Abstract
Background
Newborn screening (NBS) programs for treatable metabolic disorders have been enormously successful, but molecular-based screening has not been broadly implemented so far.
Methods
This prospective pilot study was performed within the German NBS framework. DNA, extracted from dried blood cards was collected as part of the regular NBS program. As cystinosis has a prevalence of only 1:100,000–1:200,000, a molecular genetic assay for detection of the SMN1 gene mutation with a higher prevalence was also included in the screening process, a genetic defect that leads to spinal muscular atrophy (SMA). First tier multiplex PCR was employed for both diseases. The cystinosis screening employed assays for the three most common CTNS mutations covering 75% of German patients; in case of heterozygosity for one of these mutations, samples were screened by next generation sequencing (NGS) of the CTNS exons for 101 CTNS mutations. A detection rate of 98.5% is predicted using this approach.
Results
Between January 15, 2018 and May 31, 2019, 257,734 newborns were screened in Germany for cystinosis. One neonate was diagnosed with cystinosis, consistent with the known incidence of the disease. No false positive or false negatives were detected so far. Screening, communication of findings to parents, and confirmation of diagnosis were accomplished in a multi-disciplinary setting. This program was accomplished with the cooperation of hospitals, physicians, and parents. In the neonate diagnosed with cystinosis, oral cysteamine treatment began on day 18. After 16 months of treatment the child has no clinical signs of renal tubular Fanconi syndrome.
Conclusions
This pilot study demonstrates the efficacy of a molecular-based neonatal screening program for cystinosis using an existing national screening framework.
Abbreviations: NBS, newborns screening; SMA, spinal muscular atrophy; NGS, next generation sequencing; QA/QC, quality assurance, quality control; SMN, survival motor neuron protein; MS-MS, tandem mass spectroscopy
Keywords: Cystinosis, Molecular-based newborn screening, Efficacy, Follow-up
1. Introduction
Population-based newborn screening (NBS) is an important public health program that has vastly improved the course of several diseases through early detection [1]. The selection of screened disorders generally follows the principles outlined by Wilson and Jungner in 1968 (Supplement Table 1) [2]. Specifically, newborn screening for a target disease should only be offered when high sensitivity and specificity is ensured by an analytic process of high quality, including appropriate QA/QC, confirmatory testing, approved therapeutics, availability of therapy, clinical follow-up, genetic counselling, and regulatory oversight [3]. In Germany, NBS has been a voluntary National Health Service program since 1969, with legislation-based policy in all 16 states currently offering screening for 14 disorders [4,5]. Current NBS methods, which employ tandem mass-spectrometric analysis of newborn dried blood spots, cannot detect many potentially treatable genetic conditions [6]. At the same time, molecular-based NBS is increasingly feasible because DNA can be extracted from a dried blood spot, next generation sequencing has become economical, and molecular diagnostics have greater reliability and increased validity as genetic databases become more refined and comprehensive [7].
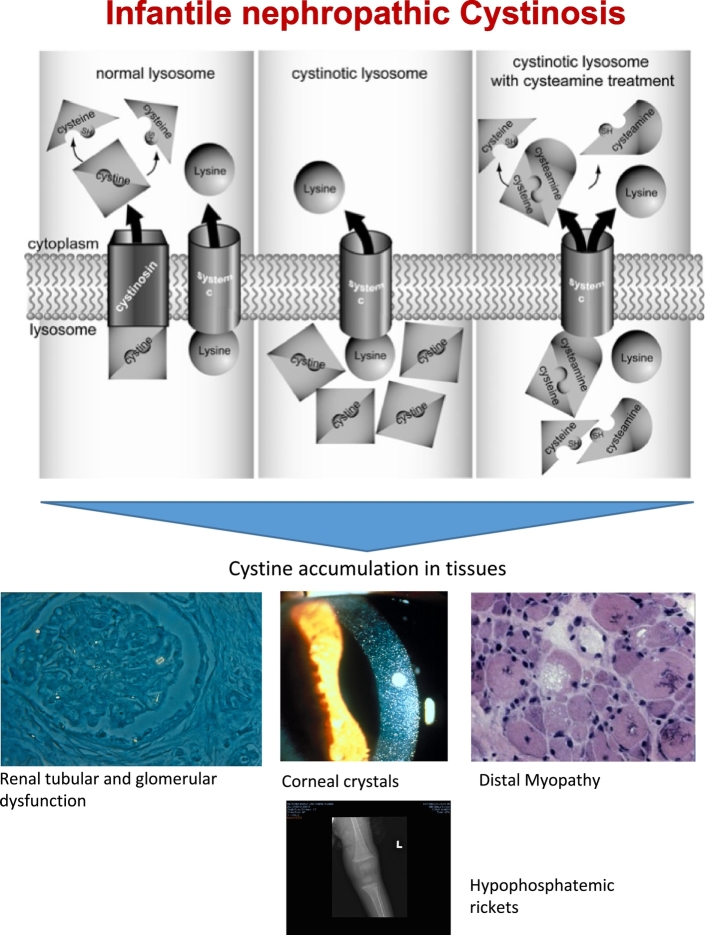
Two genetic disorders, nephropathic cystinosis and spinal muscular atrophy (SMA), are suitable for molecular based NBS because they now have effective therapies. Nephropathic cystinosis, with an incidence of 1: 100–200,000, results from biallelic mutations in CTNS, which encodes a protein (cystinosin) that transports cystine out of lysosomes (Fig. 1) [8,9]. In cystinosis, the lysosomal accumulation of cystine damages kidneys, eyes, muscle, liver, pancreas, and brain [10]. A small free thiol, cysteamine, depletes cells of cystine and has salutary effects on renal function, growth, and nonrenal complications, but by the time cystinosis is diagnosed (mean age, 14 months), substantial renal damage has already occurred [[11], [12], [13]]. The diagnosis of cystinosis is based on finding elevated cystine levels in white blood cells; however, this method is unsuitable for screening [9].
Fig. 1.
Clinical characteristics of individuals with cystinosis.
(Top) Cystinosis patients lack a functional cystine transporter in the lysosomal membrane, so cystine remains inside the lysosome. Cysteamine converts cystine to cysteine and cysteine-cysteamine mixed disulfide, which freely exits the cystinotic lysosome via the lysine transporter. (Bottom) The biallelic mutations of the CTNS gene lead to elevated cystine levels in nearly all cells, resulting in renal damage corneal cystine crystals, myopathy, and rickets due to hypophosphatemia. (Figures used with permission of [[21], [25]]).
SMA, with an incidence of 1: 6–10,000, is a neuromuscular disease mainly caused by homozygous deletion of exon 7 in the SMN1 gene [14]. Nusinersen, an antisense oligonucleotide that modifies SMN2-splicing to include exon 7, has dramatically changed the clinical course of SMA, but treatment must be initiated early in life [15,16].
This NBS pilot study employed the existing German NBS framework to incorporate first tier, high-throughput molecular genetic screening for cystinosis and SMA in newborns. The SMA molecular diagnostic methods and NBS results have been described elsewhere [17]. The overall NBS protocol and cystinosis NBS results are reported here.
2. Methods
2.1. Patients and consent
The study protocol was approved by the Ethics Committee of the Bayerische Landesärztekammer (BLAEK, Ethic permit No.16125) and for cystinosis only by the Ethics Committee of Hannover Medical School (No. 7772_BO_K_2018). An information sheet (Supplement Form 1) described the study and a parent and the attending physician signed an informed consent document (Supplement Form 2).
2.2. Sampling and DNA extraction
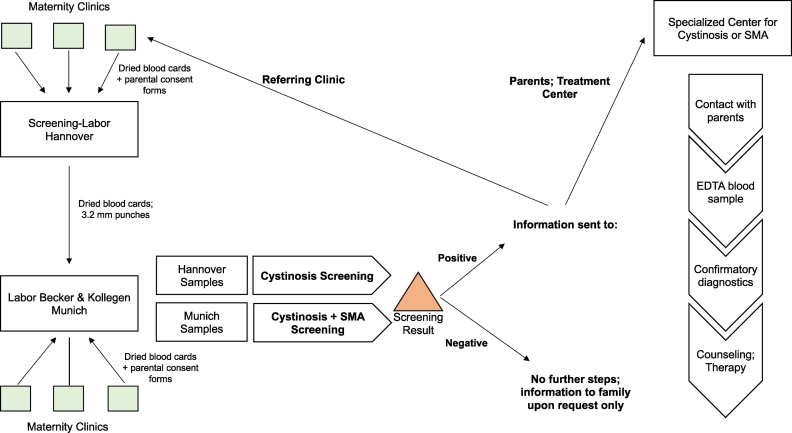
Fig. 2 shows the study workflow. Dried blood spot cards, obtained 36–72 h after birth under the German Children's Guidelines for NBS, were collected; DNA was extracted from 3.2 mm punches of the card (Supplement). All probes were analysed at the laboratory Becker& Kollegen, Munich; those sent directly to the Munich lab were screened for both cystinosis and SMA, while Hannover laboratory samples were screened for cystinosis only. In the Munich lab, 500–1000 dried blood cards were processed daily; in the Hannover lab, blood cards were processed three times a week.
Fig. 2.
Flowchart for pilot molecular newborn screening for cystinosis and spinal muscular atrophy (SMA).
Heelstick blood spots were obtained from newborns with parental consent and processed in one of two laboratories. Positive results were conveyed to the referring clinicians and to the parents, immediately for referral to a center with expertise in cystinosis or SMA.
2.3. Mutation screening
The detailed methodology for detecting CTNS variants, reported elsewhere (Fleige et al., submitted), involved first tier, multiplex PCR for 3 common CTNS mutations predicted to identify 75% of all individuals with cystinosis in Germany. Heterozygous samples were submitted to amplicon-based next-generation sequencing for 101 pathogenic CTNS mutations (Fleige T, Burggraf S, Czibere L, Häring J, Glück B, Keitel LM,Olfert L, Harms E, Hohenfellner K, Durner J, Roeschinger W, Becker M et al., submitted; www.hgmd.cf.ac.uk). For neonates with biallelic mutations in CTNS, the diagnosis was confirmed by determining the leucocytic cystine level from a blood sample obtained in the first 14 days of life.
2.4. Follow-up
Negative screening findings were communicated in writing to the responsible physician. Parents were informed of a negative result only upon written request. For positive findings, the lab first informed the hospital or local pediatrician and then the appropriate expert. A neonate with cystinosis was referred to the nearest Pediatric Metabolic Center and cysteamine therapy was begun immediately after a confirmatory diagnosis.
3. Results
3.1. Participation and adherence rate
Between January 15, 2018 and May 31, 2019, 257,734 newborns were screened for cystinosis and 200,901 for SMA in Germany. The Munich Laboratory received dried blood cards from 59 hospitals, covering approximately 150,000 newborns per year in Bavaria and North Rhine-Westphalia. Three hospitals did not participate in the pilot study. The Hannover laboratory entered the pilot study in May of 2018, and 128 hospitals participated.
The most recent rates of adherence to the established NBS program in these regions, from 2016, ranged from 97.7% to 98.0%; adherence to the pilot project involving explicit genetic screening was 88% in Bavaria. The pilot project did not influence parental acceptance of the regular NBS program.
For the pilot study, the Munich laboratory processed 200,901 samples for cystinosis and SMA and the Hannover laboratory processed 56,833 samples for cystinosis No sample required repeat testing from a new blood card due to an ambiguous result. Results of the first year of SMA screening and follow up are reported elsewhere [17] (Vill et al. submitted).
3.2. Cystinosis findings
Of the 257,734 total blood cards examined for cystinosis, one child, homozygous for 57 kb deletion, was identified and confirmed genetically in a diagnostic reference laboratory; this corresponds with the published frequencies and known incidence for cystinosis. A second neonate, screened as heterozygous for the common 57 kb deletion, was found to harbor an additional promoter variant (c.-512G>C) in CTNS previously reported as disease causing. However, according to current ACMG schemes the respective promoter variant needs to be reclassified as a non-pathogenic change. Genetic analysis revealed no additional mutation.
Indeed, other evidence indicates that the variant may not be pathogenic [18]. In fact, the infant showed no biochemical evidence of cystinosis, with a normal leucocyte cystine level, i.e., <0.2 nmol cystine/mg protein. According to a questionnaire-based survey carried out in pediatric nephrology departments in the screened regions of Germany (n = 11), no patient with nephropathic cystinosis was missed by newborn-screening.
In total, 696 heterozygous mutations were identified; 574 carried the 57 kb deletion, 72 the c.18_21del GACT (=357delGACT), and 50 the c.926dupG (=1261InsG). Second tier, next generation sequencing revealed no pathogenic second mutations except the promoter variant mentioned above.
3.3. Clinical and laboratory data of the neonate with cystinosis
The affected neonate started cysteamine treatment on day 18, with an increasing dosage from 10 to 80 mg/kg/day by 8 weeks of life with leucocytic levels <0,5 mmol cystine/mg protein. At age 16 months, serum creatinine was 0.3 mg/dl and spot urine analysis showed no signs of proximal tubular deterioration. Physical development was according to the 45th percentile for age.
4. Discussion
In 2017 alone, 786 of the 784,900 children born in Germany were diagnosed by NBS using enzymatic tests, immunochemistry as well as mass spectrometry and were treated beginning shortly after birth [19]. Molecular-based NBS, however, has not been broadly implemented; in Germany, it plays only a confirmatory, third-tier role in screening for cystic fibrosis [20]. Therefore, we selected two rare genetic diseases, nephropathic cystinosis and spinal muscular atrophy, to pilot first-tier molecular screening using the country's established neonatal screening program. Neither disease can currently be identified by the established screening method of tandem mass spectrometry.
This pilot study produced encouraging results in several domains. The program was successfully integrated into the existing German NBS infrastructure, collecting blood spots from ~20% of all neonates born in the country in 2018 and processing them within 24 h. Parents were generally informed of “screen-positive” results by a specialist on the same day that the result was obtained, with the recommendation to present the child promptly in a designated treatment center. In all cases, arrival at a treatment facility occurred within 6 days of the time that the parents were informed.
Detection rates approximated the known incidences of the two screened disorders. False positive results were not detected. According to a questionnaire-based survey carried out in pediatric nephrology departments, no patient with nephropathic cystinosis was missed by newborn-screening, in the screened areas. It is too early to be certain that no false negatives occurred, and technical and analytical errors resulting in missed diagnoses are always possible.
This pilot program clearly benefitted every newborn whose disease was detected. Cystinosis patients are generally diagnosed at 12–18 months of age, by which time significant renal tubular and glomerular damage has occurred. Treatment with oral cysteamine has salutary effects on preservation of renal function, growth, and prevention of late complications of the disease, such as myopathy [21]. For the few infants treated shortly after birth because an older sibling had cystinosis, even the renal tubular Fanconi syndrome that presents in the first months of life was ameliorated [22,23]. The newborn identified with the homozygeous 57 -kb deletion started cystine depletion therapy as a neonate and has no signs of proximal tubular involvement at age 16 months.
The second patient with a 57-kb variant and a heterozygous promotor variant c.-512G>C, was detected by second tier strategy. This promotor change was described in three cystinosis patients, two with ocular cystinosis and one with infantile cystinosis. According to the leucocyte cystine measurements the neonate was found to have no cystinosis. The laboratory findings are supported by the public databases for c.-512G>C, which revealed the variant in about 1.5% of the overall population (in 31.340 alleles 468 heterozygous and 12 homozygous entries; gnomAD), and an allele frequency of about 6.5% in the Asian population. This finding supports the use of ACMG classification of described genetic variants in available databases.
This pilot study also addressed the issue of how the public and physicians feel about the use of molecular genetics for NBS. In fact, participation in the regular NBS program did not change, and acceptance for this study appeared to be high; 88% participated in the screening. Only three hospitals and 3.2% of parents in Bavaria declined to participate. Being informed about carrier status for cystinosis was a different matter. Individuals heterozygous for a pathogenic CTNS mutation do not show clinical disease manifestations, so parents of neonates with monoallelic mutations were not automatically informed about such findings. NBS in Germany is regulated by the German Genetic Diagnostic's Act: German citizens have a “Right Not To Know” and a person must legally agree to genetic testing for her/himself or to benefit a family member [24]. For infants, a health advantage must be demonstrated, and parents can choose whether to be informed about risk factors in their children's genomes. In our study, parents were given the opportunity to be informed in writing about the heterozygosity status of their newborn; so far, no parent has made use of this opportunity.
5. Conclusion
Using cystinosis and SMA as models of treatable, very rare and rare genetic disorders, respectively, we demonstrated that efficient molecular-based NBS for single gene disorders can be achieved in a socially acceptable fashion, using an informed consent process. This pilot study took place in Bavaria, Germany, with a population of ~13 million; scaling up to the United States population of ~320 million could prove challenging. However, the success of this program provides proof of principle for molecular-based NBS and for detection of pathogenic CTNS variants.
Acknowledgments
Acknowledgments
The project was funded by the Cystinosis Foundation Germany, the Dietmar Hopp Foundation Germany, Cystinosis Research Network, USA and Sternstunden Germany.
Declaration of competing interest
Katharina Hohenfellner has previously received a fee for speaking at a symposium from Orphan Europe. Carsten Bergmann, Tobias Fleige, Nils Janzen, Siegfried Burggraf, Bernd Olgemöller, William A. Gahl, Ludwig Czibere, Sonja Froschauer, Wulf Röschinger, Katharina Vill, Erik Harms, Uta Nennstiel have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2019.100514.
Contributor Information
Katharina Hohenfellner, Email: katharina.hohenfellner@ro-med.de.
Carsten Bergmann, Email: carsten.bergmann@uniklinik-freiburg.de.
Tobias Fleige, Email: t.fleige@labor-becker.de.
Nils Janzen, Email: n.janzen@metabscreen.de.
Siegfried Burggraf, Email: s.burggraf@labor-becker.de.
Bernd Olgemöller, Email: bernd@lb-olegmoeller.de.
William A. Gahl, Email: gahlw@mail.nih.gov.
Ludwig Czibere, Email: l.czibere@labor-becker.de.
Sonja Froschauer, Email: sonja.froschauer@cystinose-stiftung.de.
Wulf Röschinger, Email: w.roeschinger@labor-becker.de.
Katharina Vill, Email: Katharina.vill@med.uni-muenchen.de.
Erik Harms, Email: harms@uni-muenster.de.
Uta Nennstiel, Email: uta.nennstiel@lgl.bayern.de.
Appendix A. Supplementary data
Supplementary material
References
- 1.Newborn screening: A blueprint for the future executive summary: newborn screening task force reportPediatrics. 2000;106(2 Pt 2):386–388. [PubMed] [Google Scholar]
- 2.Wilson J., Jungner Y. Principles and practice of mass screening for disease. Boletín De La Oficina Sanit Panam Pan Am Sanit Bureau. 1968;65(4):281–393. [PubMed] [Google Scholar]
- 3.Friedman J.M., Cornel M.C., Goldenberg A.J. Genomic newborn screening: public health policy considerations and recommendations. BMC Med. Genet. 2017;10(1):9. doi: 10.1186/s12920-017-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekanntmachung eines Beschlusses des Gemeinsamen Bundesausschusses über eine Änderung der Richtlinien des Bundesausschusses der Ärzte und Krankenkassen über die Früherkennung von Krankheiten bei Kindern bis zur Vollendung des 6. Lebensjahres (Kinder-Richtlinien) zur Einführung des erweiterten Neugeborenen-Screenings. BAnz. Nr. 60 vom 31.03.2005, 4833–8. Fassung vom: 18.06.2015 BAnz AT 18.08.2016 B1, Letzte Änderung: 19.10.2017 BAnz AT 15.03.2018 B2, In Kraft getreten am: 16.03.2018 https://www.g-ba.de/downloads/39-261-1251/2010-12-16_Kinder-RL_Anpassung%20GenDG_BAnz.pdf
- 5.Beschluss Kinder-Richtlinie: Screening von Neugeborenen zur Früherkennung voSCID. Beschlussdatum: 22.11.2018; Inkrafttreten: 09.02.2019 https://www.g-ba.de/downloads/40-268-5426/2018-11-22_Kinder-RL_SCID-Screening_ZD.pdf
- 6.Landau Y.E., Lichter-Konecki U., Levy H.L. Genomics in newborn screening. J. Pediatr. 2014;164(1):14–19. doi: 10.1016/j.jpeds.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Adams D.R., Eng C.M. Next-generation sequencing to diagnose suspected genetic disorders. N. Engl. J. Med. 2018;379:1353–1362. doi: 10.1056/NEJMra1711801. [DOI] [PubMed] [Google Scholar]
- 8.Gahl W.A., Bashan N., Tietze F., Bernardini I., Schulman J.D. Lysosomal cystine transport is defective in cystinosis. Science. 1982;217:1263–1265. doi: 10.1126/science.7112129. [DOI] [PubMed] [Google Scholar]
- 9.Town M., Jean G., Cherqui S., Attard M., Forestier L., Whitmore S.A. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. M. Town, G. Jean, S. Cherqui, M. Attard, et al. [DOI] [PubMed] [Google Scholar]
- 10.Gahl W.A., Thoene J.G., Schneider J.A. Cystinosis. N. Engl. J. Med. 2002;347(2):111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 11.Thoene J., Oshima R., Crawhall J., Olson D., Schneider J. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J. Clin. Invest. 1976;58(1):180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gahl W.A., Reed G.F., Thoene J.G., Schulman J.D., Rizzo W.B., Jonas A.J., Denman D.W., Schlesselman J.J., Corden B.J., Schneider J.A. Cysteamine therapy for children with nephropathic cystinosis. N. Engl. J. Med. 1987;316:971–977. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 13.Markello T.C., Bernardini I.M., Gahl W.A. Improved renal function in children with cystinosis treated with cysteamine. N. Engl. J. Med. 1993;328:1157–1162. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre S., Bürglen L., Reboullet S. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 15.Finkel R.S., Chiriboga C.A., Vajsar J. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet Lond. Engl. 2017;388 doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 16.Glascock J., Sampson J., Haidet-Phillips A. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J. Neuromuscul. Dis. 2018;5:145–148. doi: 10.3233/JND-180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czibere L., Burggraf S., Fleige T. High-throughput genetic newborn screening for spinal muscular atrophy by rapid nucleic acid extraction from dried blood spots and 384-well qPCR. Eur. J. Hum. Genet. 2019 doi: 10.1038/s41431-019-0476-4. Jul 30 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason S., Pepe G., Dall'Amico R. Mutational spectrum of the CTNS gene in Italy. Eur. J. Hum. Genet. 2003;11(7):503–508. doi: 10.1038/sj.ejhg.5200993. [DOI] [PubMed] [Google Scholar]
- 19.Nationaler Screening Report, 07.2019. http://www.screening-dgns.de/Pdf/Screeningreports/DGNS-Screeningreport-d_2015.pdf
- 20.Naehrig S., Chao C.-M., Naehrlich L. Cystic fibrosis. Dtsch. Arztebl. Int. 2017;114(33–34):564–574. doi: 10.3238/arztebl.2017.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesterova G., Gahl W.A. Cystinosis: the evolution of a treatable disease. Pediatr. Nephrol. 2013;28(1):51–59. doi: 10.1007/s00467-012-2242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleta R., Bernardini I., Ueda M. Long-term follow-up of well-treated nephropathic cystinosis patients. J. Pediatr. 2004;145(4):555–560. doi: 10.1016/j.jpeds.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 23.Da Silva V.A., Zurbrügg R.P., Lavanchy P. Long-term treatment of infantile nephropathic cystinosis with cysteamine. N. Engl. J. Med. 1985;313(23):1460–1463. doi: 10.1056/NEJM198512053132307. N. Engl. J. Med., 313 (1985), pp. 1460–1463. [DOI] [PubMed] [Google Scholar]
- 24.Gendiagnostikgesetz vom 31. Juli 2009 (BGBl. I S. 2529, 3672), das zuletzt durch Artikel 2 Absatz 1 des Gesetzes vom 4. November 2016 (BGBl. I S. 2460) geändert worden ist. https://www.gesetze-im-internet.de/gendg/GenDG.pdf
- 25.Wilmer M.J. Cystinosis:practical tools for diagnosis and treatment. Pediatr. Nephrol. 2011;26:205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material