Highlights
-
•
77-Year-old female patient with the diagnosis of pancreatic head insulinoma.
-
•
We used near infrared light to detect synchronous pancreatic tumors.
-
•
Near infrared light was used to detect secondary lymph node, liver metastasis.
-
•
Was perform cephalic pancreatoduodenectomy.
-
•
Evolution was favorable.
Keywords: Insulinoma, Near infrared light, Indocyanine green, Pancreatic neuroendocrine tumors, Case report
Abstract
Introduction
We report the case of a 77-year-old female patient with the diagnosis of pancreatic head insulinoma, in whom we used near infrared light (NIR) to detect synchronous pancreatic tumors and potential secondary lymph node or liver involvement. The patient presented with hypoglycemia manifesting by lipothymia. With the diagnosis of secretory neuroendocrine tumor (insulinoma) of the pancreatic head, cephalic pancreatoduodenectomy with the preservation of the pylorus was performed after NIR visualization of the pancreatic tumor mass. At 6, 12, 18 months postoperatively, the patient no longer had hypoglycemia and her general state was good.
Conclusion
NIR with indocyanine green (ICG) evidences pancreatic neuroendocrine tumors, as well as possible synchronous tumors and secondary lymph node or liver involvement.
1. Introduction
Pancreatic insulinomas are benign tumors in 90% of the cases. Sporadic insulinoma occurs in the pancreas in 98% of cases and is usually small (0.5 cm–3 cm), but may have malignant characteristics if its size exceeds 2.5 or 3 cm. Surgery is the only curative treatment. The examinations used to evidence the location of these neuroendocrine tumors preoperatively are somatostatin receptor scintigraphy, echoendoscopy, F dihydroxyphenylalanine (F-DOPA) PET CT. Somatostatin receptor scintigraphy (SRS) has a sensitivity of about 50–60% in detecting benign insulinomas. Echoendoscopy (EES) has an 86% (57–100%) rate of detection, while intraoperative ultrasound identifies 92% (84–100%) of all insulinomas. If the preoperative location of insulinoma is difficult, F dihydroxyphenylalanine (F-DOPA) PET CT is indicated. Another examination to detect secretory pancreatic neuroendocrine tumors (PanNET) is glucagon-like peptide-1 PET CT, which is useful particularly in MEN-1 (1). Intraoperatively, PanNET can be examined by palpation, intraoperative ultrasound (IOUS) or near infrared (NIR) light [1].
A 77-year-old female patient was treated in the Surgical Department of the Cluj-Napoca Clinical Emergency Hospital.
The aim of this case report is to demonstrate whether ICG administration and NIR examination in pancreatic insulinoma are beneficial for patients.
The work in this case has been reported in line with the SCARE criteria [2].
Surgical Clinic II, “Iuliu Hatieganu” UMF Cluj-Napoca, Romania.
2. Case report
Caucasian, female patient NM aged 77 years, from an urban area, retired, was admitted to the Surgical Clinic II by transfer from the Clinic of Diabetes Mellitus and Nutrition Diseases for repeated lipothymia, symptomatic hypoglycemia. The patient’s personal pathological history included antral gastritis, Helicobacter pylori infection and hypertriglyceridemia. She was on hypolipidemic medication and she had never smoked. Objective examination showed an overweight patient with a BMI of 28.3 kg/m2, dark circles under eyes, moderately altered general status, hot, dehydrated skin, murmur in the aortic focus, diminished vesicular murmur bilaterally and left basal crackles, kyphotic chest, painless abdomen on superficial and deep palpation.
The diagnosis of pancreatic insulinoma was confirmed by Whipple’s triad: low glycemia, hypoglycemia-associated symptoms and disappearance of symptomatology when glycemia returns to normal (ingestion of sugar).
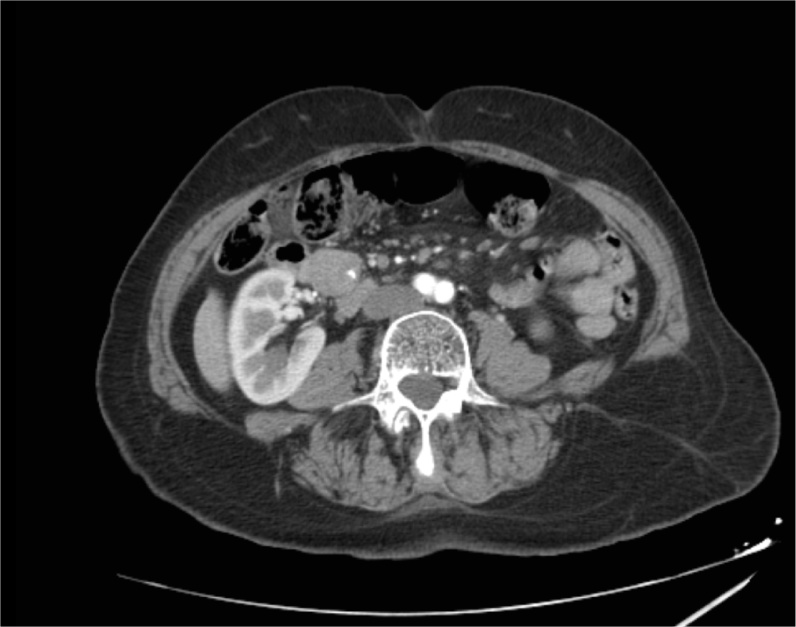
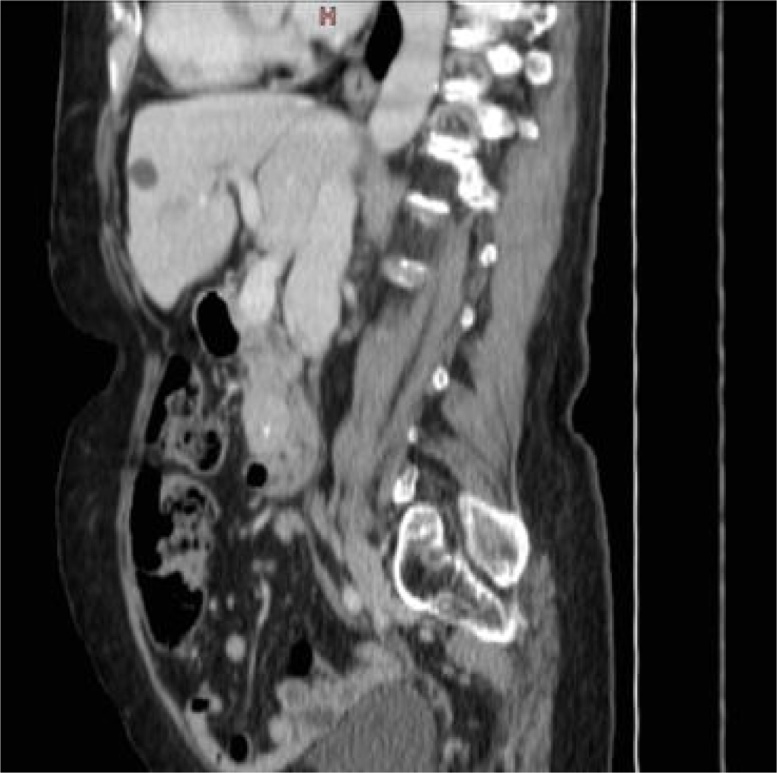
Laboratory examinations showed glycemia 110 mg/dl under 20% glucose 3 vials 500 ml/day, glycosylated hemoglobin 4.37%, hypertriglyceridemia, hepatic and renal tests within normal limits, serum cortisol within normal limits, thyroid hormones within normal limits, parathormone within normal limits. Insulinemia in the hypoglycemic crisis was 28.7 μg/l (NV 2.6–24.9 μg/l). Imaging explorations (abdominopelvic CT with iv contrast, abdominal ultrasound) detected a hypoechogenic tumor mass in the pancreatic uncinate process, with gross calcification and peripheral vascularization 3.4/2 cm in diameter, without causing a mass effect in the pancreatic duct (Fig. 1, Fig. 2). Echoendoscopy-guided fine-needle aspiration puncture biopsy was performed. The histopathological result revealed moderately differentiated neuroendocrine pancreatic tumor (G2) (highly positive diffuse CD56), and Ki-67 was 6%.
Fig. 1.
Arterial phase abdominal CT – tumor mass in the pancreatic uncinate process with inner calcification of 3.4/2 cm.
Fig. 2.
Abdominal CT, sagittal section: tumor mass of the pancreatic uncinate process.
The near infrared (NIR) detection device in the reported case was NIRF-800 probe, ArteMIS Handheld System, Quest Medical Imaging BV, Wieringerwerf, the Netherlands, which detects NIR between 790 nm and 805 nm, and indocyanine green (ICG) (25 mg/20 ml VERDYE). Five boluses of 2 ml from the 2.5 mg/ml ICG solution were administered.
The exclusion criteria (contraindications) for ICG administration in the current case were allergy to ICG and shellfish; hepatic or renal failure, hyperthyroidism, criteria that the patient denied. The patient’s informed consent for this procedure was obtained.
With the diagnosis of insulinoma of the pancreatic uncinate process, surgery was performed under general anesthesia. Intraoperative ultrasound evidenced a pancreatic uncinate process tumor of 3.3/2 cm, which entered into contact with the pancreatic duct without having a mass effect on it. No other pancreatic or hepatic tumor masses were ultrasonographically detected.
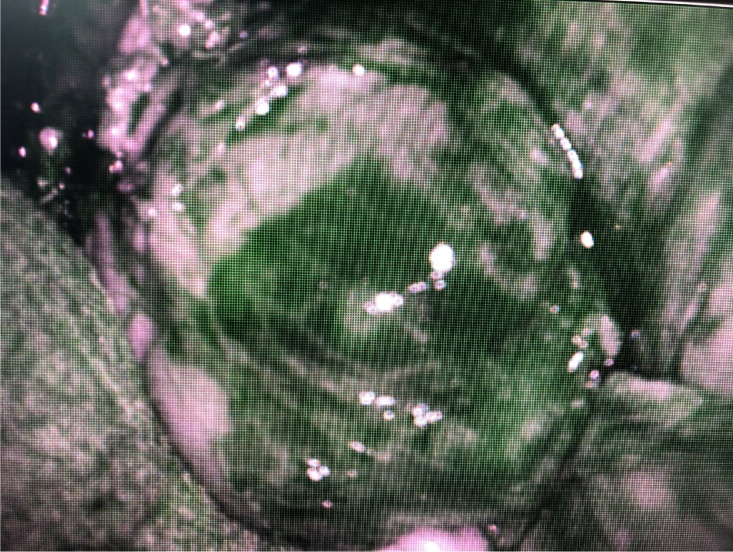
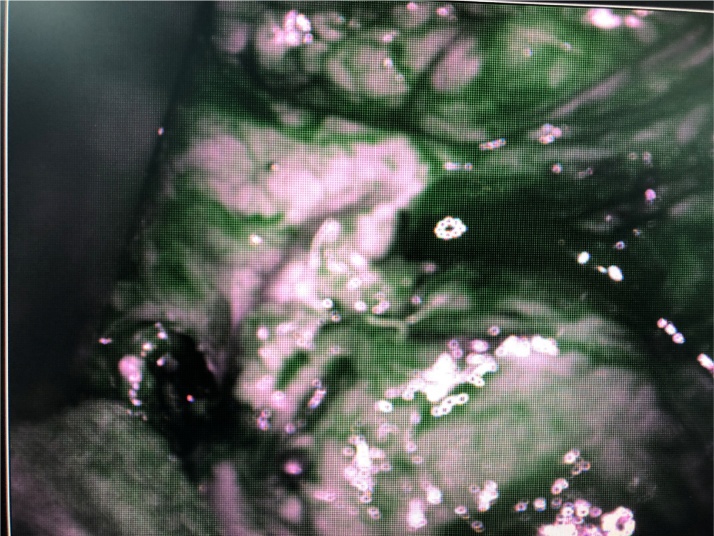
Intraoperatively, after Kocher procedure, a tumor mass in the uncinate process was detected. To explore a possible multicentric insulinoma or secondary lymph node involvement in the hepatic hilum or liver metastases, ICG injection and examination with a NIR camera were carried out. No other tumor masses were found in the tail of the pancreas and no secondary hepatic or lymph node involvement was detected (Fig. 3, Fig. 4). With the diagnosis of secretory pancreatic neuroendocrine tumors (PanNET) located in the head of the pancreas in contact with the Wirsung duct, cephalic pancreatoduodenectomy with the preservation of the pylorus was performed. The operating time was 3 h; blood loss was 500 ml.
Fig. 3.
Tumor mass taking up ICG, situated in the pancreatic head.
Fig. 4.
Exploration of the left pancreas with ICG and NIR.
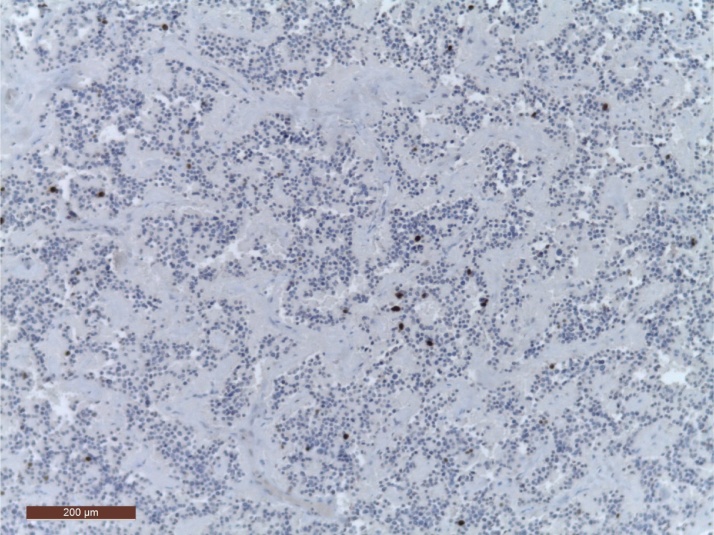
Postoperative evolution was favorable (Clavien-Dindo grade I), the patient did not require 20% glucose intake, and discharge was on the 12th postoperative day. The histopathological result was G2 neuroendocrine tumor consistent in the clinical context with a pT2N0M0L0V0R0 insulinoma with a 3% Ki67 index (Fig. 5).
Fig. 5.
HE staining with Ki67 IHC.
Evolution at 30 days was favorable, without ultrasonographically detected recurrences, and computed tomography with iv contrast detected no tumor recurrences and no secondary lymph node or liver involvement.
3. Discussions
The incidence of PanNET has increased over the last two decades [1].
Given the size of the tumor and its relationship with the pancreatic duct (the tumor was in direct connection with the main pancreatic duct), limited tumor resection could not be performed. The appropriateness of extended pancreatic resection was also supported by the high mitotic index (Ki67 3%).
3.1. Functional neuroendocrine tumors of the pancreas
In cases of insulinomas, surgery is the only curative treatment [4,5]. Because of their small size and low risk of malignancy, enucleation is the treatment of choice and this operation can be performed via laparoscopy if the location of the tumor is known and favorable (superficial lesion, located more than 2 or 3 mm from the main pancreatic duct (MPD) [5]. A formal resection pancreatoduodenectomy (PD), median or distal pancreatectomy can be necessary if enucleation is not possible because of the proximity of the lesion to the MPD or malignancy (10%) requiring lymphadenectomy (lymph node “picking” can be associated with enucleation). Preoperatively, important management elements include “patient education”, specifically frequent snacks and medical treatment to control hypoglycemia, whereas intravenous injection of glucose is usually not necessary. Diazoxide, a hyperglycemic sulfonamide (50–300 mg/day, a dose that can be increased up to 600 mg/day), is the most efficient treatment [5]. In case of undesirable effects or inefficacy, this drug can be associated with or replaced by others, in particular mTOR inhibitors (everolimus, rapamycin) [5].
Multiple endocrine neoplasia (MEN) should be excluded in the case of gastrinoma because, while long-term recovery is possible after R0 surgery for sporadic gastrinoma (20–45%), complete cure is very rare when gastrinoma is a component of MEN 1 (<10% when PD is not performed).
The other functional PanNET (glucagonoma, VIPoma, somatostatinoma, GRFoma, ACTHoma, PTH-rP secreting tumors or calcitoninoma) are even rarer. These tumors are usually voluminous, malignant, with locoregional or liver extension [5].
3.2. What type of pancreatic resection?
The type of surgery depends essentially on the size of the tumor and its location, but also on the relationship with nearby structures, the existence of synchronous lymph node metastases (LNM) or liver metastases (LM), patient co-morbidities, and possible hormonal secretion.
Pancreatoduodenectomy with tumor-free margins and lymphadenectomy is the carcinological operation for PanNET of the pancreatic head. The expected risk/benefit ratio has to be measured with extreme caution, particularly if the lesion is benign or of borderline malignancy [6]. This operation has at least three disadvantages. First, it is associated with the highest postoperative mortality among all pancreatic operations. Secondly, because of the volume of the resection, the patient is at risk for (mainly exocrine) pancreatic insufficiency [7]. Thirdly, this operation requires a bilioenteric anastomosis, which contraindicates or at least increases the risks of future procedures such as (chemo) embolization, or radiofrequency or microwave ablation of LM.
Distal pancreatectomy is the carcinological operation for lesions in the body or tail of the pancreas. This procedure, especially when performed for proximal lesions, removes the major part of the gland with a risk of pancreatic insufficiency (mainly endocrine) leading to de novo postoperative diabetes in about 15% of patients [8]. According to the characteristics of the PanNET (size of the lesion, locoregional involvement, tumor aggressiveness), the spleen can be preserved when its ablation is not indispensable for a R0 resection and/or lymphadenectomy. A tumor size greater than 3 cm seems to be a predictive factor for failure of preservation of the splenic vessels during PD [9].
In patients with a low carcinological risk, it is appropriate to preserve as much pancreatic parenchyma as possible, to avoid the risks of endocrine and/or exocrine insufficiency, particularly when the tumor is small, benign or of low-grade malignancy [10,11]. Enucleation can be performed via laparoscopy, with decreased duration of hospital stay, convalescence and abdominal parietal injury [13]. Because there is no tumor-free margin or routine lymphadenectomy, enucleation should be limited to benign or low-grade malignant tumors: insulinoma or small non-secretory PanNET (less than 2 or even 3 cm), without suspected lymph node or liver involvement on preoperative imaging. In case of isthmic or isthmo-corporeal PanNET, located to the left of the gastroduodenal artery, when enucleation is not possible, it is possible to perform a central pancreatectomy (CP) to avoid an extended left pancreatectomy [7].
In the absence of liver metastases, PanNET and metastatic lymph node resection is the only accepted curative treatment [3]. In a literature review, Jilesen et al. reported a 5-year mortality rate of 90% for completely resected, non-metastatic PanNET tumors [4].
Shirata et al. demonstrated that 100% of PanNET were visualized using fluorescence imaging [3].
Guarantying free resection margins and oncological radical resections, laparoscopy is becoming widely preferred over open surgery for the treatment of small benign or indeterminate pancreatic lesions, such as PanNET, the body–tail of the gland [6,14].
One of the most challenging aspects of the surgical plan is the exact localization of the tumors. Considering small PanNET, the reported preoperative and intraoperative detection rate is up to 92% [15], even when using palpation and IOUS, and it decreases to 50% for tumors below 1 cm. Even in high-volume centers, the intraoperative detection rate does not reach 100% [3].
Fluorescence-guided surgery has emerged as a novel intraoperative modality to assist surgeons to visualize tumors, sentinel lymph nodes, and vital structures in real time [11,12]. A vital dye, such as ICG administered iv, turns into fluorescent when excited by a light with specific wavelength, in the NIR spectrum.
Detection of PanNET with NIR prolongs surgery by approximately 8 min, but brings important benefits for visualizing the tumor [15].
Paiella et al. report a 0% reintervention and mortality rate in 10 cases operated by laparoscopy with a mean size of 2.4 cm (1–4 cm) and a Ki-67 index of 6% (2–12%). Nine patients underwent corporeo-caudal pancreatectomy and for one patient, tumor enucleation was performed. Eight PanNETs were non-secretory and two were insulinomas [15].
4. Conclusion
NIR by ICG administration is a method with almost 100% sensitivity and specificity for the intraoperative exploration of secretory PanNET and for the identification of other synchronous lesions, secondary lymph node involvement or liver metastases, which does not significantly prolong surgery.
Sources of funding
No founding received.
Ethical approval
Not applicable. The study is exempt from ethical approval in our institution.
Consent
Consent has been obtained from the patient. No identifying details have been used in the article.
Author’s contribution
V. Muntean: study concept, and final approval. V. Muntean, D. Constantinescu, R. Ciocan, D. Pintea treated the patient. D. Constantinescu, M. Constantinescu, S. Chiorescu wrote the first draft of the manuscript and the final draft of the manuscript, M. Pop photoediting, in editing manuscript. All authors read and approved the final manuscript.
Registration of research studies
Not available.
Guarantor
Valentin Muntean.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of Competing Interest
None of the authors have any conflict of interest.
References
- 1.Deguelte S., Mestier L., Hentic O., Cros J., Lebtahi R., Hammel P., Kianmanesh R. Preoperativ imaging and pathologic classifiaction for pancreatic neuroendocrine tumors. J. Visc. Surg. 2018;155:117–125. doi: 10.1016/j.jviscsurg.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Shirata C., Kawaguchi Y., Kobayashi K. Usefulness of indocyanine green-fluorescence imaging for real-time visualization of pancreas neuroendocrine tumor and cystic neoplasm. J. Surg. Oncol. 2018;118:1012–1020. doi: 10.1002/jso.25231. [DOI] [PubMed] [Google Scholar]
- 4.TNCD: The Version Finale 2016. http://snfge.org/sites/default/files/SNFGE/TNCD/tncdtnefinale10-3-16.pdf.
- 5.Jensen R.T., Cadiot G., Brandi M.L. ENSTS consensu guidelines for the management of patients with digestive neuroendocrine neoplasm: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jilesen A.P.J., van Eijk C.H.J., in’t Hof K.H. Post-operative complications in-hospital mortality and 5-year survival after surgical resection for patients with a pancreatic neuroendocrine tumor: a sistematic review. World J. Surg. 2016;40:729–748. doi: 10.1007/s00268-015-3328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguelte S., Mestier L., Hentic O., Cros J., Lebtahi R., Hammel P., Kianmanesh R. Sporadic pancreatic neuroendocrine tumor: surgery of the primary tumor. J. Visc. Surg. 2018:1878–7886. doi: 10.1016/j.jviscsurg.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 8.De Bruijn K.M.J., van Eijck C.H.J. New-onset diabetes after distal pancreatectomy: a systematic review. Ann. Surg. 2015;261:854–861. doi: 10.1097/SLA.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 9.Dai M.-H., Shi N., Xing C. Splenic preservation in laparoscopic distal pancreatectomy. Br. J. Surg. 2017;104:452–462. doi: 10.1002/bjs.10434. [DOI] [PubMed] [Google Scholar]
- 10.Beger H.G., Siech M., Poch B. Limited surgery for benign tumours of the pancreas: a systematic review. World J. Surg. 2015;39:1557–1566. doi: 10.1007/s00268-015-2976-x. [DOI] [PubMed] [Google Scholar]
- 11.Falconi M., Zerbi A., Crippa S. Parenchyma-preserving resection for small non-functioning pancreatic endocrine tumors. Ann. Surg. Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 12.Sauvanet A., Partensky C., Sastre B. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the Franch Pancreas Club. Surgery. 2002;132:836–843. doi: 10.1067/msy.2002.127552. [DOI] [PubMed] [Google Scholar]
- 13.Faitot F., Gaujoux S., Barbier L. A single center experience of 126 pancreatic enucleation: indication, short and long-term outcome. Surgery. 2015;158:201–210. doi: 10.1016/j.surg.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Pavel M., O`Toole D., Costa F. ENETS consensus guide-line update for the management of distant metastatic disease of intestinal, pancreatic, bronchial, neuroendocrine neoplasm (NEN) of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 15.Paiella S., De Pastena M., Landoni L., Esposito A. Is the role for near-infrared technology in laparoscopic resection pancreatic neuroendocrine tumors? Result of COLOPLAN “color and resect the pancreas” study. Surg. Endosc. 2017;31:4478–4484. doi: 10.1007/s00464-017-5501-5. [DOI] [PubMed] [Google Scholar]