Abstract
Background
India has one of the largest population of heart failure (HF) patients in the world; yet only limited information is available about HF in India.
Methods
This observational study was performed at Medanta- The Medicity, a large, tertiary-care institute in the National Capital Region of India. Records of HF patients with reduced left ventricular ejection fraction (LVEF) registered at Medanta HF clinic during the period early 2014 to mid-2017 were reviewed. Disease characteristics and one-year mortality details were collected.
Results
Mean age of the subjects (n = 5590) was 59.1 ± 11.8 years with 83.0% males. Mean LVEF was 30.0 ± 6.6%. Coronary artery disease (CAD) was the dominant cause of HF, accounting for 77.8% of the total population. Most patients received guideline-directed medical therapy with a beta blocker being prescribed to 81.8% subjects. The one-year all-cause mortality was 17.6%. On multivariate analysis, age, usage of loop diuretics and ivabradine, and serum creatinine were independently associated with one-year mortality, whereas rheumatic etiology had an inverse association.
Conclusions
This represents the largest single-center data of HF patients reported so far and the largest study describing clinical outcomes from HF patients in India. Our patients were younger, had high proportion of CAD, and there was higher usage of beta-blockers. Despite this, the one-year mortality was substantial. Given the enormous magnitude of HF burden in India and the paucity of information on this subject, these findings should be of help in identifying key problem areas and potential solutions for management of HF in India.
Keywords: Heart failure, Heart failure with reduced ejection fraction, Heart failure with preserved ejection fraction, Ambulatory heart failure, Beta blockers, Cardiovascular disease
1. Introduction
Heart failure (HF) is a major public health problem, accounting for considerable morbidity and mortality worldwide. Despite improvements in overall healthcare, ironically, the prevalence of HF continues to rise in most countries1 because of rising prevalence of HF risk factors [e.g. hypertension, diabetes, coronary artery disease (CAD), etc.], improved survival of patients with acute myocardial infarction (MI), and increasing overall lifespan of individuals.
India is currently in the midst of an epidemiological transition, resulting in rapid increase in the incidence of cardiovascular risk factors, cardiovascular disease, and HF.2, 3 Some investigators have projected the total HF burden in India to be close to 20 million,4, 5 making it one of the largest HF populations in the world. Despite this enormous burden, only very limited data are currently available about HF in India.6, 7, 8 Previous few registries involving Indian patients6, 7, 8 and a meta-analysis9 of studies conducted in other low- to middle-income countries (LMIC) have revealed wide heterogeneity in the epidemiology of HF among different nations. This variability has necessitated development of population-specific databases to define the etiology and risk factors of HF, presentation patterns, treatment practices, and overall clinical outcomes of HF in different ethnic/geographic groups. Such information is critical to inform policy-making processes, improve management practices, and identify future research opportunities. With this objective, we describe here clinical characteristics and one-year survival among patients with HF with reduced ejection fraction (HFrEF), registered at Medanta Heart Failure Clinic (MHFC) in India. This would be the largest single-center data on HF patients to be reported so far and the largest study describing clinical outcomes in HF patients from India.
2. Methods
This is an observational study performed at Medanta- The Medicity, which is a large, tertiary-care, multispecialty, healthcare institute located in the National Capital Region of India. The hospital is a major referral center for patients from most parts of North India, apart from also receiving patients from other parts of the country as well as outside India.
The Department of Cardiology at Medanta- The Medicity has been running a dedicated heart failure clinic—MHFC—for several years. The patients presenting to the cardiac specialty of the hospital (inpatient or outpatient) with a diagnosis of heart failure and/or left ventricular (LV) systolic dysfunction are referred to MHFC for further evaluation, management, and follow-up.
In the beginning of 2014, two research nurses were entrusted with the responsibility to enter details of all the new patients in an Excel database. They collected information from patient prescriptions/files/electronic hospital information system (eHIS) and recorded these details on daily basis. Simultaneously, they were also asked to record one-year mortality status of these patients on a time-to-time basis.
For the purpose of this study, records of all the patients registered at MHFC during the period early 2014 to mid-2017 with a diagnosis of HFrEF [defined as HF with LV ejection fraction (LVEF) ≤40%] were reviewed. The diagnosis of HF was based on clinical assessment, and a single echocardiographic recording of LVEF ≤40% was sufficient for inclusion in the study. For these subjects, information was collected about the demographic details, clinical characteristics (cardiovascular risk factors, underlying etiology, previous cardiac procedures, functional class, vital signs, etc.), laboratory investigation details (hemoglobin, serum creatinine, LVEF, etc.), and treatment details (usage of various cardiac drugs, cardiac rhythm devices implantation, etc.). One-year survival status was ascertained from hospital records or, as needed, from telephonic follow-up. The patients who had visited the hospital for any purpose any time after 1 year of their initial visit were automatically marked ‘alive’ (this information was retrieved from the eHIS). For the remaining subjects, phone calls were made to inquire about the same. For those subjects for whom this information could not be gathered telephonically, their mortality status was left undetermined and such subjects were excluded from the analysis. Apart from one-year survival, other clinical follow-up details were not collected.
Ethics committee approval and informed consent were not required because the study primarily involved retrospective review of the patient case records.
2.1. Statistical analysis
The baseline characteristics and other descriptive variables were summarized using standard statistical tools such as mean ± standard deviation, median and interquartile range, or counts and proportions as appropriate. The categorical variables were compared using Chi-square test and continuous variables using independent t-Test or Mann–Whitney U test, as applicable. A multivariate binary logistic regression analysis was performed to determine the independent predictors of one-year all-cause mortality. Two-sided P-value <0.05 was considered statistically significant. All analyses were performed using SPSS version 20.0.
3. Results
Beginning 2014, through June 2017, a total of 6111 subjects were available for inclusion in the study. Of these, 302 subjects were excluded because of incomplete laboratory parameters. Of the remaining subjects, one-year survival status could be ascertained for 5590 (96.2%) subjects. This report describes clinical profile and one-year all-cause mortality among these 5590 patients.
3.1. Clinical and biochemical characteristics
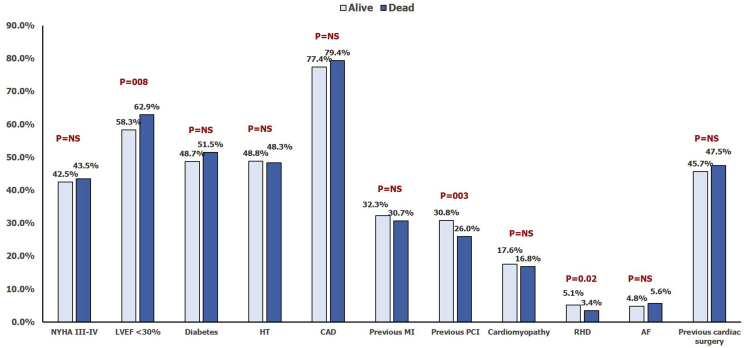
Table 1 and Fig. 1 provide baseline clinical and biochemical characteristics of the study subjects. Mean age of the subjects was 59.1 ± 11.8 years with 83.0% males. Mean LVEF was 30.0 ± 6.6%, and 59.1% of the subjects had LVEF ≤30%. CAD was the main cause of HF, accounting for nearly three-fourths of the total population.
Table 1.
Clinical and laboratory characteristics of the study participants.
| Characteristic | All subjects (5590) |
Survival status at 1 year after the index visit |
||
|---|---|---|---|---|
| Alive (4606) | Dead (984) | P-value | ||
| Age, years | 59.1 ± 11.8 | 58.6 ± 11.6 | 61.5 ± 12.2 | <0.001 |
| Age 50 years or below | 1173 (21.0) | 1012 (22.0) | 161 (16.4) | <0.001 |
| Male gender | 4637 (83.0) | 3807 (82.7) | 830 (84.3) | 0.20 |
| Heart rate, beats/min | 80 ± 11 | 80 ± 11 | 80 ± 12 | 0.46 |
| Blood pressure, mmHg | ||||
| Systolic | 114 ± 15 | 114 ± 15 | 113 ± 16 | 0.054 |
| Diastolic | 71 ± 9 | 71 ± 9 | 71 ± 9 | 0.079 |
| NYHA class | 0.045 | |||
| I | 435 (7.8) | 340 (7.4) | 95 (9.7) | |
| II | 2772 (49.6) | 2311 (50.2) | 461 (46.8) | |
| III | 2098 (37.5) | 1726 (37.5) | 372 (37.8) | |
| IV | 285 (5.1) | 229 (5.0) | 56 (5.7) | |
| Cardiovascular risk factors | ||||
| Diabetes | 2749 (49.2) | 2242 (48.7) | 507 (51.5) | 0.11 |
| Hypertension | 2733 (48.9) | 2248 (48.8) | 485 (49.3) | 0.78 |
| Smoking/tobacco use | 466 (8.3) | 376 (8.2) | 90 (9.1) | 0.31 |
| Alcohol consumption | 231 (4.1) | 190 (4.1) | 41 (4.2) | 0.95 |
| Underlying disease characteristics | ||||
| Coronary artery disease | 4348 (77.8) | 3567 (77.4) | 781 (79.4) | 0.19 |
| Previous myocardial infarction | 1790 (32.0) | 1488 (32.3) | 302 (30.7) | 0.32 |
| Previous PCI | 1676 (30.0) | 1420 (30.8) | 256 (26.0) | 0.003 |
| Cardiomyopathy | 977 (17.5) | 812 (17.6) | 165 (16.8) | 0.52 |
| Rheumatic heart disease | 266 (4.8) | 233 (5.1) | 33 (3.4) | 0.02 |
| LVEF, % | 30.0 ± 6.6 | 30.2 ± 6.6 | 29.2 ± 6.8 | <0.001 |
| LVEF 30% or below | 3304 (59.1%) | 2685 (58.3) | 619 (62.9) | 0.008 |
| Atrial fibrillation | 278 (5.0) | 223 (4.8) | 55 (5.6) | 0.33 |
| Previous cardiac surgery | 2574 (46.0) | 2107 (45.7) | 467 (47.5) | 0.33 |
| Blood investigation findings | ||||
| Hemoglobin, g/dL | 11.9 ± 2.0 | 11.9 ± 2.0 | 11.7 ± 2.0 | 0.01 |
| Blood urea, mg/dLa | 37.0 (28.0–53.0) | 37.0 (28.0–51.0) | 43.0 (31.0–66.0) | <0.001 |
| Serum creatinine, mg/dLa | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 1.1 (0.8–1.5) | <0.001 |
| Estimated GFR, mL/min/1.73 m2 | 76.1 ± 27.7 | 77.7 ± 26.9 | 68.5 ± 30.2 | <0.001 |
Continuous values are reported as mean ± standard deviation and categorical values as actual numbers with percentages in parentheses. Bold values in P-value column indicate statistically significant differences.
GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Reported as median with interquartile range because of non-normal distribution.
Fig. 1.
Baseline clinical characteristics of the study subjects according to survival status at one year. AF, atrial fibrillation; CAD, coronary artery disease; HT, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infraction; NS, not significant; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RHD, rheumatic heart disease.
Mean hemoglobin was 11.9 ± 2.0 gm/dL, and 65.8% subjects were found to be having anemia (defined as a hemoglobin concentration <13.0 g/dL in men and <12.0 g/dL in women10). Median serum creatinine was 1.0 mg/dL (interquartile range 0.8–1.3), with 7.8% subjects having values ≥ 2.0 mg/dL. Estimated glomerular filtration rate (estimated using Chronic Kidney Disease Epidemiology Collaboration equation11) was 76.1 ± 27.1 mL/min/1.73 m2, with 37.0% having values above 90 mL/min/1.73 m2, 56.7% between 30 and 89.9 mL/min/1.73 m2, and the remaining 6.3% below 30 mL/min/1.73 m2.
3.2. Treatment details
Table 2 summarizes treatment details of the study subjects at the baseline. The medication details represent treatment advice provided at the clinic, and it is not known whether these medications were continued as advised, changed, or stopped during the subsequent one year.
Table 2.
Pharmacological and device therapy details of the study participants.
| Characteristic | All subjects (5590) |
Survival status at 1 year after the index visit |
||
|---|---|---|---|---|
| Alive (4606) | Dead (984) | P-value | ||
| Drugsa | ||||
| Beta blockers | 4574 (81.8) | 3824 (83.0) | 750 (76.2) | <0.001 |
| ACEI | 2796 (50.0) | 2342 (50.8) | 454 (46.1) | 0.007 |
| ARB | 953 (17.0) | 772 (16.8) | 181 (18.4) | 0.22 |
| ACEI or ARB | 3680 (65.8) | 3062 (66.5) | 618 (62.8) | 0.027 |
| Loop diuretics | 4441 (79.4) | 3610 (78.4) | 831 (84.5) | <0.001 |
| Antiarrhythmic drugs | 677/4891 (13.8) | 533/4080 (13.1) | 144/811 (17.8) | <0.001 |
| Ivabradine | 971/4892 (19.8) | 786/4081 (19.3) | 185/811 (22.8) | 0.021 |
| Statin | 4205 (75.2) | 3491 (75.8) | 714 (72.6) | 0.033 |
| Digoxin | 247/4548 (5.4) | 195/3867 (5.0) | 52/681 (7.6) | 0.006 |
| Cardiac rhythm device therapy | ||||
| Overall usage | 585 (10.5) | 493 (10.7) | 92 (9.3) | 0.21 |
| Device type | 0.25 | |||
| CRT/CRTD | 240 (4.3) | 207 (4.5) | 33 (3.4) | |
| Dual-chamber pacemaker | 106 (1.9) | 84 (1.8) | 22 (2.2) | |
| AICD | 239 (4.3) | 202 (4.4) | 37 (3.8) | |
All values are reported as actual numbers with percentages in parentheses. Bold values in P-value column indicate statistically significant differences.
ACEI, angiotensin-converting enzyme inhibitor; AICD, automatic implantable cardioverter defibrillator; ARB, angiotensin receptor blocker; CRT, cardiac resynchronization therapy; CRTD, cardiac resynchronization therapy with defibrillator.
The medication details represent treatment advise provided at the clinic, and it is not known whether these medications were continued as advised, changed, or stopped during the subsequent one year.
3.3. All-cause mortality at one year
Of the total 5590 subjects, 984 subjects died by the end of one year, yielding an all-cause mortality rate of 17.6%. The clinical, biochemical, and treatment details of those who died versus those who remained alive are presented in Table 1, Table 2. Compared with those who remained alive, those who died were older in age (61.5 ± 12.2 years vs. 58.6 ± 11.6 years, P < 0.001) and were less likely to have RHD (3.4% vs. 5.1%, P = 0.02). Mean LVEF was lower in the deceased group (29.2 ± 6.8% vs. 30.2 ± 6.6%, P < 0.001) with 62.9% having LVEF ≤30% as compared with 58.3% in the other group (P = 0.008). The patients who died also had lower hemoglobin (11.7 ± 2.0 gm/dL vs. 11.9 ± 2.0 gm/dL, P = 0.01) and estimated glomerular filtration rate (eGFR) (68.5 ± 30.2 mL/min/1.73 m2 vs. 77.7 ± 26.9 mL/min/1.73 m2, P < 0.001).
The patients who died were less likely to be prescribed a beta blocker (76.2% vs. 83.0%, P < 0.001), ACEI (46.1% vs. 50.8%, P = 0.007), and statin (72.6% vs. 75.8%, P = 0.033) but were more likely to be prescribed a loop diuretic (84.5% vs. 78.4%, P < 0.001), antiarrhythmic drug (17.8% vs. 13.1%, P < 0.001), ivabradine (22.8% vs. 19.3%, P = 0.021), and digoxin (7.6% vs. 5.0%, P = 0.006). There was no difference in the usage of cardiac rhythm device therapy between the two groups.
On multivariate logistic regression analysis (Table 3), age, serum creatinine, and usage of loop diuretics and ivabradine were independently associated with one-year mortality, whereas rheumatic etiology had an inverse association. Beta blockers and previous PCI showed a trend in favor, whereas digoxin and antiarrhythmic drugs showed a trend toward an inverse relationship with one-year mortality.
Table 3.
Multivariate logistic regression analysis for prediction of one-year all-cause mortality.
| Parameter | Unstandardized coefficient B | Standard error | Wald | Odds ratio (95% confidence interval) | P-value |
|---|---|---|---|---|---|
| Age | 0.016 | 0.004 | 17.895 | 1.016 (1.009–1.024) | <0.001 |
| Left ventricular ejection fraction | −0.010 | 0.007 | 2.179 | 0.990 (0.978–1.003) | 0.140 |
| Previous percutaneous coronary intervention | −0.179 | 0.097 | 3.383 | 0.836 (0.691–1.012) | 0.066 |
| Rheumatic heart disease | −0.604 | 0.264 | 5.236 | 0.546 (0.326–0.917) | 0.022 |
| Beta blockera | −0.202 | 0.109 | 3.423 | 0.817 (0.660–1.012) | 0.064 |
| Angiotensin-converting enzyme inhibitora | −0.008 | 0.088 | 0.008 | 0.992 (0.835–1.179) | 0.927 |
| Loop diuretica | 0.323 | 0.121 | 7.088 | 1.382 (1.089–1.753) | 0.008 |
| Antiarrhythmic druga | 0.222 | 0.117 | 3.608 | 1.248 (0.993–1.569) | 0.057 |
| Ivabradinea | 0.228 | 0.105 | 4.727 | 1.256 (1.023–1.542) | 0.03 |
| Statina | −0.109 | 0.115 | 0.901 | 0.896 (0.715–1.123) | 0.343 |
| Digoxina | 0.316 | 0.172 | 3.362 | 1.372 (0.978–1.922) | 0.067 |
| Hemoglobin | 0.015 | 0.022 | 0.432 | 1.015 (0.971–1.060) | 0.511 |
| Serum creatinine | 0.266 | 0.048 | 30.343 | 1.305 (1.187–1.434) | <0.001 |
| Constant | −2.966 | 0.485 | 37.456 | 0.052 | <0.001 |
Bold values in P-value column indicate statistically significant differences.
The medication details represent treatment advise provided at the clinic, and it is not known whether these medications were continued as advised, changed, or stopped during the subsequent one year.
4. Discussion
The present study represents the largest single-center data of HF patients reported so far and the largest study involving HF patients in India. The salient findings of our study are- (1) consistent with the previous small reports from India, our patients were much younger than the western HF patients and CAD was the dominant cause of HF; (2) compared with previous reports, we reported a higher usage of beta blockers and more frequent history of previous cardiac procedures in our patients; despite this, the one-year mortality was substantial; and (3) increasing age, renal dysfunction, and usage of loop diuretics and ivabradine were independently associated with increased risk of mortality in this cohort, whereas rheumatic etiology had a favorable impact.
Callender et al recently performed a meta-analysis of 53 studies on HF patients from LMIC.9 They found that the presentation, underlying causes, management, and outcomes of HF varied substantially across LMICs. This heterogeneity across nations underscores the need to develop regional HF databases to gain insights into the characteristics and outcomes of HF in different ethnic/geographic groups. A few registries have subsequently included Indian patients, including Asian Sudden Cardiac Death in Heart Failure registry (ASIAN-HF),7 International Congestive Heart Failure registry (INTER-CHF),8, 12 and Trivandrum Heart Failure Registry (THFR).6, 13 Apart from these, the American College of Cardiology's Practice Innovation And Clinical Excellence (PINNACLE) India Quality Improvement Program also collected data on prescription of guideline-directed medical therapy to ambulatory HF patients.14 However, this survey had limited scope in terms of the information collected and did not report mortality outcomes. With this background, our study provides additional information that should be of help in further defining changing epidemiological profile of HF in India, identifying key problem areas, and developing strategies required to deal with the problem. Importantly, as discussed below, we found high one-year mortality among our patients despite high usage of beta blockers and coronary revascularization procedures. This finding once again reiterates the urgent need to implement strategies required to prevent development of HF and greater allocation of resources to such programs.
4.1. Comparison with previous HF registries
4.1.1. Clinical profile of HF
Previous studies have shown that Indian HF patients are approximately 10 years younger than their western counterparts.13, 15, 16, 17, 18 Consistent with these observations, we also found mean age of our patients to be 59 years, which is similar to that reported in the THFR13 and INTER-CHF,8 but much less than the same in the USA-based17, 18 and European registries15, 16 (Table 4). The lower age of our patients reflects development of CAD at a younger age in Indians, as has been demonstrated in several previous studies.19, 20, 21
Table 4.
Comparison of our findings with those of major previous registries.
| Studyparameters | Medanta |
INTER-CHF8 |
THFR6 |
ASIAN-HF7 |
ADHERE (only HFrEF)17 | ADHERE-AP23 | OPTIMIZE-HF (only HFrEF)18 | GWTG-HF (HFrEF)22 | EHFS II16 |
ESC-HF Pilot15 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Indian cohort | Overall | HFrEF | Overall | Southeast Asia (India) | Acute HF | Chronic HF | |||||||
| N | 5590 | 5283 | 858 | 1205 | 894 | 5275 | 1436 | 25865 | 10171 | 20118 | 15716 | 3580 | 1892 | 3226 |
| Regions | Predominantly North India | Africa, Asia, the Middle East, and South America | India | Trivandrum (South India) | 11 Asian countries including India | India | USA | 8 Asia–Pacific countries (India not included) | USA | USA | Europe | Europe | ||
| Time Frame | 2014–2017 | 2012–2014 | 2013 | 2012–2015 | 2001–2004 | 2006–2008 | 2002–2005 | 2005–2012 | 2004–2005 | 2009–2010 | ||||
| Presentation | Ambulatory HF patients | Ambulatory as well as hospitalized patients | Ambulatory as well as hospitalized patients | Hospitalized patients | Hospitalized patients | Ambulatory as well as hospitalized patients | Ambulatory as well as hospitalized patients | Hospitalized HF | Hospitalized HF | Hospitalized HF | Hospitalized HF | Hospitalized HF | Hospitalized HF | Ambulatory HF |
| HFrEF | 100 | 50 | 53 | 74.1a | 100a | 100 | 100 | 100 | 53 | 100 | 100 | 66a | 64a | 69a |
| LVEF, % | 30.0 ± 6.6 | 28 (22, 33) | 29 (25, 33) | 34 ± 16 | – | 24 ± 8 | 25 (20, 30) | 38 ± 15 | 38 ± 14 | 38 ± 13 | ||||
| Age, years | 59 ± 12 | 59 ± 15 | 56 ± 15 | 61 ± 14 | 62 ± 13 | 60 ± 13 | 58 ± 13 | 70 ± 14 | 67 | 70 ± 14 | 79 (72–85) | 70 ± 13 | 69 ± 13 | 66 ± 13 |
| Male, % | 83.0 | 61 | 62 | 69.2 | 72.1 | 78.2 | 75.7 | 60 | 57 | 62 | 60 | 61 | 63 | 70 |
| Ischemic etiology/CAD | 77.8 | 39 | 46 | 71.9 | 75.7 | 50.2 | 51.1 | 59 | 50 | 64 | 58 | 54 | 51 | 41 |
| Prior MI | 32.0 | 36 | – | – | 24.9 | |||||||||
| Hypertension | 48.9 | 57.8 | 58.7 | 51.9 | 37.9 | 69 | 64 | 66 | 73.1 | 63 | 62 | 58 | ||
| Diabetes mellitus | 49.2 | 29 | 26 | 54.9 | 56 | 40.4 | 37.1 | 40 | 45 | 39 | 39.3 | 33 | 35 | 29 |
| RHD | 4.8 | 7.9 | 3.9 | |||||||||||
| Atrial fibrillation/flutter | 5.0 | 14.7 | 11.7 | 17.9 | 4.2 | 31 | 24 | 28 | 36.1 | 39 | 44 | 39 | ||
| CKDb | 7.8 | 8 | 3 | 17.9 | 17.8 | 18 | 22 | – | 20.9 | 17 | 23 | 18 | ||
| Heart rate, beats/min | 80 ± 11 | 98 ± 22 | 98 ± 22 | 80 ± 16 | 82 ± 16 | 93 ± 23 | – | 89 ± 22 | 82 (71–98) | 95 (77–114) | 88 ± 24 | 72 ± 14 | ||
| Systolic BP, mmHg | 114 ± 15 | 125 ± 23 | 125 ± 21 | 129 ± 32 | 128 ± 30 | 118 ± 20 | 116 ± 19 | 139 ± 31 | – | 136 ± 31 | 132 (115–151) | 135 (110–160) | 133 ± 29 | 125 ± 20 |
| Serum creatinine, mg/dL | 1.0 (0.8–1.3) | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.6 ± 1.3 | – | 1.4 (1.1, 1.9) | 1.4 (1.0–1.8) | |||||||
| Beta blockers | 81.8 | 67 | 57 | 58 | 60 | 79 | 67 | 63 | 41 | 73 | 73 | 61.4 | 66–85 | 87–92 |
| ACEI/ARB | 65.8 | 74 | 68 | 49 | 50 | 75 | 78 | 71 | 63 | 73 | 88 | 80.2 | 70–79 | 84–91 |
| Digoxin | 5.4 | 26 | 25 | 28 | 29 | 44.1 | 34 | 38 | 26.6 | |||||
| Ivabradine | 19.8 | |||||||||||||
| Statin | 75.2 | |||||||||||||
| CRT/AICD/Pacemaker | 10.5 | 14.3 | 7 | 9.3 | 23.1 | |||||||||
| 1-yr all-cause mortality | 17.6 | 16.5 | 23.3 | 30.8 | 32.0 | 6.9c | 5.4c | 9.8d | 37.5 | 17.4 | 7.2 | |||
Continuous values are reported as mean ± standard deviation or as median with interquartile range, and categorical values are reported as actual numbers with percentages in parentheses.
ACEI, angiotensin-converting enzyme inhibitor; ADHERE, Acute Decompensated Heart Failure National Registry; ADHERE-AP, Acute Decompensated Heart Failure National Registry International–Asia Pacific; AICD, automatic implantable cardioverter-defibrillator; ARB, angiotensin II receptor blockers; ASIAN–HF, Asian Sudden Cardiac Death in Heart Failure; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; EHFS, EuroHeart Failure Survey; ESC-HF Pilot, European Society of Cardiology Heart Failure Pilot; GWTG–HF, Get With The Guidelines–Heart Failure; HFrEF, heart failure with reduced ejection fraction; INTER–CHF, International Congestive Heart Failure; LVEF, left ventricular ejection fraction; MI, myocardial infraction; OPTIMIZE–HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure; RHD, rheumatic heart disease; THFR, Trivandrum Heart Failure Registry.
In these registries, HFrEF was defined as LVEF <45%, unlike other registries which used <40% as the cut-off.
CKD defined in most registries as serum creatinine >2 mg/dL or need for dialysis. The exact definition not provided in INTER-CHF, THFR, and GTWG-HF, whereas ESC-HF Pilot used >1.5 mg/dL as the cut-off for serum creatinine.
6-months mortality only.
90-days mortality only.
More than 80% of our subjects were males, which is higher than most of the previous registries.8, 15, 16, 17, 18, 22, 23, 24 This likely represents a gender bias in patients seeking healthcare in our community, which is not uncommon in many of the developing nations.
CAD was present in three-fourths of our subjects, which is similar to that seen in THFR,6 but much greater than in most other registries.7, 8, 15, 16, 17, 18, 24 While this may partly be because of the profile of the patients presenting to our center, equally high prevalence of CAD in THFR suggests that this more likely reflects the current epidemiological scenario of CAD in India. The prevalence of CAD has risen sharply in India over the last three decades, which is now driving the rise in the prevalence of HF. In contrast to this, RHD was seen in only a few patients, once again reiterating the declining incidence of RHD in India. Although the proportion of RHD patients would be higher at public hospitals, CAD is the dominant etiology of HF there too.
In our study, hypertension was less common but diabetes more common than in most of the western registries.15, 16, 17, 18 The lower prevalence of hypertension can be explained by the younger age of our subjects and the fact that we did not include subjects with HF with preserved EF (HFpEF), which has a stronger association with hypertension. The higher prevalence of diabetes is likely to be due to high prevalence of the same in our community. Notably, India has the second largest population of diabetes patients in the world at present.25
Atrial fibrillation/flutter were seen in <5% of our subjects, which is similar to the prevalence reported in the ASIAN-HF registry,7 but much lower than in the western registries.15, 16, 17, 18 The lower prevalence of atrial arrhythmias was expected given the younger age of our subjects and non-inclusion of HFpEF subjects, both of which are associated with high incidence of atrial fibrillation.
4.1.2. Treatment pattern
We report a high usage of beta blockers and low usage of digoxin, both reflecting better adherence to the current practice recommendations.26 The beta blocker usage in our patients was, in fact, higher than even in the registries from Europe or the USA16, 17, 18, 23 (Table 4). Additionally, as most of our patients had underlying CAD, a high proportion of subjects received statin also. The usage of ACEI/ARB was, however, lower than some of the previous registries.7, 15, 16, 17, 18, 22 The lesser usage of ACEI/ARB possibly reflects lower prevalence of hypertension and lower systolic blood pressure in our patients.
A high proportion of our subjects had undergone a cardiac surgery or PCI in past. This is likely to be due to high prevalence of CAD in our subjects and also the fact that ours is a large, tertiary-care referral center. The use of cardiac rhythm devices was seen in roughly 10% subjects, which is lower than the average usage in the registries involving ambulatory HF patients, such as ASIAN-HF7 and the European Society of Cardiology HF Pilot (ESC-HF Pilot).15 The lower usage of these devices in India is because of cost limitations as well as reluctance on the part of the patients/treating physicians toward an invasive procedure, especially for primary prevention purpose.
4.1.3. Mortality rate
We reported one-year all-cause mortality of 17.6% which is much higher than that reported in ASIAN-HF7 or ESC-HF Pilot (ambulatory HF cohort)15 but lower than that observed in Indian patients in INTER-CHF8 or THFR6 (Table 4). The high mortality in our subjects is surprising, considering greater use of beta blockers and high proportion of subjects with previous coronary bypass surgery or PCI. The mean LVEF was also not different in our subjects as compared to ASIAN-HF.7 The higher mortality could be because of significantly higher prevalence of CAD in our subjects. Unlike HF resulting from cardiomyopathy, HT or diabetes, LV systolic dysfunction due to previous MI and underlying CAD is often irreversible and could be responsible for a higher mortality rate.
The multivariate analysis showed that age, serum creatinine, and usage of loop-diuretics and ivabradine were associated with higher mortality, whereas the rheumatic etiology was associated with lower mortality. Serum creatinine has been consistently shown to be an independent predictor of mortality in almost all the registries.6, 8, 15, 17 Similarly, several cohort studies have suggested that the use of loop diuretics in chronic HF is associated with increased mortality.27, 28, 29, 30 It has been further suggested that their usage represents a true risk factor for increased mortality, rather than just a disease severity marker.31 The relationship with ivabradine is more complex. Higher resting heart rate is a well-established predictor of mortality in HF.32, 33, 34, 35 Therefore, the higher mortality in our patients on ivabradine likely reflects the effect of higher baseline heart rate in these patients (84 ± 12 beats/min vs. 79 ± 10 beats/min, P < 0.001) who were sicker and could not receive larger doses of beta blocker. We also found trends toward favorable impact of beta blockers and adverse impact of digoxin usage on mortality, which is consistent with the current understanding of their role in the management of HF.
4.2. Limitations
Our study has several limitations that need to be acknowledged. First, this was a hospital-based study and, therefore, may not be reflective of the actual scenario of HF in the community. However, while population-based surveys and epidemiological studies are likely to yield more accurate information, such studies are impractical given the challenges inherent in diagnosing HF in the community. Accordingly, most of the published data on HF have been derived from hospital-based registries only.6, 7, 8, 15, 16, 17, 18, 24, 36, 37, 38 Such hospital-based registries add to our knowledge regarding the behavior of the disease and offer a reality check about the implementation of guideline-directed medical therapies. They also identify the utilization of device-based therapies and hurdles in their widespread use. Outcomes with various therapies can be identified, and they bring out regional differences in management and outcomes.
Second, ours was a single-center study which introduces an inevitable bias. However, the large sample size and the fact that our hospital receives patients from most parts of North India (both rural and urban) partially mitigate this limitation.
Third, a large proportion of our subjects had undergone previous cardiac procedures, reflective of our center being a referral center. This does introduce a selection bias, which was inevitable. However, at the same time, this allowed us to study clinical outcomes of HF despite the availability of best resources. As mentioned previously, the high mortality rate in our study reinforces the need to prevent development of HF, rather than to treat it- an important message in the context of rapidly rising incidence and prevalence of CAD in our country.
Finally, due to the largely retrospective nature of our study and logistic constraints, we were not able to collect detailed information about the clinical characteristics at baseline (e.g. duration of HF), treatment prescribed (e.g. dosages of various medications used in our patients, usage of mineral ocorticoid receptor antagonists, etc.), and clinical outcomes during follow-up (e.g. change in functional class, LVEF, etc.), except for one-year mortality. Although, many of the previous large-scale prospective registries have also not reported these details, this does remain an important limitation. We are now in the process of collecting these details prospectively.
5. Conclusions
In this largest study of HF patients from India, we describe clinical characteristics and one-year mortality in ambulatory patients with HFrEF. Given the enormous magnitude of HF burden in India and the paucity of information on this subject, our study provides data that should be of help in identifying key problem areas, potential solution to mitigate those challenges, and the future research priorities. Such information should be useful to inform policy formulation in our country, besides being of interest to HF specialists at large.
Funding
No external funding was received for this work.
Conflict of interest
All authors have none to declare.
Acknowledgments
The authors thank Mr Manish Kumar Singh, Biostatistician, Medanta - The Medicity, for his help in data compilation and analysis.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Huffman M.D., Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India. 2010;23:283–288. [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran D., Jeemon P., Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605–1620. doi: 10.1161/CIRCULATIONAHA.114.008729. [DOI] [PubMed] [Google Scholar]
- 4.Pais P., Xavier D. Heart failure in India: an area of darkness. Natl Med J India. 2011;24:53. [PubMed] [Google Scholar]
- 5.Pillai H.S., GanaPathi S. Heart failure in South Asia. Curr Cardiol Rev. 2013;9:102–111. doi: 10.2174/1573403X11309020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanjay G., Jeemon P., Agarwal A. In-hospital and three-year outcomes of heart failure patients in South India: the Trivandrum Heart Failure Registry. J Card Fail. 2018;24:842–848. doi: 10.1016/j.cardfail.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam C.S., Teng T.K., Tay W.T. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Feart Failure registry. Eur Heart J. 2016;37:3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 8.Dokainish H., Teo K., Zhu J. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017;5:e665–e672. doi: 10.1016/S2214-109X(17)30196-1. [DOI] [PubMed] [Google Scholar]
- 9.Callender T., Woodward M., Roth G. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001699. e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanc B., Finch C.A., Hallberg L., Herbert V., Lawkowicz W., Layrisse M. Nutritional anaemias. Report of a WHO scientific group. WHO Tech Rep Ser. 1968:1–40. [Google Scholar]
- 11.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dokainish H., Teo K., Zhu J. Heart failure in Africa, Asia, the Middle East and South America: the INTER-CHF study. Int J Cardiol. 2016;204:133–141. doi: 10.1016/j.ijcard.2015.11.183. [DOI] [PubMed] [Google Scholar]
- 13.Harikrishnan S., Sanjay G., Anees T. Clinical presentation, management, in-hospital and 90-day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17:794–800. doi: 10.1002/ejhf.283. [DOI] [PubMed] [Google Scholar]
- 14.Pokharel Y., Wei J., Hira R.S. Guideline-directed medication use in patients with heart failure with reduced ejection fraction in India: American College of cardiology's PINNACLE India Quality Improvement Program. Clin Cardiol. 2016;39:145–149. doi: 10.1002/clc.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maggioni A.P., Dahlstrom U., Filippatos G. EURObservational research programme: regional differences and 1-year follow-up results of the Heart Failure Pilot survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen M.S., Brutsaert D., Dickstein K. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 17.Yancy C.W., Lopatin M., Stevenson L.W. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow G.C., Stough W.G., Abraham W.T. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 19.Joshi P., Islam S., Pais P. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. J Am Med Assoc. 2007;297:286–294. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 20.Mohanan P.P., Mathew R., Harikrishnan S. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xavier D., Pais P., Devereaux P.J. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 22.Cheng R.K., Cox M., Neely M.L. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Atherton J.J., Hayward C.S., Wan Ahmad W.A. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific) J Card Fail. 2012;18:82–88. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Sato N., Kajimoto K., Keida T. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry) Circ J. 2013;77:944–951. doi: 10.1253/circj.cj-13-0187. [DOI] [PubMed] [Google Scholar]
- 25.IDF Diabetes Atlas. 6th ed. International Diabetes Federation; Brussels, Belgium: 2013. https://www.idf.org/component/attachments/attachments.html?id=1405&task=download [Google Scholar]
- 26.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 27.Damman K., Kjekshus J., Wikstrand J. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2016;18:328–336. doi: 10.1002/ejhf.462. [DOI] [PubMed] [Google Scholar]
- 28.Domanski M., Norman J., Pitt B. Diuretic use, progressive heart failure, and death in patients in the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 29.Hamaguchi S., Kinugawa S., Tsuchihashi-Makaya M. Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure: a report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD) Circ J. 2012;76:1920–1927. doi: 10.1253/circj.cj-11-1196. [DOI] [PubMed] [Google Scholar]
- 30.Miura M., Sugimura K., Sakata Y. Prognostic impact of loop diuretics in patients with chronic heart failure- effects of addition of renin-angiotensin-aldosterone system inhibitors and beta-blockers. Circ J. 2016;80:1396–1403. doi: 10.1253/circj.CJ-16-0216. [DOI] [PubMed] [Google Scholar]
- 31.Kapelios C.J., Malliaras K., Kaldara E., Vakrou S., Nanas J.N. Loop diuretics for chronic heart failure: a foe in disguise of a friend? Eur Heart J Cardiovasc Pharmacother. 2018;4:54–63. doi: 10.1093/ehjcvp/pvx020. [DOI] [PubMed] [Google Scholar]
- 32.Pocock S.J., Wang D., Pfeffer M.A. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 33.Fox K., Ford I., Steg P.G. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 34.McAlister F.A., Wiebe N., Ezekowitz J.A., Leung A.A., Armstrong P.W. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Kotecha D., Flather M.D., Altman D.G. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017;69:2885–2896. doi: 10.1016/j.jacc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Chioncel O., Vinereanu D., Datcu M. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162:142–153 e141. doi: 10.1016/j.ahj.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Senni M., Gavazzi A., Oliva F. In-hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. Results from IN-HF Outcome Registry. Int J Cardiol. 2014;173:163–169. doi: 10.1016/j.ijcard.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Zannad F., Mebazaa A., Juilliere Y. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]