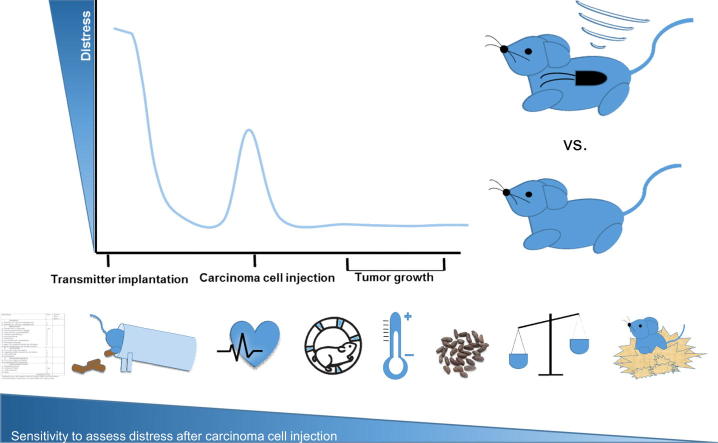
Graphical abstract
Keywords: Telemetry, Distress, Severity assessment, Mice, Animal models, Pancreatic cancer
Abbreviations: HR, heart rate; BT, body temperature; Act, activity; TI, transmitter implantation; CI, carcinoma cell injection; FCMs, faecal corticosterone metabolites; ROC, receiver operating characteristic; AUC, area under the curve; NSAIDS, non-steroidal anti-inflammatory drugs
Highlights
-
•
The suitability of methods for analysing distress in laboratory mice was assessed.
-
•
Non-invasive vs. telemetric methods were compared in an orthotopic pancreatic cancer model.
-
•
Transmitter implantation caused higher distress than laparotomy plus carcinoma cell injection.
-
•
Tumour growth provoked very mild distress.
-
•
Non-invasive methods had a better performance than telemetry for distress analysis.
Abstract
Prospective severity assessment is legally required in many countries to ensure high-quality research along with high welfare standards for laboratory animals. Mice and rats, the most common laboratory species, are prey animals that usually suppress signs of pain and suffering. Therefore, highly sensitive readout parameters are necessary to adequately quantify distress. The present study compared the performance of different non-invasive methods in determining animal distress, such as measuring body weight, distress score, faecal corticosterone metabolites, burrowing, and nesting behaviour, with continuous monitoring of heart rate, body temperature and activity by telemetry. The distress caused by two surgical interventions was compared and the burden caused by tumour growth was described. Transmitter implantation caused higher distress than laparotomy plus carcinoma cell injection into the pancreas. Surprisingly, no significant increase in distress was observed during tumour growth. The receiver operating characteristic curve analysis revealed that some non-invasive distress-parameters, i.e., distress-score and burrowing activity, exhibited slightly better performance to quantify distress than the most suitable parameters measured by telemetry. Due to the high burden caused by the implantation of the telemetric device, the use of non-invasive methods to assess distress in laboratory animals after surgical interventions should be favoured in future studies.
Introduction
Animal welfare is important for animal based biomedical research. Therefore, many countries have implemented new regulations to ensure high-quality research with minimal harm to animals. The European Union requires application of the 3Rs (replace, reduce, refine), a harm and benefit analysis and a prospective severity assessment for each animal experiment [1], [2], [3]. To adequately implement these mandatory guidelines, an evidenced-based distress analysis for laboratory animals is essential. However, guidance and examples of comprehensive distress analyses or studies that compare the suitability of different distress parameters are difficult to find in the current literature. Mice and rats are the most commonly used laboratory animals in biomedical research. These prey animals usually suppress signs of pain, suffering and weakness. Therefore, highly sensitive readout parameters are mandatory for evaluating distress or wellbeing in rodents [4].
Well-being or welfare of animals is fulfilled when the nutritional, environmental, health, behavioural and mental needs of animals are satisfied [5]. Different interventions, such as surgery or handling procedures, can provoke short time stress responses or even “distress”, where an animal is unable to adapt for a distinct period of time to the different stressors or its environment [6]. This distress can be evaluated by indicators of the physical, biochemical and psychological state of animals [7]. As indicators of the physical condition of an animal, changes in body weight and clinical scoring systems have proven useful for assessing severity and as criteria to determine a humane end point [8], [9], [10]. Changes in so-called “luxury” behaviour, i.e., burrowing and nesting activity, reflect the psychological state of animals. These natural behaviours are comparable to activities of daily living in humans, and good performance should represent well-being in mice and rats [11], [12], [13]. However, deterioration of nesting and burrowing activity might function as an indicator for neurological disorder [14], [15], [16], pain and stress [17], [18], [19]. In addition, the stress hormone corticosterone is a sensitive distress indicator, and the measurement of its metabolites in faeces represents a novel non-invasive method to assess distress by analysing the biochemical state of animals [20], [21]. In addition to these non-invasive approaches, heart rate (HR), body temperature (BT) and activity (Act) have proven to be important readout parameters for the physical state of an animal. Continuous monitoring of these parameters can be provided by telemetry using implanted radio telemetric transmitters [22], [23]. HR reportedly increases after acute restraint stress [24], [25], [26], cage change [27], and laparotomy [17]. Stress induced hypothermia was observed after handling [23], cage change [27], [28] and different injections [29]. Moreover, it is an accepted method to measure anxiety [30]. Activity of mice was reported to be enhanced after short term stressors, such as handling [23] and cage change [27] or reduced upon laparotomy and chronic tumour disease [31]. These publications indicate that HR, BT and Act might function as sensitive readout parameters for assessing animal distress. The aim of the current study was to directly compare non-invasive methods to telemetric parameters for assessing distress in a murine orthotopic pancreatic cancer model.
Material and methods
Ethical statement
All animal experiments were approved by the local authority (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern; Az. 7221.3-1-062/16). Decisions of the local authority were in accordance with the protection of animal act for Germany and the European Directive 2010/63/EU [2]. Male C57BL/6J mice between 13 and 15 weeks of age were housed separately in type III cages (dark light cycle; dark period: 7 pm-7am) with food and water ad libitum. Enrichment was provided in the form of nesting material, paper roles, and wooden sticks.
Transmitter implantation and telemetry
For transmitter implantation, each mouse was anaesthetized with 1–2 vol% isoflurane. For perioperative analgesia, 5 mg/kg carprofen was injected subcutaneously (Rimadyl®, Pfizer GmbH, Berlin, Germany). The eyes of the mouse were kept wet by using eye ointment (Jenapharm, Jena, Germany). The abdomen and the right sight of the thorax were shaved. A mid line laparotomy was performed, and the ETA-F-10 transmitter (Data Sciences International, Minnesota, USA), which is able to detect ECG signals, BT and Act of the mice, was placed in the abdominal cavity. The telemetry lead for the negative electrode was tunnelled subcutaneously from the peritoneum to the right side of the thorax, and the end of the electrode was fixed with two sutures (4–0 polyester, non-absorbable, SMI sutures, Steinberg, Belgium) in the pectoralis major muscle. The positive electrode was tunnelled subcutaneously to the left side under the costal arch and was sutured onto the external oblique muscle. The peritoneum was closed with resorbable, coated 5–0 vicryl sutures (Johnson & Johnson MEDICAL GmbH, New Brunswick, USA). Skin lesions were sewed using a 5–0 prolene suture (Johnson & Johnson MEDICAL GmbH), and mice were placed in front of a heating lamp for 30 min. Transmitter implantation surgeries lasted for 45–55 min for each mouse. For postoperative analgesia, 1250 mg/L metamizol (Ratiopharm, Ulm, Germany) was provided continuously in the drinking water until the end of the experiment. The mouse cages were placed above the receiver to detect the signals, and the parameters were monitored using the programme Ponemah (Version 5.2; Data Sciences International, Minnesota, USA). Data were saved every minute for each parameter and each mouse, and were analysed using the programme Excel (Microsoft, Redmont, USA). To analyse the ECG signals two distinct parameters, i.e. HR and heart rate variability were calculated (HRV) via SDANN-value (standard deviation of R-R intervals in 1 min intervals over on 12 h segment; 7 pm-7am). All telemetric parameters were monitored continuously for 24 h after the distinct interventions and on the recovery days (Fig. 1). However, the conclusions were based on 12-h recordings of HR, BT and Act at night during the active period of the mice to eliminate physical reactions caused by handling of the animals or disturbances in the animal facility.
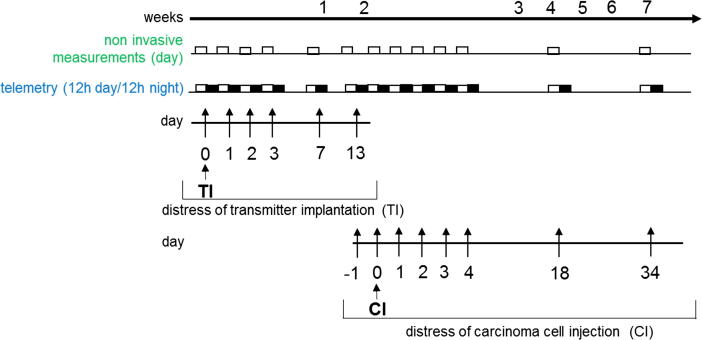
Fig. 1.
Experimental design for distress assessment. Radio telemetric transmitters were implanted on day 0 (TI). The distress of transmitter implantation (TI) was analysed using telemetry and non-invasive methods after surgery on day 0 and on recovery days 1–3, 7 and 13. The distress assessment for laparotomy plus cancer cell injection into the pancreas (CI) was performed before (−1) and after laparotomy (0), as well as on recovery day 1–3. Distress was also quantified during tumour growth on days 4, 18 and 34 after carcinoma cell injection (CI).
Syngeneic orthotopic pancreatic carcinoma model
Orthotopic injection of pancreatic cancer cells was performed as previously described by Zechner et al. [32]. In short, the mice were anaesthetized with 1.2–2.0 vol% isoflurane, and 5 mg/kg carprofen (Rimadyl®, Berlin, Pfizer GmbH) was applied by s.c. injection for analgesia. Eyes were kept wet by using eye ointment. The abdomen of the mice was shaved, and the abdominal cavity was opened by laparotomy. Then, 5 μL of cell suspension (murine cell line 6606PDA, 2.5 × 105/5 μL cells in Matrigel) was injected slowly using a 25 μL syringe (Hamilton, Reno, Nev., USA). The pancreas was placed back into the cavity. The peritoneum was closed using a coated 5–0 vicryl suture (Johnson & Johnson MEDICAL GmbH), the skin was sewed with a 5–0 prolene suture (Johnson & Johnson MEDICAL GmbH), and mice were placed in front of a heating lamp for 30 min. The duration of the surgical procedure for carcinoma cell injection was 20 min per mouse. A total of 1250 mg/L metamizol was applied daily in the drinking water until the end of the experiment (mice were euthanized 37 days after tumour cell injection). Metamizol was chosen to cover possible pain caused during the recovery days after surgery and during the tumour growth period. The benefit of metamizol is that it can be self-administered by the mice. Repetitive injection of analgesic components would cause additional distress [33], [24] and influence our data analysis. To cover possible pain after the surgical procedures, carprofen 5 mg/kg was injected subcutaneously. The application of metamizol in combination with carprofen was chosen for both surgical interventions, i.e. transmitter implantation and carcinoma cell injection, in order to directly compare those procedures in terms of distress. An opioid such as buprenorphine was intentionally not applied for analgesia because it is reported to reduce food and water consumption, activity, body weight in both mice and rats [34], [35], [36]. Compared to buprenorphine, non-steroidal anti-inflammatory drugs (NSAIDS), such as ketoprofen, indomethacin, meloxicam and carprofen, provoked fewer side effects and similar analgesic efficacy after distinct abdominal surgeries [37], [38] and after transmitter implantation [39], [40], [41] in rodents.
Assessment of non-invasive distress parameters
Non-invasive parameters were assessed during the light phase (7am-7pm). After transmitter implantation (TI), distress was analysed after surgery (0) and on recovery days 1–3, 7–8 and 13. Evaluation of distress after tumour cell injection (CI) was quantified on the day before (−1), the day after surgery (0) and on recovery day 1–3. The assessment of distress during disease progression was performed on days 4, 18 and 34 of tumour development (Fig. 1). The distress score was assessed 30 min after each surgical intervention and at the indicated recovery days according to a clinical scoring sheet, which has been published by Kumstel et al. [33] and is based on other scoring systems [42], [43], [44]. Body weight was measured 24 h after evaluating the other distress parameters to allow sufficient time for body weight adjustments to a specific level of distress. To assess body weight, mice were placed by tail handling on a scale for 5–10 s. Changes in body weight were calculated as a percentage of the value assessed 1–4 days before transmitter implantation. The weight of the transmitter (1.6–1.7 g dependent on the electrode length) was subtracted from the measured body weight. Burrowing behaviour was analysed according to Deacon et al. [11] by placing a burrowing tube filled with 200 g of food pellets into the cage 3 h before the dark phase. The amount of burrowed pellets was evaluated 2 h later. To analyse nesting behaviour, a nestlet (5-cm square of pressed cotton batting, Zoonlab GmbH, Castrop-Rauxel, Germany) was provided 1 h before the dark phase. The nest was scored the following morning. In addition to the 1–5 point scale from Deacon et al. [12], 6 points were scored for a perfect nest when more than 90% of the circumference of the walls were higher than the mouse’s body height. To facilitate individual learning, burrowing and nesting were performed three times in group housing until mice were housed separately throughout the entire experiment. To measure faecal corticosterone metabolites (FCMs), 200–400 mg faeces were collected from the home cages 24 h after the interventions or on indicated days. The faeces were dried 4 h at 65 °C and stored at −20 °C. Later, 50 mg homogenized faeces were extracted with 1 mL 80% methanol and analysed by a 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay, which has been validated for use with mice [20], [45].
Data analysis
A repeated measure design was applied without using a control group, since the aim was to compare distress parameters after distinct interventions and the corresponding recovery phases. The quantification of distress after transmitter implantation was performed on 10 mice, after carcinoma cell injection on 9 mice and during tumour progression on 7 mice, since one mouse had to be euthanized due to complications after carcinoma cell injection, and two additional mice reached humane endpoint criteria during tumour progression. All data were graphed and analysed using GraphPad Prism 8.0 (GraphPad Software, San Diego, USA). Data for Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 9 are presented in line graphs, indicating mean values ± standard deviations. For assessment of normality, the Shapiro-Wilk-test was applied, and for scores (distress score, nesting-score), the Kolmogorow-Smirnov test. For parametric data a repeated measures one-way ANOVA followed by a Tukey test for multiple comparisons was performed. For non-parametric data and data in percent the Friedman test followed by Dunn’s method was applied. Data for Fig. 6, Fig. 7 are presented in point plots, indicating mean ± standard deviation. Significance was calculated either by unpaired t-test for parametric data or by Mann-Whitney test for non-parametric data or data in percent. Differences with P < 0.05 were considered significant. To evaluate the performance of each parameter to quantify distress, the area under the curve (AUC) was assessed by performing receiver operating characteristic (ROC) curve analysis on data from mice before and after laparotomy plus carcinoma cell injection. The 95% confidence intervals and P-values were calculated for each parameter.
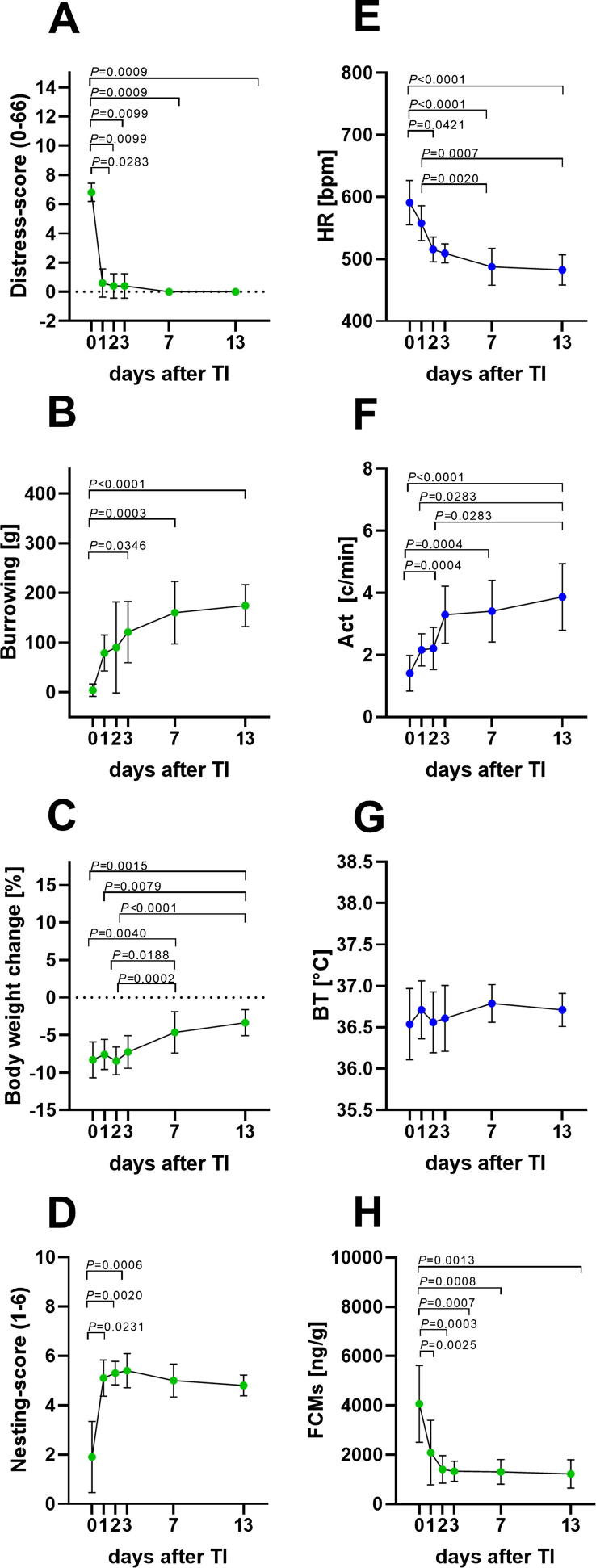
Fig. 2.
Animal distress after transmitter implantation (TI). Distress-score (A), burrowing behaviour (B), percentage of body weight change (C), nesting-score (D), heart rate (HR) (E), activity (Act) (F), body temperature (BT) (G) and faecal corticosterone metabolites (FCMs) (H) were evaluated on the day of surgery (0) and on recovery days 1–3, 7 and 13. Significant differences were determined for non-parametric data with the Friedman test and Dunn’s method for multiple comparisons (A–F). Parametric data was calculated by repeated measures one-way ANOVA followed by Tukey’s test for pairwise comparisons (G–H). P ≤ 0.05 significant to indicated day (n = 10). For details, see Table 1.
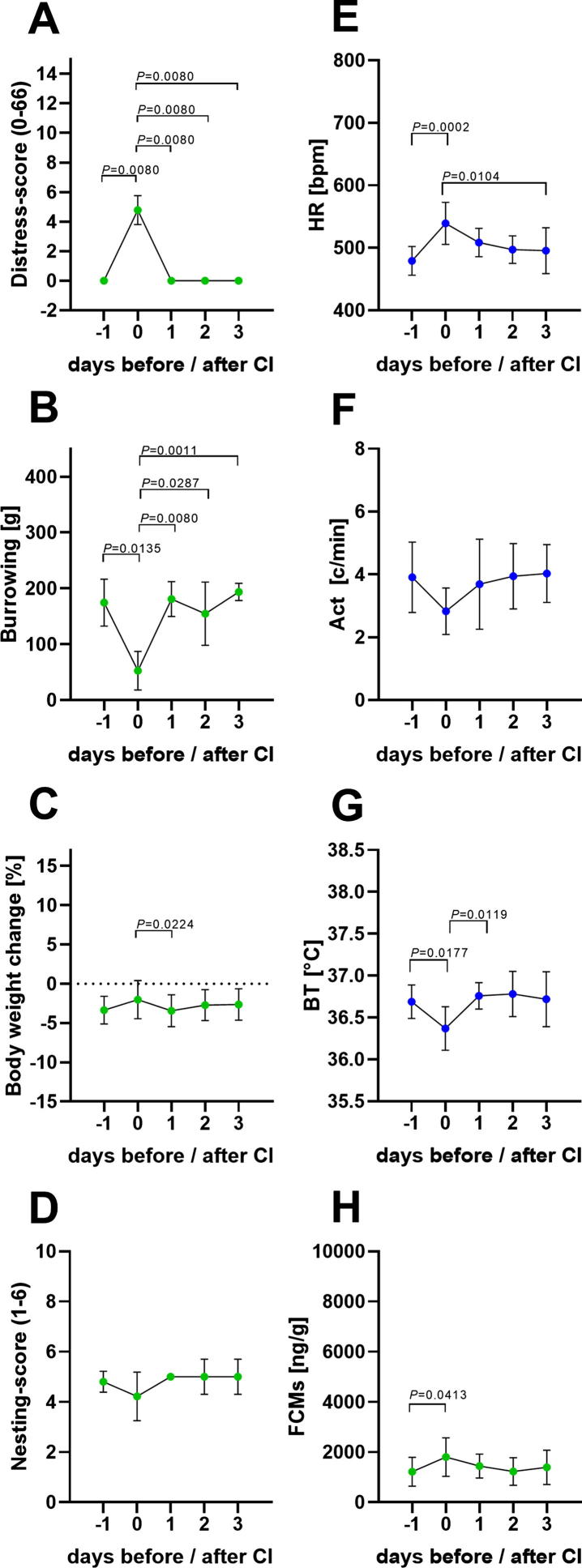
Fig. 3.
Animal distress after laparotomy plus carcinoma cell injection (CI). Distress score (A), burrowing behaviour (B), percentage of body weight change (C), nesting score (D), HR (E), Act (F), BT (G) and FCMs (H) were evaluated before (−1), on the day after surgery (0) and on recovery day 1–3. Significance was analysed for non-parametric data and data in percent with the Friedman test followed by Dunn’s method (A–E). Parametric data was determined by repeated measures one-way ANOVA followed by multiple comparisons via Tukey’s test (F-H): P ≤ 0.05 significant to indicated day (n = 9). For details, see Table 2.
Fig. 4.
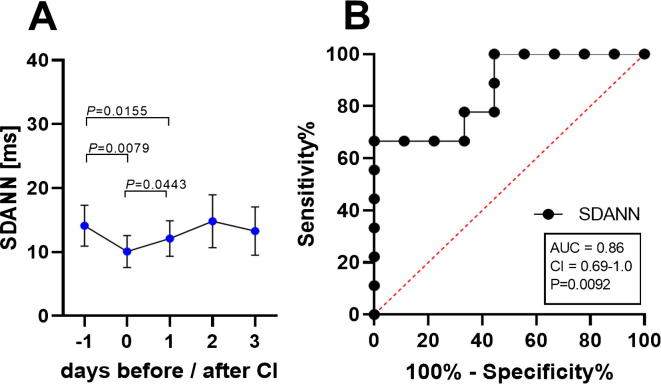
Heart rate variability (HRV) before and after carcinoma cell injection (CI). HRV calculated as SDANN in ms (standard deviation of R-R intervals in 1 min segments of 12 h during the dark phase, 7pm-7am) before surgery, after carcinoma cell injection (CI) on day 0 and on the recovery day 1–3 (A). To evaluate the performance of HRV the area under the curve (AUC), the 95% confidence interval (CI) and the P-value (p) were assessed by performing receiver operating characteristic curve (ROC) analysis on data from mice before (−1) and after carcinoma cell injection (0) (B). Significance was determined for these parametric data by One Way ANOVA for repeated measurement, followed by multiple comparison via the Tukey test (A): P ≤ 0.05 (n = 9). For details of the data, see Table 3.
Fig. 5.
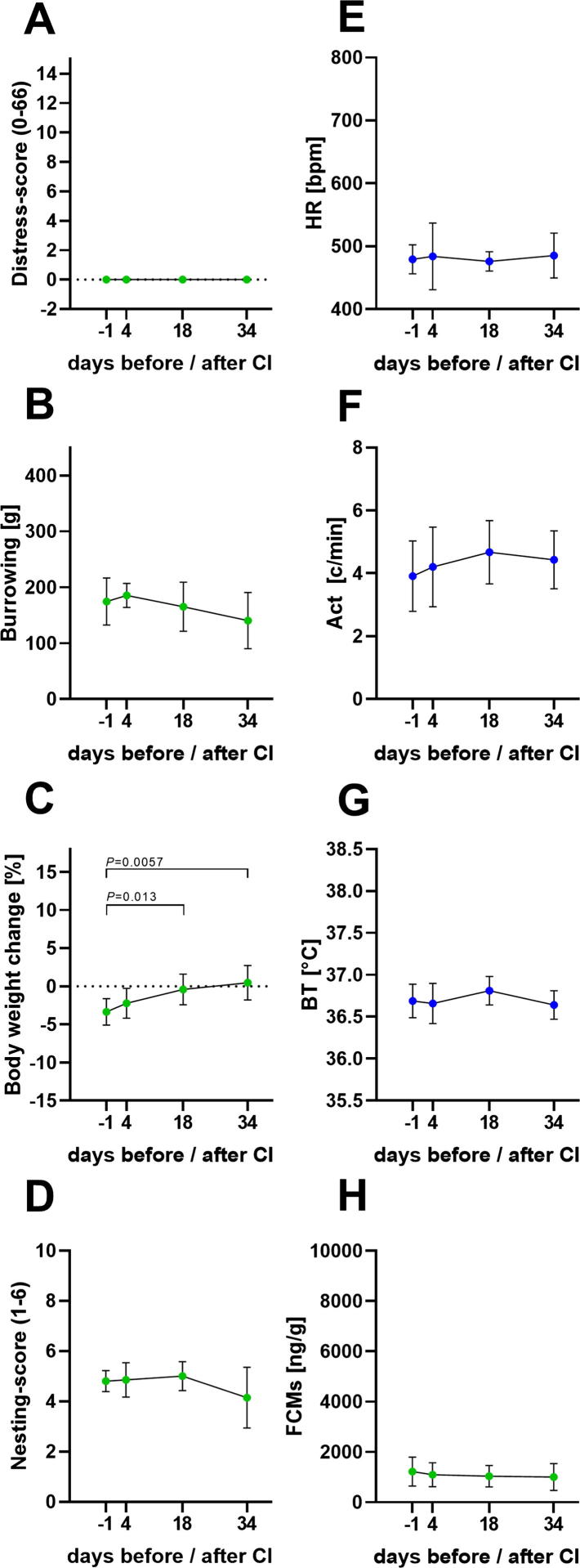
Animal distress before and during tumour growth. Distress score (A), burrowing behaviour (B), percentage of body weight change (C), nesting score (D), HR (E), Act (F), BT (G) and FCMs (H) were evaluated before (−1) carcinoma cell injection (CI) and on days 4, 18, 34 after cancer cell injection. Significant differences of non-parametric data and data in percent were evaluated with the Friedman test followed by Dunn’s method (A-E). Parametric data was determined by repeated measures one-way ANOVA followed by pairwise comparison via Tukey’s test (F–H): P ≤ 0.05 significant to indicated day (n = 7). For details of the data, see Table 4.
Fig. 9.
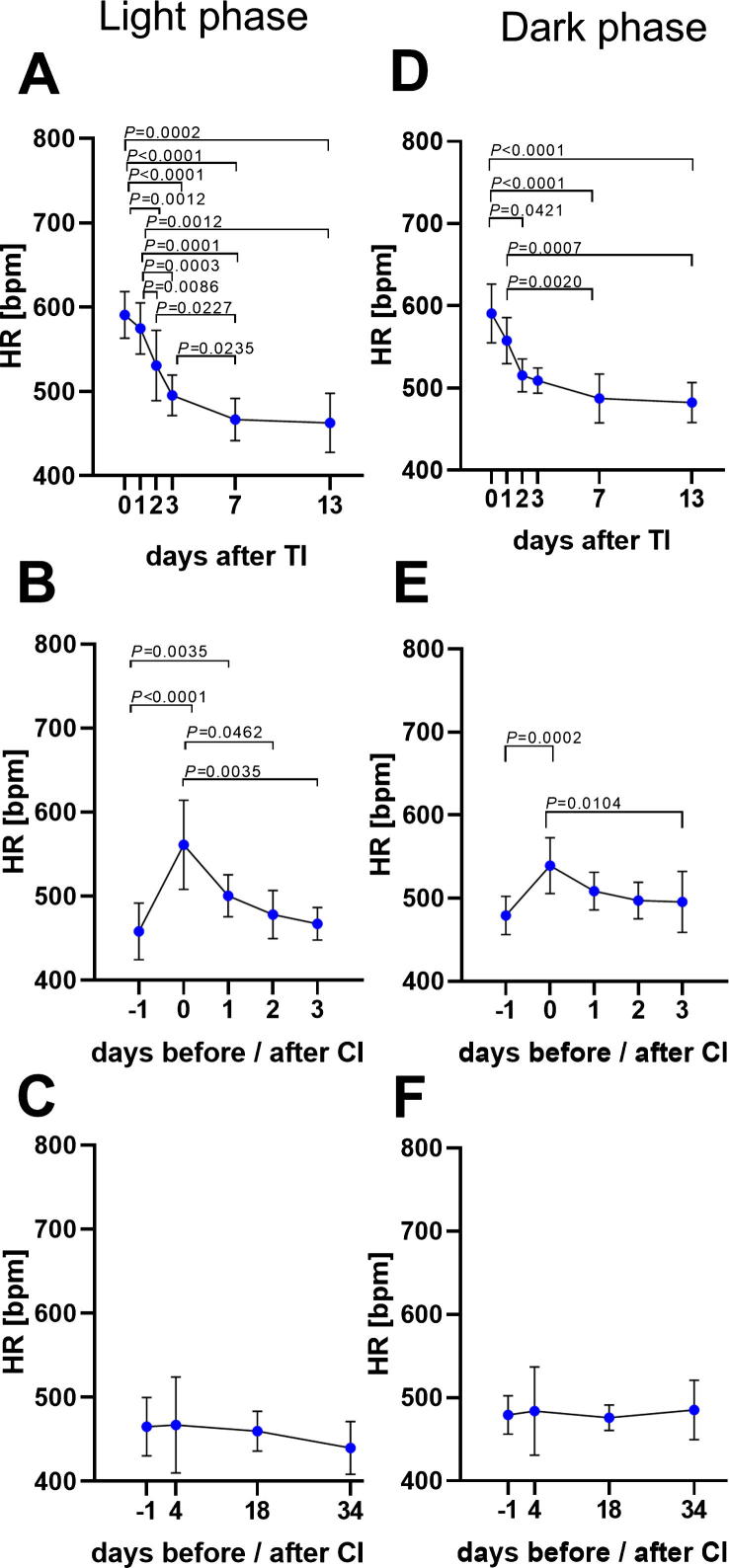
Comparison of mean HR during light and dark phase. Mean HR continuously monitored during 12 h light phase (7am-7pm, except on surgery days: 4pm-7pm), on the days after transmitter implantation (TI) (A), before and after carcinoma cell injection (CI) (B), as well as before and during tumour growth (C). Mean HR evaluated during the 12 h dark phase (7pm-7am) after transmitter implantation (D), on the days before and after carcinoma cell injection (E), as well as during tumor growth (F). Significance was analysed for parametric data by One Way ANOVA for repeated measurement, followed by multiple comparison via the Tukey test (A). Non-parametric data were evaluated with the Friedman test and followed by Dunn’s method (B–F): P ≤ 0.05 (TI: n = 10, CI: n = 9, TP: n = 7). For details of data see Table 7.
Fig. 6.
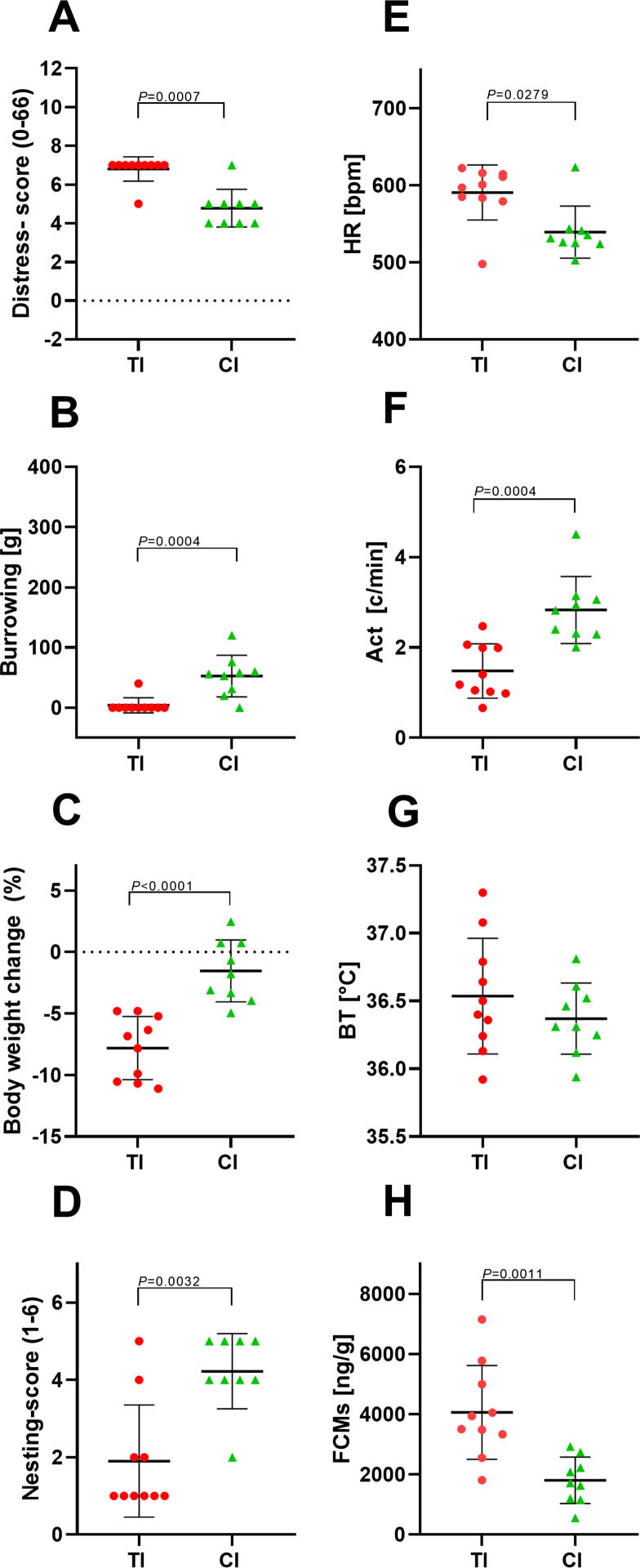
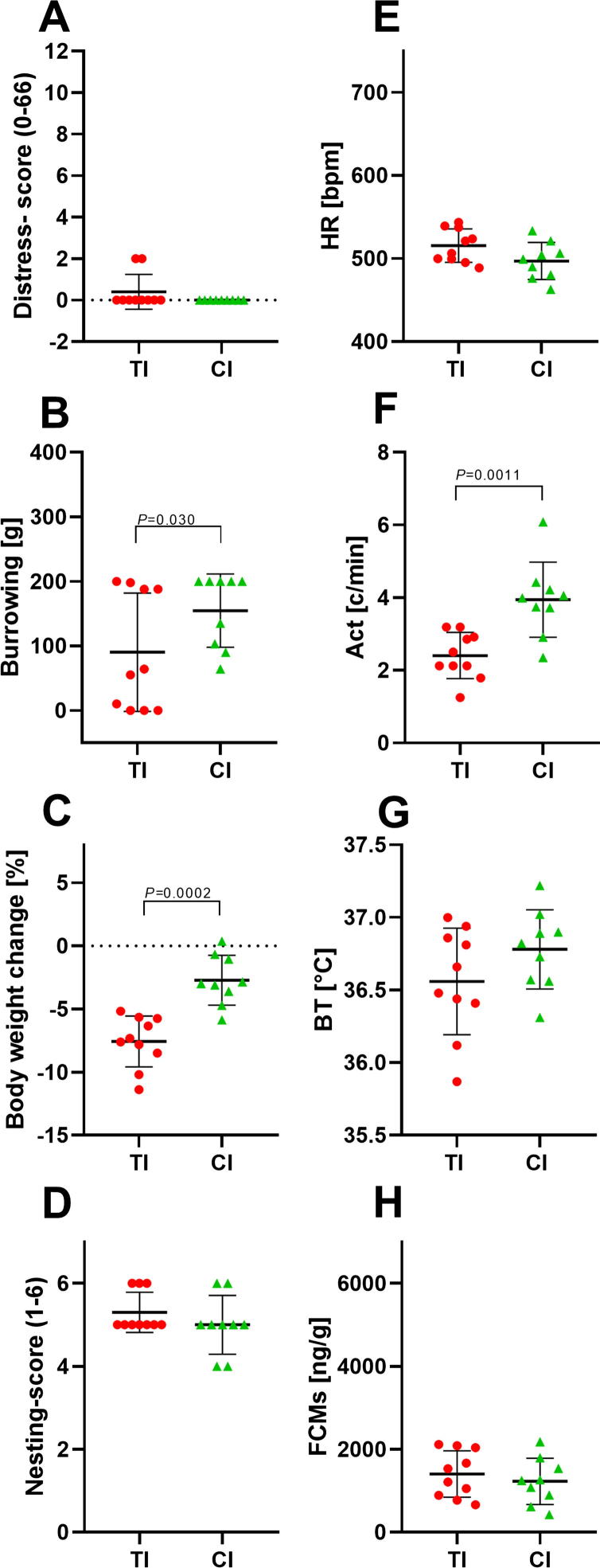
Comparison of animal distress after transmitter implantation and carcinoma cell injection. Distress score (A), burrowing behaviour (B), percentage of body weight change (C), nesting score (D), HR (E), Act (F), BT (G) and FCMs (H) were evaluated on days of transmitter implantation (TI) and carcinoma cell injection (CI). The point plots indicate mean ± standard deviation. Significance was determined for non-parametric data and data in percent with the Mann-Whitney test (A–E) and for parametric data with the unpaired t-test (F–H): TI: n = 10; CI: n = 9. For details of the data, see Table 5.
Fig. 7.
Comparison of animal distress on recovery day 2 after transmitter implantation and carcinoma cell injection. Distress-score (A), burrowing behaviour (B), the percentage of body weight change (C), nesting-score (D), HR (E), Act (F), BT (G) and FCMs (H) were evaluated on the recovery day two after transmitter implantation (TI) and recovery day two after carcinoma cell injection (CI). The point plots indicate mean ± standard deviation. Significance was determined for non-parametric data and data in percent with the Mann-Whitney test (A–D) and parametric data with the unpaired t-test (E–H): TI: n = 10; CI: n = 9. For details of data see Table 6.
Results
Animal distress after transmitter implantation
To assess the distress of mice after transmitter implantation, non-invasive and telemetric parameters were quantified after surgery (0), as well as on recovery day 1–3, 7 and 13 (Fig. 1). The distress score was significantly increased on days after the intervention, due to observations of abnormal posture and passive spontaneous and flight behaviour, as previously published [33]. However, mice recovered within one day (Fig. 2A). Compared to the day of surgery, a significant increase in burrowing behaviour was observed on day 3, 7 and 13 after transmitter implantation, indicating a steady recovery of mice (Fig. 2B). A reduction in body weight of 8% average was observed in animals after transmitter implantation. A slow body weight gain was characterized by a significant increase on recovery days 7 and 13. However, within the two weeks of recovery, mice did not return to their initial body weights (Fig. 2C). The day after surgery, nesting scores were low but recovered within one day (Fig. 2D). Mean HR during the night after surgery (day 0) was significantly elevated compared to recovery days 3, 7 and 13. Additionally, a significant reduction from recovery day 1 to day 7 and 13 was noticed (Fig. 2E). Compared to day 0, a significant increase in Act was observed on post-surgical days 3, 7 and 13 (Fig. 2F). Significant differences could even be observed when comparing day 1 and 2 to day 13. This indicates that mice needed more than two days to recover (Fig. 2F). No significant differences were noticed in BT after surgery or during the recovery period (Fig. 2G). However, a significant increase of FCM concentrations after surgery compared to all recovery days was quantified (Fig. 2H). All parameters, with the exception of BT, demonstrated recovery of mice after transmitter implantation. However, the timing of recovery, especially between day 1 and day 3, was different between parameters. All parameters approached a plateau phase of recovery on day 7–13, indicating stabilization of the physical, physiological and psychological state of the mice.
Table 1.
Data analysis for distress parameter after transmitter implantation (n = 10).
| Days after transmitter implantation (TI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 7 | 13 | |||
| Figs. | Distress parameter | |||||||
| 2A | Distress-score (0–66) |
Mean ±SD |
6.80 0.63 |
0.60a 0.97 |
0.40a 0.84 |
0.40a 0.84 |
0.00a 0.00 |
0.00a 0.00 |
| 2B | Burrowing [g] | Mean ±SD |
4.0 12.6 |
78.9 36.3 |
90.3 91.6 |
121.1a 61.6 |
160.3a 63.0 |
174.4a 42.0 |
| 2C | Body weight change [%] | Mean ±SD |
−8.30 2.39 |
−7.58 2.02 |
−8.45 1.86 |
−7.25 2.18 |
−4.65a, b, c 2.76 |
−3.36a, b, c 1.75 |
| 2D | Nesting-score (1–6) | Mean ±SD |
1.90 1.45 |
5.10a 0.74 |
5.30a 0.48 |
5.40a 0.70 |
5.00 0.67 |
4.80 0.42 |
| 2E | HR [bpm] | Mean ±SD |
590.75 35.86 |
557.82 27.89 |
515.44 20.12 |
509.01a 15.59 |
487.45a, b 29.58 |
482.45a, b 24.31 |
| 2F | Act [c/min] | Mean ±SD |
1.41 0.57 |
2.16 0.52 |
2.21 0.68 |
3.30a 0.92 |
3.41a 0.99 |
3.87a, b, c 1.07 |
| 2G | BT [°C] | Mean ±SD |
36.54 0.43 |
36.71 0.35 |
36.56 0.37 |
36.61 0.40 |
36.79 0.23 |
36.71 0.20 |
| 2H | FCMs [ng/g] | Mean ±SD |
4061.98 1560.77 |
2085.26a 1311.05 |
1402.17a 560.25 |
1325.48a 413.48 |
1301.45a 500.32 |
1220.90a 576.03 |
P < 0.05 compared to day 0.
P < 0.05 compared to day 1.
P < 0.05 compared to day 2.
Animal distress after carcinoma cell injection
For assessment of distress after orthotopic injection of pancreatic cancer cells, all non-invasive and telemetric distress parameters were quantified before (-1), on the same day of carcinoma cell injection (0) and on recovery day 1–3 after cell injection (Fig. 1). Distress score and burrowing behaviour were significantly altered on day 0, indicating increased distress of the mice that exhibited fast recovery within one day (Fig. 3A and B). A significant reduction in the percentage of body weight was noticed on the first recovery day compared to the day of surgical intervention (Fig. 3C). A minor non-significant reduction of nesting behaviour was observed on day 0 (Fig. 3D). HR was significantly elevated after laparotomy, followed by a steady reduction until recovery day 3 (Fig. 3E). Act was slightly and non-significantly reduced after surgery (Fig. 3F). However, BT was significantly reduced, while FCMs were significantly increased, after carcinoma cell injection, followed by rapid recovery within one day (Fig. 3G and H). In addition to HR, the HRV was calculated from the ECG signals and a significant reduction in HRV after carcinoma cell injection was quantified (Fig. 4A). The analysis of the discriminatory power of HRV revealed a lower AUC (AUC = 0.86, P = 0.0092) compared to HR (AUC = 0.95, P = 0.0013) (Fig. 4B). The following analysis, therefore, focused on the HR values. The non-invasive methods distress score, burrowing behaviour and FCMs, as well as the telemetric data for HR and BT, indicated increased distress of mice after carcinoma cell injection followed by rapid recovery within one day.
Table 2.
Data analysis for distress parameter after carcinoma cell injection (n = 9).
| Days before/after carcinoma cell injection (CI) |
|||||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |||
| Figs. | Distress parameter | ||||||
| 3A | Distress-score (0–66) | Mean ±SD |
0.00a 0.00 |
4.78 0.97 |
0.00a 0.00 |
0.00a 0.00 |
0.00a 0.00 |
| 3B | Burrowing [g] | Mean ±SD |
174.40a 41.99 |
52.67 34.43 |
181.00a 31.14 |
154.67a 56.72 |
193.78a 15.42 |
| 3C | Body weight change [%] | Mean ±SD |
−3.36 1.75 |
−2.02 2.43 |
−3.44a 2.02 |
−2.72 1.98 |
−2.63 2.00 |
| 3D | Nesting-score (1–6) | Mean ±SD |
4.80 0.42 |
4.22 0.97 |
5.00 0.00 |
5.00 0.71 |
5.00 0.71 |
| 3E | HR [bpm] | Mean ±SD |
479.09a 23.19 |
539.29 33.82 |
508.47 22.71 |
497.03 22.27 |
495.44a 36.56 |
| 3F | Act [c/min] | Mean ±SD |
3.91 1.12 |
2.83 0.74 |
3.69 1.43 |
3.94 1.04 |
4.03 0.92 |
| 3G | BT [°C] | Mean ±SD |
36.69a 0.20 |
36.37 0.26 |
36.76a 0.16 |
36.78 0.27 |
36.72 0.33 |
| 3H | FCMs [ng/g] | Mean ±SD |
1220.90a 579.03 |
1800.68 772.92 |
1445.50 476.36 |
1226.45 556.10 |
1392.81 686.63 |
P < 0.05 compared to day 0.
Table 3.
Data analysis for HRV before and after carcinoma cell injection (CI) (n = 9).
| Days before/after carcinoma cell injection (CI) |
|||||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |||
| Fig. | Distress parameter | ||||||
| 4A | SDANN [ms] | Mean ±SD |
14.12a, b 3.22 |
10.08 2.49 |
12.12a 2.77 |
14.83 4.15 |
13.28 3.76 |
P < 0.05 compared to day 0.
P < 0.05 compared to day 1.
To evaluate animal distress during tumour growth, all parameters were assessed one day before and on days 4, 18 and 34 after carcinoma cell injection. No significant alterations in distress score or burrowing behaviour were noticed during this period (Fig. 5A and B). The percentage changes in body weight increased significantly during tumour growth (Fig. 5C). No significant alterations were observed either in the other non-invasive parameters, nesting-activity, FCMs, or in any parameters measured by telemetry, such as HR, Act, or BT (Fig. 5D–H). In conclusion the applied methods do not indicate a significant increase in distress during tumour growth.
Table 4.
Data analysis for distress parameter during tumor progression (n = 7).
| Days before/after carcinoma cell injection (CI) |
||||||
|---|---|---|---|---|---|---|
| −1 | 4 | 18 | 34 | |||
| Fig. | Distress parameter | |||||
| 5A | Distress-score | Mean ± SD |
0.00 0.00 |
0.00 0.00 |
0.00 0.00 |
0.00 0.00 |
| 5B | Burrowing [g] | Mean ± SD |
174.40 41.99 |
185.57 21.57 |
165.14 44.09 |
140.29 50.41 |
| 5C | Body weight change | Mean ± SD |
−3.36 1.75 |
−2.24 1.97 |
−0.41a 2.01 |
0.48a 2.28 |
| 5D | Nesting-score | Mean ± SD |
4.8 0.42 |
4.86 0.69 |
5.0 0.58 |
4.14 1.21 |
| 5E | HR [bpm] | Mean ± SD |
479.09 23.19 |
483.89 53.11 |
475.77 15.43 |
485.04 35.92 |
| 5F | Act [c/min] | Mean ± SD |
3.91 1.12 |
4.20 1.27 |
4.67 1.01 |
4.43 0.92 |
| 5G | BT [°C] | Mean ± SD |
36.69 0.20 |
36.66 0.24 |
36.81 0.17 |
36.64 0.17 |
| 5H | FCMs [ng/g] | Mean ± SD |
1220.90 579.03 |
1097.52 480.24 |
1038.54 428.81 |
1003.62 534.43 |
P < 0.05 compared to day −1.
Comparison of animal distress after surgical procedures
To compare the distress of transmitter implantation to laparotomy plus orthotopic carcinoma cell injection, we graphed all distress parameters measured on the same day (0) after each intervention. All non-invasive parameters and most parameters measured by telemetry (HR, Act but not BT) demonstrated significantly more distress after transmitter implantation compared to carcinoma cell injection (Fig. 6A–H). Even on recovery day 2 after both surgical interventions, distress parameters such as burrowing, body weight change and Act indicate significantly more distress after transmitter implantation compared to carcinoma cell injection (Fig. 7).
Table 5.
Data analysis for comparison of animal distress after transmitter implantation (TI) and carcinoma cell injection on day 0.
| TI | CI | |||
|---|---|---|---|---|
| Fig. | Distress parameter | |||
| 6A | Distress-score | Mean ±SD n |
6.80a 0.63 10 |
4.78 0.97 9 |
| 6B | Burrowing [g] | Mean ±SD n |
4.0a 12.6 10 |
52.67 34.43 9 |
| 6C | Body weight change | Mean ± SD n |
−8.30a 2.39 10 |
−2.02 2.43 9 |
| 6D | Nesting-score | Mean ± SD n |
1.90a 1.45 10 |
4.22 0.97 9 |
| 6E | HR [bpm] | Mean ± SD n |
590.75a 35.86 10 |
539.29 33.82 9 |
| 6F | Act [c/min] | Mean ± SD n |
1.41a 0.57 10 |
2.83 0.74 9 |
| 6G | BT [°C] | Mean ± SD n |
36.54 0.43 10 |
36.37 0.26 9 |
| 6H | FCMs [ng/g] | Mean ± SD n |
4061.98a 1560.77 10 |
1800.68 772.92 9 |
P < 0.05 compared to CI.
Table 6.
Data analysis for comparison of animal distress after transmitter implantation (TI) and carcinoma cell injection on recovery day 2.
| TI | CI | |||
|---|---|---|---|---|
| Fig. | Distress parameter | |||
| 7A | Distress-score (0–66) | Mean ± SD n |
0.40 0.84 10 |
0.00 0.00 9 |
| 7B | Burrowing [g] | Mean ± SD n |
90.30a 91.63 10 |
154.67 56.72 9 |
| 7C | Body weight change [%] | Mean ± SD n |
−7.25a 2.18 10 |
−2.72 1.98 9 |
| 7D | Nesting-score (1–6) | Mean ± SD n |
5.30 0.48 10 |
5.00 0.71 9 |
| 7E | HR [bpm] | Mean ± SD n |
515.44 20.12 10 |
497.03 22.27 9 |
| 7F | Act [c/min] | Mean ± SD n |
2.41a 0.64 10 |
3.94 1.04 9 |
| 7G | BT [°C] | Mean ± SD n |
36.56 0.37 10 |
36.78 0.27 9 |
| 7H | FCMs [ng/g] | Mean ± SD n |
1402.17 560.25 10 |
1226.45 556.10 9 |
P < 0.05 compared to CI.
The AUC of each parameter for predicting animal distress
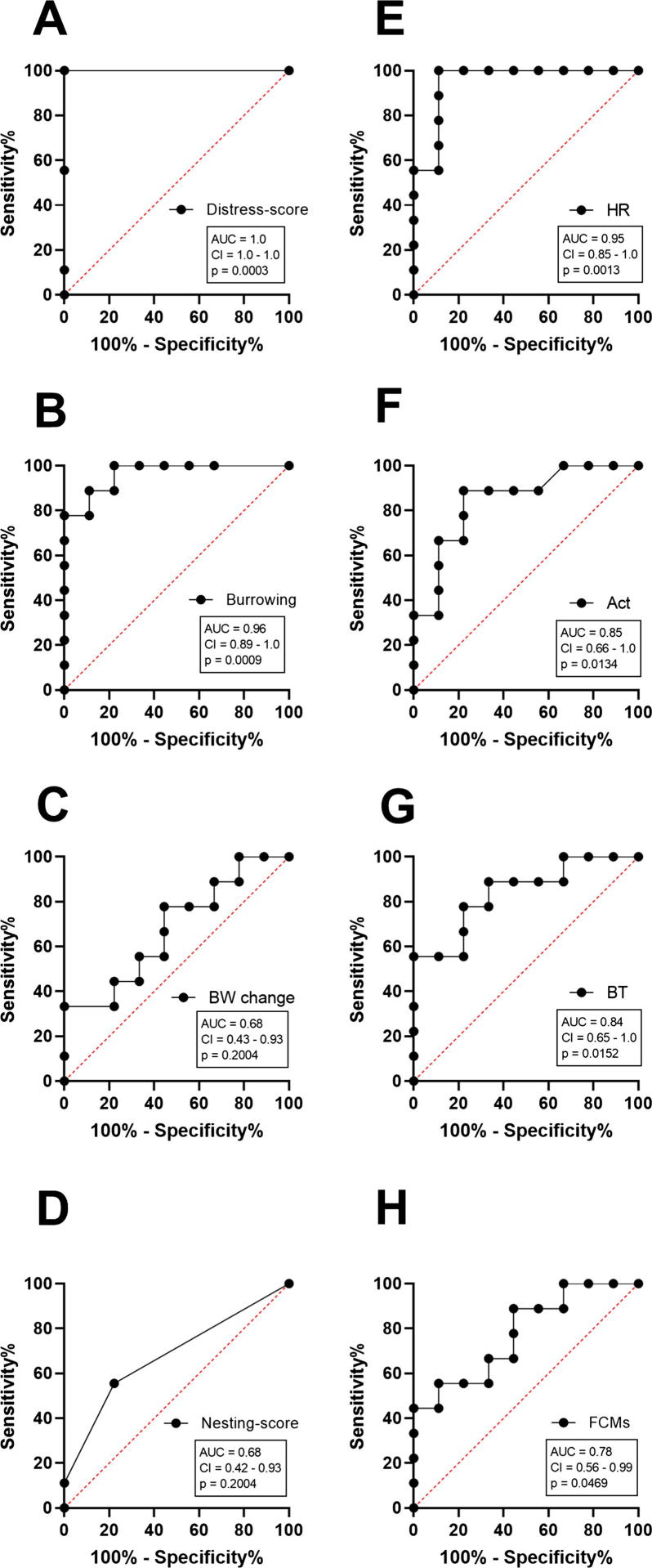
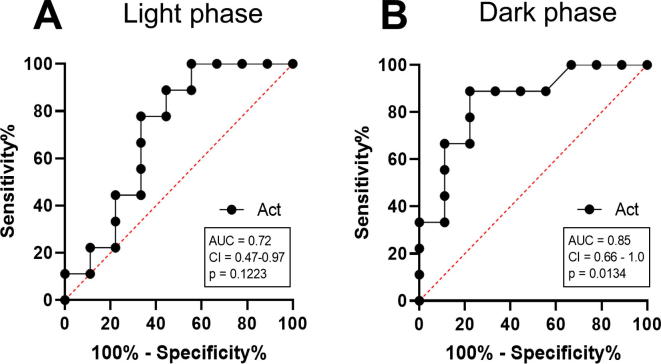
The performance of each readout parameter to diagnose distress was evaluated by applying ROC curve analysis. Therefore, data from all animals were used from the day before (−1) and after carcinoma cell injection (0). ROC curves are typically used to graph the performance of diagnostic tests [46]. An area under the curve (AUC) of 1.0 represents high discriminatory power to differentiate between animals before and after carcinoma cell injection, while a value of 0.5 demonstrates no discriminatory power for this parameter. According to this analysis, the highest discriminatory power was obtained for the distress score (AUC = 1.0, P = 0.0003) (Fig. 8A). Burrowing behaviour also displayed a good performance, with a high AUC of 0.96 and a P-value of 0.0009 (Fig. 8B). In contrast, percentage of body weight change (AUC = 0.68, P = 0.2004) and nesting behaviour (AUC = 0.68, P = 0.2004) demonstrated low discriminatory power for distress assessment (Fig. 8C and D). Telemetric monitoring of HR (AUC = 0.95, P = 0.0013), Act (AUC = 0.85, P = 0.0134) and BT (AUC = 0.84, P = 0.0152) also indicated high AUC to discriminate between animals before and after surgery (Fig. 8E–G). FCMs also exhibited discriminatory power to differentiate between animals before and after carcinoma cell injection (AUC = 0.78, P = 0.0469) (Fig. 8H). When comparing the distress after all interventions by HR during light and dark phase, more significant changes during the recovery phase were observed in the light phase (Fig. 9). However, the AUC of Act to detect distress after CI proved to be better during the dark phase (AUC = 0.85, P = 0.0134) compared to the light phase (AUC = 0.72, P = 0.1223) (Fig. 10).
Table 7.
Data analysis for HR values during light and dark phase.
| Days after transmitter implantation (TI) | 0 | 1 | 2 | 3 | 7 | 13 | ||
|---|---|---|---|---|---|---|---|---|
| Fig. | Distress parameter | |||||||
| 9A | HR [bpm] | Mean ± SD n |
590.67 27.96 10 |
574.87 30.26 10 |
530.96a,b 41.65 10 |
495.63a,b 24.05 10 |
466.94a,b,c,d 25.07 10 |
462.95a,b 35.08 10 |
| 9D | HR [bpm] | Mean ± SD n |
590.75 35.86 10 |
557.82 27.89 10 |
515.44 20.12 10 |
509.01a 15.59 10 |
487.45a,b 29.58 10 |
482.45a,b 24.31 10 |
| Days before/after carinoma cell injection (CI) | −1 | 0 | 1 | 2 | 3 | |||
| Fig. | Distress parameter | |||||||
| 9B | HR [bpm] | Mean ± SD n |
458.25a,b 33.70 9 |
561.19 52.86 9 |
500.54 25.2s0 9 |
478.03a 28.94 9 |
467.33a 19.47 9 |
|
| 9E | HR [bpm] | Mean ± SD n |
479.09a 23.19 9 |
539.29 33.82 9 |
508.47 22.71 9 |
497.03 22.27 9 |
495.44a 36.56 9 |
|
| Days before/after carinoma cell injection (CI) | −1 | 4 | 18 | 34 | ||||
| Fig. | Distress parameter | |||||||
| 9C | HR[bpm] | Mean ± SD n |
465.26 34.80 7 |
467.19 56.86 7 |
459.74 23.72 7 |
439.95 31.50 7 |
||
| 9F | HR[bpm] | Mean ± SD n |
482.94 24.67 7 |
483.89 53.11 7 |
475.77 15.43 7 |
485.04 35.92 7 |
||
P < 0.05 compared to day 0.
P < 0.05 compared to day 1.
P < 0.05 compared to day 2.
P < 0.05 compared to day 3.
Fig. 8.
Performance of each parameter to quantify distress after carcinoma cell injection. To evaluate the performance of each parameter, the area under the curve (AUC), the 95% confidence interval (CI) and the P-value (p) was assessed by performing receiver operating characteristic curve (ROC) analysis on data from mice before (−1) and after tumour cell injection (0). The distress score (A), burrowing behaviour (B), body weight change (BW change) (C), nesting behaviour (D), HR (E), Act (F), BT (G) and FCMs (H) were evaluated (n = 9).
Fig. 10.
Comparison of AUC of activity (Act) during light and dark phase. To evaluate the performance of Act to determine distress the area under the curve (AUC), the 95% confidence interval (CI) and the P-value (p) were assessed by performing receiver operating characteristic curve (ROC) analysis on data from mice before (−1) and after tumour cell injection (0), during 3–12 h light phase (A) and 12 h dark phase (B).
Discussion
This study compared the suitability of non-invasive to telemetry based methods when evaluating distress in mice. The animal distress was quantified after transmitter implantation, carcinoma cell injection and during cancer growth. Transmitter implantation caused significantly more burden to mice when compared to laparotomy plus carcinoma cell injection (Fig. 6). In contrast, no significant induction of distress was detected during tumour growth (Fig. 5). In addition, ROC curve analysis revealed that the non-invasive methods, distress score and burrowing behaviour displayed slightly higher AUC than HR, BT and Act for defining distress after carcinoma cell injection.
Body weight change is an important parameter for indicating the physical state of mice. A significant reduction of body weight was, for example, observed after colitis [47] or intra-bone marrow transplantation [48]. In these studies, body weight loss correlated well with other distress parameters, such as burrowing [47] or FCMs [48]. Burrowing behaviour was also affected after laparotomy in other animal models [19] and was reported to be more sensitive than nesting [49]. An increase of the telemetric parameters HR, BT and Act was observed upon stressors such as resident-intruder test [50], handling, disturbance and cage change [27]. The above-cited literature, as well as our results, support the hypothesis that these non-invasive and telemetric parameters are able to score distress in response to different interventions.
When comparing these parameters after telemeter implantation and cell injection, it can be concluded that transmitter implantation caused significantly more distress than laparotomy plus carcinoma cell injection, as indicated by seven (out of eight) significantly altered parameters (Fig. 6). This statement is also supported by a sustained recovery period after transmitter implantation (cf. Fig. 2, Fig. 3).
Both interventions included laparotomy; however, the higher distress after transmitter implantation might be due to the second incision and implantation of the telemetric device, which weights 1.7 g and is quite heavy for a mouse. Furthermore, the duration of surgical procedures (45 to 55 min for telemeter implantation versus 20 min for carcinoma cell injection) might influence distress in mice. Even anaesthesia alone without surgery is reported to provoke significant alterations of HR, BT, ACT and burrowing as well as nesting activity [18], [51], [52]. The measured read out parameters in this manuscript are, therefore, influenced by anesthesia, but also analgesia and surgical interventions or tumor growth. A distinction between distress caused by anaesthesia, analgesia or the surgical procedure was not made, since anesthesia and analgesia are mandatory. Another limitation is that it could not be clarified how much pain the mice experienced, since the distress parameters utilized are not specific for pain. In addition, animals with analgesia could not be compared to animals that did not receive analgesia because exploring distress without analgesia would cause unintentional suffering for the animals and is not in accordance with our animal welfare guidelines. However, in future studies, these sensitive distress parameters will be used to compare different analgesia methods after distinct interventions. According to the EU-Directive 2010/63/EU, Annex VIII [2], surgical interventions, including laparotomy and transmitter implantation, should be classified as “moderate” severity. This rating is in line with our assessed distress score after both surgical interventions (total score 5–15: moderate distress [33]). After transmitter implantation, we observed a maximal body weight loss of 11.1%, which can be classified as moderate distress [53]. Additionally, seven parameters clearly indicated that transmitter implantation leads to more distress than laparotomy plus cancer cell injection (Fig. 6). It does not seem correct that both interventions have the identical severity classification. These data may suggest that more than 4 classes (non-recovery, mild, moderate and severe) or subclasses are necessary to generate a widely applicable system for the guidance of ethical review and monitoring of animal experiments.
In contrast to the surgical interventions, no distress was observed with the applied methods during cancer growth, although mice carried quite huge pancreatic tumours with an average weight of 494 mg (standard deviation: ±285 mg) on day 37 after carcinoma cell injection. Since the tumour weight only minimally contributes to the measured body weight (mean: 1.43%, standard deviation: ±0.76%), the observed body weight gain of mice during the phase of tumor growth (mean: 2.79%, standard deviation: ±2.19%), is only partially caused by increased tumour weight, but is also caused by an increase of the actual body weight. However, it is well accepted that body weight change alone can be a poor distress indicator for tumour mouse models [43].
Thus, it can be suggested that tumour growth in this animal model causes mild distress. Consistent with the present data, similar minor alterations in burrowing behaviours, FCMs, nest building activity and body weight loss were observed upon repeated anaesthesia, which is also classified as a “mild” procedure [49], [54]. Another interpretation could be that the utilized distress parameter might also not be sensitive enough to assess distress caused by the internal tumour growth.
When comparing all read-out parameters, it was observed that distress score and burrowing activity exhibited the highest AUC for determining distress after cancer cell injection (AUC of parameters: distress-score > burrowing > HR > Act > BT > FCMs > body weight change = nesting). Thus, distress score and burrowing behaviour indicated a better performance for distress determination than telemetric monitoring of HR, Act or BT. However, since the confidence intervals of all assessed parameters overlap, no distress parameter is significantly better for distress quantification. Therefore, all parameters are useful to quantify distress after laparotomy.
A limitation of the clinical score is that it was initially developed to cover several murine gastrointestinal disease models, and its assessment is always influenced by the subjective perception of the researcher. However, this scoring system proved to be sensitive to detect distress after surgery but did not detect distress symptoms during tumour progression or recovery days (see Figs. 2A, 3A and 5A). Interestingly burrowing behaviour, as one objective distress indicator, showed higher AUC than the telemetric parameters, and the AUC of FCMs is comparable to BT and Act (Fig. 8).
Another limitation of our results section might be that the average HR, BT and Act was calculated only during the active phase in mice (dark phase, 7pm-7am). In order to address this issue, comparison of HR during the light phase (7am-7pm) with the dark phase (7pm-7am) was additionally provided for all interventions and the following recovery days (Fig. 9). In fact, more significant differences were observed during the light phase caused by lower baseline values of the resting heart rate. Moreover, telemetric parameters during the light phase were also influenced by the assessment of burrowing behaviour, as well as by disturbances of personnel in the animal facility. It was previously reported that an adequate assessment of HR by telemetry should always be performed in undisturbed animals [55]. Additionally, the AUC of measuring Act was lower during the light phase, when compared to the dark phase. (Fig. 10). Based on the above mentioned reasons, the light phase was intentionally not used for the assessment of telemetric parameters.
In addition, to the slightly reduced AUC for distress prediction after surgery, another disadvantage of using telemetry is the relatively high distress caused by transmitter implantation, which also demands a long recovery period. A recovery period of 2 weeks after transmitter implantation was estimated, in accordance with other studies [27], [50]. However, the time to fully regain body weight proved to be too short (Fig. 2C). Even 13 days after transmitter implantation, the body weight of mice was still reduced by 3%. Thus, a recovery period of greater than 2 weeks can be recommended. Another disadvantage of telemetry is the expensive hardware, including receiver, matrix and transmitter and that surgical implantation of the transmitter should be performed by trained personnel with micro-surgical skills to minimize tissue trauma related distress [31]. These disadvantages might argue against using telemetry for distress assessment if other sensitive and non-invasive methods are applicable.
However, one major benefit of telemetry is the high temporal resolution of data assessment. In particular, HR proved to be a very useful readout parameter for analysis after short time stressors. For example, handling, injection and cage changes increased HR for 30–70 min [27], [24]. Such a short-term stress would be difficult to assess using behavioural methods. In addition, HR was reported to be a very sensitive indicator of stress, since not only direct stress induction but also just witnessing stress of other mice [56] or being singly housed increased HR [55]. Thus, telemetric analysis might provide valuable insight when analysing temporary or mild distress. Telemetric data acquisition is also helpful when quantifying stress induced alterations of circadian rhythm and sleep patterns [31], [57]. Hence, there are some situations where distress analysis by telemetry might be very useful.
Conclusions
In conclusion, this study revealed that non-invasive distress parameters, such as distress score and burrowing behaviour, had a slightly better performance than telemetric monitoring of HR, Act and BT in terms of distress assessment in a murine pancreatic cancer model. Considering the additional distress caused by transmitter implantation, the expensive hardware and required micro-surgical skills, the use of non-invasive parameters rather than telemetry can be recommended for evidence-based severity assessment.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of animals were followed.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The work from Edith Klobetz-Rassam for analysing FCMs samples was appreciated, as well as the technical support concerning the telemetry system from Stefano Gaburro, Christina Baciu (Data Science International) and Ralf G?l. This study was supported by the Deutsche Forschungsgemeinschaft (DFG research group FOR 2591, project number: 321137804, ZE 712/1-1 and VO 450/15-1). P.V. is supported by the German Federal Ministry of Education and Research (BMBF) and FORUN. R.D. is supported by BMBF, the German Research Foundation (DFG), the DAMP-Foundation and the State of Mecklenburg-West Pomerania via European Social Funds (ESF 14/BM-A55-0024/18).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Russell W.M.S., Burch R.L. University Federation for Animal Welfare; 1992. The Principles of Humane Experimental Technique. [Google Scholar]
- 2.The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 of September 2010 on the protection of animals used for scientific purposes: 2010/63/EU; 2010.
- 3.Graham M.L., Prescott M.J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur J Pharmacol. 2015;759:19–29. doi: 10.1016/j.ejphar.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stasiak K.L., Maul D., French E., Hellyer P.W., VandeWoude S. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci. 2003;42:13–20. [PubMed] [Google Scholar]
- 5.Mellor D.J., Reid C.S.W. Australian and New Zealand Council for Care of Animals in Research and Teaching (ANZCCART) 1994. Concepts of animal well-being and predicting the impact of procedures on experimental animals. Improving the Well-Being of Animals in Research Environment; pp. 3–18. [Google Scholar]
- 6.Institute for Laboratory Animal Research (U.S.). Recognition and alleviation of distress in laboratory animals. Washington, D.C: National Academies Press; 2008. [PubMed]
- 7.Hawkins P., Morton D.B., Burman O., Dennison N., Honess P., Jennings M. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: eleventh report of the BVAAWF/FRAME/RSPCA/UFAW joint working group on refinement. Lab Anim. 2011;45:1–13. doi: 10.1258/la.2010.010031. [DOI] [PubMed] [Google Scholar]
- 8.Nunamaker E.A., Artwohl J.E., Anderson R.J., Fortman J.D. Endpoint refinement for total body irradiation of C57BL/6 mice. Comp Med. 2013;63:22–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Redgate E.S., Deutsch M., Boggs S.S. Time of death of CNS tumor-bearing rats can be reliably predicted by body weight-loss patterns. Lab Anim Sci. 1991;41:269–273. [PubMed] [Google Scholar]
- 10.Häger C., Keubler L.M., Talbot S.R., Biernot S., Weegh N., Buchheister S. Running in the wheel: defining individual severity levels in mice. PLoS Biol. 2018;16:e2006159. doi: 10.1371/journal.pbio.2006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deacon R.M.J. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- 12.Deacon R.M.J. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 13.Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139–146. doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Deacon R.M.J., Reisel D., Perry V.H., Nicholas J., Rawlins P. Hippocampal scrapie infection impairs operant DRL performance in mice. Behav Brain Res. 2005;157:99–105. doi: 10.1016/j.bbr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Felton L.M., Cunningham C., Rankine E.L., Waters S., Boche D., Perry V.H. MCP-1 and murine prion disease: separation of early behavioural dysfunction from overt clinical disease. Neurobiol Dis. 2005;20:283–295. doi: 10.1016/j.nbd.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Yuan D., Liu C., Wu J., Hu B. Nest-building activity as a reproducible and long-term stroke deficit test in a mouse model of stroke. Brain Behav. 2018;8:e00993. doi: 10.1002/brb3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arras M., Rettich A., Cinelli P., Kasermann H.P., Burki K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res. 2007;3:16. doi: 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jirkof P., Fleischmann T., Cesarovic N., Rettich A., Vogel J., Arras M. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim. 2013;47:153–161. doi: 10.1177/0023677213475603. [DOI] [PubMed] [Google Scholar]
- 19.Jirkof P., Cesarovic N., Rettich A., Nicholls F., Seifert B., Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touma C., Palme R., Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Palme R. Non-invasive measurement of glucocorticoids: advances and problems. Physiol Behav. 2018;199:229–243. doi: 10.1016/j.physbeh.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Kramer K., van Acker S.A.B.E., Voss H.-P., Grimbergen J.A., van der Vijgh W.J.F., Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods. 1993;30:209–215. doi: 10.1016/1056-8719(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 23.Clement J.G., Mills P., Brockway B. Use of telemetry to record body temperature and activity in mice. J Pharmacol Methods. 1989;21:129–140. doi: 10.1016/0160-5402(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 24.Meijer M.K., Spruijt B.M., van Zutphen L.F.M., Baumans V. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim. 2006;40:382–391. doi: 10.1258/002367706778476370. [DOI] [PubMed] [Google Scholar]
- 25.Chen D., La Greca L., Head G.A., Walther T., Mayorov D.N. Blood pressure reactivity to emotional stress is reduced in AT1A-receptor knockout mice on normal, but not high salt intake. Hypertens Res. 2009;32:559–564. doi: 10.1038/hr.2009.59. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Su H., Copenhagen L.D., Vaishnav S., Pieri F., Shope C.D. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Bogaert M.J.V., Groenink L., Oosting R.S., Westphal K.G.C., van der Gugten J., Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 28.Keeney A.J., Hogg S., Marsden C.A. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav. 2001;74:177–184. doi: 10.1016/s0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- 29.Brodkin J., Bradbury M., Busse C., Warren N., Bristow L.J., Varney M.A. Reduced stress-induced hyperthermia in mGluR5 knockout mice. Eur J Neurosci. 2002;16:2241–2244. doi: 10.1046/j.1460-9568.2002.02294.x. [DOI] [PubMed] [Google Scholar]
- 30.Adriaan Bouwknecht J., Olivier B., Paylor R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Cesarovic N., Jirkof P., Rettich A., Arras M. Implantation of radiotelemetry transmitters yielding data on ECG, heart rate, core body temperature and activity in free-moving laboratory mice. J Vis Exp. 2011 doi: 10.3791/3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zechner D., Bürtin F., Albert A.-C., Zhang X., Kumstel S., Schönrogge M. Intratumoral heterogeneity of the therapeutical response to gemcitabine and metformin. Oncotarget. 2016;7:56395–56407. doi: 10.18632/oncotarget.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumstel S., Tang G., Zhang X., Kerndl H., Vollmar B., Zechner D. Grading distress of different animal models for gastrointestinal diseases based on plasma corticosterone kinetics. Animals. 2019;9:145. doi: 10.3390/ani9040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chappell M.G., Koeller C.A., Hall S.I. Differences in postsurgical recovery of CF1 mice after intraperitoneal implantation of radiotelemetry devices through a midline or flank surgical approach. J Am Assoc Lab Anim Sci. 2011;50:227–237. [PMC free article] [PubMed] [Google Scholar]
- 35.Goecke J.C., Awad H., Lawson J.C., Boivin G.P. Evaluating postoperative analgesics in mice using telemetry. Comp Med. 2005;55:37–44. [PubMed] [Google Scholar]
- 36.Hayes K.E., Raucci J.A., Gades N.M., Toth L.A. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci. 2000;39:18–23. [PubMed] [Google Scholar]
- 37.Roughan J.V., Flecknell P.A. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol. 2004;15:461–472. doi: 10.1097/00008877-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Cooper D.M., Hoffman W., Wheat N., Lee H.-Y. Duration of effects on clinical parameters and referred hyperalgesia in rats after abdominal surgery and multiple doses of analgesic. Comp Med. 2005;55:344–353. [PubMed] [Google Scholar]
- 39.Sharp J., Zammit T., Azar T., Lawson D. Recovery of male rats from major abdominal surgery after treatment with various analgesics. Contemp Top Lab Anim Sci. 2003;42:22–27. [PubMed] [Google Scholar]
- 40.Blaha M.D., Leon L.R. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci. 2003;47:8–19. [PMC free article] [PubMed] [Google Scholar]
- 41.Rätsep M.T., Barrette V.F., Winterborn A., Adams M.A., Croy B.A. Hemodynamic and behavioral differences after administration of meloxicam, buprenorphine, or tramadol as analgesics for telemeter implantation in mice. J Am Assoc Lab Anim Sci. 2013;52:560–566. [PMC free article] [PubMed] [Google Scholar]
- 42.Koch A., Gulani J., King G., Hieber K., Chappell M., Ossetrova N. Establishment of early endpoints in mouse total-body irradiation model. PLoS ONE. 2016;11:e0161079. doi: 10.1371/journal.pone.0161079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paster E.V., Villines K.A., Hickman D.L. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med. 2009;59:234–241. [PMC free article] [PubMed] [Google Scholar]
- 44.Morton D., Griffiths P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 45.Touma C., Sachser N., Möstl E., Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- 46.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 47.Jirkof P., Leucht K., Cesarovic N., Caj M., Nicholls F., Rogler G. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim. 2013;47:274–283. doi: 10.1177/0023677213493409. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffenberger U., Yau T., Fink D., Tichy A., Palme R., Egerbacher M. Assessment and refinement of intra-bone marrow transplantation in mice. Lab Anim. 2015;49:121–131. doi: 10.1177/0023677214559627. [DOI] [PubMed] [Google Scholar]
- 49.Hohlbaum K., Bert B., Dietze S., Palme R., Fink H., Thöne-Reineke C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-assessing the degree of distress. PLoS ONE. 2017;12:e0179588. doi: 10.1371/journal.pone.0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouwknecht J.A., Hijzen T.H., van der Gugten J., Maes R.A., Hen R., Olivier B. Absence of 5-HT(1B) receptors is associated with impaired impulse control in male 5-HT(1B) knockout mice. Biol Psych. 2001;49:557–568. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- 51.Cesarovic N., Nicholls F., Rettich A., Kronen P., Hässig M., Jirkof P. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim. 2010;44:329–336. doi: 10.1258/la.2010.009085. [DOI] [PubMed] [Google Scholar]
- 52.Jirkof P., Cesarovic N., Rettich A., Fleischmann T., Arras M. Individual housing of female mice: influence on postsurgical behaviour and recovery. Lab Anim. 2012;46:325–334. doi: 10.1258/la.2012.012027. [DOI] [PubMed] [Google Scholar]
- 53.Jones H., Oates J., Trussell B. An applied approach to the assessment of severity: humane endpoints in animal experiments for biomedical research. J R Soc Med. 1998:40–47. [Google Scholar]
- 54.Hohlbaum K., Bert B., Dietze S., Palme R., Fink H., Thöne-Reineke C. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS ONE. 2018;13:e0203559. doi: 10.1371/journal.pone.0203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Späni D., Arras M., König B., Rülicke T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab Anim. 2003;37:54–62. doi: 10.1258/002367703762226692. [DOI] [PubMed] [Google Scholar]
- 56.Gilmore A.J., Billing R.L., Einstein R. The effects on heart rate and temperature of mice and vas deferens responses to noradrenaline when their cage mates are subjected to daily restraint stress. Lab Anim. 2008;42:140–148. doi: 10.1258/la.2007.06030e. [DOI] [PubMed] [Google Scholar]
- 57.Logan R.W., Edgar N., Gillman A.G., Hoffman D., Zhu X., McClung C.A. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psych. 2015;78:249–258. doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]