithelioid hemangioendothelioma of the distal radius: a case report. Radiol Case Rep. 2007;2(4):119. doi: 10.2484/rcr.v2i4.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissferdt A., Moran C.A. Epithelioid hemangioendothelioma of the bone: a review and update. Adv Anat Pathol. 2014;21(4):254–259. doi: 10.1097/PAP.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 13.Requena L., Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30(1):29–44. doi: 10.1053/j.semdp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Flucke U., Vogels R.J., de Saint Aubain Somerhausen N., Creytens D.H., Reidl R.G., van Gorp J.M. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleer C.G., Unni K.K., McLeod R.A. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20(11):1301–1311. doi: 10.1097/00000478-199611000-00001. [DOI] [PubMed] [Google Scholar]