Abstract
Objective
To evaluate the predictive value of asymptomatic early house dust mite sensitization on allergic outcomes and pulmonary functions in 7-year olds.
Study design
The Prediction of Allergies in Taiwanese Children (PATCH) birth cohort study recruited healthy newborns at birth. At age 1.5–2 years, a Dermatophagoides pteronyssinus-specific immunoglobulin E level ≥ 0.35 kU/L was defined as early sensitization. At age 7 years, allergic outcomes were evaluated by pediatric allergists and pulmonologists, and fractional exhaled nitric oxide and pulmonary functions were measured.
Results
At age 1.5–2 years, 28.0% of toddlers were sensitized to D. pteronyssinus. Among them, 68.2% had no allergic symptoms at that time. At age 7 years, the children with early sensitization had higher risks of asthma (OR = 13.4, 95% CI, 1.2 to 153.0; P = 0.037), allergic rhinitis (OR = 10.2, 95% CI, 2.1 to 49.6; P = 0.004), and atopic dermatitis (OR = 38.5, 95% CI, 2.1 to 696.4; P = 0.014). Notably, even the asymptomatic toddlers with early D. pteronyssinus sensitization had higher probabilities of asthma (12.5% vs. 1.7%, P = 0.040), allergic rhinitis (83.3% vs. 43.1%, P = 0.009), and atopic dermatitis (20.8% vs. 0.0%, P < 0.001) at age 7 years. The asymptomatic toddlers with early sensitization also had higher exhaled nitric oxide levels and higher prevalence of airway hyperresponsiveness at age 7 years.
Conclusion
Asymptomatic toddlers with early house dust mite sensitization have higher risks of developing asthma, allergic rhinitis, atopic dermatitis, and abnormal lung functions at age 7 years.
Keywords: Birth cohort, House dust mite, Pediatric asthma, Pulmonary function
Abbreviations: ATS, American Thoracic Society; CI, Confidence interval; ERS, European Respiratory Society; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in the first second; FVC, Forced vital capacity; HDM, House dust mite; IgE, Immunoglobulin E; IQR, Interquartile range; ISAAC, International Study of Asthma and Allergies in Childhood; OR, Odds ratio; PATCH, Prediction of Allergies in Taiwanese Children; PC20, Provocative concentrations causing a 20% fall in forced expiratory volume in the first second
Introduction
House dust mite (HDM) is one of the common aeroallergens that cause chronic allergic inflammation through both innate and adaptive immunity routes.1, 2 Allergic immune responses lead to mucosal or epithelial damage accompanied by atopic symptoms and signs. In cross-sectional studies, sensitization to HDM was clearly related to asthma,3 increased fractional exhaled nitric oxide (FeNO), and decreased pulmonary functions.4 However, The relationship between the development of atopic diseases and early exposure to HDM is controversial.5, 6, 7 The longitudinal data about allergic outcomes of asymptomatic infants or toddlers with early HDM sensitization is inadequate.
Due to the humid environment in Taiwan, HDM is the leading allergen that may contribute toward worsening of asthma, allergic rhinitis, and atopic dermatitis.8 The most common HDM in Taiwan is Dermatophagoides pteronyssinus. The Prediction of Allergies in Taiwanese Children (PATCH) study is a healthy birth cohort study, focusing on the epidemiology and risk factors of allergies and asthma in Taiwanese children. In our previous study, around age 2 years, the prevalence of sensitization to aeroallergen became predominent.9 Approximately 30% of children were already sensitized to D. pteronyssinus at the age of 1.5–2 years, but more than two-thirds of them presented without any atopic symptoms or diseases. Long-term exposure and sensitization to HDM even without symptoms could cause chronic upper airway mucosal inflammation.10 Therefore, we attempted to evaluate the influence of early asymptomatic HDM sensitization on allergic diseases and lung functions in this cohort. At age 7 years, the relationship between early HDM sensitization and physician-diagnosed asthma was assessed. Other objective tools such as FeNO and pulmonary function tests were also implemented.
Methods
Study design and study population
This PATCH study is a general population cohort, and not a cohort of high-risk newborns. The enrollment for the PATCH study was conducted at Keelung Chang Gung Memorial Hospital from October 2007 to September 2010. The detailed enrollment process, including inclusion and exclusion criteria, has been described previously.11 In brief, pregnant mothers were invited to join the study at a gestational age of 32 weeks. After informed consent was obtained, detailed prenatal information was collected. Healthy newborns with more than 34 weeks of gestation were enrolled, and cord blood was collected at birth. The study was approved by the Human Research Ethics Committee of Chang Gung Memorial Hospital (No. 100–0201B).
These children received a follow-up at age 6 months, 1 year, 1.5 years, 2 years, and then yearly thereafter. At each visit, parents complete a questionnaire, modified from the International Study of Asthma and Allergies in Childhood (ISAAC) study, to gather information about allergic symptoms. Children were evaluated by pediatric allergists and pulmonologists. We defined the children with D. pteronyssinus sensitization at 1.5–2 years of age as the early HDM sensitized group.
At age 7 years, atopic diseases were diagnosed by pediatric allergists and pulmonologists. Asthma diagnosis was based on the 2017 Global Initiative for Asthma guideline.12 For uncertain cases, repeated pulmonary function tests were performed. If necessary, bronchodilator tests or methacholine challenge tests were conducted. Allergic rhinitis was diagnosed based on typical symptoms, physical examinations, and allergy tests.13 Atopic dermatitis was diagnosed by characteristic distributions of recurrent pruritic rashes with exudates, dryness, and/or lichenification, as described by Hanifin.14
Measurement of specific IgE to D. pteronyssinus, vitamin D, and soluble Fas ligand
D. pteronyssinus-specific IgE was measured by a fluorescence enzyme immunoassay (ImmunoCAP®; detection limit 0.1 kU/L; Phadia, Uppsala, Sweden). Values of ImmunoCAP ≥ 0.35 kU/L (≥ class 1) were indicative of allergic sensitization.15 Serum levels of 25(OH)D were measured by an automated electrochemiluminescence-based assay (Elecsys® Vitamin D Total assay; Roche Diagnostics, Mannheim, Germany). Cord blood soluble Fas ligand levels were measured by an enzyme-linked immunosorbent assay (Human Fas Ligand/TNFSF6 DuoSet ELISA; R & D Systems, Inc., Minneapolis, MN, USA).
Measurement of fractional exhaled nitric oxide
Fractional exhaled nitric oxide (FeNO) was measured by using a hand-held electrochemical analyzer (NIOX MINO; Aerocrine AB, Sweden) following the 2005 American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations for standardized single-breath online measurement.16 Children were refrained from eating, drinking, and vigorous exercise for at least 1 hour before FeNO measurement; nitrate or nitrate-containing foods were avoided the night before measurement.
Measurement of pulmonary function tests
Pulmonary functions were measured using spirometry (Spirolab II®; Medical International Research, Roma, Italy) following the ATS/ERS recommendations,17 and 3 acceptable tests were recorded. The highest forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1) were documented, and percentages of the predicted values were calculated.
For bronchodilator reversibility tests, the children received 200 μg of fenoterol (Berotec®, 100 μg per puff, metered-dose inhaler; Boehringer Ingelheim, Germany) through a valved spacer with a face mask. Two separate doses were delivered at 30-second intervals. FEV1 and FVC values were recorded by spirometry after 2 doses of bronchodilator.
Methacholine challenge tests were performed according to the 2017 ERS technical standard.18 Provocative concentrations causing a 20% fall in FEV1 (PC20) were calculated. PC20 ≤ 4 mg/mL was defined as airway hyperresponsiveness according to the ERS technical standard.
Statistical analysis
The relationship between sensitization to D. pteronyssinus and atopic diseases was analyzed using a Chi-square test. Univariate and multivariate logistic regression were applied to analyze the risk factors for allergic diseases outcomes at age 7 years. During univariate logistic regression, variables with a P value less than 0.1 were included in the subsequent multivariate logistic regression. While calculating odds ratios within the atopic dermatitis group, we used Haldane correction because all children with atopic dermatitis at age 7 years already had D. pteronyssinus sensitization at age 1.5–2 years. In several birth cohort studies, cord blood soluble Fas ligand and serum vitamin D levels were associated with allergic sensitization and atopic diseases.19, 20 Therefore, both of them were considered as confounding factors during analysis. Group comparisons were performed using the Mann-Whitney U test for FeNO and lung function test results. All hypothesis testing was two-sided with a priori levels of significance set at P < 0.05. Statistical analyses were performed with IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, N·Y., USA).
Results
Subject characteristics
A total of 258 newborns were enrolled at birth. At age 7 years, 132 children were evaluated. Ten (7.6%) children were diagnosed with asthma, 77 children (58.3%) had allergic rhinitis, and 9 children (6.8%) had atopic dermatitis. Nine of 10 asthma children had allergic rhinitis. All cases with atopic dermatitis had allergic rhinitis. There was no overlap between asthma and atopic dermatitis cases. The baseline characteristics between the 132 children at age 7 years and all 258 children enrolled initially had no statistically significant difference (Table 1).
Table 1.
Comparison of basic characteristics of 132 children at age 7 years and the total 258 children enrolled initially.
| Characteristics | Age 7 years (n = 132) | Total (n = 258) | P value |
|---|---|---|---|
| Family | |||
| Maternal atopic diseases | 53 (40.2%) | 103 (40.1%) | 0.985 |
| Eczema | 10 (7.6%) | 19 (7.4%) | 0.943 |
| Rhinitis | 35 (26.5%) | 75 (29.2%) | 0.535 |
| Asthma | 2 (1.5%) | 11 (4.3%) | 0.147 |
| Paternal atopic diseases | 70 (53.0%) | 127 (49.4%) | 0.501 |
| Eczema | 12 (9.1%) | 18 (7.0%) | 0.463 |
| Rhinitis | 49 (37.1%) | 94 (36.6%) | 0.923 |
| Asthma | 5 (3.8%) | 10 (3.9%) | 0.961 |
| Smoking exposure | |||
| Maternal smoking during pregnancy | 1 (0.8%) | 8 (3.1%) | 0.154 |
| Passive smoking | 32 (24.2%) | 77 (30.0%) | 0.229 |
| Older siblings | 51 (38.6%) | 113 (44.0%) | 0.308 |
| Household annual income | 0.139 | ||
| Low ≤ 500,000 NTD | 40 (30.3%) | 95 (36.8%) | |
| Medium 500,000–1,000,000 NTD | 63 (47.7%) | 115 (44.6%) | |
| High > 1,000,000 NTD | 29 (22.0%) | 48 (18.6%) | |
| Infant | |||
| Sex, male | 72 (54.5%) | 131 (50.8%) | 0.489 |
| Delivery mode, vaginal | 81 (61.4%) | 159 (61.9%) | 0.924 |
| Gestational age (wk) | 38.1 ± 1.8 | 38.1 ± 1.7 | 0.728 |
| Birth body weight (gm) | 3082 ± 501 | 3041 ± 413 | 0.480 |
| Breast feeding | 94 (71.2%) | 194 (75.2%) | 0.396 |
| Toddler | |||
| 1.5–2 yr atopic diseases | 24 (20.9%) | 41 (22.5%) | 0.746 |
| 1.5–2 yr D. pteronyssinus sensitization | 34 (32.7%) | 44 (28.0%) | 0.418 |
Data shown are mean ± SD or number (%) of patients as appropriate.
NTD, New Taiwan Dollar; yr, years old; wk, week
The prevalence data of D. pteronyssinus sensitization
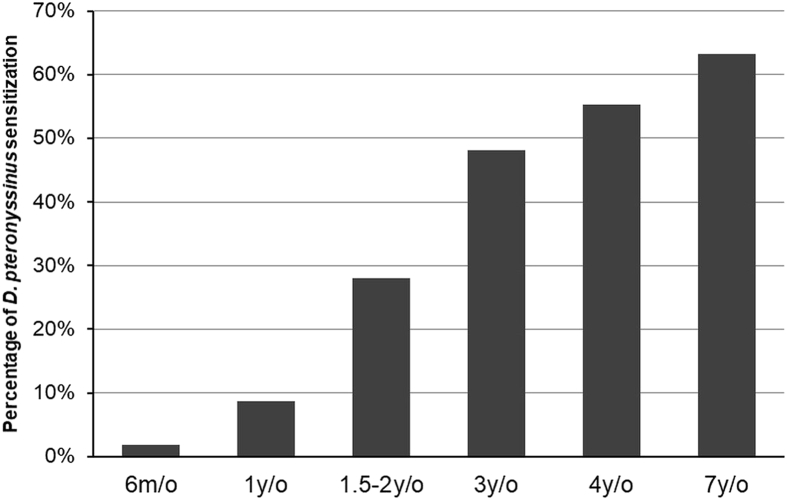
The prevalence of D. pteronyssinus sensitization increased gradually from 1.9% at age 6 months, 8.7% at age 1 year, 28.0% at age 1.5–2 years, 48.0% at age 3 years, 55.2% at age 4 years, and 63.2% at age 7 years (Fig. 1). Among the D. pteronyssinus sensitized toddlers at age 1.5–2 years, 68.2% had no allergic symptoms. Sensitization to D. pteronyssinus at age 1.5–2 years was not associated with any atopic diseases at age 1.5–2 years. Of 44 toddlers sensitized to D. pteronyssinus at age 1.5–2 years, 43 (97.7%) continued to be sensitized to D. pteronyssinus at age 7 years. Among 113 non-sensitized toddlers at age 1.5–2 years, 32 cases (27.8%) became sensitized to D. pteronyssinus at age 7 years. Up to 78.7% of children with atopic diseases at age 7 years were sensitized to D. pteronyssinus.
Fig. 1.
The sensitization rate of D. pteronyssinus at different ages. m/o: months of age; y/o: years of age
Association of early HDM sensitization and atopic diseases at age 7 years
The toddlers with early HDM sensitization, compared with the non-sensitized toddlers, had higher probabilities of asthma (14.7% vs. 2.9%, P = 0.025), allergic rhinitis (85.3% vs. 45.7%, P < 0.001), and atopic dermatitis (20.6% vs. 0.0%, P < 0.001) at age 7 years. In multivariate logistic regression, early HDM sensitization was associated with higher risks of asthma (OR = 13.4, 95% CI, 1.2 to 153.0; P = 0.037) and allergic rhinitis (OR = 10.2, 95% CI, 2.1 to 49.6; P = 0.004) at age 7 years after we adjusted for confounding factors, including gender, birth condition, atopic family history, environmental risk factors, and socioeconomic backgrounds (Table 2). All children with atopic dermatitis at age 7 years had D. pteronyssinus sensitization at age 1.5–2 years (OR = 38.5, 95% CI, 2.1 to 696.4; P = 0.014, after Haldane correction).
Table 2.
The effect of early HDM sensitization on 7-year-old asthma and allergic rhinitis.
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| 7-year-old asthma | ||||
| Early HDM sensitization | 5.86 (1.08–31.90) | 0.041* | 13.35 (1.17–153.02) | 0.037* |
| Maternal asthma | 13.44 (0.78–233.2) | 0.074 | 30.44 (0.81–1143.6) | 0.065 |
| Preeclampsia during pregnancy | 13.44 (0.78–233.2) | 0.074 | 30.44 (0.81–1143.6) | 0.065 |
| 7-year-old allergic rhinitis | ||||
| Early HDM sensitization | 6.89 (2.39–19.86) | <0.001* | 10.18 (2.09–49.57) | 0.004* |
| Cord blood soluble Fas ligand level (pg/mL) | 1.01 (1.00–1.01) | 0.043* | 1.01 (1.00–1.02) | 0.055 |
| 1.5–2 year-old vitamin D level (ng/mL) | 0.96 (0.92–0.99) | 0.038* | 0.93 (0.87–0.98) | 0.011* |
| Minimizing objects that accumulate dust | 0.38 (0.18–0.90) | 0.026* | 0.20 (0.04–0.89) | 0.034* |
OR: odds ratio, 95% CI: 95% confidence interval, HDM: house dusts mite.
*P value < 0.05 (by logistic regression analysis)
During logistic regression analysis, we noticed other factors were associated with allergic rhinitis at age 7 years. Living in houses with minimized dust accumulating objects was a protective factor for allergic rhinitis at age 7 years (Table 2). Other measures to eliminate HDM such as a dehumidifier, air cleaner, avoidance of plush toys, and allergen-impermeable mattress covers showed no protective effects. Toddlers with higher vitamin D levels at age 1.5–2 years had a significantly lower risk of allergic rhinitis at age 7 years (Table 2). In univariate logistic regression, soluble Fas ligand levels were significantly associated with allergic rhinitis at age 7 years. However, in multivariate logistic regression soluble Fas ligand levels did not reach a priori levels of significance.
Association of early HDM sensitization and lung function changes at age 7 years
At age 7 years, the children with early HDM sensitization appeared to have higher FeNO levels in comparison with the non-sensitized children (18.0 ppb [interquartile range (IQR): 11.0 to 31.0] vs. 9.0 ppb [IQR: 7.0 to 12.5], P < 0.001). In bronchodilator reversibility tests, changes of FEV1 were significantly higher in the children with early HDM sensitization comparing to the non-sensitized children (7.0% [IQR: 3.5 to 12.5] vs. 4.0% [IQR: 2.0 to 9.0], P = 0.018). Higher percentages of the early HDM sensitized children had airway hyperresponsiveness (PC20 ≤ 4 mg/mL) compared with the non-sensitized children (24.1% vs. 8.3%, P = 0.041).
Statistical analyses of children with asymptomatic early HDM sensitization
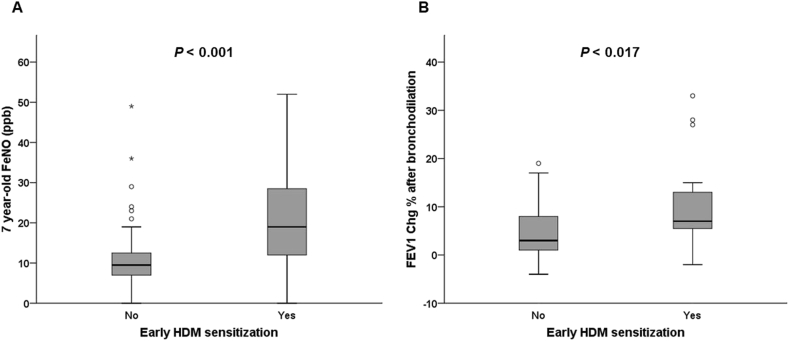
Asymptomatic toddlers with early HDM sensitization, compared with non-sensitized asymptomatic toddlers, still have higher risks of asthma (12.5% vs. 1.7%, P = 0.040), allergic rhinitis (83.3% vs. 43.1%, P < 0.001), and atopic dermatitis (20.8% vs. 0.0%, P < 0.001) at age 7 years. Likewise, asymptomatic toddlers with early HDM sensitization, compared with the non-sensitized asymptomatic toddlers, had higher FeNO levels (19.0 ppb [IQR: 12.0 to 28.5] vs 9.5 ppb [IQR: 7.0 to 12.3], P < 0.001, Fig. 2A) and higher changes of FEV1 (7.0% [IQR: 5.5 to 13.0] vs 4.0% [IQR: 1.5 to 9.0], P = 0.017, Fig. 2B) after bronchodilation at age 7 years. The asymptomatic toddlers with early HDM sensitization also had higher probabilities to have airway hyperresponsiveness than the non-sensitized asymptomatic children (25.0% vs. 6.3%, P = 0.030).
Fig. 2.
7-year-old FeNO levels and changes of FEV1 in bronchodilator reversibility tests at the asymptomatic early HDM-sensitized and non-sensitized group. (A) FeNO levels. (B) Changes of FEV1. Circles mark the mild outliers, and asterisks mark the extreme outliers. HDM: House dust mite; FeNO: Fractional exhaled nitric oxide; FEV1 Chg: Changes of forced expiratory volume in the first second
Discussion
Although HDM is a common trigger to induce acute deterioration of asthma or atopic dermatitis, its role in the initiation of asthma and other atopic diseases is still controversial. While some birth cohort studies demonstrated that early exposure to HDM increased risks of asthma and atopic dermatitis development,5, 6 other studies have questioned this correlation.7, 21 The relationship between exposure and sensitization to HDM is not straightforward and could be influenced by genetic background, the time of exposure, and environmental factors.22 In children with atopic dermatitis, skin barrier dysfunction can lead to increased penetration of allergens transcutaneously, with subsequent potential sensitization to both food and aeroallergens, including house dust mite.23 Early sensitization to HDM caused chronic inflammation and type 2 immune responses even in asymptomatic individuals.10 In the German Multicenter Allergy Study, sensitization to HDM before age 3 years, rather than exposure to HDM, was associated with wheezing and bronchial hyperresponsiveness at age 7 years.21 Our data showed that early sensitization to HDM, before symptoms or diseases developed, resulted in asthma, lung function deterioration, and other atopic diseases at age 7 years.
The correct interpretation of early HDM sensitization results is important for clinicians because sensitization to HDM before age 2 years is not a rare occurrence. In Belgium, a study reported that 28% of children aged 0–2 years were sensitized to aeroallergens, and the most commonly identified allergen was HDM.24 Clinicians often face a dilemma when they try to explain positive HDM allergy test results to parents whose children are asymptomatic. Based on our data, clinicians may need to explain the results cautiously and monitor toddlers with early HDM sensitization on a consistent basis, specifically in countries with a high prevalence of HDM allergy.
HDM sensitization deteriorates lung functions in atopic patients. In adults, a cross-sectional study reported that HDM sensitization caused patients to have a decreased FEV1, increased PC20 concentration, and increased FeNO levels.4 In children, early wheezers at preschool age without atopy had normal lung functions at school age. By contrast, the children sensitizing to HDM before age 3 years had deteriorated lung functions after age 7 years,25 comparable with the findings in this study.
Increasing evidence shows that vitamin D acts as a hormone and mediates cardiovascular, oncological, autoimmune, and allergic disease manifestations in humans.26 Previously, our groups demonstrated that cord blood and maternal vitamin D levels were associated with allergic sensitization and atopic manifestation in preschool-aged children.27, 28 The longitudinal trajectory of vitamin D deficient patterns was clearly associated with HDM sensitization and eosinophilia.20 This study further demonstrated that children with low vitamin D levels at age 1.5–2 years had higher risk to develop allergic rhinitis at age 7 years.
One limitation of this study is that the PATCH birth cohort study is a relatively small cohort. Only 10 cases with asthma and 9 cases with atopic dermatitis were identified at age 7 years. Larger birth cohorts are required to validate the findings observed in this study. Another limitation is that a high percentage of participants withdrew voluntarily or were lost to follow-up. The loss to follow-up is a common challenge to many birth cohort studies. The follow-up rate of the PATCH birth cohort study was comparable to the rates of other birth cohorts, which included serial blood samplings and lung function tests in their protocols.21, 29 In this study, the basic characteristics of the children evaluated at age 7 years remained similar to the population enrolled initially.
The strength of this study is that the PATCH birth cohort study is a general population cohort, and not a cohort of high-risk newborns. In addition, the diagnosis of asthma and other atopic diseases was based on long-term histories and physician's diagnosis, which make atopic outcomes more accurate. The lung function tests and FeNO were implemented to provide subjective parameters about the allergic outcomes.
In conclusion, children with early sensitization to D. pteronyssinus were at risk of having asthma, allergic rhinitis, and abnormal lung functions at age 7 years, despite being asymptomatic before the age 2 years. Clinicians need to closely monitor the asymptomatic toddlers with early HDM sensitization in order to provide prompt diagnosis and treatment when they develop atopic diseases.
Funding source
A research grant (CMRPGG3E1195) from Chang Gung Memorial Hospital, Chang Gung Medical Foundation, Taiwan.
Consent for publication
All authors consent to the publication of the manuscript in World Allergy Organization Journal.
Conflict of interest
The authors have no competing interests to disclose.
Ethics approval
The study was approved by the Human Research Ethics Committee of Chang Gung Memorial Hospital (No. 100–0201B).
Acknowledgments
We appreciate those children and their parents participating in the PATCH birth cohort study. This study was sponsored by a research grant (CMRPGG3E1195) from Chang Gung Memorial Hospital, Chang Gung Medical Foundation, Taiwan.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Jing-Long Huang, Email: long@adm.cgmh.org.tw.
Kuo-Wei Yeh, Email: kwyeh@adm.cgmh.org.tw.
References
- 1.Wang J.Y. The innate immune response in house dust mite-induced allergic inflammation. Allergy Asthma Immunol Res. 2013;5:68–74. doi: 10.4168/aair.2013.5.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory L.G., Lloyd C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miraglia Del Giudice M., Pedulla M., Piacentini G.L. Atopy and house dust mite sensitization as risk factors for asthma in children. Allergy. 2002;57:169–172. doi: 10.1034/j.1398-9995.2002.1s3252.x. [DOI] [PubMed] [Google Scholar]
- 4.Langley S.J., Goldthorpe S., Craven M., Morris J., Woodcock A., Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112:362–368. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 5.Huang J.L., Chen C.C., Kuo M.L., Hsieh K.H. Exposure to a high concentration of mite allergen in early infancy is a risk factor for developing atopic dermatitis: a 3-year follow-up study. Pediatr Allergy Immunol. 2001;12:11–16. doi: 10.1034/j.1399-3038.2001.012001011.x. [DOI] [PubMed] [Google Scholar]
- 6.Sporik R., Holgate S.T., Platts-Mills T.A., Cogswell J.J. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 7.Casas L., Sunyer J., Tischer C. Early-life house dust mite allergens, childhood mite sensitization, and respiratory outcomes. Allergy. 2015;70:820–827. doi: 10.1111/all.12626. [DOI] [PubMed] [Google Scholar]
- 8.Yeh K.W., Chiang L.C., Huang J.L. Epidemiology and current status of asthma and associated allergic diseases in Taiwan- ARIA Asia-Pacific Workshop report. Asian Pac J Allergy Immunol. 2008;26:257–264. [PubMed] [Google Scholar]
- 9.Chiu C.Y., Huang Y.L., Tsai M.H., Tu Y.L., Hua M.C., Yao T.C. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS One. 2014;9:e102809. doi: 10.1371/journal.pone.0102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciprandi G., Buscaglia S., Pesce G. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995;96:971–979. doi: 10.1016/s0091-6749(95)70235-0. [DOI] [PubMed] [Google Scholar]
- 11.Su K.W., Tu Y.L., Chiu C.Y., Huang Y.L., Liao S.L., Chen L.C. Cord blood soluble CD14 predicts wheeze and prolonged cough in young children: the PATCH study. Int Arch Allergy Immunol. 2016;169:189–197. doi: 10.1159/000445501. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma . 2017. Global Strategy for Asthma Management and Prevention. [Google Scholar]
- 13.Bousquet J., Khaltaev N., Cruz A.A. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World health organization, GA(2)len and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin J.M. Atopic dermatitis. J Allergy Clin Immunol. 1984;73:211–226. doi: 10.1016/s0091-6749(84)80008-1. [DOI] [PubMed] [Google Scholar]
- 15.Sunyer J., Anto J.M., Castellsague J., Soriano J.B., Roca J. Total serum IgE is associated with asthma independently of specific IgE levels. The Spanish Group of the European Study of Asthma. Eur Respir J. 1996;9:1880–1884. doi: 10.1183/09031936.96.09091880. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic S., European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 17.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Coates A.L., Wanger J., Cockcroft D.W., Culver B.H., the Bronchoprovocation Testing Task Force. Kai-Hakon C., Diamant Z. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49 doi: 10.1183/13993003.01526-2016. [DOI] [PubMed] [Google Scholar]
- 19.Su K.W., Chen P.C., Wang I.J. Cord blood soluble Fas ligand and pediatric atopic dermatitis. Allergy Asthma Proc. 2011;32:366–371. doi: 10.2500/aap.2011.32.3468. [DOI] [PubMed] [Google Scholar]
- 20.Chiu C.Y., Su K.W., Tsai M.H., Hua M.C., Liao S.L., Lai S.H. Longitudinal vitamin D deficiency is inversely related to mite sensitization in early childhood. Pediatr Allergy Immunol. 2018;29:254–259. doi: 10.1111/pai.12846. [DOI] [PubMed] [Google Scholar]
- 21.Lau S., Illi S., Sommerfeld C. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 22.Custovic A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin Exp Allergy. 2015;45:54–62. doi: 10.1111/cea.12450. [DOI] [PubMed] [Google Scholar]
- 23.Irvine A.D., McLean W.H., Leung D.Y. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 24.de Bilderling G., Mathot M., Agustsson S., Tuerlinckx D., Jamart J., Bodart E. Early skin sensitization to aeroallergens. Clin Exp Allergy. 2008;38:643–648. doi: 10.1111/j.1365-2222.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 25.Illi S., von Mutius E., Lau S. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 26.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 27.Chiu C.Y., Huang S.Y., Peng Y.C., Tsai M.H., Hua M.C., Yao T.C. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol. 2015;26:337–343. doi: 10.1111/pai.12384. [DOI] [PubMed] [Google Scholar]
- 28.Chiu C.Y., Yao T.C., Chen S.H., Tsai M.H., Tu Y.L., Hua M.C. Low cord blood vitamin D levels are associated with increased milk sensitization in early childhood. Pediatr Allergy Immunol. 2014;25:767–772. doi: 10.1111/pai.12304. [DOI] [PubMed] [Google Scholar]
- 29.Cox D.W., Mullane D., Zhang G.C. Longitudinal assessment of airway responsiveness from 1 month to 18 years in the PIAF birth cohort. Eur Respir J. 2015;46:1654–1661. doi: 10.1183/13993003.00397-2015. [DOI] [PubMed] [Google Scholar]