Abstract
Background
International guideline-recommended on-demand treatments for hereditary angioedema (HAE) include: C1-esterase inhibitor (plasma-derived or recombinant), or bradykinin-receptor antagonists. In most low- and middle-income countries (LMIC) these products are not registered or are unaffordable. Solvent-detergent, fresh or freeze-dried plasma therapy is thus the only available on-demand treatment in these settings; but published data on efficacy and safety are limited. This study evaluated the efficacy and safety of on-demand plasma treatment of acute HAE in two LMICs.
Methods
A retrospective folder or patient registry review of acute swelling episodes necessitating emergency room attendance amongst known HAE patients was conducted at treatment centers in South Africa and Iran. Data collected included the site of angioedema, timing and amount of fresh frozen plasma (FFP) administered, time-to-resolution, hospital length of stay and adverse events.
Results
There were 176 acute swelling episodes amongst 43 HAE patients; 98 were treated with FFP. The face, upper airway, and abdomen were involved in 15.3% (15/98), 53.1% (52/98) and 29.6% (29/98) of episodes treated with FFP respectively. Median (interquartile range ([IQR]) of FFP administered was 400 (280–560) mLs. In all episodes except two, FFP led to resolution, with median (IQR) hours to resolution 4 (2–12). Five transfusion reactions occurred, with one case of anaphylaxis and no deaths; giving an adverse reaction rate of 5%. Differences between South Africa and Iran included: (1) proportion of HAE type II(2) median (IQR) hours to FFP administration and hospitalization, (3) number of intubations after FFP infusion. Healthcare cost for FFP treatment was USD369- 791 in South Africa and USD275-550 in Iran, largely influenced by hospital length of stay.
Conclusions
Plasma (fresh-frozen) remains the only available effective on-demand treatment for acute HAE in many countries. FFP is effective and safe, but time-to-resolution is slower and adverse events are more frequent than published data on targeted therapies. Overall healthcare cost of FFP approaches that of targeted therapies – now available through global access programs – when hospitalization is prolonged.
Keywords: Hereditary angioedema, Fresh frozen plasma, Treatment
Abbreviations: HAE, hereditary angioedema; LMIC, low- and middle-income country; FFP, fresh frozen plasma; IQR, interquartile range; USD, United States dollar; C1–INH, C1-esterase inhibitor; SA, South Africa(n); WAO, World Allergy Organisation; TEAE, treatment-emergent adverse event; CTCAE, common terminology criteria for adverse events; SAE, serious adverse event; FAST, for angioedema subcutaneous treatment; USA, United States of America
Introduction
Hereditary angioedema (HAE) is an autosomal dominant disorder characterized by acute episodes of angioedema, which can be intensely distressing, and life-threatening.1 HAE is a global disease, reported amongst varied ethnicities. Prevalence reports range from 1:10 000 to 1:150000, with data from South Africa and Iran estimating 1:140 000 and 1:1000000 respectively, although underreporting is a problem.2, 3 HAE treatment consists of 3 aspects: treatment of acute swelling episodes, short-term prophylaxis, and long-term prophylaxis.4 International guideline-recommended treatment options for on-demand therapies include: C1-INH concentrates (either plasma-derived or recombinant), bradykinin B2 receptor antagonist (icatibant), and the injectable plasma kallikrein inhibitor, ecallantide.1 Targeted therapies are prohibitively expensive and/or unlicensed in many low- and middle-income countries (LMIC); in fact, less than 20% of the global HAE population may have access to these therapies (calculation based on global prevalence and current product registration information). Solvent-detergent, frozen or freeze-dried plasma, which contains C1–INH, is thus the only on-demand therapy available for acute swelling episodes for patients with HAE in such settings.
Solvent-detergent, frozen or freeze-dried plasma is used in many countries for on-demand therapy, yet there is limited published data on efficacy and safety with a total of only 60 acute swelling episodes in patients with HAE treated with plasma therapy reported.5, 6, 7, 8 Concerningly, it has been suggested that plasma therapy may worsen angioedema due to containing potentially harmful substrates. Prematta et al. found 12 case reports in the literature and reported 11 instances of fresh frozen plasma (FFP) use for acute swelling episodes at the Hershey Medical Centre, in which one patient showed no improvement, and two had transient worsening of symptoms that may or may not have been related to FFP.5 Winnewisser et al. found no adverse reactions in 30 cases treated at their institution.6 In addition to reviewing the same literature as Prematta, Tang, Chen and Zhang examined 4 unique episodes from 2 patients at the Peking Union Medical College Hospital, with 1 transfusion reaction.7 Pekdemir et al. published 3 cases of HAE successfully treated with FFP, including 1 patient with an urticarial transfusion reaction that responded well to treatment.8 Thus, there is very limited published data on plasma therapy for on-demand treatment of HAE despite widespread use. No direct comparative data is available between targeted and plasma therapy. We thus conducted a study to examine the efficacy and safety of FFP for acute swelling episode treatment of HAE in 2 LMICs where FFP is commonly used.
Methods
Study design and setting
A retrospective patient folder review, as well as registry data, was used to extract data on the management of acute swelling episodes in patients with HAE treated in 6 South African and 1 Iranian National Reference center for HAE management respectively. In South Africa, folders of known HAE patients at 6 independent treatment centers were reviewed.1, 9 In Iran, HAE patients detailed in an Iranian HAE registry were reviewed. Additionally, in South Africa HAE patients treated in the private healthcare sector were contacted via email and asked to provide any information available regarding any acute swelling episodes of HAE treated with FFP. Recorded acute swelling episodes spanned a period from 2001 to 2017, the year in which the data were collected.
Data were extracted and tabulated in an Excel spreadsheet. Data variables included: date of birth; gender; age of HAE diagnosis/episode; HAE type; family history of HAE; date of the first episode of angioedema; date of an episode requiring hospital (emergency room) visit; time of symptom onset; time from symptom onset to arrival at the emergency room (ER); location of the angioedema, FFP treatment (yes/no), time FFP initiated; FFP dose in units and mL/kg; use of repeat dosages of FFP and time from initial dose; patient admission ward and total hospital length of stay; time from FFP infusion to resolution: time from emergency unit admission to start of FFP infusion; need for endotracheal intubation before or after FFP; treatment other than FFP; FFP adverse events and if they necessitated treatment and/or stopping the FFP infusion.
The method of recording time-to-resolution between South Africa and Iran differed substantially. South Africa's time-to-resolution was based on the nursing notes from patient folders, while Iran, based on patient recall, included an initial and final time-t-resolution for some episodes. As a result, time-to-resolution is not comparable between countries.
Statistical analysis
Categorical variables were summarized as frequencies and percentages. Parametric and non-parametric continuous data are presented using mean ± standard deviation, and medians and interquartile ranges (IQR) respectively. Data were analyzed in Microsoft Excel and Stata 14•2 (Stata Corp., College Station, TX).
Results
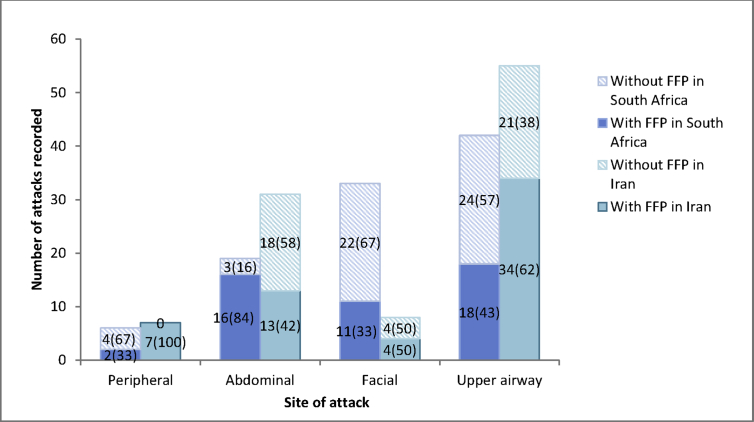
Table 1 details all of the episodes of HAE presenting for ER treatment in South Africa and Iran. Overall, there were 176 episodes of HAE necessitating hospital treatment amongst 43 HAE patients; 81 and 95 episodes from 27 South African and 16 Iranian patients respectively. The anatomical site of angioedema episodes necessitating ER visits included: 55% (97/176) upper airway, 28% (50/176) abdominal, 24% (42/176) face, and 7% (12/176) peripheries. Iranian patients presented for on-demand treatment more frequently with abdominal and South African patients with facial angioedema. Only 56% (98/176) were treated with FFP; 54% (44/81) and 57% (54/95) in South Africa and Iran respectively. Fig. 1 shows the use of FFP therapy depending on the site of angioedema.
Table 1.
Details of angioedema episodes requiring emergency room (ER) treatment stratified by country, IQR = interquartile range. Potentially life-threatening episodes considered as episodes involving the upper airway.
| Total | South Africa | Iran | ||
|---|---|---|---|---|
| Number of patients | 43 | 27 | 16 | |
| Females:males | 30:13 | 18:9 | 12:4 | |
| Mean age | 29 ± 15 | 26 ± 16 | 34 ± 12 | |
| HAE type diagnosed | Type 1 | 35 | 26 | 9 |
| Type 2 | 7 | 0 | 7 | |
| Unknown | 1 | 1 | 0 | |
| Patients with HAE family history | 34 | 22 | 12 | |
| Number of episodes | 176 | 81 | 95 | |
| Number of episodes per location, N (%) | Peripheral | 13 (7) | 6 (7) | 7 (7) |
| Abdominal | 50 (28) | 19 (23) | 31 (33) | |
| Facial | 41 (23) | 33 (41) | 8 (8) | |
| Upper airway | 97 (55) | 42 (52) | 55 (58) | |
| Number of episodes, N (%) | Treated with FFP | 98 (56) | 44 (54) | 54 (57) |
| Treated without FFP | 78 (44) | 37 (46) | 41 (43) | |
| Time from symptom onset to ER arrival in hours | Within 12 h | 74 | 22 | 52 |
| After 12 h | 20 | 18 | 2 | |
| Median time from admission to FFP infusion in hours (IQR) | 2 (0.5–3.0) | 3 (2–5) | 1 (0.3–2.5) | |
| Median first dose of FFP in mL (IQR) | 400 (280–560) | 280 (280–560) | 400 (200–1000) | |
| Number of episodes requiring repeat dose, N (%) | 7 (6) | 6 (14) | 1 (2) | |
| Number of potentially life-threatening episodes not treated with FFP, N (%) | 45 (46) | 24 (57) | 21 (38) | |
| Number of episodes requiring intubation, N (%) | Before FFP infusion | 5 (5) | 5 (11) | 0 |
| After FFP infusion | 2 (2) | 1 (2) | 1 (2) | |
| Median time-to-resolution in hours (IQR) | 4 (2–12) | 9.3 (5–12.3) | Initial | 2 (0.5–2.5) |
| Finala | 48 (0.5–72) | |||
| Median length of stay at hospital in hours (IQR) | Without FFP | 18 (4–48) | 24 (18–60) | 6 (3–48) |
| With FFP | 12 (5–36) | 36 (24–72) | 5 (4–9) | |
| Number of transfusion reactions from infusions, N (%) | 5 (5) | 2 (5) | 3 (6) |
Iranian initial and final time-to-resolution not comparable to South Africa's single figure
Fig. 1.
Relationship between angioedema site and decision to treat with FFP
Of patients treated with FFP, 75.5% (74/98), 20.4% (20/98), and 4.1% (4/98) arrived at the ER within 12 hours of symptom onset, after 12 hours from symptom onset, and after an unknown time period respectively. Of note is that of the South African episodes, 43.1% (18/44) patients arrived after 12 hours from symptom onset. Time from arrival at the emergency room to the start of FFP infusion was a median (IQR) of 2 hours (0.5–3); with longer delay inSouth Africa than Iran [South Africa: 3 (2–4.9) hours vs. Iran: 1 (0.3–2.5) hours. Standard unit sizes of FFP are 280 mLs and 200 mLs in South Africa and Iran, respectively. Overall, the median (IQR) volume of the first dose of FFP was 400 mLs (280–560 mLs). A repeat dose of FFP was only required in 7 episodes (6/7 in SA), all of which had originally only been treated with a median (IQR) of 280 mLs (280–560 mLs) of FFP (1 unit), and which were treated with a second dose of a median (IQR) of 280 mLs (280-280 mLs) per episode. Notably, 46% (45/97) of life-threatening upper airway angioedema episodes, considered as mandatory for on-demand therapy by World Allergy Organization (WAO) guidelines,1 were not treated with FFP. Prior to being able to commence FFP treatment, 5 South African patients with upper airway episodes required intubation for airway protection, whereas no Iranian patients required intubation. Two patients required intubation after the start of commencing FFP: 1 South African patient secondary to anaphylaxis; and 1 Iranian patient with severe upper airway oedema not responding to FFP.
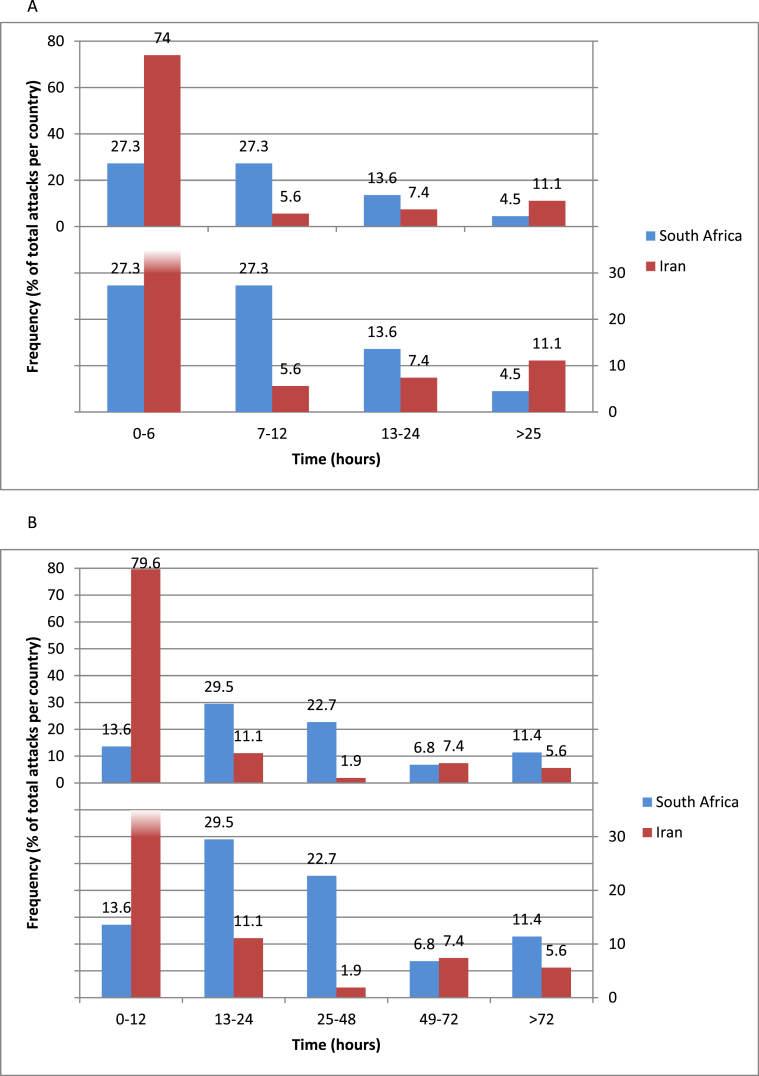
Fig. 2 shows the time-to-resolution of angioedema following the commencement of FFP infusion (A) and the duration of hospitalization associated with observation and treatment for an angioedema episode (B). Overall, the median (IQR) hours to resolutions was 4 hours (2–12). Overall, 79.6% (43/54) Iran vs. 54.5% (24/44) South African patients resolved in less than 12 hours (Fig. 2A)]. Patients treated with FFP spent a median of 12 (5–36) hours in hospital. South African patients spent a longer number of hours than Iranian patients [SA: 36 (24–72) vs. 5 (4–9)]; similar differences in hospitalization time were noted between the countries for episodes not treated with FFP. In 2 episodes, FFP infusion did not seem to improve angioedema (0 in South Africa, 2 in Iran), with prolonged hospital stays of 144 and 72 hours. Hospital length of stay was not affected by receipt of FFP [Without FFP 18 (4–48) hours vs. with FFP: 12 (5–36) hours (Fig. 2B).
Fig. 2.
A. Time-to-resolution after FFP, stratified by country; B. Time in hospital per episode treated with FFP stratified by country
Table 2 details the 5 treatment-emergent adverse events (TEAEs) to FFP infusion; 3 were grade 2, 1 was grade 3 and 1 was grade 4 according to the National Cancer Institute's common terminology criteria for adverse events (CTCAE).10 All reactions necessitated stopping FFP infusions but were successfully treated and no fatalities occurred. The overall rate of transfusion reactions was 5%. There was no difference in adverse reactions necessitating treatment interruption between SA and Iran. However, one Iranian patient, who had experienced adverse reactions to FFP prior to the acute swelling episodes recorded in this review, and the 23-year-old female patient listed as one of the TEAEs, received premedication with antihistamine with/without corticosteroids prior to subsequent FFP infusions, and this was successful in preventing subsequent TEAEs. Premedication was given for a total of 14 of the reported episodes in the Iranian cohort.
Table 2.
Transfusion reactions secondary to FFP administration.
| Source of data | Sex/age (yr) | Date of episode | Site of oedema | Dose (mL) | Adverse events | Treatment for side effects | Time until resolution (hours) | Length of stay in hospital (hours) | CTCAE grade10 |
|---|---|---|---|---|---|---|---|---|---|
| South Africa | F/31 | 05/2017 | Upper airway | 800 | Urticaria | Antihistamine | 24 | 72 | 2 |
| M/40 | 12/2015 | Upper airway | 800 | Anaphylaxis | Adrenaline, antihistamine, corticosteroid, intubation | 46 | 125 | 4 | |
| Iran | F/23a | 2015 | Abdominal | 200 | Urticaria and dyspnoea | Steroids, cetirizine, adrenaline | 0.5 | 5 | 3 |
| F/40 | 05/2017 | Upper airway | 900 | Urticaria | Hydroxyzine | – | 72 | 2 | |
| 08/2017 | Upper airway | 1350 | Urticaria | Hydroxyzine | 5 | 12 | 2 |
This patient had subsequent FFP-treated HAE acute episodes; receipt of premedication with antihistamines resulted in no subsequent TEAE
Table 3 shows the health provider estimated cost of hospitalization and FFP treatment for an acute swelling episode in a patient with HAE in South Africa (A) and Iran (B), using an ingredients approach. In South Africa the cost of treatment was between USD369 and USD791 using the median dose of FFP, with the differences related to hospital length of stay. Similarly, in Iran, the cost of treatment was between USD275 and USD550.
Table 3.
Ingredients costing of treatment with FFP in (A) South Africa (USD1 = R14) and (B) Iran (USD1 = 40 000 Rials).
| Fee category | Fee subcategory | Time in hospital |
||
|---|---|---|---|---|
| 12 h | 24 h | 36 h | ||
|
A |
||||
| Emergency consultation | Facility fee | 16 | 32 | 48 |
| Specialist medical practitioner | 26 | 52 | 78 | |
| Nursing practitioner | 7 | 14 | 21 | |
| Inpatient high care | Facility fee | 151 | 302 | 453 |
| Specialist medical practitioner | 11 | 22 | 33 | |
| Medication fee | Facility fee | 3 | 3 | 3 |
| FFPa | 96 | 96 | 96 | |
| Minor procedure cat A (infusion) | Facility fee | 38 | 38 | 38 |
| Specialist medical practitioner | 21 | 21 | 21 | |
| Total cost (USD) |
369 |
580 |
791 |
|
|
B |
||||
| Emergency consultation | Facility fee | 71 | 71 | 142 |
| Specialist medical practitione | 10 | 10 | 20 | |
| Nursing practitioner | 5 | 5 | 10 | |
| Inpatient high care | Facility fee | 164 | 164 | 328 |
| Specialist medical practitioner | 10 | 10 | 20 | |
| Medication fee | Facility fee | 3 | 3 | 6 |
| FFPb | 10 | 10 | 10 | |
| Minor procedure cat A (infusion) | Facility fee and Specialist medical practitioner | 2 | 2 | 4 |
| Total cost (USD) | 275 | 275 | 550 | |
The cost indicated is for two units of FFPs, the median dose required by SA patients (~280 mLs). Certain patients require additional units at a cost of USD96/unit.
The cost indicated is for two units of FFPs, the median dose required by Iran patients (~400 mLs). Certain patients require additional units at a cost of USD5/unit
Discussion
The latest WAO guidelines for on-demand HAE treatment recommends targeted therapies (C1-INH concentrates, bradykinin-B2 receptor antagonists, and plasma kallikrein inhibitors) or solvent-detergent, frozen or freeze-dried plasma. Compared to the large randomized controlled trials conducted for targeted therapies, published data on plasma therapies is restricted to 60 episodes. Yet, this remains the only available on-demand treatment for the majority of global HAE patients, especially those in LMIC countries. Our study reports 98 acute swelling episodes in patients with HAE treated with FFP across two LMICs where targeted therapies are not registered and unaffordable to the majority of HAE patients. The key findings of our study include: (1) FFP is effective, although the time-to-resolution is considerably slower than reported for targeted therapies; (2) FFP has a TEAE rate of ~5%, and premedication may reduce TEAEs; (3) setting-specific differences in acute HAE management and FFP use exist including speed of access to FFP therapy, treatment thresholds, dosing and time-to-resolution, and hospital length of stay; and (4) direct healthcare costs of FFP treatment with prolonged hospital length of stay approaches that of targeted therapy increasingly available through global access initiatives.
Our study of 98 acute HAE episodes treated with FFP across two LMICs is a substantive increase to the published literature evaluating the use of plasma therapy in acute HAE. In both South Africa and Iran, FFP therapy led to resolution in all but 2 cases (2%). Time-to-resolution was a median of 4 hours; consistent with existing data7 but nearly double that reported for targeted therapies – including plasma-derived and recombinant C1-inhibitor, and icatibant.11, 12, 13 In addition, in contrast to targeted therapy, FFP therapy cannot be administered at home, nor is it immediately available on arrival to hospital as FFP specific to the patient's blood group must be ordered. In our study patients waited a median of 2 hours from admission to the start of plasma therapy. There is no clear recommendation about the per kilogram dosing for FFP in the literature, with estimates of 1–4 units or 20mLs/kg of FFP being effective5; notably in our study 280 mL (1 unit) and 400 mL (2 units) of FFP were required in the majority of cases in Iran and South Africa respectively (estimated as less than 10mLs/kg given the majority were adults >40 kgs).
FFP use is safe for the majority of patients, but serious adverse events (SAEs) can occur. In our study there were 5% TEAEs, including one SAE (life-threatening anaphylaxis); similar to that reported in the HAE literature (3.3%)5, 6, 7, 8; and from other studies of FFP use in the general population (3.4%).16 In comparison, there was no or a very low rate of SAEs reported for trials of plasma-derived C1-inhibitor (0.19% of a total of 3196 infusions17); and in the bradykinin-receptor antagonist trials (no SAEs in FAST-1, 2 or 318, 19, 20). Two Iranian patients, 1 with past adverse reactions to FFP and 1 with a TEAE captured in Table 2, were given antihistamine or corticosteroid prophylaxis prior to 14 subsequent acute episodes of FFP infusion without developing further TEAE; this suggests that premedication may be effective in reducing the incidence of TEAE in HAE patients receiving FFP infusions.
Prescribing practices for FFP use in acute HAE are based on a limited evidence base and consequently local practice is based on expert experience. This is the first study to compare FFP use for acute HAE in two LMICs. Substantial differences were noted including: (1) the anatomical locations of angioedema resulting in presentation to emergency care (South Africa higher facial and Iran higher abdominal episodes); (2) speed of access to hospital treatment (slower in South Africa); (3) proportion of hospitalized episodes treated with FFP (fewer in South Africa); (4) FFP single unit volume and hence initial dosing used; (5) reported time-to-resolution, and (6) hospital length of stay. There are a number of possible explanations for these differences. There was a weak correlation between longer time to access FFP treatment and time-to-resolution. Delay in the administration of FFP treatment (and lower dose units) may contribute to adverse events in the South Africa setting.2, 21 Notably, a higher proportion of SA (5) than Iranian (1) patients required intubation prior to FFP therapy. Iranian patient feedback also indicated that a shorter stay in hospital was due to: a preference to leave the hospital quickly to attend to other commitments; a feeling that no effective treatment was provided at the hospital; and a sense that physicians did not take problems seriously, especially in the initial stage of an acute swelling episode. Finally, given the retrospective nature of the study, recall bias may also have contributed. These differences highlight a need for HAE experts in LMICs to produce a consensus document on the use of plasma therapy for HAE.
The major argument for the use of FFP or solvent-detergent plasma in LMIC settings is the high cost of targeted therapies. However, our ingredients costing data indicate that when all direct costs such as hospitalization and doctor time are included, the total cost to public or private healthcare funders is substantial. Global access initiatives, such as the one driven by HAE International,23 will increasingly mean that targeted therapies can be accessed more affordably (~USD800/adult treatment dose). If these products could be administered at home in LMIC, they may be cost neutral for healthcare funders and yet save patients substantial indirect cost; in 2019 the annual indirect costs for HAE patients in the USA were estimated to be USD52600.24 Local indirect costing is not available for either South Africa or Iran, but from our clinical experience we know that for certain indigent patients, even the cost burden of transport to and from emergency care is a significant economic burden.
This study has a number of strengths including the largest number of acute swelling episodes treated with FFP, as well as the inclusion of two different LMIC treatment settings. However, its major limitation remains that it was a retrospective study. There were thus missing data and possible recall bias. There may also have been differences in interpretation that could have affected study findings; for instance the time-to-resolution of symptoms which may have been recorded by some as the time to the start of symptoms resolution and not to that of complete resolution – patient notes often lacked clarity in this regard. Every effort was made to interrogate nursing charts to ensure accurate data and to limit missing information.
Conclusion
In summary, our study indicates that FFP is an effective and relatively safe therapy for on-demand treatment of swelling attacks in patients with HAE. The logistics of administration, the need to access hospital services, a slower time-to-resolution and consequent prolonged hospitalization, and the higher TEAE rate mean that targeted therapies remain preferable. Considerable differences in practice are evident, and to standardize treatment would be of considerable benefit where FFP may remain the only available therapy for many affected patients. Local experts should also conduct setting specific costings to motivate to public and private health care funders that, given improved pricing and global access programs, targeted therapy may, in fact, have a net economic benefit. Ideally a head-to-head comparison of a targeted versus a plasma-product in an LMIC with robust economic analysis would help provide a robust evidence base.
Declarations
Ethics approval and consent to participate
This study was approved by the “University of Cape Town” and “Immunology, Asthma and Allergy Institute (IAARI), Tehran University of Medical Sciences” Human Research ethics committees (HREC). The approval number for the UCT HREC was 513/2016 and approval number for IAARI HREC was IR.TUMS.IAARI.REC.1396.2066.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This study did not receive any external funding.
Authors' contributions
NW participated in Capetonian data collection, data analysis and writing the manuscript. AP aided in Capetonian data collection. MA, ZP and MRF collected the Iranian data, and SDN collected the data for KwaZulu-Natal. DH, PP and ML assisted with the editing of the first draft of the manuscript. JP supervized and coordinated the research, including data collection, and writing the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Not applicable.
References
- 1.Maurer M., Mageri M., Ansotegui I. The international WAO/EAACI guideline for the management of hereditary angioedema- the 2017 revision and update. Allergy. 2017;73:1575–1596. doi: 10.1111/all.13384. [DOI] [PubMed] [Google Scholar]
- 2.Coovadia K.M., Chothia M.-Y., Baker S.G., Peter J.G., Potter P.C. Hereditary angio-oedema in the western Cape Province, South Africa. S Afr Med J. 2018;108(4):283–290. doi: 10.7196/SAMJ.2017.v108i4.12823. [DOI] [PubMed] [Google Scholar]
- 3.Immunology, Asthma and Allergy I congress Selected abstracts of the third international congress of Immunology. Asthma and Allergy. 2017;17(1):1–96. Iran. Iran J Allergy Asthma Immunol. [Google Scholar]
- 4.Gómez-Traseira C., Pérez-Fernández E., López-Serrano M.C. Clinical pattern and acute and long-term management of hereditary angioedema due to C1-esterase inhibitor deficiency. J Investig Allergol Clin Immunol. 2015;25(5):358–364. [cited 2018, Dec 05] [PubMed] [Google Scholar]
- 5.Prematta M., Gibbs J.G., Pratt E.L., Stoughton T.R., Craig T.J. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2007;98(4):383–388. doi: 10.1016/S1081-1206(10)60886-1. [DOI] [PubMed] [Google Scholar]
- 6.Winnewisser J., Rossi M., Path P. Type I hereditary angioedema: variability of clinical presentation and course within two large kindreds. J Intern Med. 1997;241:39–46. doi: 10.1046/j.1365-2796.1997.76893000.x. [DOI] [PubMed] [Google Scholar]
- 7.Tang R., Chen S., Zhang H. Fresh frozen plasma for the treatment of hereditary angioedema acute attacks. Chin Med Sci J. 2012;27(2):92–95. [cited 2018, Dec 05] [PubMed] [Google Scholar]
- 8.Pekdemir M., Ersel M., Aksay E., Yanturali S., Akturk A., Kiyan S. Effective treatment of hereditary angioedema with fresh frozen plasma in an emergency department. J Emerg Med. 2007;33(2):137–139. doi: 10.1016/j.jemermed.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Ntshalintshali S.D., Manzini T.C., Buldeo S. Hereditary angioedema type 1 experience in KwaZulu-Natal. Current Allergy and Clinical Immunology. 2018;31(4):258–262. [Google Scholar]
- 10.National Cancern Institute . 2017. Common terminology criteria for adverse events (CTCAE) version 5.0.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf [2019 April 12]. Available from: [Google Scholar]
- 11.Zuraw B., Cicardi M., Levy R.J. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010;126(4):821–827. doi: 10.1016/j.jaci.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Lumry W.R., Farkas H., Moldovan D. Icatibant for multiple hereditary angioedema attacks across the controlled and open-label extension Phases of FAST-3. Int Arch Allergy Immunol. 2015;168(1):44–55. doi: 10.1159/000441060. [DOI] [PubMed] [Google Scholar]
- 13.Cicardi M., Levy R.J., McNeil D.L. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363(6):523–531. doi: 10.1056/NEJMoa0905079. [DOI] [PubMed] [Google Scholar]
- 16.Meza B.P., Lohrke B., Wilkinson R. Estimation of the prevalence and rate of acute transfusion reactions occurring in Windhoek, Namibia. Blood Transfus. 2014;12(3):352–361. doi: 10.2450/2013.0143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busse P., Bygum A., Edelman J. Safety of C1-esterase inhibitor in acute and prophylactic therapy of hereditary angioedema: findings from the ongoing international Berinert patient registry. J Allergy Clin Immunol Pract. 2015;3(2):213–219. doi: 10.1016/j.jaip.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Malbrán A., Riedl M., Ritchie B. Repeat treatment of acute hereditary angioedema attacks with open-label icatibant in the FAST-1 trial. Clin Exp Immunol. 2014;177(2):544–553. doi: 10.1111/cei.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baş M., Greve J., Hoffmann T.K. Repeat treatment with icatibant for multiple hereditary angioedema attacks: FAST-2 open-label study. Allergy. 2013;68(11):1452–1459. doi: 10.1111/all.12244. [DOI] [PubMed] [Google Scholar]
- 20.Lumry W.R., Li H.H., Levy R.J. Randomized placebo-controlled trial of the bradykinin B₂ receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107(6):529–537. doi: 10.1016/j.anai.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Potter P., Peter J. Life-threatening hereditary angio-oedema: challenges of care in South Africa. S Afr Med J. 2018;108(4):254–255. doi: 10.7196/SAMJ.2017.v108i4.12824. [DOI] [PubMed] [Google Scholar]
- 23.HAEi HAEi. 2018. Global Access Program [Internet]https://haei.org/hae/global_access_program/ [cited 2018, Dec 05]. Available from: [Google Scholar]
- 24.Castaldo A.J., Jervelund C., Kirk A.R. A comprehensive approach to assessing the value of prophylactic therapy for the ultra rare disease hereditary angioedema using real World patient data. J Allergy Clin Immunol. 2019;143(2) AB426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.