Abstract
Background
The natural history of allergic sensitization in childhood, and its impact on allergic disease development, needs to be clarified. This study aims to identify allergic sensitization and morbidity patterns during the first 8 years of life.
Methods
The study was conducted in the on-going population-based prospective Pollution and Asthma Risk: an Infant Study (PARIS) birth cohort. Sensitization profiles were identified by k-means clustering based upon allergen-specific IgE levels measured at 18 months and 8/9 years. Allergic morbidity profiles were identified by latent class analysis based on symptoms, symptom severity, treatments, and lifetime doctor-diagnoses of asthma, allergic rhinitis, and atopic dermatitis and on lower respiratory infections before 2 years.
Results
Five sensitization and 5 allergic morbidity patterns were established in 714 children. Children not sensitized or with isolated and low allergen-specific sensitization were grouped together (76.8%). A profile of early and transient sensitization to foods that increased the risk of asthma later in childhood was identified (4.9%). Children strongly sensitized (≥3.5 kUA/L) to house dust mite at 8/9 years (9.0%) had the highest risk of asthma and allergic rhinitis. Finally, timothy grass pollen at 8/9 years sensitization profile (5.3%) was related to respiratory allergic diseases, as was early onset and persistent sensitization profile (4.1%), this latter being also strongly associated with atopic dermatitis.
Conclusions & Clinical Relevance
We show that accurate assessment of the risk of allergic disease should rely on earliness and multiplicity of sensitization, involved allergens, and allergen-specific IgE levels, and not considering solely allergic sensitization as a dichotomous variable (allergen-specific IgE ≥0.35 kUA/L), as usually done. This is particularly striking for house dust mite. We are hopeful that, pending further confirmation in other populations, our findings will improve clinical practice as part of an approach to allergic disease prevention.
Keywords: Allergic morbidity, Birth cohort, Cluster analysis, Latent class analysis, Specific IgE levels
Abbreviations: AIC, Akaike Information Criteria; BAMSE, Stockholm Children Allergy and Environmental Prospective Birth Cohort; BIC, Bayesian Information Criteria; BMI, body mass index; COPSAC2000, Copenhagen Prospective Study on Asthma in Childhood 2000; IgE, Immunoglobulin E; ISAAC, International Study of Asthma and Allergies in Childhood; LCA, latent class analysis; LRI, lower respiratory infections; MAS, Multicenter Allergy Study; MeDALL, Mechanisms of the Development of ALLergy; OR(a), (adjusted) odds ratio; PARIS, Pollution and Asthma Risk: an Infant Study; PASTURE, Protection Against Allergy: Study in Rural Environments; SES, socio-economic status
Introduction
Prevalence of allergic diseases, namely asthma, allergic rhinitis, atopic dermatitis, and food allergy, has critically increased worldwide in childhood over the past decades.1, 2 However, allergic sensitization, biologically defined by high IgE production and involving inflammatory cells and mediators, occurs before the onset of allergic disease is clinically signalled.3, 4
The natural history of allergic sensitization is unique to each individual. Firstly, although exposure to allergens is universal, only a part of the population acquires sensitization. Secondly, sensitization can come and go over a lifetime, and particularly in infancy.5 Thirdly, sensitization can differ between subjects with regard to the number of allergen-specific sensitizations5, 6 and allergen-specific IgE levels.6, 7 Lastly, allergic sensitization can go unnoticed, with sensitized people never declaring an allergic disease.8 Unfortunately, the determinants and consequences of allergic sensitization are still little understood and therefore merit further investigation.
In response to this, several teams have recently identified allergic sensitization phenotypes using unsupervised methods.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 In these studies, early or multiple sensitizations were sometimes highly involved in allergic disease development.10, 11, 12, 13, 14, 15, 16 Allergen-specific IgE levels are, however, almost unexplored,18 which highlights the need for new insights into the understanding of allergic sensitization, the first biological step towards clinical disease.
The present study aims to identify allergic sensitization and morbidity patterns during childhood, and to assess associations between them. It was conducted as part of Pollution and Asthma Risk: an Infant Study (PARIS), a population-based prospective birth cohort especially designed to explore the development of allergic diseases.19
Methods
Study design and population
This study forms part of the PARIS follow-up until 8/9 years of age. PARIS is a French ongoing population-based prospective birth cohort.
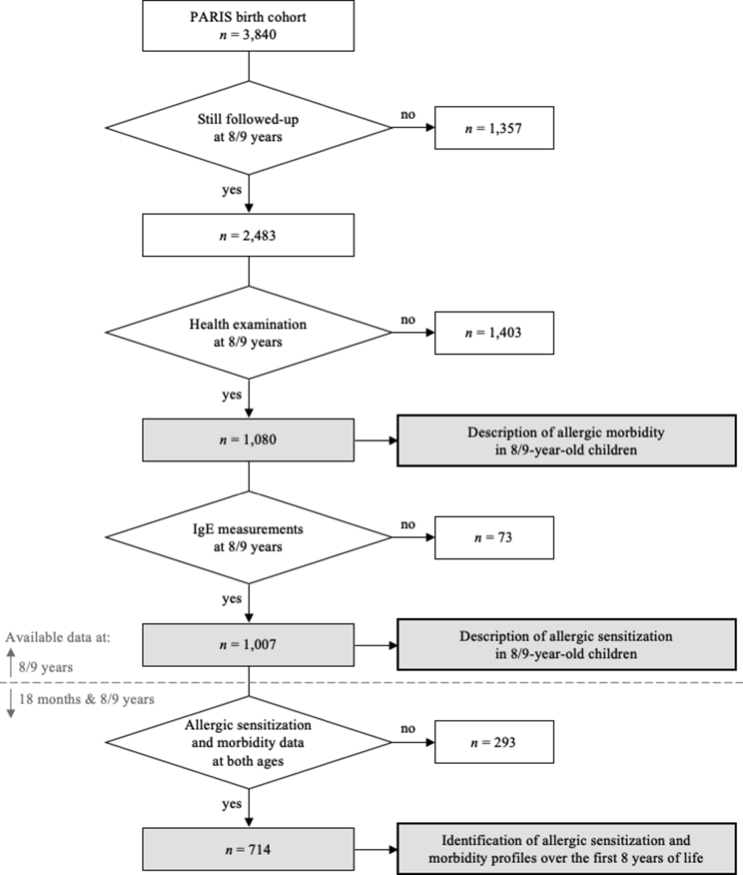
As previously described,19 the PARIS birth cohort consists of 3840 full-term and healthy singletons, born from parents living in Paris or close suburbs. These newborns were recruited soon after birth from 5 Paris maternity hospitals between 2003 and 2006. At 8/9 years of age, 2483 (64.7%) of them were still being followed-up. The present study deals with those who participated in a free health examination at 8/9 years of age, held between 2011 and 2015 (Fig. 1).
Fig. 1.
Flow chart of PARIS birth cohort children participating in the health examination when 8/9 years old
Data collection
All children were offered a biological-clinical examination at ages 18 months and 8/9 years, which took place in the Paris Health Centre of the National French Health Insurance System and in the Necker and Armand-Trousseau children's hospitals, respectively. A physician examined the child and gave a face-to-face interview to his/her parents. A blood sample was also taken from each child and analyzed for IgE measurements.
Other data on child's environment of life, lifestyle, and habits were prospectively collected by questionnaires. Gestational age, season of birth, and the number of siblings were recorded at recruitment. Breastfeeding duration and habits (exclusive or not) were prospectively documented. Trained nurses measured and weighed children at birth and at 8/9 years, and body mass index (BMI) at 8/9 years was calculated. Parental history of eczema, asthma, and allergic rhinitis was recorded at recruitment and when the child was 8/9 years old. Family socio-economic status (SES) was assessed according to the highest occupation of the two parents, and categorised as either low, intermediate, or high. As smoking exposure was documented at each point of the child's follow-up, three patterns were considered in this study: prenatal (active maternal), postnatal (anybody at home, one month after birth), and current (anybody at home, at 8/9 years). Furred pet (cat and/or dog) ownership was also prospectively documented.
Allergic sensitization
IgE were firstly analyzed in sera with ImmunoCAP Trophatop® fx26, fx27, and fx28 and Phadiatop® (Phadia, Thermo Fisher Scientific, Uppsala, Sweden). In case of positive result of Trophatop®, allergen-specific IgE towards all respective food items were measured: egg white, cow's milk, peanut, and mustard for fx26, fish, wheat, soya, and hazelnut for fx27, and sesame, shrimp, beef, and kiwi for fx28. Similarly, a positive result using Phadiatop® led to specific IgE measurement towards: 4 aeroallergens (house dust mite, cat, cocksfoot, and alternaria) and 7 aeroallergens (house dust mite, cat, dog, timothy grass, birch, alternaria, and cockroach) at ages 18 months and 8/9 years, respectively. Otherwise, in case of negative result of Trophatop® or Phadiatop® (all included allergen-specific IgE <0.35 kUA/L) or in case of allergen-specific IgE concentration below the detection limit (0.35 kUA/L for tests used at 18 months and 0.10 kUA/L for those used at 8/9 years), half of the threshold was set.
An allergen-specific IgE ≥0.35 kUA/L defined sensitization and at least two allergen-specific IgE above this threshold defined multi-sensitization.
Allergic morbidity
Health data were prospectively collected using standardized questionnaires taken from the International Study of Asthma and Allergies in Childhood (ISAAC) protocol, or from the Mechanisms of the Development of ALLergy (MeDALL) project.20 Thus, current (i.e. in the past 12 months) symptoms, symptom severity, and medication intake as well as lifetime doctor-diagnoses of atopic dermatitis, asthma, and allergic rhinitis were recorded.
Atopic dermatitis was assessed by a doctor diagnosis or an intermittent itchy rash affecting the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or face. Appropriate topical steroid treatments were considered, and the disease was regarded as severe when the itchy rash kept the child awake at night.
Asthma was evidenced by a doctor diagnosis or respiratory symptoms such as wheezing and/or tightness of chest and/or asthma attack, or medication intake such as bronchodilators, inhaled/oral steroids, or specific oral treatment improving child's breathing. Four signs of asthma severity were taken into account: wheezing severe enough to limit the child's speech to only 1 or 2 words at a time, wheezing interfering with the child's daily activities, wheezing or whistling in the chest accompanied by an attack of breathlessness, and the child's sleep disturbed due to wheezing.
Allergic rhinitis was assessed by a doctor diagnosis or sneezing or runny/blocked nose or rhino-conjunctivitis when the child did not have a cold or the flu. Appropriate nasal treatments were also considered. Allergic rhinitis severity was defined by the child's daily activity disturbance.
Furthermore, the onset of atopic dermatitis and asthma was considered to be early when the disease was diagnosed before median age of diagnosis, i.e. 2 and 3 years in the study population, respectively.
Prevalent atopic dermatitis, asthma, and allergic rhinitis were assessed according to MeDALL consensual definitions, respectively: 1) skin symptoms in the past 12 months; 2) two criteria among the triad of respiratory symptoms (wheezing and/or tightness of chest and/or asthma attack), doctor diagnosis, and medication intake; 3) nose symptoms (when the child did not have a cold or the flu) with concomitant positive Phadiatop®.
In addition, lower respiratory infections (LRI) in the first 2 years of life were prospectively documented, and whether or not the child had at least 2 episodes was noted.
Statistical analyses
All statistical analyses were performed using R software version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and Stata® version 11.2 (StataCorp, College Station, Texas).
Allergen-specific IgE levels were categorized according to the IgE distribution in sensitized children at each age. Thus, 3 sensitization classes were defined in toddlers considering the median: “no” (<0.35), “low” (0.35–1.05), and “high” (≥1.05 kUA/L). Similarly, 4 sensitization classes were defined at 8/9 years by taking into account tertiles: “no” (<0.35), “low” (0.35–3.5), “intermediate” (3.5–35), and “high” (≥35 kUA/L).
First, prevalence of allergic sensitization and morbidity at age 8/9 years was estimated.
Then, profiles of allergic sensitization and of allergic morbidity were identified over the first 8 years of life when data were available at both ages 18 months and 8/9 years.
K-means method (using “kmeans.ddR” R package) was performed to identify allergic sensitization profiles in childhood. Clustering was based on all available allergen-specific IgE levels (towards 16 and 19 allergens at ages 18 months and 8/9 years, respectively). The optimal number of clusters, in a range of 2–7 clusters, was determined according to the within groups sum of squares.
In parallel, allergic morbidity profiles in childhood were identified by latent class analysis (LCA, using “poLCA” R package) based on symptoms, symptom severity, and medication intake, all recorded during health examinations at ages 18 months and 8/9 years, and lifetime doctor-diagnoses of atopic dermatitis, asthma, and allergic rhinitis, and early LRI. Allergic morbidity models of 2–7 latent classes were assessed for best fit using bootstrap likelihood ratio test and parsimony Akaike/Bayesian Information Criteria (AIC and BIC). Mean and standard deviation of morbidity class membership probabilities, overall and in each latent class, were calculated for classification homogeneity assessment.
Finally, associations between profiles of allergic sensitization and profiles of allergic morbidity were assessed by multinomial logistic regression weighted by morbidity class membership probabilities (estimated from LCA), and adjusted for potential confounders.
Known and suspected risk factors according to the literature, as well as variables associated with profiles of allergic morbidity in univariate analyses with a p-value ≤0.20, were introduced in the multivariate model.
Results
Study population
The present study deals with the 1080 PARIS children who participated in the health examination at 8/9 years of age (Fig. 1), of whom 1007 had available allergen-specific IgE measurements at this age. Their mean age was 8.5 ± 0.6 years and the sex ratio was 1.09. Profiles of allergic sensitization and morbidity over the first 8 years of life could be identified, and relations between them assessed, in 714 children.
Participants were more likely to be born from high socio-economic status (SES) families and to be breastfed for longer (see Table E1). There was no significant difference between groups with regard to gender, older siblings, parental history of allergy, and exposure to tobacco smoking.
Allergic sensitization
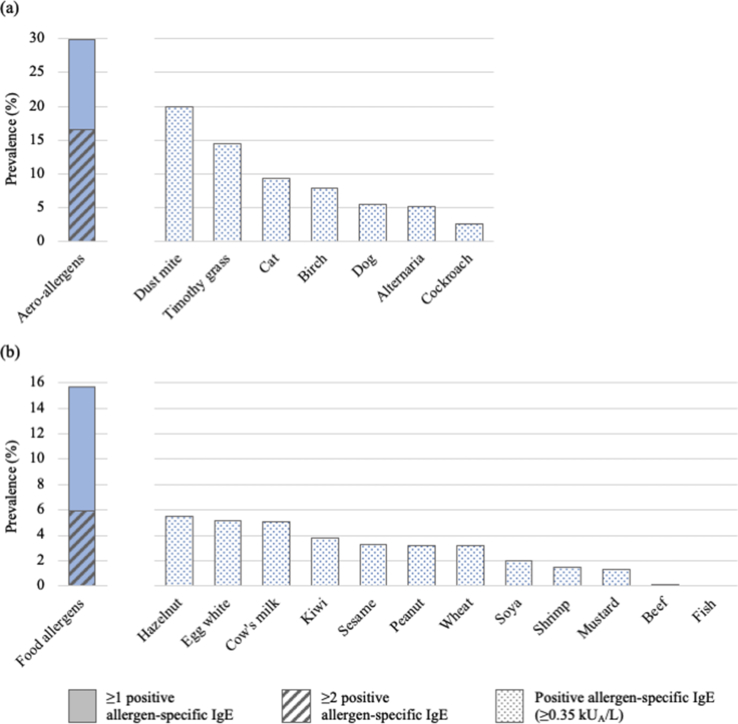
At age 8/9 years, 34.5% of children were sensitized to at least 1 allergen, and 19.8% were multi-sensitized. Sensitization to inhalant and food allergens concerned 29.9% and 15.8% of children, respectively; the most prevalent allergen-specific sensitization was towards house dust mite (19.9%; Fig. 2).
Fig. 2.
Sensitization to (a) inhalant and (b) food allergens at 8/9 years of age in PARIS birth cohort children (N = 1007)
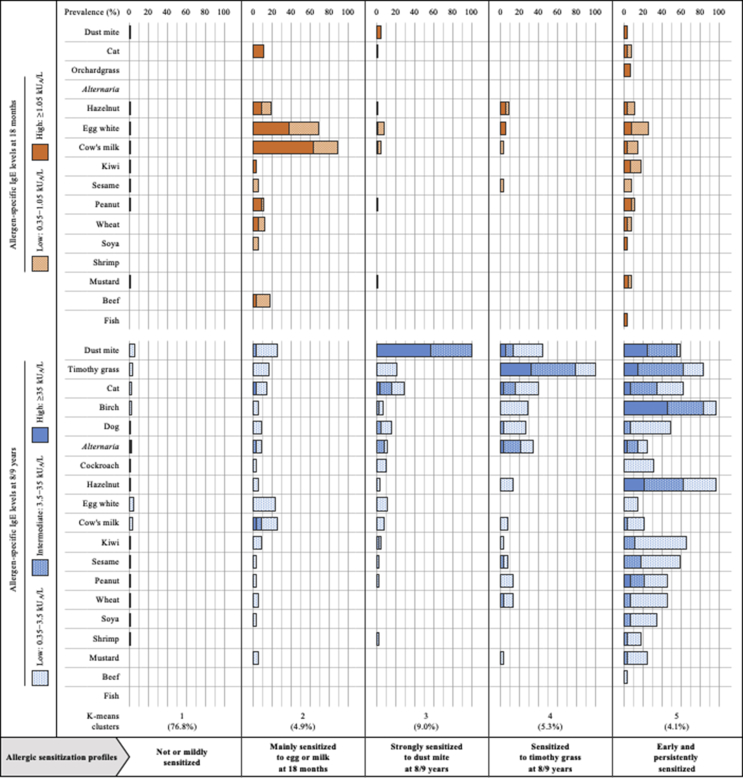
Cluster analysis led to the unsupervised identification of 5 patterns for allergic sensitization over the first 8 years of life (Fig. 3). Allergic sensitization in the first cluster (n = 548, 76.8%) was rare and low throughout childhood: only 4.1% and 16.8% of sensitized children at age 18 months and 8/9 years, respectively, with low IgE levels in more than 98% of cases. All children in the second cluster (n = 35, 4.9%) were characterized by an allergic sensitization in infancy, with high IgE levels to egg white and/or cow's milk in 88.6% of cases, that tended to disappear later in childhood. The third cluster (n = 64, 9.0%) involved children all strongly sensitized to house dust mite. Children belonging to the fourth cluster (n = 38, 5.3%) had a general sensitization to timothy grass pollen, often combined with other aeroallergens (84.2% of multi-sensitization cases). In the fifth cluster (n = 29, 4.1%), allergic sensitization was early, progressive throughout childhood, and strong towards a wide range of allergens, particularly to birch pollen (82.8%) and food allergens (79.3%) at 8/9 years of age.
Fig. 3.
Unsupervised identification of allergic sensitization profiles over the first 8 years of life in PARIS birth cohort children, identification based upon allergen-specific IgE levels at both ages 18 months and 8/9 years (N = 714)
Allergic morbidity
Fig. 4 shows, according to the considered variable (current symptoms, lifetime diagnoses, or current medication), between 24.2% and 31.7% of 8/9-year-old children were affected by an allergic disease. Taking into consideration MeDALL consensual definitions,20 27.0% and 5.2% were affected by allergic morbidity and multi-morbidity, respectively, and atopic dermatitis, asthma, and allergic rhinitis affected 12.8%, 8.6%, and 11.3% of children, respectively.
Fig. 4.
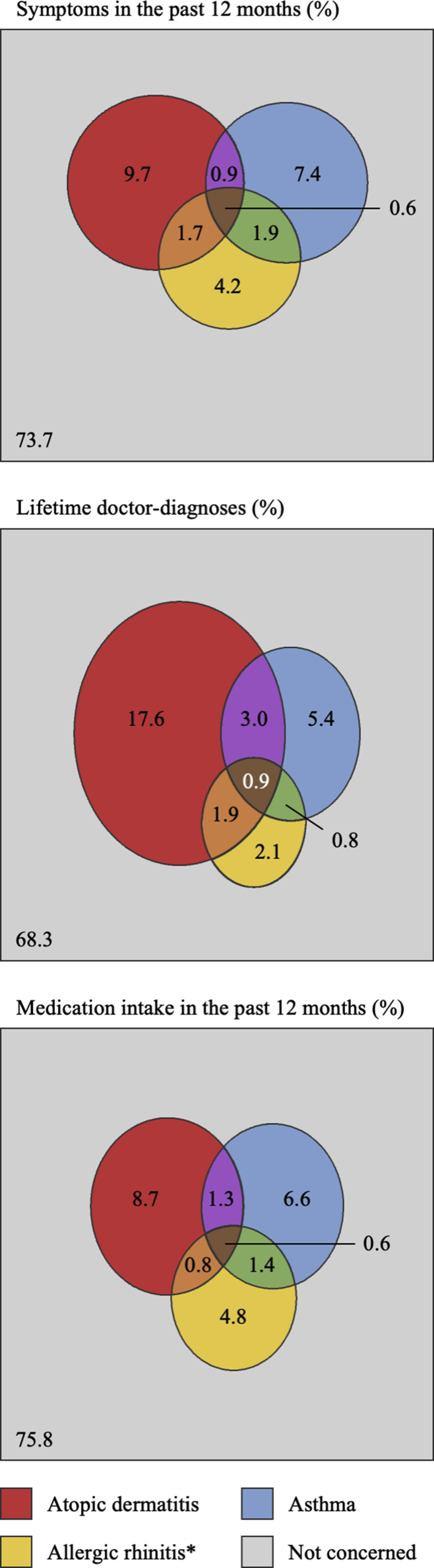
Allergic morbidity at 8/9 years of age in terms of current symptoms, lifetime doctor-diagnoses, and current medication intake, in PARIS birth cohort children (N = 1080). ∗Concerning symptoms, only when accompanied by itchy/watery eyes
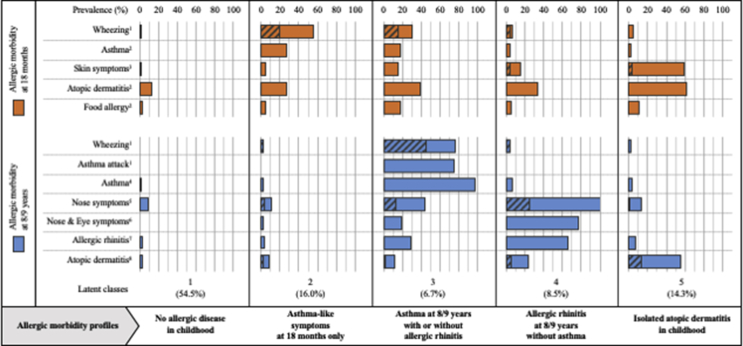
Unsupervised identification of allergic morbidity latent structure over the first 8 years of life assigned children to 5 distinct classes (Fig. 5): no allergic disease in childhood (n = 389, 54.5%), asthma-like symptoms at 18 months only (n = 114, 16.0%), asthma at 8/9 years with or without allergic rhinitis (n = 48, 6.7%), allergic rhinitis at 8/9 years without asthma (n = 61, 8.5%), and isolated atopic dermatitis in childhood (n = 102, 14.3%). Indeed, in the first class, only 1.9% and 7.4% of parents reported symptoms of atopic dermatitis, asthma, or allergic rhinitis at 18 months and 8/9 years, respectively. The second class comprised children with wheezing (55.7%, of which 35.6% were severe), frequent LRI (80.7%), who were treated for breathing difficulties (95.6%) in infancy but almost asthma-free later in childhood (only 1.8% of those concerned). The third class grouped children suffering from asthma at 8/9 years (97.8%) with wheezing reported as early as infancy for 30.4% (of which 50% were severe). At 8/9 years, 29.2% of children in this group were affected by allergic rhinitis. In the fourth class, nose symptoms were found in all children at age 8/9 years, 76.7% having eye problems. Furthermore, atopic dermatitis affected 33.9% and 24.1% of infants and children, respectively, whilst asthma was almost absent (3.6% and 6.8%, respectively). Lastly, concerning the fifth class, atopic dermatitis has been diagnosed in 91.2% of 8/9-year-old children, while asthma and allergic rhinitis lifetime doctor-diagnosis prevalence was 11.2% and 10.8%, respectively. Overall, the homogeneity of these classes was very satisfactory (see Table E2).
Fig. 5.
Unsupervised identification of allergic morbidity profiles over the first 8 years of life in PARIS birth cohort children, identification based upon symptoms, symptom severity, doctor-diagnoses, and medication intake at both ages 18 months and 8/9 years (N = 714). Crosshatched: severe symptoms. 1Occurred in the past 12 months. 2Lifetime doctor-diagnosis. 3Intermittent itchy rash affecting the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears, or face in the past 12 months. 4Epidemiologic definition: two criteria among the triad of respiratory symptoms (wheezing and/or tightness of chest and/or asthma attack) in the past 12 months, lifetime doctor diagnosis, and medication intake in the past 12 months. 5Sneezing or runny/blocked nose when the child did not have a cold or the flu in the past 12 months. 6Nose symptoms accompanied by itchy/watery eyes in the past 12 months. 7Epidemiologic definition: nose symptoms in the past 12 months with concomitant positive Phadiatop®. 8Epidemiologic definition: skin symptoms in the past 12 months
Associations between profiles
Table 1 lists statistical associations assessed between allergic sensitization profiles and allergic morbidity profiles. No sensitization profile was associated with the asthma-like symptoms at 18 months only profile. Children from the mainly to egg or milk at 18 months sensitization profile were more at risk of belonging to the asthma at 8/9 years with or without allergic rhinitis profile and not significantly, to the allergic rhinitis at 8/9 years without asthma and the isolated atopic dermatitis in childhood profiles. The timothy grass pollen at 8/9 years sensitization profile was related to the asthma and allergic rhinitis profiles, as was the early onset and persistent sensitization profile, this latter being also strongly associated with the atopic dermatitis profile. Finally, children strongly sensitized to house dust mite at 8/9 years were the most likely to suffer from asthma and allergic rhinitis at 8/9 years.
Table 1.
Associations between allergic sensitization and morbidity profiles over the first 8 years of life, assessed by a weighted multinomial logistic regression model, in PARIS birth cohort children (N = 714).
| Asthma-like symptoms at 18 months only |
Asthma at 8/9 years with or without allergic rhinitis |
Allergic rhinitis at 8/9 years without asthma |
Isolated atopic dermatitis in childhood |
|||||
|---|---|---|---|---|---|---|---|---|
| aORa (95%CI) | p-value | aORa (95%CI) | p-value | aORa (95%CI) | p-value | aORa (95%CI) | p-value | |
| Allergic sensitization profiles | ||||||||
| Not and mildly | 1 | 1 | 1 | 1 | ||||
| Mainly at 18 months | 1.47 (0.61–3.53) | 0.39 | 4.59 (1.34–15.69) | 0.015 | 3.23 (0.98–10.63) | 0.054 | 2.43 (1.00–5.92) | 0.051 |
| Strongly to house dust mite at 8/9 years | 1.64 (0.67–4.01) | 0.28 | 10.46 (4.40–24.88) | <0.001 | 19.44 (9.15–41.30) | <0.001 | 2.42 (1.10–5.31) | 0.027 |
| To timothy grass pollen at 8/9 years | 1.16 (0.39–3.42) | 0.79 | 5.84 (1.96–17.41) | 0.002 | 13.42 (5.37–33.51) | <0.001 | 1.62 (0.58–4.48) | 0.36 |
| Early and persistently | −b | −b | 5.22 (1.32–20.57) | 0.018 | 14.33 (4.84–42.46) | <0.001 | 5.11 (2.01–13.00) | 0.001 |
In bold: p-value <0.05.
OR were adjusted for maternal history of allergy, paternal history of allergy, socio-economic status, gestational age, season of birth, gender, birth weight, body mass index at 8/9 years, exclusive breastfeeding, smoking patterns (prenatal, postnatal, and/or current), and furred pet ownership.
OR could not be assessed due to a lack of subjects.
Discussion
Using data-driven methods, 5 allergic sensitization profiles and 5 allergic morbidity profiles have been established over the first 8 years of life. We clearly show that allergen-specific IgE levels deserve to be considered in addition to sensitization multiplicity and the earliness of sensitization with regard to the risk of allergic disease development. Indeed, children not sensitized or with an isolated and low allergen-specific sensitization are grouped in the same profile. In addition, we show a profile for children strongly sensitized to house dust mite with the highest risk for asthma and allergic rhinitis at age 8/9 years. We also highlight that early sensitization to egg white or cow's milk, even transient, significantly increases the risk of asthma later in childhood.
Strengths and limitations
The present study draws its strength from the 2 unsupervised classifications performed in parallel without a priori for both allergic sensitization and morbidity profile identification. Furthermore, this study is innovative in using specific IgE levels for a large panel of allergens, measured in infancy and in childhood. Our results are supported by the quality of the data, exclusively collected during the two health examinations in the prospective follow-up of the population-based PARIS birth cohort. All allergy data were validated by a pediatrician, which limited misclassification and reporting bias. Nevertheless, food allergy was not investigated at 8/9 years.
The substantial attrition rate at age 8/9 years (n = 1357/3840) might be, however, a limitation. This could be explained by the study area: Paris is the largest French urban area, where young couples often start their careers before moving away as family size increases. An exit from the study area was indeed the first cause of follow-up discontinuation in our population (53.1%). Another limitation could be the health examination participation rate at 8/9 years (n = 1080/2483), which included a blood sample that could have discouraged children. Participating children were from families with a higher SES and were breastfed for longer than non-participants. Parents with a higher SES might have been more likely to participate in longitudinal studies, and to take their child to health examinations.21 As in our population, breastfeeding duration is generally related to SES.22 In any case, SES and exclusive breastfeeding are not related to allergic sensitization in our study (data not shown). There was no other difference between participants and non-participants, especially concerning parental history of allergy. Consequently, the aforementioned differences should not greatly affect our results.
Allergic sensitization profiles in relation to allergic morbidity profiles
Firstly, it is important to emphasize that, using unsupervised clustering based on specific IgE levels, children not sensitized or with an isolated and low allergen-specific sensitization were grouped in a same profile, named here not or mildly sensitized. This pattern questions the generally used definition of allergic sensitization (at least one allergen-specific IgE ≥0.35 kUA/L).
A second profile of children, strongly sensitized to egg or milk but only at 18 months, was identified, possibly due to the high prevalence of these two allergen-specific sensitizations in infancy. In children belonging to this profile, the risk of asthma at 8/9 years with or without allergic rhinitis was significantly higher while the risk of allergic rhinitis at 8/9 years without asthma and isolated atopic dermatitis in childhood also tended to be increased. Using data-driven method, Havstad et al. (2014)10 previously reported a similar profile in toddlers, which was related to atopic dermatitis but not to asthma at 4 years. Similarly, Hose et al. (2017)18 identified early persistent food allergen sensitization trajectories in MAS (Multicenter Allergy Study) and PASTURE (Protection Against Allergy: Study in Rural Environments), which were however not associated with allergic morbidity. Nonetheless, food sensitization in infancy was related to wheezing phenotypes by an unsupervised approach in a few cohorts.23, 24 Our findings are in accordance with a meta-analysis recently performed on 13 birth cohort studies that highlights an increased risk of asthma, allergic rhinitis, and atopic dermatitis in 4-to-7-year-old children sensitized early to foods.25 Furthermore, our findings confirm a previous work in the PARIS cohort, showing that infants sensitized, mainly to foods at an early age, experienced allergic morbidity more often when 6 years old.12
Another child profile, early and persistently sensitized, was characterized by early, progressive, and multiple allergic sensitization towards a wide range of allergens, particularly birch pollen and many foods at 8/9 years of age. The identification of this profile is consistent given the high exposure to birch pollen in French non-coastal cities (http://www.pollens.fr/en/). This profile was strongly associated with increased risk of all allergic diseases, and among all sensitization profiles, this one has the strongest link with atopic dermatitis. This small profile of multi-sensitized children has already been described in several unsupervised studies,9, 10, 11, 12, 14, 15, 16, 18 and shown as the most at risk of asthma,9, 10, 14, 15, 16, 18 allergic rhinitis,16, 18 and atopic dermatitis.10, 11, 16, 18 Simpson et al. (2010)14 also showed an increased risk in hospital admissions for wheezing or asthma in children belonging to this sensitization profile. Just et al. (2014)26 identified 2 multi-sensitization profiles among asthmatic schoolchildren, and interestingly allergic morbidity and asthma severity were higher only when total IgE was increased. Our results emphasize the IgE level relevancy. Moreover, the progressive character of allergic sensitization in our profile and the fact that atopic dermatitis can precede sensitization support the dual-allergen-exposure hypothesis proposed by Lack (2008).27
Children sensitized to timothy grass pollen at 8/9 years were grouped in a fourth profile, which is associated with asthma and allergic rhinitis at age 8/9 years, but not with isolated atopic dermatitis in childhood. It can be noted that, in a previous data-driven study, the earlier the timothy grass pollen specific sensitization, the higher the risk of asthma, allergic rhinitis, atopic dermatitis, and multi-morbidity.13 Unfortunately, however, this aspect cannot be assessed in our two-point follow-up study. Our profile of children sensitized to timothy grass but not to birch pollen differs from a few data-driven studies, which sometimes report a pattern joining specific sensitizations to pollens.9, 17 Nevertheless, the identification of a profile of birch and food sensitizations – hazelnut mainly – is biologically plausible due to structure resemblance in the molecular allergen family (PR-10), known to be responsible for many cross-reactions.28
Finally, the identification of a profile grouping children strongly sensitized to house dust mite at 8/9 years makes sense given the urban character of our population. In addition, this characteristic of the families involved in PARIS cohort, who rarely owned pets (18.3% versus 32.5% on average in European birth cohorts29), could explain the absence of a profile of sensitization to animal allergens as previously described in COPSAC2000 (Copenhagen Prospective Studies on Asthma in Childhood) and BAMSE (Children, Allergy, Milieu, Stockholm, Epidemiology) cohorts.17 In our study, risks of asthma and allergic rhinitis at 8/9 years were highest in children strongly sensitized to house dust mite, which contrasts with previous unsupervised classifications. Indeed, although a profile of isolated house dust mite sensitization was identified several times with dichotomized sensitization variables (commonly, allergen-specific IgE < or ≥0.35 kUA/L), this profile was moderately9, 14, 16 or not17 linked to asthma. Taking into account allergen-specific IgE levels, children strongly sensitized to house dust mite could be distinguished from the others. To the best of our knowledge, the present study is the first to describe such a sensitization profile, which appears to be the most at risk of respiratory allergic diseases in 8/9-year-old children. Hose et al. (2017),18 the only ones who also performed a data-driven classification based on specific IgE levels, identified a house dust mite sensitization profile strongly associated to current wheeze and lifetime asthma in 6-year-old children. However, contrary to our study, this profile mixed low and strong house dust mite specific sensitizations, a discrepancy that might be explained by different IgE level categorization. Our results are consistent with the study of Custovic et al. (2015),13 which reported the highest risk of asthma and allergic multi-morbidity in the profile containing the most of house dust mite specific sensitizations among 4 trajectories identified by LCA based on 7 microarray house dust mite specific IgE measurements. In addition, Mohammad et al. (2016)30 recently showed in 3-to-8-year-old children the higher the sum of mite/cat/dog specific IgE levels, the higher the risk of current wheezing and asthma.
Overall, no sensitization profile was associated with the asthma-like symptoms at 18 months profile. Such a morbidity profile – not related to sensitization – was previously reported in other unsupervised studies that focused on wheezing in childhood.23, 24 As in our study, 90% of non-atopic wheeze disappeared after age 5 years in MAS birth cohort.31 Symptoms seemed to be caused by early life virus LRI, and therefore independent of allergy.
Conclusions
Using an unsupervised approach, we have shown how allergic sensitization follows 5 patterns during the first 8 years of life, and is influenced by specific IgE levels in both infancy and childhood. These profiles have proven to be distinctly, but not specifically, linked to allergic morbidity. Moreover, this study highlights the complexity of the allergic disease development. Aside from major contributors like involved allergens and earliness and multiplicity of sensitization, allergen-specific IgE levels should be taken into account in the allergic disease risk assessment. This is particularly striking for house dust mite. Further studies are thus needed to precisely place of these IgE levels in the different allergic morbidities. We are hopeful that, pending further confirmation in other populations, our findings will improve clinical practice as part of an approach to allergic disease prevention.
Declarations
Ethics approval and consent to participate
The French Ethics Committees approved the research protocol (permissions no. 031153, no. 051289, and ID-RCB: 2009-A00824-53) and written informed consent was obtained from all parents.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the personal and health information included in these datasets.
Funding
This survey, which was led as part of the first author's doctoral thesis (freely available here, in French: https://www.theses.fr/2017USPCB031), was supported by the Paris Municipality Social, Childhood and Health Direction (DASES). It was also funded by the French Agency for Food, Environmental and Occupational Health & Safety (Anses) as part of the PNR EST [National Research Programme in Environment-Health-Work] (grants no.2015/1/082). The free-of-charge health examinations took place in the Paris Health Centre of the National French Health Insurance System, the Paris Council Saint Marcel Centre, and the Necker and Armand-Trousseau children's hospitals (AP-HP).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SG participated in the present study design, did the statistical analyses, participated in the interpretation of data and results, and drafted the article.
FR participated in the present study design and in the interpretation of data and results, and revised the article critically for important intellectual content.
JJ participated in the present study design and in the interpretation of data and results, and revised the article critically for important intellectual content.
JdB participated in the interpretation of data and results, and revised the article critically for important intellectual content.
GL participated in the interpretation of data and results, and revised the article critically for important intellectual content.
FA participated in the interpretation of data and results, and revised the article critically for important intellectual content.
NS participated in the present study design and in the interpretation of data and results, supervised analyses and drafting, and revised the article critically for important intellectual content.
IM participated in the present study design and in the interpretation of data and results, supervised analyses and drafting, and revised the article critically for important intellectual content.
All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the Paris Municipality administrative staff, Dominique Viguier, Marianne Bijou, and Bruno Métivier, for their involvement in the PARIS birth cohort follow-up. We are thankful to the Paris Health Centre of the National French Health Insurance System, the Paris Council Saint Marcel's Centre, and the Necker and Armand-Trousseau children's hospitals that received the children for the health examinations at ages 18 months and 8/9 years. We thank the physicians, Dr Michèle Boulé, Dr Bernard Boutin, Dr Mathieu Pellan, Dr Rym Belmir, Dr Michaela Semeraro, Dr Isabelle Haegy, Dr Wajed Aljundi, Dr Candice Meyzer, Dr Eric Daireaux, Dr Anne-Marie Le Marec, and Dr Sofia Kalaboka, the nurses, Eve Thioux, Patricia Laskowsky, Dorothée Nguyen Van Suong, and Charlotte Pellerin, and the all hospital administrative staff members for their fruitful collaboration. Finally, we are grateful to all parents and children who participated in this study and for their continuous involvement in the PARIS birth cohort over the years.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100057.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Asher M.I., Stewart A.W., Wong G., Strachan D.P., García-Marcos L., Anderson H.R. Changes over time in the relationship between symptoms of asthma, rhinoconjunctivitis and eczema: a global perspective from the International Study of Asthma and Allergies in Childhood (ISAAC) Allergol Immunopathol. 2012;40:267–274. doi: 10.1016/j.aller.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.-L., Brisman J., Åberg M.A., Forslund H.B., Winkvist A., Torén K. Trends in the prevalence of asthma, rhinitis, and eczema in 15 year old adolescents over an 8 year period. Respir Med. 2014;108:701–708. doi: 10.1016/j.rmed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Arshad S.H., Tariq S.M., Matthews S., Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 Years: a whole population birth cohort study. Pediatrics. 2001;108 doi: 10.1542/peds.108.2.e33. e33–e33. [DOI] [PubMed] [Google Scholar]
- 4.Pinart M., Benet M., Annesi-Maesano I. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014;2:131–140. doi: 10.1016/S2213-2600(13)70277-7. [DOI] [PubMed] [Google Scholar]
- 5.Baatenburg de Jong A., Dikkeschei L.D., Brand P.L.P. Sensitization patterns to food and inhalant allergens in childhood: a comparison of non-sensitized, monosensitized, and polysensitized children: sensitization patterns to food and inhalant allergens. Pediatr Allergy Immunol. 2011;22:166–171. doi: 10.1111/j.1399-3038.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 6.Wickman M., Asarnoj A., Tillander H. Childhood-to-adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol. 2014;133:580–582. doi: 10.1016/j.jaci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Matricardi P.M., Bockelbrink A., Keil T. Dynamic evolution of serum immunoglobulin E to airborne allergens throughout childhood: results from the Multi-Centre Allergy Study birth cohort. Clin Exp Allergy. 2009;39:1551–1557. doi: 10.1111/j.1365-2222.2009.03348.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J., Anto J.M., Akdis M. Paving the way of systems biology and precision medicine in allergic diseases: the MeDALL success story. Allergy. 2016;71:1513–1525. doi: 10.1111/all.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson A., Lazic N., Belgrave D.C.M. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol. 2015;136:1224–1231. doi: 10.1016/j.jaci.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havstad S., Johnson C.C., Kim H. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.01.022. 722–727.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amat F., Saint-Pierre P., Bourrat E. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? Results from the ORCA cohort. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131369. e0131369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabet S., Just J., Couderc R., Bousquet J., Seta N., Momas I. Early polysensitization is associated with allergic multimorbidity in PARIS birth cohort infants. Pediatr Allergy Immunol. 2016;27:831–837. doi: 10.1111/pai.12622. [DOI] [PubMed] [Google Scholar]
- 13.Custovic A., Sonntag H.-J., Buchan I.E., Belgrave D., Simpson A., Prosperi M.C.F. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136 doi: 10.1016/j.jaci.2015.03.041. 1645–1652.e8. [DOI] [PubMed] [Google Scholar]
- 14.Simpson A., Tan V.Y.F., Winn J. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 15.Lazic N., Roberts G., Custovic A. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68:764–770. doi: 10.1111/all.12134. [DOI] [PubMed] [Google Scholar]
- 16.Garden F.L., Simpson J.M., Marks G.B. Caps Investigators. Atopy phenotypes in the Childhood Asthma Prevention Study (CAPS) cohort and the relationship with allergic disease. Clin Exp Allergy. 2013;43:633–641. doi: 10.1111/cea.12095. [DOI] [PubMed] [Google Scholar]
- 17.Schoos A.-M.M., Chawes B.L., Melén E. Sensitization trajectories in childhood revealed by using a cluster analysis. J Allergy Clin Immunol. March 2017 doi: 10.1016/j.jaci.2017.01.041. Published Online First. [DOI] [PubMed] [Google Scholar]
- 18.Hose A.J., Depner M., Illi S. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. 2017;139 doi: 10.1016/j.jaci.2016.08.046. 1935–1945.e12. [DOI] [PubMed] [Google Scholar]
- 19.Clarisse B., Nikasinovic L., Poinsard R., Just J., Momas I. The Paris prospective birth cohort study: which design and who participates? Eur J Epidemiol. 2007;22:203–210. doi: 10.1007/s10654-007-9109-2. [DOI] [PubMed] [Google Scholar]
- 20.Hohmann C., Pinart M., Tischer C. The development of the MeDALL core questionnaires for a harmonized follow-up assessment of eleven European birth cohorts on asthma and Allergies. Int Arch Allergy Immunol. 2014;163:215–224. doi: 10.1159/000357732. [DOI] [PubMed] [Google Scholar]
- 21.Guxens M., Ballester F., Espada M. Cohort profile: the INMA - INfancia y medio ambiente - (environment and childhood) project. Int J Epidemiol. 2012;41:930–940. doi: 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- 22.Logan C., Zittel T., Striebel S. Changing societal and lifestyle factors and breastfeeding patterns over time. Pediatrics. 2016;137 doi: 10.1542/peds.2015-4473. e20154473–e20154473. [DOI] [PubMed] [Google Scholar]
- 23.Lodge C.J., Zaloumis S., Lowe A.J. Early-life risk factors for childhood wheeze phenotypes in a high-risk birth cohort. J Pediatr. 2014;164 doi: 10.1016/j.jpeds.2013.09.056. 289–294.e2. [DOI] [PubMed] [Google Scholar]
- 24.Savenije O.E., Granell R., Caudri D. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127 doi: 10.1016/j.jaci.2011.02.002. 1505–1512.e14. [DOI] [PubMed] [Google Scholar]
- 25.Alduraywish S.A., Lodge C.J., Campbell B. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 26.Just J., Saint-Pierre P., Gouvis-Echraghi R. Childhood allergic asthma is not a single phenotype. J Pediatr. 2014;164:815–820. doi: 10.1016/j.jpeds.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331–1336. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Hirschwehr R., Valenta R., Ebner C. Identification of common allergenic structures in hazel pollen and hazelnuts: a possible explanation for sensitivity to hazelnuts in patients allergic to tree pollen. J Allergy Clin Immunol. 1992;90:927–936. doi: 10.1016/0091-6749(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 29.Lødrup Carlsen K.C., Roll S., Carlsen K.-H. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043214. e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad H.R., Belgrave D., Kopec Harding K., Murray C.S., Simpson A., Custovic A. Age, sex and the association between skin test responses and IgE titres with asthma. Pediatr Allergy Immunol. 2016;27:313–319. doi: 10.1111/pai.12534. [DOI] [PubMed] [Google Scholar]
- 31.Illi S., von Mutius E., Lau S. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the personal and health information included in these datasets.