Graphical abstract
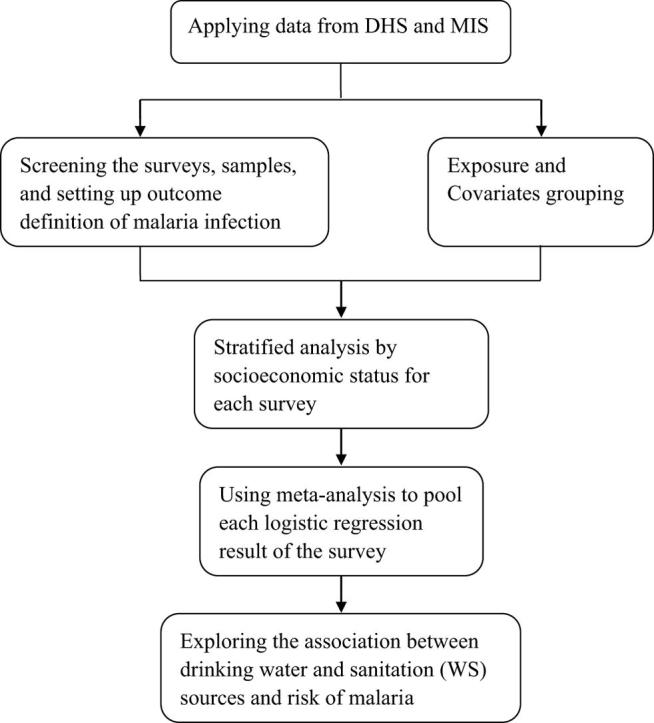
Flowchart of the method to explore the association between the type of WS and malaria infection among children under five years across sub-Saharan Africa.
Keywords: Drinking water, Sanitation, Malaria, Risk, Children, Sub-Saharan Africa
Abbreviations: SSA, sub-Saharan Africa; LLINs, long-lasting insecticidal mosquito nets; ITNs, insecticide treated nets; IRS, indoor residual spraying; WHO, World Health Organization; WASH, water, sanitation, and hygiene; NTDs, neglected tropical diseases; WS, drinking water and sanitation; SDGs, sustainable development goals; DHS, Demographic and Health Survey; MIS, Malaria Indicator Surveys; RDT, rapid diagnostic test; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; STHs, soil transmitted helminth diseases
Highlights
-
•
Drinking water and sanitation is a risk factor to malaria infection.
-
•
Wealth brought mixed effects of the relationship between WS and malaria.
-
•
The associations between WS and malaria were more pronounced among the non-poor children.
-
•
This pooling multi-country data eliminates many bias seen in traditional meta-analysis.
-
•
Improved drinking water and sanitation seemed to be promising in preventing malaria.
Abstract
Current efforts for the prevention of malaria have resulted in notable reductions in the global malaria burden; however, they are not enough. Good hygiene is universally considered one of the most efficacious and straightforward measures to prevent disease transmission. This work analyzed whether improved drinking water and sanitation (WS) conditions were associated with a decreased risk of malaria infection. Data were acquired through surveys published between 2006 and 2018 from the Demographic and Health Program in sub-Saharan Africa (SSA). Multiple logistic regression was used for each national survey to identify the associations between WS conditions and malaria infection diagnosed by microscopy or a malaria rapid diagnostic test (RDT) among children (0–59 months), with adjustments for age, gender, indoor residual spraying (IRS), insecticide-treated net (ITN) use, house quality, and the mother’s highest educational level. Individual nationally representative survey odds ratios (ORs) were combined to obtain a summary OR using a random-effects meta-analysis. Among the 247,440 included children, 18.8% and 24.2% were positive for malaria infection based on microscopy and RDT results, respectively. Across all surveys, both unprotected water and no facility users were associated with increased malaria risks (unprotected water: aOR 1.17, 95% CI 1.07–1.27, P = 0.001; no facilities: aOR 1.35, 95% CI 1.24–1.47, P < 0.001; respectively), according to microscopy, whereas the odds of malaria infection were 48% and 49% less among piped water and flush-toilet users, respectively (piped water: aOR 0.52, 95% CI 0.45–0.59, P < 0.001; flush toilets: aOR 0.51, 95% CI 0.43–0.61, P < 0.001). The trends of individuals diagnosed by RDT were consistent with those of individuals diagnosed by microscopy. Risk associations were more pronounced among children with a “nonpoor” socioeconomic status who were unprotected water or no facility users. WS conditions are a vital risk factor for malarial infection among children (0–59 months) across SSA. Improved WS conditions should be considered a potential intervention for the prevention of malaria in the long term.
Introduction
Malaria is one of the most severe public health problems, posing significant risks to the lives of children, especially in sub-Saharan Africa (SSA). Although cases of malaria have decreased by an estimated 20 million since 2010 [1], there was no significant progress in reducing the number of global cases from 2015 to 2017 [1]. Current efforts to prevent malaria mainly include preventive and symptomatic treatment with antimalarial compounds, consisting of artemisinin-based combination therapies [2], as well as vector control with long-lasting insecticidal mosquito nets (LLINs) and indoor residual spraying (IRS) [3], [4]; these methods have resulted in reductions in case incidence and mortality. However, increasing evidence has revealed that these efforts can only go so far [1], [5]. Therefore, we need to determine and invest in additional effective measures to tackle the complex challenges.
Good hygiene is universally known as one of the most efficacious and straightforward measures to prevent disease transmission [6]. To date, the water, sanitation and hygiene (WASH) component of the strategy has received little attention, and the potential to link WASH efforts with malaria and neglected tropical disease (NTD) transmission has been largely untapped [7]. Some studies explored the effect of water and sanitation (WS) on malaria in Ethiopia and Kenya on a small scale [8], [9], [10], [11], but there are no clear existing studies that have comprehensively evaluated the association between different types of WS conditions and malaria infection among children under five years old across a broad epidemic region, such as SSA. Considering the target date for the malaria roadmap and for the Sustainable Development Goal (SDG) of universal access to basic WASH in communities, schools, and health care facilities is both 2030 [7], [12], the primary hypothesis was whether the redoubling of efforts to improve WS and its recognition as a new policy for the prevention and control of malaria transmission can contribute to the achievement of malaria elimination targets from 2016 to 2030.
It is well known that Demographic and Health Survey (DHS) and Malaria Indicator Survey (MIS) are national cross-sectional surveys that provide data for many indicators in the areas of health, populations, and nutrition [13], [14], [15]. Each DHS survey usually takes an average of 18–20 months and is executed in four phase [13]. Although most of the collected variables are different in each survey [14], [15], the types of WS sources used by children under five years old are meticulously classified, and the available data provide a convenient condition to comprehensively evaluate the effect of WS conditions on the risk of malaria on a large scale.
In this study, using all the available data derived from DHS and MIS in SSA, a model analysis of the relationship between WS and malaria was performed. Specifically, the hypothesis that the odds of malaria infection in children under 5 years old with access to improved WS conditions across SSA are lower than those in children with access to unimproved WS conditions across SSA was tested. This is the most comprehensive study of the relationship between WS conditions and malaria across SSA to date, and it is also the first to demonstrate the effects between drinking water and sanitation use in relation to malaria prevalence stratified by household socioeconomic status on a large scale.
Methods
Study design and data sources
A model analysis of individual-level data that were acquired through surveys published between 2006 and 2018 and performed by the DHS Program in SSA was conducted. The cross-sectional survey data used in this study had been provided by the DHS Program. First, surveys were excluded if the data on malaria infection in children or information on WS conditions were not complete. Second, participants in each survey were excluded if there was no data or ambiguous data on their WS use (these variables in the DHS and MIS were always represented in the form of “do not know” or “others”) or if their age was over 59 months. Only children under five years old were included in this study because they (including infants) are the most vulnerable group, especially in high-transmission areas of the world [16]. More importantly, only this age group was tested for malaria infection during all the DHS and MIS surveys. Then, each national DHS and MIS survey on the exposure to various WS conditions and risk of malaria was separately analyzed for the outcome definition, exposure and covariate groupings, and stratified analysis by household socioeconomic status. Finally, to obtain a summary OR, individual national survey ORs obtained by multivariable logistic regression were synthesized through a random-effects meta-analysis.
Outcome definition
The endpoint was the participants’ malaria status as measured by a malaria rapid diagnostic test (RDT) or microscopy using thick or thin blood smears. A positive result by either of these two test methods indicated a malaria case. Because the microscopy results of the participants from Angola 2015–2016, Angola 2006–2007, Cameroon 2011, Liberia 2016, Mozambique 2015, Tanzania 2017, and Uganda 2016 were not available, only the RDT results for these participants were recorded in the aforementioned years.
Exposure: drinking water and sanitation (WS)
The DHS and MIS classified drinking water sources into five groups (piped water, tube well water, dug well, surface water, others), and they categorized sanitation sources into three groups (flush or pour flush-toilet, pit latrine toilet, and no facility). In this study, the DHS/MIS sanitation classifications were used. However, drinking water sources were condensed into three groups (piped water in accordance with the DHS/MIS definition, protected water, and unprotected water) [10]. Protected water was obtained from a tube well or borehole, protected well, protected spring, tanker truck, cart with a small tank, bicycle with jerrycans, bottles, or sachets [10]. Unprotected water was obtained from an unprotected well, unprotected spring, river, dam, lake, pond, stream or the rain [10].
Covariates
Information on the participants’ age, gender, IRS in the past 12 months, insecticide-treated net (ITN) use, house quality, mother’s highest educational level, and socioeconomic status was collected. For these covariates, age (in months) was treated as a continuous variable. Gender was categorized into two groups (male versus female). IRS in the past 12 months was treated as a dichotomized variable (yes/no). ITN use was grouped into three categories (ITNs or LLINs, untreated nets, or no nets). Specifically, if ITNs were >1 year old or were not retreated within a year before the survey [13], [17] or if LLINs were 3 years old at the time of survey, these nets were considered “untreated nets” [13], [18], [19], [20]. House quality was divided into two groups (modern versus traditional). Houses built with finished walls, a finished roof, and a finished floor were categorized as “modern”, while all other houses were categorized as “traditional” [13]. Mother’s highest educational level was classified into four groups (no education, primary, secondary, or higher), which were in accordance with the DHS/MIS definitions. The DHS and MIS classified the population’s socioeconomic status into five categories, namely, “poorest”, “poor”, “middle”, “rich”, and “richest”. In this study, the total population was classified into two groups for further stratified analyses, namely, “poor” (poorest + poor) and “nonpoor” (middle + rich + richest). No missing values were observed for all the other covariates in each survey, except for IRS in the past 12 months and mother’s highest educational level in some surveys (no data on IRS in the past 12 months in Angola 2011, DRC 2013–2014, Kenya 2015, Liberia 2009, Madagascar 2016, Malawi 2017, Rwanda 2014–2015, Rwanda 2010, Tanzania 2017, Togo 2017, Togo 2013–2014, Uganda 2009; no data on mother’s highest educational level in Rwanda 2017).
Stratified analyses by household socioeconomic status
For descriptive analyses, chi-square (χ2) tests or Fisher’s exact tests were used for each survey to compare the prevalence of unprotected water and piped water with that of protected water, and the prevalence of flush toilets and no facility sources with that of pit latrine toilets among the total population. Chi-square (χ2) tests or Fisher’s exact tests were also used to compare the proportion of “poor” associated with different WS conditions for each survey.
Second, a logistic regression model was used to conduct the primary analysis of the total population to estimate the adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) of the associations between different WS conditions and malaria infection for each survey, considering protected water and pit latrine toilets as reference. In these regression analyses, aORs were adjusted for (i) age in months, (ii) gender, (iii) IRS in the past 12 months, (iv) ITN use, (v) house quality, and (vi) mother’s highest educational level. The main reasons for the retention of the above covariables in the “best” model were based on clinical or statistical significance in previous studies [13], [17], [21]. Furthermore, for the stratified analyses, the population was first categorized into two groups, namely, “poor” children and “nonpoor” children in each survey. Then, the aORs revealing the associations between WS conditions and the odds of malaria infection in children aged 0–59 months in a logistic regression model were performed for each DHS/MIS survey among those who were “poor” and “nonpoor”, respectively, adjusting for the above confounding factors.
Finally, a meta-analysis method was performed to combine data from independent scientific trials as well as observational studies. In this study, each national survey was conducted independently. Using national survey data based on a random-effects meta-analysis might eliminate many biases typically related to pooling observational data, such as publication, selection, and measurement biases and selective outcome reporting bias. In this study, to determine the overall and the stratified aORs for WS and malaria risks among all the surveys, random-effect models in the meta-analysis were used to pool logistic regression results for the surveys which were calculated among total children, “poor” children, and “nonpoor” children, respectively. Furthermore, to investigate the heterogeneity among the survey-specific effects, Tau-squared statistics, I2 statistics and P-values were analyzed with chi-square and Cochran’s Q tests.
All analyses were conducted using SPSS Statistics version 22.0 (IBM Co., Armonk, NY, USA), except for the meta-analysis and forest plots, which were performed using STATA version 15.0 (StataCorp, College Station, TX, 77845, USA) and relating line diagrams and bar charts in GRAPHPAD PRISM version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 for each overall aOR was considered statistically significant.
Results
Study population
After screening 189 identified surveys (136 DHS, 27 MIS, and 26 others) published between 2006 and 2008, none of 138 surveys met the inclusion criteria because they did not document malaria infection status (Additional file 1). After the removal of 138 surveys, 2 surveys were further excluded because they did not contain data on WS use (Additional file 1). Finally, 49 surveys (23 DHS, 24 MIS, and 2 others) including data for 307,365 individuals from 23 countries (Additional file 1) were identified. Among the identified individuals, 6,058 did not record information on WS use, and the age of 53,867 individuals was over 59 months; thus, these 59,925 individuals were excluded (Additional file 1). Overall, 49 eligible surveys comprising data for 247,440 individuals were included in the analysis (Additional file 1).
Table 1 provides the descriptive statistics for the health outcomes and covariates. Of the included individuals, 213,920 children aged 0–59 months were tested for malaria infection using microscopy, with a prevalence of 18.8%, whereas 59,988 (24.2%) positive cases were identified in 247,440 children by RDTs (Table 1). Across all surveys, the average age of the children was 32.6 months, and 50.2% were male (Table 1). Nearly half (47.3%) of the mothers had no education, this proportion ranged from 10.1% (Malawi 2017) to 83.0% (Burkina Faso 2010). With regard to preventive measures targeting vectors, data on the use of ITNs and IRS for each survey were extracted. As shown in Table 1, it is clear that ITN usage was less than half (45.8%) overall and ranged from 15.2% (Cameroon 2011) to 71.5% (Burkina Faso 2014). Among the households surveyed, 12.5% experienced IRS in the past 12 months. With regard to house quality, the majority of the overall houses were traditional (69.7%), ranging from 38.1% (Ghana 2014) to 100% (Uganda 2009).
Table 1.
Characteristics of children under five years old across SSA who were included in the analysis.
| Country and year | N | Mean age (Months) | Male (%) | Mother's highest educational level (no education valid percent)* | ITN use (%) | IRS in Past 12 mo (Valid Percent)* | Traditional house (%) | Socioeconomic status (the poor percent) | Parasite rate (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Microscopy | RDT | |||||||||
| Angola 2015–2016 | 6746 | 31.9 | 50.4 | 36.8 | 21.2 | 1.4 | 71.2 | 53.3 | – | 16.5 |
| Angola 2011 | 3259 | 32.1 | 48.1 | 35.4 | 21.9 | – | 69.8 | 47.1 | 9.8 | 12.5 |
| Angola 2006–2007 | 2573 | 32.2 | 44.1 | 32.3 | 17.8 | 4.2 | 61.6 | 54.4 | – | 22.2 |
| Benin 2011–2012 | 3709 | 33.2 | 51.7 | 74.7 | 69.6 | 12.6 | 62.3 | 44.9 | 29.9 | 27.1 |
| Burkina Faso 2014 | 6090 | 32.5 | 50.8 | 81.6 | 71.5 | 0.7 | 82.4 | 44.7 | 47.6 | 64.5 |
| Burkina Faso 2010 | 6088 | 32.1 | 51.4 | 83.0 | 44.5 | 1.6 | 77.5 | 40.9 | 65.0 | 75.6 |
| Burundi 2016–2017 | 5755 | 32.5 | 50.3 | 44.0 | 36.8 | 0.8 | 84.3 | 40.0 | 24.4 | 34.8 |
| Burundi 2012 | 3710 | 32.8 | 50.3 | 47.6 | 48.0 | 4.5 | 86.2 | 42.0 | 16.2 | 20.5 |
| Cameroon 2011 | 5367 | 31.7 | 49.1 | 23.3 | 15.2 | 3.1 | 63.0 | 43.1 | – | 32.6 |
| Coate D Ivoire 2011–2012 | 3762 | 31.6 | 43.6 | 67.9 | 37.0 | 1.4 | 43.0 | 50.5 | 16.1 | 42.0 |
| DRC 2013–2014 | 8159 | 32.5 | 49.8 | 22.0 | 46.0 | – | 89.8 | 49.9 | 26.3 | 35.9 |
| Gambia 2013 | 3104 | 31.4 | 52.0 | 66.0 | 38.1 | 59.1 | 47.5 | 54.4 | 0.5 | 1.8 |
| Ghana 2016 | 3071 | 32.3 | 51.2 | 34.8 | 52.0 | 18.8 | 58.8 | 55.5 | 23.0 | 32.5 |
| Ghana 2014 | 2705 | 32.7 | 52.1 | 36.8 | 38.9 | 21.6 | 38.1 | 54.0 | 28.8 | 40.8 |
| Guinea 2012 | 3192 | 32.3 | 52.3 | 79.7 | 20.3 | 1.8 | 57.8 | 44.3 | 43.8 | 45.7 |
| Kenya 2015 | 3352 | 33.3 | 50.5 | 21.3 | 45.1 | – | 98.5 | 53.0 | 5.3 | 9.4 |
| Liberia 2016 | 2569 | 33.3 | 49.6 | 43.5 | 39.2 | 0.8 | 67.5 | 54.7 | – | 50.3 |
| Liberia 2011 | 2888 | 33.1 | 50.5 | 49.9 | 32.8 | 10.3 | 75.7 | 61.4 | 32.5 | 52.3 |
| Liberia 2009 | 4766 | 32.5 | 49.5 | 54.4 | 25.0 | – | 77.1 | 55.7 | 33.3 | 37.4 |
| Madagascar 2016 | 6734 | 32.5 | 51.6 | 26.8 | 69.6 | – | 90.3 | 50.1 | 5.5 | 3.7 |
| Madagascar 2013 | 5322 | 32.7 | 50.9 | 32.3 | 37.7 | 41.4 | 92.6 | 47.6 | 6.5 | 7.5 |
| Madagascar 2011 | 6132 | 33.7 | 50.6 | 32.6 | 70.5 | 50.7 | 90.2 | 50.0 | 4.1 | 6.2 |
| Malawi 2017 | 2295 | 33.7 | 50.2 | 10.1 | 54.6 | – | 65.5 | 31.8 | 16.9 | 26.0 |
| Malawi 2014 | 1893 | 32.4 | 50.5 | 12.7 | 62.4 | 7.0 | 71.0 | 38.2 | 26.0 | 29.9 |
| Malawi 2012 | 2074 | 32.3 | 47.1 | 18.3 | 44.4 | 8.9 | 74.9 | 37.8 | 24.6 | 37.8 |
| Mali 2015 | 7277 | 32.7 | 50.9 | 78.0 | 62.8 | 6.6 | 78.2 | 43.5 | 35.0 | 31.5 |
| Mali 2012–2013 | 4653 | 33.1 | 50.9 | 82.9 | 62.4 | 8.3 | 84.1 | 41.3 | 48.7 | 44.1 |
| Mozambique 2015 | 4429 | 32.4 | 48.8 | 27.1 | 38.3 | 15.1 | 74.8 | 36.7 | – | 31.7 |
| Mozambique 2011 | 4874 | 31.8 | 49.0 | 34.8 | 28.6 | 23.3 | 79.9 | 36.9 | 29.9 | 34.0 |
| Nigeria 2015 | 5530 | 32.8 | 50.4 | 44.0 | 34.2 | 1.6 | 49.6 | 40.2 | 27.3 | 41.3 |
| Nigeria 2010 | 4907 | 32.6 | 50.7 | 47.3 | 27.5 | 1.0 | 58.5 | 37.5 | 38.3 | 46.3 |
| Rwanda 2017 | 2615 | 32.2 | 52.1 | – | 58.9 | 17.2 | 75.9 | 40.3 | 6.6 | 10.9 |
| Rwanda 2014–2015 | 3416 | 32.1 | 51.0 | 14.9 | 55.8 | – | 82.1 | 45.9 | 2.2 | 7.6 |
| Rwanda 2010 | 3931 | 33.4 | 50.6 | 19.0 | 63.2 | – | 87.2 | 43.3 | 1.2 | 2.4 |
| Senegal 2017 | 9772 | 32.6 | 50.7 | 60.8 | 57.6 | 8.7 | 49.1 | 55.2 | 0.6 | 1.6 |
| Senegal 2016 | 12,091 | 32.9 | 50.7 | 71.4 | 57.2 | 10.0 | 52.9 | 59.6 | 1.0 | 1.4 |
| Senegal 2015 | 6046 | 32.8 | 50.5 | 71.6 | 51.5 | 9.7 | 50.6 | 58.0 | 0.4 | 1.0 |
| Senegal 2014 | 12,118 | 32.5 | 50.3 | 72.2 | 42.2 | 15.6 | 55.9 | 57.7 | 2.8 | 2.9 |
| Senegal 2012–2013 | 5889 | 32.2 | 50.1 | 72.1 | 44.7 | 18.4 | 55.5 | 53.7 | 3.7 | 4.1 |
| Senegal 2010–2011 | 3852 | 32.6 | 52.4 | 74.9 | 39.0 | 14.8 | 58.4 | 56.4 | 3.7 | 3.3 |
| Sierra Leone 2016 | 6328 | 32.1 | 50.5 | 64.2 | 36.9 | 1.3 | 66.7 | 51.5 | 41.9 | 56.3 |
| Tanzania 2017 | 7125 | 32.4 | 50.3 | 24.7 | 44.9 | – | 69.0 | 47.4 | – | 8.4 |
| Tanzania 2015–2016 | 10,047 | 35.7 | 50.1 | 21.9 | 45.7 | 9.3 | 66.7 | 43.6 | 5.1 | 12.7 |
| Tanzania 2011–2012 | 7361 | 32.1 | 50.6 | 24.7 | 59.7 | 27.6 | 76.6 | 44.2 | 4.7 | 10.0 |
| Togo 2017 | 3174 | 32.3 | 49.7 | 44.8 | 59.9 | – | 46.8 | 54.8 | 29.6 | 47.2 |
| Togo 2013–2014 | 3181 | 32.5 | 50.6 | 47.5 | 29.9 | – | 59.0 | 53.2 | 37.8 | 39.3 |
| Uganda 2016 | 4711 | 32.5 | 50.4 | 13.3 | 44.3 | 11.3 | 75.9 | 47.2 | – | 33.2 |
| Uganda 2014–2015 | 4831 | 30.2 | 49.0 | 22.8 | 67.3 | 8.6 | 80.1 | 52.7 | 19.9 | 32.6 |
| Uganda 2009 | 3967 | 30.2 | 49.5 | 23.6 | 28.0 | – | 100.0 | 46.2 | 43.6 | 53.1 |
| Total | 247,440 | 32.6 | 50.2 | 47.3 | 45.8 | 12.5 | 69.7 | 48.6 | 18.8 | 24.2 |
All surveyed children were 0–59 months.
Valid percent was measured among the valid records because some records on the mother’s highest educational level and IRS were missing in some surveys. RDT = Rapid Diagnostic Test; DRC = Democratic Republic of the Congo. ITN = Insecticide-treated Net; IRS = Indoor Residual Spraying.
Drinking water and sanitation (WS) and household socioeconomic status
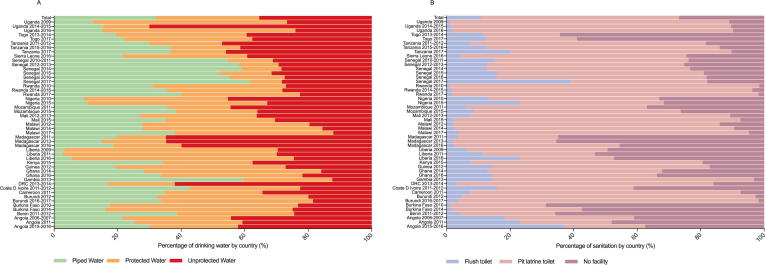
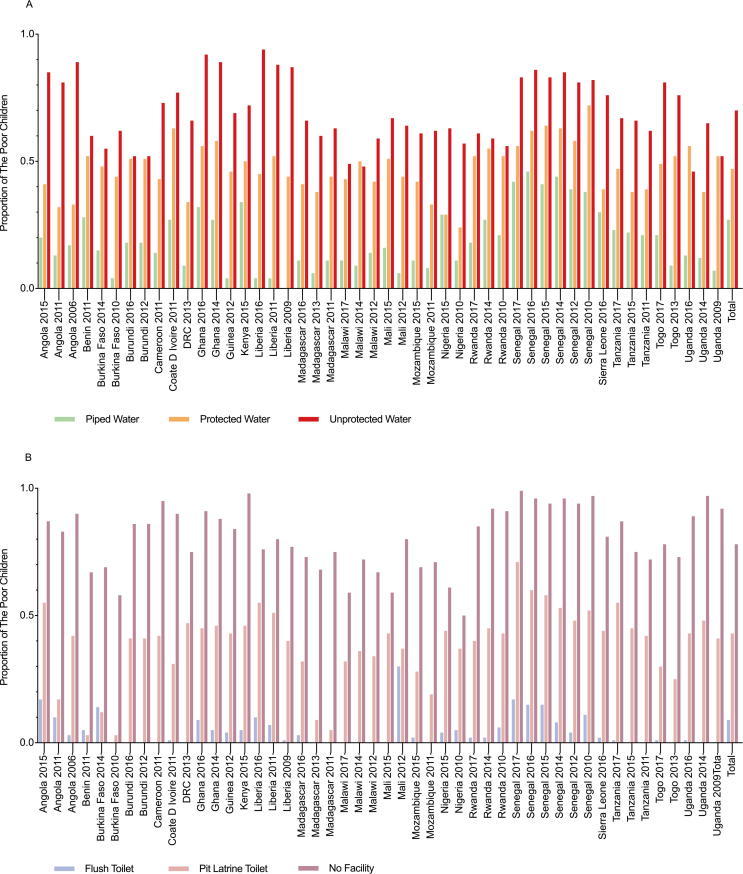
Fig. 1 presents the proportion of WS in the 23 countries in this study. Across all surveys, 35.4% of the included children had access to unprotected water, followed by protected water (32.5%) and piped water (32.1%) (Fig. 1A). Additionally, Fig. 1B demonstrates that most children utilized pit latrine toilets (62.4%), followed by no facilities (26.8%) and flush toilets (10.8%). The proportion of households with a “poor” (versus “nonpoor”) socioeconomic status was 48.6% overall and ranged from 31.8% (Malawi 2017) to 61.4% (Liberia 2011) (Table 1). The greatest proportion of children who were classified as having a “poor” socioeconomic status were unprotected water users (69.6%), followed by protected water users (46.5%) and piped water users (26.7%) (P < 0.001) (Fig. 2A). Additionally, Fig. 2B illustrates that the proportion of children with “poor” socioeconomic status who were no facility users (77.7%) was higher than the proportions of those who were pit latrine toilet users (42.6%) and flush-toilet users (8.6%) (P < 0.001).
Fig. 1.
Proportion of children under 5 years old who used various WS conditions. (A) drinking water, (B) sanitation.
Fig. 2.
The percentage of children with a “poor” socioeconomic status and different WS sources for each national survey. (A) The association between socioeconomic status and drinking water sources. (B) The association between socioeconomic status and sanitation conditions. Chi-square (χ2) tests were used for assessing the differences in the proportion of children with a “poor” socioeconomic status among the various WS conditions. The P-values of all the χ2 tests in Fig. 2 were less than 0.001. WS = Drinking Water and Sanitation.
Association between drinking water and sanitation (WS) and malaria infection
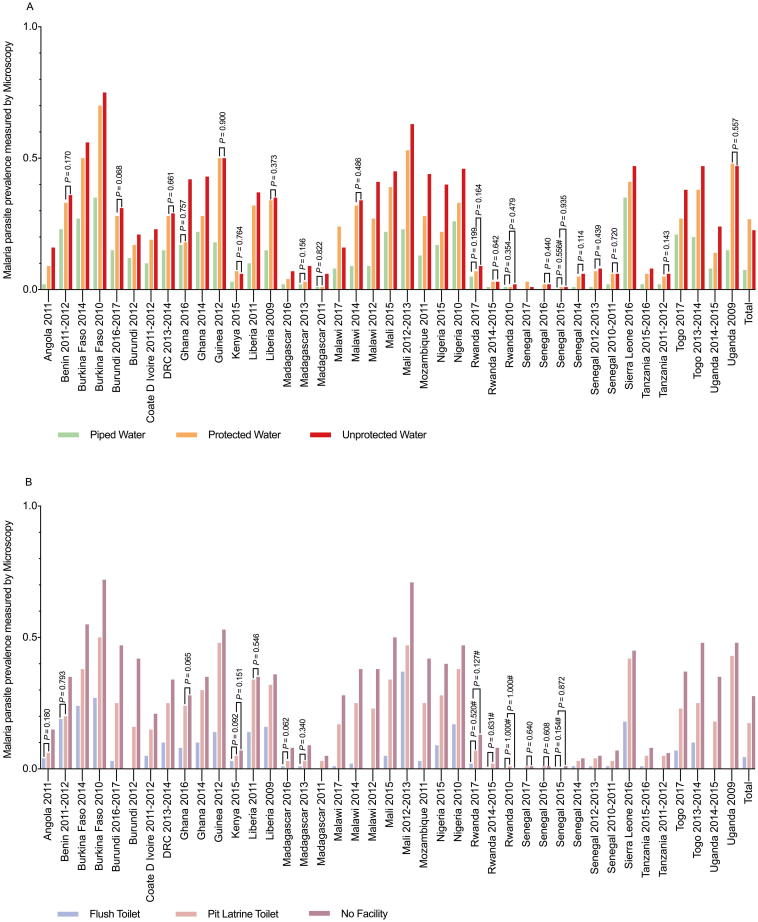
Across all surveys, the comparison of malaria infections diagnosed by microscopy among individuals with different WS access in different countries revealed that the prevalence rates of malaria among the unprotected water users (22.6%) and piped water users (7.5%) were both significantly lower the prevalence rate among the protected water users (22.6% versus 26.8%, p < 0.001; 7.6% versus 26.8%, P < 0.001); however, this trend was not always consistent in all the surveys (Fig. 3A). Children who used no facilities were more likely to have malaria than children who used pit latrine toilets (Fig. 3B) according to microscopy (27.7% versus 17.4%, P < 0.001), whereas children who used flush toilets had a low tendency of malaria infection (4.5% versus 17.4%, P < 0.001); this trend was consistent in each survey (Fig. 3B). Data on malaria infections measured by RDTs in exposed and unexposed groups were provided by a survey, as shown in Additional file 2.
Fig. 3.
Prevalence of malaria infection in different WS users identified by microscopy for each national survey. (A) The association between malaria prevalence and different drinking water sources. (B) The association between malaria prevalence and different sanitation conditions. Chi-square (χ2) tests or Fisher’s exact tests were used to assess the differences in malaria infection between the various WS users. The infections were determined by microscopy. #P-values were obtained with Fisher’s exact test. P-values (>0.05) were obtained with χ2 tests or Fisher’s exact tests; all unmarked P-values are less than 0.001. WS = Drinking Water and Sanitation.
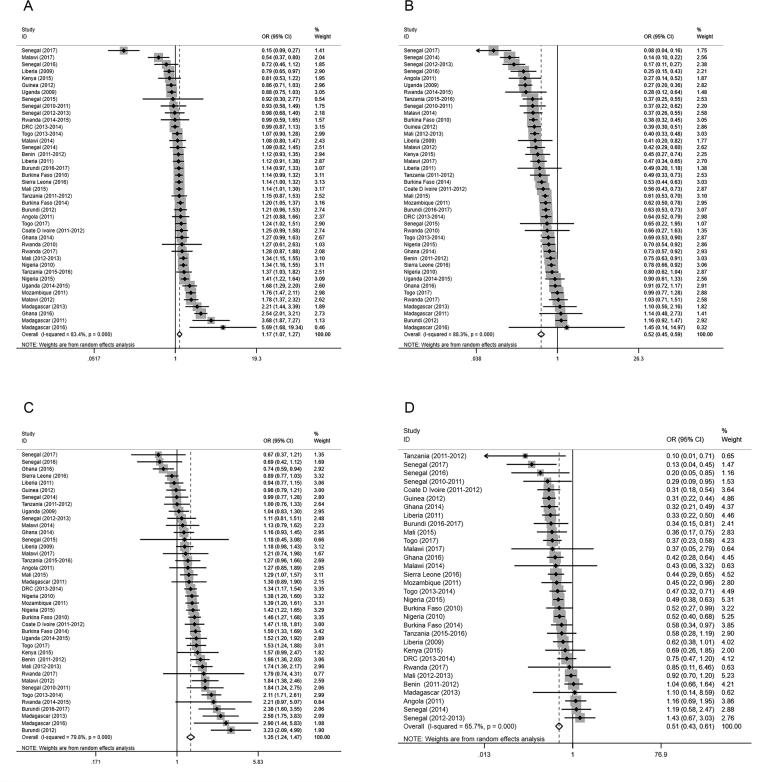
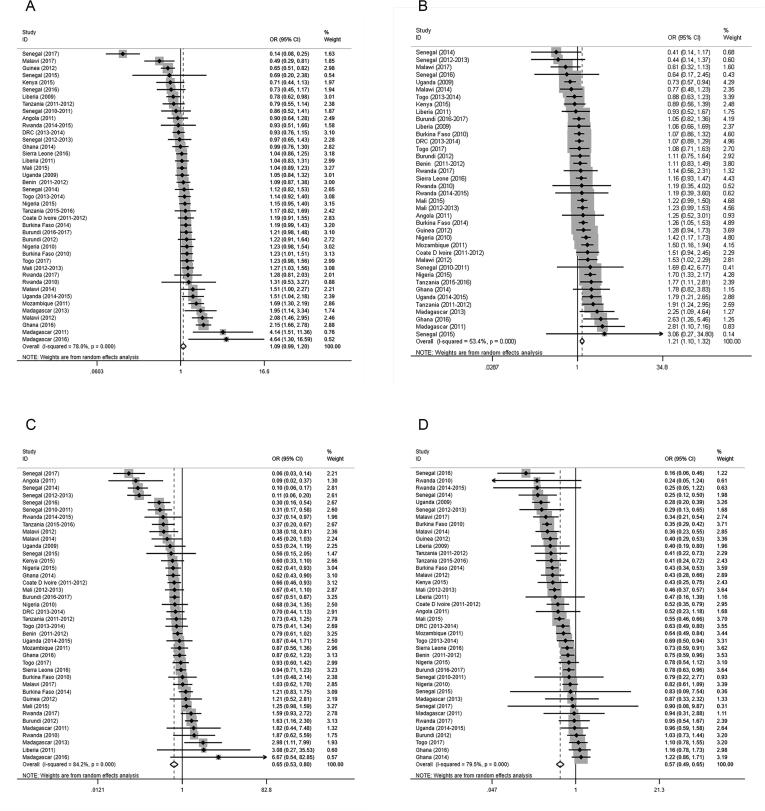
For the total population, the specific regression results for each survey based on the logistic regression model are shown in the forest plot (Fig. 4, Additional file 3). Across all surveys, unprotected water users were associated with a significantly increased prevalence of malaria (aOR 1.17, 95% CI 1.07–1.27, P = 0.001) as measured by microscopy (Table 2, Fig. 4A), while piped water users were associated with a significantly decreased prevalence of malaria (aOR 0.52, 95% CI 0.45–0.59, P < 0.001) as measured by microscopy (Table 2, Fig. 4B). Both results were retained when adjustments were made for age, gender, IRS in the past 12 months (when measured), ITN use, house quality, and mother’s highest educational level (when measured). Moreover, no facility users had increased odds and flush-toilet users had decreased odds of malaria risk as measured by microscopy (Table 2, Fig. 4C, D). The overall aORs for no facility users and flush-toilet users were 1.35 (95% CI 1.24–1.47, P < 0.001), and 0.51 (95% CI 0.43–0.61, P < 0.001), respectively (Table 2, Fig. 4C, D). The trends of individuals diagnosed by RDTs were consistent with those of microscopy (Table 2, Additional file 3).
Fig. 4.
Forest plots of the effects of WS conditions on malaria infection among the total children diagnosed by microscopy. The ORs and 95% CIs for the risk of infection as determined by microscopy in relation to (A) Unprotected Water, (B) Piped Water, (C) No Facility, and (D) Flush toilets in each survey were measured by logistic regression models with adjustments for age, gender, IRS, ITN use, house quality, and mother’s highest educational level. The datapoints, lines, boxes, and vertical dashed lines present the ORs, 95% CIs, weight that each survey contributed to the overall OR, and overall 95% CIs, respectively. WS = Drinking Water and Sanitation; OR = Odds Ratio; 95% CI = 95% Confidence Interval.
Table 2.
Meta-analysis of the associations between WS conditions and malaria infections among the total children, children with a “poor” socioeconomic status, and children with a “poor” socioeconomic status.
| Number of surveys* | Total children OR (95%CI) | Number of surveys* | Poor children OR (95%CI) | Number of surveys* | Non-poor children OR (95%CI) | |
|---|---|---|---|---|---|---|
| Microscopy | ||||||
| Protected water (Reference) | – | 1.00 | – | 1.00 | – | 1.00 |
| Unprotected water | 41 | 1.17 (1.07, 1.27) | 41 | 1.09 (0.99, 1.21) | 39 | 1.21 (1.10, 1.32) |
| Piped water | 41 | 0.52 (0.45, 0.59) | 40 | 0.65 (0.53, 0.80) | 40 | 0.57 (0.49, 0.65) |
| Pit latrine (Reference) | – | 1.00 | – | 1.00 | – | 1.00 |
| No facility | 40 | 1.35 (1.24, 1.47) | 39 | 1.14 (1.03, 1.26) | 35 | 1.46 (1.32, 1.61) |
| Flush toilet | 32 | 0.51 (0.43, 0.61) | 14 | 0.80 (0.55, 1.17) | 32 | 0.57 (0.49, 0.66) |
| RDT | ||||||
| Protected water (Reference) | – | 1.00 | – | 1.00 | – | 1.00 |
| Unprotected water | 48 | 1.11 (1.02, 1.22) | 48 | 1.02 (0.93, 1.13) | 47 | 1.24 (1.11, 1.38) |
| Piped water | 47 | 0.49 (0.43, 0.57) | 46 | 0.68 (0.56, 0.82) | 47 | 0.53 (0.46, 0.60) |
| Pit latrine (Reference) | – | 1.00 | – | 1.00 | – | 1.00 |
| No facility | 48 | 1.38 (1.27, 1.50) | 48 | 1.15 (1.05, 1.25) | 42 | 1.54 (1.38, 1.72) |
| Flush toilet | 44 | 0.46 (0.39, 0.53) | 24 | 0.71 (0.56, 0.91) | 44 | 0.53 (0.47, 0.60) |
Some surveys were excluded in the meta-analysis due to the unavailability of logistic regression results. Each logistic regression model was adjusted for age, gender, IRS, ITN use, house quality, and mother’s highest educational level. OR = Odds Ratio; 95% CI = 95% Confidence Interval; WS = Drinking Water and Sanitation; RDT = Rapid Diagnostic Test.
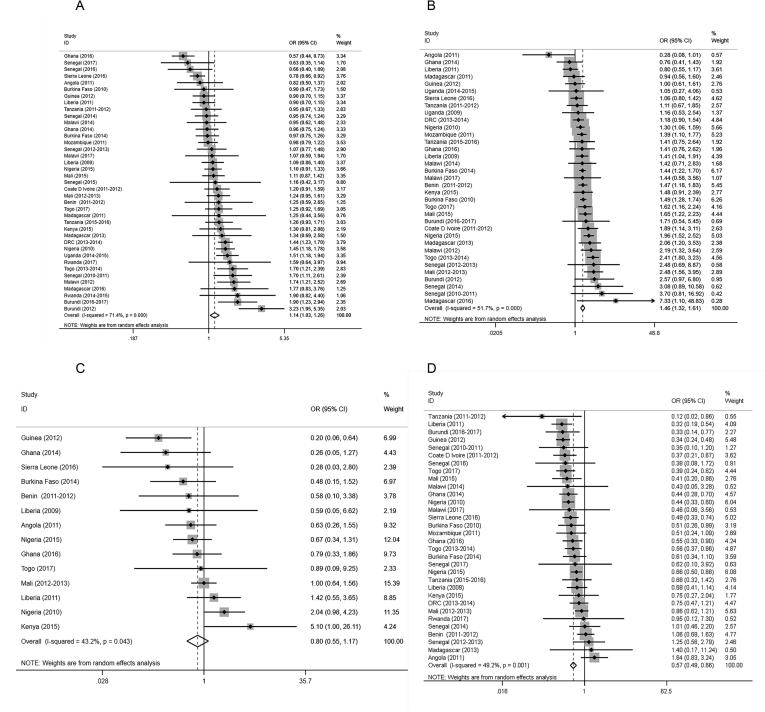
For the stratified results, the specific regression results for each survey stratified by household socioeconomic status are shown in the forest plot (Fig. 5, Fig. 6, Additional files 4, 5). In children with a “poor” socioeconomic status, no overall associations with malaria risk were observed in the unprotected water users compared to protected water users (microscopy: aOR 1.09, 95% CI 0.99–1.21, P = 0.083; RDT: aOR 1.02, 95% CI 0.93–1.13, P = 0.652) (Fig. 5A, Additional file 4A), whereas in children with a “nonpoor” socioeconomic status, the risk of malaria in the unprotected water users was more pronounced than that in protected water users (microscopy: aOR 1.21, 95% CI 1.10–1.32, P < 0.001; RDT: aOR 1.24, 95% CI 1.11–1.38, P < 0.001) (Fig. 5B, Additional file 4B). In children with a “poor” socioeconomic status, the protective effects of piped water were still significant, and the overall aORs of the piped water users were 0.65 (95% CI 0.53–0.80, P < 0.001) in those diagnosed by microscopy (Fig. 5C) and 0.68 (95% CI 0.56–0.82, P < 0.001) in those diagnosed by RDTs (Additional file 4C). In children with a “nonpoor” socioeconomic status, the aORs of the piped water users were 0.57 (95% CI 0.49–0.65, P < 0.001) in those diagnosed by microscopy (Fig. 5D) and 0.53 (95% CI 0.46–0.60, P < 0.001) in those diagnosed by RDTs (Additional file 4D)
Fig. 5.
Forest plots of the effects of drinking water sources on malaria infection diagnosed by microscopy based on socioeconomic status. (A) Unprotected Water among children with a “poor” socioeconomic status, (B) Unprotected Water among children with a “nonpoor” socioeconomic status, (C) Piped Wateramongchildrenwitha“poor”socioeconomicstatus, (D) Piped Water among children with a “nonpoor” socioeconomic status. Malaria infections were determined by microscopy. Datapoints, lines, boxes, and vertical dashed lines represent ORs, 95%CIs,weight that each survey contributed to the overall OR, and overall 95% CIs, respectively. OR = Odds Ratio; 95% CI = 95% Confidence Interval..
Fig. 6.
Forest plots of the effects of sanitation conditions on malaria infection diagnosed by microscopy based on socioeconomic status. (A) No Facility among children with a “poor” socioeconomic status, (B) No Facilityamongchildrenwitha“nonpoor”socioeconomicstatus, (C) Flush toilet among children with a “poor” socioeconomic status, (D) Flush toilets among children with a “nonpoor” socioeconomic status. Malaria infections were diagnosed by microscopy. Datapoints, lines, boxes, and vertical dashed lines represent ORs, 95% CIs, weight that eachsurvey contributed to the overall OR, and overall 95% CIs, respectively. OR = Odds Ratio; 95% CI = 95% Confidence Interval.
For children with a “poor” socioeconomic status who were pit latrine toilet users, the overall aORs of the no facility users were 1.14 (95% CI 1.03–1.26, P = 0.010) in those diagnosed by microscopy (Fig. 6A) and 1.15 (95% CI 1.05–1.25, P = 0.002) in those diagnosed by RDTs (Additional file 5A); for the children with a “nonpoor” socioeconomic status, the aORs were 1.46 (95% CI 1.32–1.61, P < 0.001) in those diagnosed by microscopy (Fig. 6B) and 1.54 (95% CI 1.38–1.72, P < 0.001) in those diagnosed by RDTs (Additional file 5B). Additionally, in children with a “poor” socioeconomic status, the flush-toilet users did not have significant protection from malaria infection according to microscopy; the aOR of the flush-toilet users was 0.80 (95% CI 0.55–1.17, P = 0.250) (Fig. 6C). In the children with a “nonpoor” socioeconomic status, the protective effects of flush-toilets (considering both microscopy and RDTs) were significant (microscopy: aOR 0.57, 95% CI 0.49–0.66, P < 0.001; RDT: aOR 0.53, 95% CI 0.47–0.60, P < 0.001) in relation to malaria risk (Fig. 6D, Additional file 5D).
Discussion
To our knowledge, this is the first analysis of the associations between WS conditions and the risk of malaria among children under five years old across SSA employing data from multi-country, cross-sectional surveys. This analysis of 49 surveys (23 DHS, 24 MIS, and 2 others) found that compared to protected water and pit latrine toilets, piped water and flush toilets were associated with significantly reduced malaria prevalence rates, whereas unprotected water and no facilities were related to an increased risk of malaria after adjusting for potential confounders. However, this association was mostly influenced by the household socioeconomic status. In children with a “poor” socioeconomic status, no significant associations were observed between unprotected water and flush toilets in relation to malaria infection, whereas in children with a “nonpoor” socioeconomic status, the associations between unimproved WS conditions (including unprotected water or no facilities) and the risk of malaria appeared to be pronounced.
These findings are in line with several previous studies [8], [9], [10], [11], [22], [23]; for example, Ayele et al. assessed various WS conditions as indicators of socioeconomic status on the prevalence of malaria in Ethiopia from December 2006 to January 2007 using a generalized additive mixed model, generalized linear mixed model with spatial covariance structure, and generalized linear mode [8], [9], [10]. All of these studies found that malaria disproportionately affected people who had a poor socioeconomic status and limited access to clean drinking water sources [8], [9], [10]. Similarly, Kinuthia et al. also observed an increased number of malaria cases associated with inappropriate WS conditions in Njoro District, Kenya, using chi-squared tests and confidence limits [11]. Furthermore, Hasyim et al. indicated that individuals who lived in unimproved sanitation environments were more frequently infected with malaria than those who lived in improved sanitation environments, even though the association between environmental sanitation and malaria prevalence was not statistically significant (OR 1.13, 95% CI 0.99–1.31, P = 0.081) [22]. Finally, as Hasyim et al. also suggested, most individuals who used open sewage systems (domestic wastewater or municipal wastewater) at home and those who did not have a sewage system were at higher risk of malaria infection (OR 1.250, 95% CI 1.095–1.427, P = 0.001) than those who used closed sewage systems, further highlighting the significance of potential larval habitats near houses [23]. The results of all of these studies were in line with our results; due to closed systems, improved WS users had a decreased risk of malaria infection.
It is well known that mosquitoes and their ecosystems are significant spatial drivers of malaria transmission. Potential larval habitats may occur due to the physical disturbances created by human fetching or storing of unimproved drinking water (e.g., splashing water on the ground when fetching or storing unimproved water results in shallow puddles or footprints; additionally, storing unimproved drinking water creates stagnant water sources for nearby households), further increasing mosquito breeding and adult vector densities near households. The top three vector species of human malaria in our study area included Anopheles gambiae, An. arabiensis, and An. funestus (Additional file 6; the data sources were derived from country profiles based on the World Health Organization (WHO) database online because the DHS and MIS did not include entomological surveys). Among these Anopheles species, An. gambiae and An. arabiensis prefer to inhabit sunlit, shallow, temporary bodies of fresh water, such as puddles, pools, ground depressions, and hoof prints [24]. In addition, water in these larval sites is often turbid or polluted [25], [26], [27]. In contrast, An. funestus inhabits permanent or semipermanent bodies of fresh water with emergent vegetation, such as swamps, ponds, and lake edges [24]. This evidence suggests that closed systems with improved water are relatively inappropriate environments for Anopheles.
The association between improved WS (including protected and piped water; pit latrines and flush toilets) and the reduced risk of malaria in this study could be explained by several potential mechanisms. There are data that indicate that wealth is probably protective against malaria risk [28], [29], [30], [31], [32], [33], [34], as prevention and treatment are affordable [35], [36], [37]. In this study, among the total participants, socioeconomic status (a confounder) determined access to improved water, sanitation and hygiene practices and malaria prevention practices, all of which affected the level of malaria risk [8], [9], [10]. We can easily see that the highest proportion of children with a “poor” socioeconomic status were unimproved WS users (Fig. 2). To address the confounding nature of socioeconomic status, the results of WS conditions and prevalence of malaria in children under five years old were stratified by household socioeconomic status, and the aORs within each socioeconomic level were calculated. In the stratified results, the mixed effects of wealth weighed heavily upon the WS conditions related to malaria risk in the children with a “poor” socioeconomic status (Table 2). This nonsignificant phenomenon was mostly attributed to the decreased proportion of improved water access in children with a “poor” socioeconomic status (Fig. 2). This result simply showed that malaria infection rates were the highest among the poorest populations who had little or no access to safe drinking water and toilets.
Regarding the overall OR results between children with a “poor” or “nonpoor” socioeconomic status, the effects of WS and malaria infections were more obvious in the children with a “nonpoor” socioeconomic status (Table 2), demonstrating that it is urgent to improve WS conditions in nonpoor populations if economic circumstances permit. The important finding in this study was that in the children with a “nonpoor” socioeconomic status, the effects of WS conditions were still significant even without the confounding effects of socioeconomic status. This may be explained by the fact that unimproved WS users may indirectly increase the likelihood of contracting Plasmodium falciparum by increasing the risk of other waterborne parasitic diseases, such as soil transmitted helminth diseases (STHs, such as hookworm, Strongyloides stercoralis) or Schistosoma haematobium infections directly [38], [39], [40], [41], [42].
According to previous studies, we hypothesize that children who have STHs or schistosomiasis may be more susceptible to malaria infection [38], [39], [40], [41], [42], [43], [44], [45]. There are many mechanisms to support this theory. For example, Strongyloides stercoralis could increase the risk of Plasmodium infection because of the predominance of Th2 responses in young children [38], [39]. Furthermore, schistosomiasis infection alone or in combination with trichiasis or hookworm infection can apparently increase the risk of P. falciparum by modulating the immune system [41], [42], [43]. Additionally, helminth-infected individuals can present decreased cutaneous reactivity to anopheline bites, which may theoretically facilitate the success of sporozoite introduction [44], [45]. There are also many previous studies exploring the risk factors of STH or Schistosoma haematobium and malaria coinfections, and all these articles indicate that unsafe WASH conditions are the primary risk factors associated with such coinfections [38], [46], [47], suggesting that clean WS conditions can help to prevent malaria infections. Finally, the most important distinction between unimproved water and improved water is whether drinking water is treated. In this study, it was apparent that a high proportion of disposed unprotected water was linked to a relatively low prevalence of malaria (Additional file 7).
The strength of this study includes the large and comprehensive dataset obtained from the DHS and MIS. The analysis aimed to elucidate the influence of household WS on malaria risk stratified by household socioeconomic status on a large scale for the first time. Some studies have indicated that many high-income countries eliminated malaria without malaria-specific interventions; for example, malaria in Europe and North America declined as a result of improved living conditions and increased wealth [48]. As Lucy Tusting et al. stated, halting existing malaria control efforts is not recommended; however, we believe there is a need to increase investment in interventions that support socioeconomic development [33]. Although wealth status is a combination of multiple factors, it is important to know which specific aspect of wealth affects malaria infection. In this study, the mixed effects of socioeconomic status were eliminated, and we focused on exploring the relationship between WS and malaria. Water-associated vector-borne diseases (including malaria and many NTDs) continue to be a major public health problem in many developing countries [7]. However, remarkable and significant progress in the prevention and control of water-related vector-borne diseases has been made in many regions, primarily through the strengthening of vector control strategies, case detection, and treatment methods [1], [7]. These present strategies must be expanded. Strengthening of intersectoral links with improving WASH may provide a method to increase the pace of malaria elimination. Although the SDGs have offered unprecedented opportunities to improve health by dramatically increasing the availability and use of WASH services [7], the coverage of safe WASH in SSA is still very low. These findings suggest that efforts should be redoubled to improve WS conditions, which should be considered an important component of malaria prevention and control. Finally, the use of pooled observational multicountry data eliminated many biases, including publication, selection, and measurement biases and selective outcome reporting, which are typically presented in traditional systematic reviews and meta-analyses.
This study has several limitations. First, it did not explore the association between drinking water storage sites and malaria infection. However, in this study data on drinking water storage sites were absent in many surveys, making it too difficult to link the various types of drinking water sources with their storage sites. Further studies are needed to investigate the influence of storage sites in depth. Second, although the results of WS conditions and malaria prevalence among children under 5 years old were stratified by household socioeconomic level, the stratification (“poor” versus “nonpoor”) in this study was not very prudent because of the original stratifications in the DHS and MIS were grouped into five categories, namely, “poorest”, “poor”, “middle”, “rich”, and “richest”. There may still be residual confounding caused by wealth status in our study. However, considering the proportion of children with a “poor” socioeconomic status (approximately 50%) (Table 1), this study classified the total children into two groups to avoid an uneven sample distribution. Furthermore, entomological surveys, particularly among unimproved drinking water sources and unimproved sanitation facilities in SSA, are important to understand how the type and the behavior of Anopheles species affect malaria transmission and to assist in addressing confounding factors involving the various ecological niches of distinct species. Unfortunately, entomological surveys were not conducted in the DHS and MIS surveys. Finally, due to the lack of examination in the DHS Program of other parasitic diseases, such as STHs or schistosomiasis, the proposed effect of coinfections is still under speculation in this study. It would be beneficial to add coinfection investigations to the DHS and MIS in the future.
Conclusions
In conclusion, WS conditions were important risk factors for malaria among children under five years old across SSA after adjustments for age, gender, IRS in the past 12 months and insecticide-treated use, house quality, and mother’s highest educational level. Unimproved WS access (unprotected water; no facility) was related to a relatively high risk of malaria. Furthermore, this association was mostly influenced by socioeconomic status. However, the malaria risk associated with unimproved WS was more pronounced among the children with a “nonpoor” socioeconomic status. These findings indicated incremental improvements to WS in SSA might be considered a potential intervention for the prevention and control of malaria in the long term.
Compliance with Ethics Requirements
The DHS Program has the compliance with ethics requirements.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgements
We are very grateful for the Demographic and Health Program for making the survey data available and it provided a convenient condition for comprehensively evaluating the associations of WS on malaria infection. Additionally, all authors thank Dr. Yan Zhao, Dr. Qiao He, and Dr. Zhuo Zuo for giving the constructive suggestions on the manuscript revision.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2019.09.001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.World malaria report 2018. [https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1].
- 2.Mathenge P.G., Low S.K., Vuong N.L., Mohamed M.Y.F., Faraj H.A., Alieldin G.I. Efficacy and resistance of different artemisinin-based combination therapies: a systematic review and network meta-analysis. Parasitol Int. 2019;101919 doi: 10.1016/j.parint.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Sluydts V., Durnez L., Heng S., Gryseels C., Canier L., Kim S. Efficacy of topical mosquito repellent (picaridin) plus long-lasting insecticidal nets versus long-lasting insecticidal nets alone for control of malaria: a cluster randomised controlled trial. Lancet Infect Dis. 2016;16(10):1169–1177. doi: 10.1016/S1473-3099(16)30148-7. [DOI] [PubMed] [Google Scholar]
- 4.Pinder M., Jawara M., Jarju L.B., Salami K., Jeffries D., Adiamoh M. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet (London, England) 2015;385(9976):1436–1446. doi: 10.1016/S0140-6736(14)61007-2. [DOI] [PubMed] [Google Scholar]
- 5.Ghebreyesus T.A., Admasu K. Countries must steer new response to turn the malaria tide. Lancet (London, England) 2018;392(10161):2246–2247. doi: 10.1016/S0140-6736(18)32943-X. [DOI] [PubMed] [Google Scholar]
- 6.WHO: 2.1 billion people lack safe drinking water at home, more than twice as many lack safe sanitation; 2017. http://www.who.int/mediacentre/news/releases/2017/water-sanitation-hygiene/en/
- 7.WHO: Water, sanitation and hygiene strategy 2018-2025; 2018. https://apps.who.int/iris/bitstream/handle/10665/274273/WHO-CED-PHE-WSH-18.03-eng.pdf?ua=1
- 8.Ayele D.G., Zewotir T.T., Mwambi H.G. Prevalence and risk factors of malaria in Ethiopia. Malar J. 2012;11:195. doi: 10.1186/1475-2875-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayele D.G., Zewotir T.T., Mwambi H.G. Spatial distribution of malaria problem in three regions of Ethiopia. Malar J. 2013;12:207. doi: 10.1186/1475-2875-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayele D.G., Zewotir T.T., Mwambi H.G. Semiparametric models for malaria rapid diagnosis test result. BMC Public Health. 2014;14:31. doi: 10.1186/1471-2458-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinuthia G.K., Gicheru M.M., Ngure P.K., Kabiru E.W. Lifestyles and practices that enhance malaria and typhoid fever in Njoro District, Kenya. J Community Health. 2012;37(1):224–233. doi: 10.1007/s10900-011-9440-0. [DOI] [PubMed] [Google Scholar]
- 12.WHO: Global technical strategy for malaria 2016-2030; 2015. https://apps.who.int/iris/bitstream/handle/10665/176712/9789241564991_eng.pdf?sequence=1 [DOI] [PMC free article] [PubMed]
- 13.Tusting L.S., Bottomley C., Gibson H., Kleinschmidt I., Tatem A.J., Lindsay S.W. Housing improvements and malaria risk in Sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 2017;14(2):e1002234. doi: 10.1371/journal.pmed.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The DHS Program, Survey search, 2017, ICF International; Rockville (Maryland). http://dhsprogram.com/What-We-Do/survey-search.cfm.
- 15.Fund UNCs: MICS surveys. New York: United Nations Children’s Fund; 2016. http://mics.unicef.org/surveys.
- 16.WHO: Malaria: High-risk groups. https://wwwwhoint/malaria/areas/high_risk_groups/en/; August 21, 2019.
- 17.Fullman N., Burstein R., Lim S.S., Medlin C., Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J. 2013;12:62. doi: 10.1186/1475-2875-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaxman A.D., Fullman N., Otten M.W., Menon M., Cibulskis R.E., Ng M. Rapid scaling up of insecticide-treated bed net coverage in Africa and its relationship with development assistance for health: a systematic synthesis of supply, distribution, and household survey data. PLoS Med. 2010;7(8):e1000328. doi: 10.1371/journal.pmed.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for health metrics and evaluation: Bednet tracking database. Seattle, Washington, USA: IHME; 2012.
- 20.Burgert CR BS, Eckert E. Improving estimates of insecticide treated mosquito net coverage from household surveys: using geographic coordinates to account for endemicity and seasonality. DHS Analytical Studies 32 Calverton (Maryland): ICF International; 2012. [DOI] [PMC free article] [PubMed]
- 21.Njau J.D., Stephenson R., Menon M.P., Kachur S.P., McFarland D.A. Investigating the important correlates of maternal education and childhood malaria infections. Am J Trop Med Hyg. 2014;91(3):509–519. doi: 10.4269/ajtmh.13-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasyim H., Dale P., Groneberg D.A., Kuch U., Muller R. Social determinants of malaria in an endemic area of Indonesia. Malar J. 2019;18(1):134. doi: 10.1186/s12936-019-2760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasyim H., Dhimal M., Bauer J., Montag D., Groneberg D.A., Kuch U. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17(1):302. doi: 10.1186/s12936-018-2447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinka M.E., Bangs M.J., Manguin S., Coetzee M., Mbogo C.M., Hemingway J. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimnig J.E., Ombok M., Kamau L., Hawley W.A. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol. 2001;38(2):282–288. doi: 10.1603/0022-2585-38.2.282. [DOI] [PubMed] [Google Scholar]
- 26.Ye-Ebiyo Y., Pollack R.J., Spielman A. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am J Trop Med Hyg. 2000;63(1–2):90–93. doi: 10.4269/ajtmh.2000.63.90. [DOI] [PubMed] [Google Scholar]
- 27.Charlwood J.D., Edoh D. Polymerase chain reaction used to describe larval habitat use by Anopheles gambiae complex (Diptera: Culicidae) in the environs of Ifakara, Tanzania. J Med Entomol. 1996;33(2):202–204. doi: 10.1093/jmedent/33.2.202. [DOI] [PubMed] [Google Scholar]
- 28.Sachs J., Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 29.WHO: Global report for research on infectious diseases of poverty. Geneva: World Health Organization; 2012.
- 30.Hotez P.J. The poverty-related neglected diseases: Why basic research matters. PLoS Biol. 2017;15(11):e2004186. doi: 10.1371/journal.pbio.2004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makoge V., Vaandrager L., Maat H., Koelen M. Poverty and health among CDC plantation labourers in Cameroon: Perceptions, challenges and coping strategies. PLoS NeglTrop Dis. 2017;11(11):e0006100. doi: 10.1371/journal.pntd.0006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos-Vega M., Bouma M.J., Kohli V., Pascual M. Population density, climate variables and poverty synergistically structure spatial risk in urban Malaria in India. PLoS NeglTrop Dis. 2016;10(12):e0005155. doi: 10.1371/journal.pntd.0005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tusting L.S., Willey B., Lucas H., Thompson J., Kafy H.T., Smith R. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet (London, England) 2013;382(9896):963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 34.Utzinger J., Tanner M. Socioeconomic development to fight malaria, and beyond. Lancet (London, England) 2013;382(9896):920–922. doi: 10.1016/S0140-6736(13)61211-8. [DOI] [PubMed] [Google Scholar]
- 35.Gingrich C.D., Hanson K., Marchant T., Mulligan J.A., Mponda H. Price subsidies and the market for mosquito nets in developing countries: A study of Tanzania's discount voucher scheme. Soc Sci Med. 2011;73(1):160–168. doi: 10.1016/j.socscimed.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Matovu F., Goodman C., Wiseman V., Mwengee W. How equitable is bed net ownership and utilisation in Tanzania? A practical application of the principles of horizontal and vertical equity. Malar J. 2009;8:109. doi: 10.1186/1475-2875-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed S.M., Haque R., Haque U., Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J. 2009;8:173. doi: 10.1186/1475-2875-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salim N., Knopp S., Lweno O., Abdul U., Mohamed A., Schindler T. Distribution and risk factors for Plasmodium and helminth co-infections: a cross-sectional survey among children in Bagamoyo district, coastal region of Tanzania. PLoS NeglTrop Dis. 2015;9(4):e0003660. doi: 10.1371/journal.pntd.0003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PrabhuDas M., Adkins B., Gans H., King C., Levy O., Ramilo O. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12(3):189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 40.Babamale OA, Ugbomoiko US, Heukelbach J. High prevalence of Plasmodium falciparum and soil-transmitted helminth co-infections in a periurban community in Kwara State, Nigeria. J Infection Public Health 11(1): 48-53. [DOI] [PubMed]
- 41.Dejon-Agobé J.C., Zinsou J.F., Honkpehedji Y.J., Ateba-Ngoa U., Edoa J.R., Adegbite B.R. Schistosoma haematobium effects on Plasmodium falciparum infection modified by soil-transmitted helminths in school-age children living in rural areas of Gabon. PLoS NeglTrop Dis. 2018;12(8):e0006663. doi: 10.1371/journal.pntd.0006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ateba-Ngoa U., Jones S., Zinsou J.F., Honkpehedji J., Adegnika A.A., Agobe J.C. Associations between helminth infections, plasmodium falciparum parasite carriage and antibody responses to sexual and asexual stage malarial antigens. Am J Trop Med Hygiene. 2016;95(2):394–400. doi: 10.4269/ajtmh.15-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier JP, Pillet S, et al. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol 26(8-9): 365–69. [DOI] [PubMed]
- 44.Nacher M., Singhasivanon P., Yimsamran S., Manibunyong W., Thanyavanich N., Wuthisen R. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol. 2002;88(1):55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Hagel I., Lynch N.R., Pérez M., Di Prisco M.C., López R., Rojas E. Modulation of the allergic reactivity of slum children by helminthic infection. Parasite Immunol. 1993;15(6):311–315. doi: 10.1111/j.1365-3024.1993.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 46.M'Bondoukwé N.P., Kendjo E., Mawili-Mboumba D.P., Lengongo J.V.K., Mbouoronde C.O., Nkoghe D. Correction to: prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co-infections in different settlements of Gabon, Central Africa. Infect Diseases Poverty. 2018;7(1):38. doi: 10.1186/s40249-018-0415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anthonj C., Githinji S., Kistemann T. The impact of water on health and ill-health in a sub-Saharan African wetland: Exploring both sides of the coin. Sci Total Environ. 2018;624:1411–1420. doi: 10.1016/j.scitotenv.2017.12.232. [DOI] [PubMed] [Google Scholar]
- 48.García-Martín G. Status of malaria eradication in the Americas. Am J Tropical Med Hygiene. 1972;21(5):617–633. doi: 10.4269/ajtmh.1972.21.617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.