Abstract
Human Papillomavirus (HPV) remains one of the most commonly contracted sexually transmitted diseases around the world. There are a multitude of HPV types, some of which may never present any symptoms. Others, however, are considered high-risk types, which increase the chance of the person infected to develop cancer. In recent years, the utilization of nanotechnology has allowed researchers to employ and explore the use of nanoparticles in immunotherapies.
The new nanoparticle frontier has opened many doors in this area of research as a form of prevention, diagnosis, and treatment in cancers resulting from HPV. This review will provide a brief background of HPV, its relationship to head and neck cancer (HNC) and present some insight into the field of immunotherapeutic nanoparticles.
Keywords: Cancer, HPV, HNC, Nanoparticle, Immunotherapy
1. Introduction
Human Papillomavirus (HPV) is considered to be one of the most sexually transmitted diseases in the world.1 HPV exists on a spectrum with a multitude of types across many species, each with a wide range of symptoms. Some types may lead to esthetically displeasing oral, genital, or anal papillomas (more commonly known as warts); some may lead to life threatening cancers; others may be completely asymptomatic and will resolve on their own.2
The high-risk types discussed in this paper, such as HPV 16 and HPV 18, are classified as such due to the increased likelihood of those infected with the virus to develop cancer.3 These types have most commonly been connected to the development of cervical cancer in women, which remains one of the leading cancers in women across the globe.4 However, due to recent observations, interest in the relationship between HPV and associated head and neck cancer (HNC) has gained the interest of many scientists.
An essential tool in exploring this relationship further is made possible by nanoparticles. The structuring and development of nanoparticles for medicinal purposes has allowed researchers to not only develop more accurate diagnostic tools but has also allowed for more specialized and personalized treatment.5, 6 Like HPV, these nanoparticles also exist on a spectrum. The vast variety and ever evolving forms and techniques that nanoparticles provide allow for an individualized touch in both research approach and clinical therapies.
2. Human Papillomavirus
2.1. Biology and physiology
Papillomaviruses exist as small, non-enveloped viruses that inhabit and cause disruptions within the skin and mucous membranes.7, 8 Their genetic information is made up of circular, double-stranded DNA, whereas other viruses, like Human Immunodeficiency Virus (HIV), consist of single-stranded RNA. With HIV, the single-stranded RNA is much less stable and is constantly mutating. This makes it very difficult to study and develop treatment. The viral double-stranded DNA that exists in viruses like HPV however, allows for higher levels of stability and results in lower mutation rates.9 As a result, this presents both positive and negative aspects for the human population.
On one hand, the low mutation rate could be seen as beneficial. Due to this, the virus remains consistent over long periods of time. Increased stability allows researchers to study it more effectively and work toward treatment without viral variations and adaptations.
However, the steadiness of HPV also leaves behind a long-lasting family tree. Consequentially, scientists have been able to identify more than 200 different types of HPV genotypes that are in existence today.10 The increased level of circulating viral genotypes lends a hand to higher prevalence rates among the global population.
The surface of the papillomavirus is coated in capsid proteins known as L1 and L2 proteins. These surface proteins have key characteristics that have been used by scientist to manufacture novel immunotherapeutic approaches.
The L1 protein, also known as the major capsid protein, is the most abundant HPV capsid protein. These form pentamers that function to provide structural integrity to the virus as it matures. Dimer linkages are formed as the cysteine group of one L1 protein forms a disulfide bond with another cysteine group on a separate L1 protein. Until the formation of these cross-links is complete, the viral genetic material is exposed, leaving it extremely vulnerable. While it may be susceptible to attack it is still highly infectious. Throughout this maturation process the virus transitions from a highly flexible and vulnerable state to a rigid and securely structured virus. The ability of L1 to extemporaneously encapsulate has established the premise of virus-like particles (VLPs) in HPV-related cancer immunotherapy.11
The L2 proteins, while still not yet fully understood, are known to play a minor role as a structural protein but are more commonly studied in the infiltration of HPV into the epithelium. Structural changes in the capsid expose the L2 protein and allow for it to be proteolytically cleaved. This cleavage supports the entry of the virus into the cell. Once inside the cell, it is believed that L2 collaborates with various other factors to enter Golgi bodies, travel along the cytoskeleton, and ultimately infiltrate the cell nucleus.12
2.2. E6 and E7 peptides
Interestingly, not everyone that is infected with HPV develops cancer. This observation is what led scientists to try to discover what variables were necessary for tumorigenesis. As in most cancers, there is no single cancerous factor; it is a conglomerate of variables that ultimately results in cancer progression. As researchers began to assess this relationship more closely, it was discovered that this is also true for HPV-related cancer. This resulted in a focus on the peptides E6 and E7.
These two peptides are required elements for cancer development in high-risk infected individuals. It is important to note they are not confined to only high-risk types of HPV. They have been observed to form a deadlier partnership in these high-risk types.
It is imperative to understand the basic workings of these peptides as we move forward in research involving nanoparticles for immunotherapy in HPV-related cancers. E6 and E7 work together to not only proliferate, but to also function to resist committed cell death. Thus, by inhibiting apoptotic process and enhancing cellular proliferation these peptides stimulate the immortalization of epithelial cells – thereby resulting in development of cancer disease.
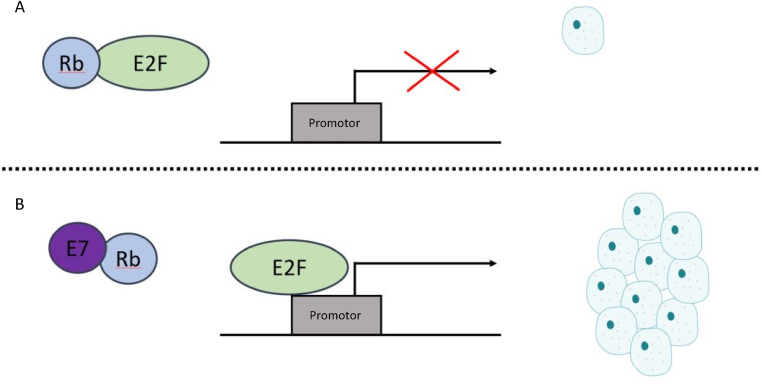
E7 functions to disrupt the cell cycle and therefore allows for an extensive proliferation of cells. This does so by directly binding to the tumor suppressor Retinoblastoma (Rb). Rb functions to bind to specific transcription factors like E2F. This Rb–E2F complex acts as a gatekeeper that prevents the cell from progressing into S phase. However, E7 competitively binds Rb over E2F, which allows for early and continuous entry of the cell into S phase which is vital for dysregulated cell growth.8 Remembering that most HPV types carry the E7 protein, we see that the high-risk types happen to bind Rb with a higher affinity than the others (Fig. 1).
Fig. 1.
(A) Healthy cell: Rb binds and inactivates transcription factor E2F, preventing E2F from binding with its promotor, thus suppressing proliferation. (B) Cancer cell: E7 peptide directly binds to Rb, preventing its interaction with E2F. This allows for E2F to bind its promotor, resulting in the dysregulation of cell proliferation.8
At this point the cell is continuously replicating. Fortunately, our body has systems in place to counteract cells that are undergoing unsupervised hyperproliferation. Typically, these cells would be targeted for apoptosis, a form of regulated cell suicide, and would no longer be considered a dangerous risk to the body. This is why the partnership between E7 and E6 is so insidious.
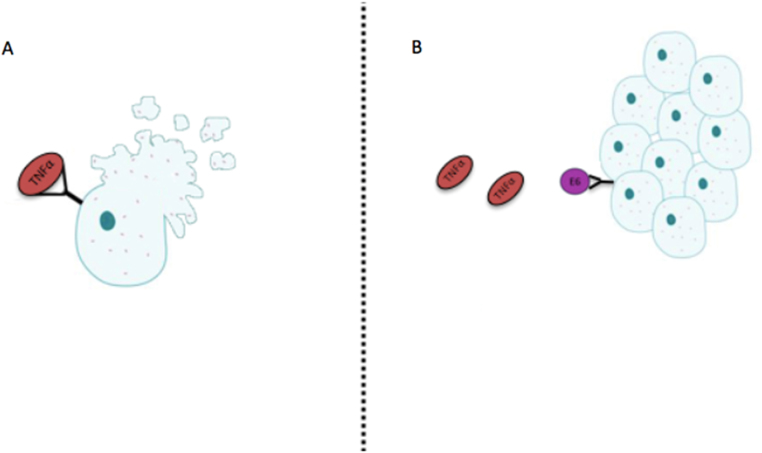
The E6 peptide suppresses apoptotic signals to bolster the extensive proliferation triggered by E7. One of the ways it is capable of doing so is by directly binding to TNFR1. TNFR1 is a transmembrane receptor that will bind the TNF-α ligand, a complex which is responsible for initiating the cascading effects of mediated cell death, more specifically tumor cell death.13 E6 will compete with TNF-α, and its binding to TNFR1 will inhibit apoptosis, further supporting the immortality of the cell (Fig. 2).
Fig. 2.
(A) Proinflammatory cytokine, TNF-α, binding to its cell surface receptor TNFR1, activating the process of apoptosis. (B) E6 binds to TNFR1 and inhibits the activation of apoptotic pathways.13
These are just a couple of ways this peptide partnership functions to induce cancer development. Other ways include standard viral traits like an integration of viral genome into host DNA8; through the obstruction of anoikis, an apoptotic pathway triggered when cells detach from the extracellular matrix (ECM)14; as well as preventing cell cycle arrest via the targeting of the tumor suppressor gene p53 to undergo proteasome-mediated degradation.15
3. Cancer
3.1. HPV and HNC
As previously discussed, some of the types of HPV present a much more clear and present danger to those infected as they are more likely to result in cancer development. These are classified as high-risk types of HPV. The two most commonly studied high-risk types (due to their even further increased risk of cancer development) are HPV 18 and HPV 16, with a specific focus on their relationship to cervical and head-and-neck cancer (HNC).16
Many factors that function in tandem ultimately are leading to the development and progression of cancer. This leads to a confounding web of variables that must be heavily researched to untangle each element. Throughout this process, studies have uncovered an overwhelming amount of cancers are able to be traced back to HPV infections.
HPV is most commonly studied in association with cervical cancer. Consequently, there have been far fewer studies that examine its relationship to HNC. This is not unsurprising when we consider how most HNC were an effect of tobacco and alcohol use. Although, with the turn of the 21st century an increase in HPV-related HNC has been observed along with a decline in HNC due to tobacco and alcohol usage.17 It can still be easy to forget HPV's connection to HNC when compared to over a half a million cases of cervical cancer worldwide.16 Nevertheless, some reports hypothesize that there is evidence that in the coming years this increase in HPV-related HNC may eventually surpass the statistics we see with HPV-associated cervical cancer.
From the information gathered thus far, there is evidence implicating HPV 16 and 18 as key players in the rising incidence of cancer of the oropharynx.9 A recent study found that nearly one third of oropharyngeal cancers can be attributed to HPV.16 These results demonstrate the importance of analyzing the effects of different types of the virus and their links to various forms of cancer.
For example, the predominance of HPV 16 in oropharynx cancer (OPC) has been observed to be around 90%, while it is involved in only about 50% of cancer in the cervix.9 This evidence may suggest a specific viral tropism of this type of HPV that varies from other types.
This is important for not only our understanding of how viral genetics play a role in infectivity, but also in how we need different approaches to study and treat HPV. Therefore, future studies need to be conducted to analyze this relationship further.
Moreover, the way we study and treat cancer is due for updates as well. While there are effective cancer therapies, the battle is still far from over. In some cases involving cancers of the head and neck, about half of the patients that receive treatments like radiation or surgical resections will relapse.18 Current therapy, like chemotherapy, while deadly to tumor cells, is also delivered in solvents that are also toxic to the human body, resulting in devastating side-effects. Additionally, chemotherapy must be repeatedly administered due to its high level of instability,19 furthering its toxic effects. All of which demonstrates that while current therapies may have success, there is always room for improvement. There is still information that escapes our grasp and lives that are consequently lost. Nanoparticles, especially when utilized as a tool in immunotherapy, seem to shine a light on some of the darkness that has shrouded therapeutic cancer tactics.
4. Nanoparticles and immunotherapy
4.1. Emergence of nanoparticles
Nanoparticles are a more recent tactical approach in the fight against cancer. They have become highly sought after for their malleability and effectiveness in infiltrating and eliminating tumor cells. Nanoparticles are easily modified to include a desired therapeutic peptide, often have little to no toxic effect when in the body, are highly stable, and are considerably more effective in being delivered to/remaining in the site of interest.20
4.2. Nanoparticle variations
There are multitudes of nanoparticles that currently exist in research, one of which includes polymeric nanoparticles. Polymeric nanoparticles are highly advantageous as they are proficient at containing therapeutics until they are appropriately stimulated for release at the site of interest. These nanoparticles are desirable due to their increased stability, adaptability to be fused with various surface proteins, and their highly manageable size.20, 21, 22 Chitosan is a common nanoparticle adjuvant used in these nanoparticles.20, 22 Nanoparticles that are chitosan-based are often favored as they are non-toxic, highly biocompatible, and have an extended retention time due to their decreased susceptibility to biodegradation.
A study by Tahamtan et al. sought to provide some further insight into the therapeutic effect of these chitosan-based nanoparticles in HPV DNA vaccines. A perk to DNA vaccines is that they are considered to be safe and are also easily prepared. However, a caveat to DNA vaccines can be observed in their form of delivery as they are often ineffectively taken up by antigen presenting cells. As a result, we may see low levels of elicited immune responses in the administering of DNA vaccines.23 Tahamtan et al. experimented with a chitosan delivery mechanism to assist in this uptake of DNA vaccines expressing HPV 16 E7, one of the discussed oncogenic peptides involved in HPV-related cancer development.
This study found that the chitosan-based nanoparticles used to deliver the DNA vaccine not only significantly increased the proliferation of lymphocytes but also significantly increased the lysis of specific cells. Furthermore, the chitosan-based nanoparticles initiated a substantial increase in the Th1 and Th2 immune responses, illustrating that this delivery mechanism could effectively increase both the activity of macrophages and antibodies against HPV tumor formation.24
Gold nanoparticles also provide promise in future endeavors associated with the development of effective vaccines. First and foremost, they have idiosyncratic optical properties. They can be adjusted to absorb a range of near-infrared (NIR) light as well as retaining the ability to scatter light; both of which are extraordinarily helpful in both diagnosis and treatment of cancer.18, 20, 25 They have also been shown to be beneficial in cancer treatment as they exhibit cytotoxic properties when exposed to oxidative stress.20 Oxidative stress has been shown to bolster the development of cancer through a variety of different mechanisms.26 Therefore, oxidative stress that drives the activation of gold nanoparticles could be highly effective in combatting tumorigenesis.
Magnetic nanoparticles have also become frontrunners in the manufacturing of nanoparticles. These are commonly characterized by their iron-oxide core which is useful in both the targeting of cancer sites, as well as their capacity to remain within the targeted tissue.27 Even more exciting, these particles remain non-toxic due to their capability to recycle in the body as hemoglobin.28
In a study directed by Melancon et al., a novel therapeutic approach to head and neck cancer is depicted through the combination of gold and magnetic nanoparticles. This novel nanoparticle manufactured an iron-oxide core that was contained within a gold nanoshell decorated with antibodies that would specifically target Epidermal Growth Factor Receptor (EGFR) in the tumor. This hybrid approach would allow nanoparticles to migrate to and be retained in the target tumor site. The ability of the nanoshell to absorb NIR light would allow for more successful image-guided laser ablation of the tumor cells without causing harm to healthy tissue.18
They found that these nanoparticles formed effectively, and each element retained full functionality. More importantly, their test results showed that these nanoparticles were drastically more successful in mediating laser-guided cell lysis when compared to other traditional techniques. This helps to lay a framework for how nanoparticles can be more impactful in how we navigate cancer therapies.
4.3. Viral nano-vaccination
There currently exists four approaches in viral vaccination: 1) live-attenuated viruses – which utilize viruses that have been weakened in a way that their infectivity is inhibited; 2) inactive viruses – which are no longer live; 3) viral subunits – or fragments of a whole virus; and 4) virus-like particles and virus-based nanoparticles, which tend to be stronger and therefore require a lower dosage. These last two novel nano-vaccination, which include virus-like and virus-based approaches, are strategies that have been used more recently in nanotechnological studies involving HPV positive cancers.
The first to be discussed are the virus-based nanoparticles (VNPs). These are typically easier to produce than VLPs as the viral genome remains intact. However, this makes them more dangerous when used in humans and are thus preferred to be studied with bacteriophages and plant viruses.29
On the other hand, there exists VLPs that belong to a group of nanoparticles that are capable of self-assembling to form a capsid. VLPs lack the viral genome, and as a result, are unable to replicate. VLPs are primary candidates for immunotherapy as they retain viral factors that allow for autonomous manufacturing but no longer exhibit infectious capabilities, which makes them less dangerous when used in human studies.22
There are currently three methods in use to genetically modify the self-assembled viral coat which allows for the attachment of a peptide of interest. These are as follows: 1) direct fusion, where a peptide is directly linked to either the C-terminus or the N-terminus of the viral coat protein; 2) linker fusion, which utilizes a short, repeated amino acid sequence (like glycine) to link the peptide to the viral coat proteins; and 3) “protein overcoat”, which employs viral catalysts like Foot and Mouth Disease Virus (FMDV) to enact a ribosomal skip in the coat protein translation.29
Direct fusion is depicted by studies done by researchers like Monroy-Garcia et al., where VLPs containing L1 proteins were additionally fused with E6 and E7 epitopes to elicit long-term protection against tumorigenic HPV. Interestingly enough, this team utilized a chimeric VLP (cVLP) derived from tomato plants. The use of tomato plants to manufacture recombinant proteins is not only a more cost effective decision, but also yields less harmful effects.30
For this study, researchers directly fused E6 and E7 T-cell epitopes from HPV 16 to the C-terminus of the cVLP. This was then compared to the untransformed plant sample, non-chimeric VLP, and the leading vaccine at the time, Gardasil.
They verified that their product was viable and unaffected by the larger amino acid sequence epitopes via electron microscopy. Further evaluation via western blotting confirmed the fusion of the high-risk HPV 16 peptides to the cVLP.
Through this study, they unveiled that mice vaccinated with the cVLP were able to bolster long-lasting immune response when compared to other VLPs. Strikingly, mice in this study that were preemptively vaccinated with cVLP developed tumors that were significantly smaller in size. Even more astonishing, mice that received an additional vaccination after a year of the primary immunization were entirely safeguarded from tumor development.
Furthermore, cVLPs in this study depicted an inhibitory effect in vivo, meaning it was able to reduce pre-existing tumor size in mice that had not been immunized with the cVLP.4
Direct fusion is again exemplified in a study performed by Tumban et al., this time to the N-terminus. Here, they sought to offer insight into a potential immunization that would effectively provide cross-protection among the high-risk types of HPV. Their study linked L2 epitopes to the N-terminus of an MS2 bacteriophage in an attempt to achieve this goal.
The successful assembly of their VLP was confirmed via Transmission Electron Microscopy (TEM) and western blotting. These confirmed that morphologies were similar to the wild type and the L2 fused MS2 VLPs were viable. However, they did note that the VLP structural integrity remained uncompromised only when linked with a sequence of 10–27 amino acids.
Through their research, they determined the following: the L2-MS2 VLP was effective at producing an immune response at levels lower than usual treatments. Also, near complete protection from HPV 16 was observed in mice treated with the L2-MS2 VLP. Another important discovery of this study was that the L2-MS2 VLP provides cross-reactivity to HPV types 1, 5, 6, 16, and 18.31 This is important as there is a wide range of HPVs; therefore, in order to ward off this ubiquitous virus, a single vaccine is required that can combat many different types.
In another study conducted by Tyler et al., we see the development of another chimeric virus-like particle. Traditionally, VLPs fused with L2 do not perform as effectively as those bound with L1. This study focuses on increasing the immunogenicity of VLP combined with the L2 epitope. However, in this instance, we see a fusion of both the HPV 16 and the HPV 18 L2 epitope.
This hybrid VLP stimulated increased levels of antibodies that were not only cross-reactive for the high-risk HPV types 16 and 18, but were also reactive against types 1, 5, and 6. Even more surprising, this cVLP (fused with L2 from HPV 16 and 18) performed more effectively when compared to immunizations that contained a mixture of HPV 16 and 18 that were not fused together. Even though both immunizations were comprised of the same contents, the hybrid VLP was significantly more successful.
This may be a result of the multivalent display of the chimeric particle. The research team believed that this led to an increase in the cross-linking abilities of B-cells, ultimately resulting in elevated antibody production due to stronger B-cell activation.32 This discovery could be effective in our future steps of vaccine development. Increasing the bound epitopes—to the extent that does not compromise the particle morphology—could be incredibly more effective in producing higher levels of cross-reactivity in antibodies against various types of HPV.
In a recent study by Sonali et al., nanoparticles were praised for their theranostic abilities. Theranostic nanomedicine is a tactic which utilizes nanoparticles to deliver both a therapeutic effect, as well as diagnostic capabilities. This practical technique has gained momentum as it helps to detect the formation or development of disease, allows for spatial targeting, enables healthcare providers and researchers alike to track the progress of the administered therapy, and possibly the most important attribute, allows for personalized medicine for individual cancer phenotypes.20
As previously discussed, the various types of just HPV alone have a multitude of malicious effects and can strike in different regions of the body. Consequently, the idea that through the use of nanoparticles patients may be able to receive a personalized treatment to combat disease specific to their needs is strongly welcomed and sets a precedence for medical approaches moving forward.
5. Future studies
So, what steps need to be taken to improve both laboratory techniques and clinical approaches as we move forward into the future of nanoparticle tumor therapy?
For starters, cancer related to viral infection is preventable. There is a huge demand to not only educate the public, but to also provide increased availability and affordability of screening procedures. Taking the initial steps to prevent the infection, or even early detection of the virus, we can expect to see a drastic decline in cancer incidence rates.
While screenings exist for cervical cancer, there is still a barrier in the detection process for HPV-related HNC. Although awareness levels are rising, there are limited studies of HPV-related cervical cancer. Techniques are still being established for screening purposes, although it is proving to be difficult to differentiate normal tissues from precancerous lesions. Understanding that tissue collection and characterization may be difficult, Kreimer et al. attempted a different diagnostic approach. Their study found that antibodies for these high-risk types of HPV could be detected up to ten years before cancer even developed.33 Establishing new ways to monitor the infections and predict the risk of the disease should help to develop our front line of defense.
Also, using nanoparticles as a resource outside of the diagnostic or therapeutic realm should be considered. Due to their size and high traceability, they could be utilized as a vessel to discover pathways and interactions of L2 once inside the cell. Although it is understood that L2 is responsible for infiltration, there is still much about this process that remains unknown. Gold nanoparticles may potentially be small enough to travel inside the cell with L2 and function to pinpoint its actions within, hopefully without hindering normal L2 processes. Further exploration of nanoparticle functionality could help identify new target mechanisms that may eliminate cancer development.
In addition, current studies are depicting the increased effectiveness in the combination of nanoparticle styles. The layering of nanoparticle categories as well as the proteins adaptations bound to their shell command higher immunogenic effects. Just as the collective efforts of cancer researchers are necessary for treatment development, the combined abilities of nanoparticles could be just as efficacious.
Conflict of interest
None declared.
Financial disclosure
None declared.
Footnotes
Article from the Special Issue on Nanoparticle and Immunotherapy
References
- 1.WHO . 2018. Human papillomavirus (HPV) Available from: http://www.who.int/immunization/diseases/hpv/en/ [Google Scholar]
- 2.WHO Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol Rec. 2017;92(19):241–268. [PubMed] [Google Scholar]
- 3.Golusinski P. Is immunohistochemical evaluation of p16 in oropharyngeal cancer enough to predict the HPV positivity? Rep Pract Oncol Radiother. 2017;22(3):237–242. doi: 10.1016/j.rpor.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monroy-Garcia A. Immunization with an HPV-16 L1-based chimeric virus-like particle containing HPV-16 E6 and E7 epitopes elicits long-lasting prophylactic and therapeutic efficacy in an HPV-16 tumor mice model. Arch Virol. 2014;159(2):291–305. doi: 10.1007/s00705-013-1819-z. [DOI] [PubMed] [Google Scholar]
- 5.Nierenberg D., Khaled A.R., Flores O. Formation of a protein corona influences the biological identity of nanomaterials. Rep Pract Oncol Radiother. 2018;23(4):300–308. doi: 10.1016/j.rpor.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto C., Linares I. Nanoparticles and nanothermia for malignant brain tumors, a suggestion of treatment for further investigations. Rep Pract Oncol Radiother. 2018;23(5):474–480. doi: 10.1016/j.rpor.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finnen R.L. Interactions between papillomavirus L1 and L2 capsid proteins. J Virol. 2003;77(8):4818–4826. doi: 10.1128/JVI.77.8.4818-4826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 9.Combes J.D., Franceschi S. Human papillomavirus genome variants and head and neck cancers: a perspective. Infect Agent Cancer. 2018;13:13. doi: 10.1186/s13027-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doorbar J. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 11.Buck C.B., Day P.M., Trus B.L. The papillomavirus major capsid protein L1. Virology. 2013;445(1–2):169–174. doi: 10.1016/j.virol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J.W., Roden R.B. L2, the minor capsid protein of papillomavirus. Virology. 2013;445(1–2):175–186. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., El-Deiry W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22(53):8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 14.Chiarugi P., Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76(11):1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M., Pim D., Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18(53):7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 16.de Martel C. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi A.K. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melancon M.P. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials. 2011;32(30):7600–7608. doi: 10.1016/j.biomaterials.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu T.T., Zhou S.H. Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int J Med Sci. 2015;12(2):187–200. doi: 10.7150/ijms.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonali Nanotheranostics: emerging strategies for early diagnosis and therapy of brain cancer. Nanotheranostics. 2018;2(1):70–86. doi: 10.7150/ntno.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamo T., Poland G.A. Nanovaccinology: the next generation of vaccines meets 21st century materials science and engineering. Vaccine. 2012;30(47):6609–6611. doi: 10.1016/j.vaccine.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 23.Pachuk C.J. DNA vaccines—challenges in delivery. Curr Opin Mol Ther. 2000;2(2):188–198. [PubMed] [Google Scholar]
- 24.Tahamtan A. Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. J Biomed Sci. 2014;21:p69. doi: 10.1186/s12929-014-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthu M.S. Nanotheranostics – application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4(6):660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuter S. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A.K., Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Pankhurst Q.A., Connolly J., Jones S.K., Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36(13):R167–R181. [Google Scholar]
- 29.Lee K.L. Virus-based nanoparticles as platform technologies for modern vaccines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(4):554–578. doi: 10.1002/wnan.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim M.A.G. Transgenic plant in therapeutically valuable protein production. Transgenic Plant J. 2007;1:256–266. [Google Scholar]
- 31.Tumban E. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One. 2012;7(11):pe49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyler M. The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol Bioeng. 2014;111(12):2398–2406. doi: 10.1002/bit.25311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreimer A.R. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]