To the Editor:
Immunoglobulin E (IgE)-mediated allergy refers to the adverse reaction in some patients caused by the crosslinking of the high-affinity IgE receptor (FcεRI) on basophils and mast cells by allergen-specific IgEs. Once the contact with allergens is established, immunomodulatory therapeutic agents to prevent the onset of allergic symptoms are currently quite limited. Blocking the interaction of specific IgE with the FcεRI that is present in basophils and mast cells is an important target in anti-allergic therapy. Clinical responses have been observed after the administration of omalizumab, a humanized anti-IgE antibody, to patients with food allergy, which correlated with the suppression of degranulation of basophils.1, 2 These results indicate that actions aimed to blocking or modulating the IgE/FcεRI axis represent a promising strategy for the treatment of allergy and anaphylaxis. Therefore, in order to develop new immunomodulatory therapies, it is very important to characterize cell surface receptors capable of modulating IgE-mediated activation threshold in basophils and mast cells.3
The human CD300 family of receptors consists of 8 receptors expressed in both myeloid and lymphoid lineages.4 It has been recently demonstrated that the phosphatidylserine and phosphatidylethanolamine binding CD300c receptor acts as a co-stimulatory molecule during basophil activation through the IgE/FcεRI axis. CD300c cross-linking significantly augmented IgE-mediated basophils degranulation and cytokine production in a process involving increased calcium mobilization and phosphorylation of signaling intermediates such as protein tyrosine kinase (Syk) and extracellular signal–regulated kinases (ERK). Moreover, it was observed that basal expression levels of CD300c on basophils from IgE-dependent cow's milk allergic children are higher than those from healthy control children, suggesting that CD300c could be used as a biomarker in the diagnosis of the IgE-dependent allergic pathology.5
In order to further assess the clinical relevance of those findings, we have studied the expression of CD300c on basophils from patients with two IgE-dependent allergies. Clinical features from patients are shown in Table 1. We collected peripheral blood samples from 3 different cohorts: 1) adults with dust mites allergy (n = 36), 2) adults with grass pollen allergy (n = 22), and 3) non-allergic control individuals (n = 26).
Table 1.
Clinical data of allergic individuals
| Patient | Gender | Age (years) | Symptoms | Severity | Total IgE (kU/L) | IgE Der p (kU/L) | IgE Phl pe (kU/L) |
|---|---|---|---|---|---|---|---|
| HCAC_001 | Female | 60 | ASTHMA + RHINITIS | Mild | 3526 | >100 | – |
| HCAC_002 | Male | 19 | ASTHMA + RHINITIS | Mild | 215 | 27.6 | – |
| HCAC_003 | Male | 34 | ASTHMA + RHINITIS | Severe | 168 | 18.4 | – |
| HCAC_004 | Female | 15 | ASTHMA + RHINITIS | Moderate | 1012 | >100 | – |
| HCAC_005 | Female | 43 | RHINITIS | Mild | 133 | 39.8 | – |
| HCAC_006 | Male | 20 | RHINITIS | Mild | 526 | >100 | – |
| HCAC_007 | Female | 31 | ASTHMA + RHINITIS | Mild | 254 | 39.2 | – |
| HCAC_008 | Male | 28 | ASTHMA + RHINITIS | Mild | 618 | >100 | – |
| HCAC_009 | Female | 36 | RHINITIS | Mild | 175 | 18.5 | – |
| HCAC_010 | Female | 20 | ASTHMA + RHINITIS | Moderate | 362 | 81.8 | – |
| HCAC_011 | Female | 31 | ASTHMA + RHINITIS | Mild | 52 | nd | – |
| HCAC_012 | Male | 41 | ASTHMA + RHINITIS | Severe | 168 | 4.46 | – |
| HCAC_013 | Female | 40 | ASTHMA + RHINITIS | Mild | 1247 | >100 | – |
| HCAC_014 | Female | 36 | ASTHMA + RHINITIS | Severe | 416 | 59.2 | – |
| HCAC_015 | Female | 78 | ASTHMA + RHINITIS | Mild | 384 | 17.3 | – |
| HCAC_016 | Female | 26 | ASTHMA + RHINITIS | Mild | 445 | 49.6 | – |
| HCAC_017 | Female | 78 | ASTHMA + RHINITIS | Mild | 361 | 5.47 | – |
| HCAC_018 | Female | 46 | RHINITIS | Moderate | 199 | 11.6 | – |
| HCAC_019 | Female | 15 | RHINITIS | Mild | ND | 80.1 | – |
| HCAC_020 | Female | 25 | ASTHMA + RHINITIS | Mild | 666 | nd | – |
| HCAC_021 | Female | 41 | ASTHMA + RHINITIS | Mild | 260 | 40.8 | – |
| HCAC_022 | Male | 18 | ASTHMA + RHINITIS | Mild | 213 | 27.3 | – |
| HCAC_023 | Female | 32 | ASTHMA + RHINITIS | Mild | 458 | 34.8 | – |
| HCAC_024 | Female | 20 | RHINITIS | Mild | 37 | 10.6 | – |
| HCAC_025 | Female | 49 | ASTHMA + RHINITIS | Mild | 18 | 4.46 | – |
| HCAC_026 | Female | 23 | RHINITIS | Mild | 38 | 1.76 | – |
| HCAC_027 | Male | 27 | RHINITIS | Mild | 44 | 13.4 | – |
| HCAC_028 | Female | 31 | RHINITIS | Mild | 67 | 8.02 | – |
| HCACGR_001 | Male | 20 | RHINITIS | Mild | 782 | 56.7 | – |
| HCACGR_002 | Female | 15 | ASTHMA + RHINITIS | Mild | 449 | 69.2 | – |
| HCACGR_003 | Male | 17 | RHINITIS | Mild | 721 | 44.4 | – |
| HCACGR_004 | Female | 16 | ASTHMA + RHINITIS | Mild | 2882 | >100 | – |
| HCACGR_006 | Male | 21 | ASTHMA + RHINITIS | Moderate | 1553 | >100 | – |
| HCACGR_007 | Female | 30 | ASTHMA + RHINITIS | Mild | 691 | 35.8 | – |
| HCACGR_008 | Female | 47 | RHINITIS | Mild | 481 | 7.2 | – |
| HCACGR_009 | Female | 29 | RHINITIS | Mild | 302 | nd | 12.6 |
| HCACGR_010 | Female | 45 | ASTHMA + RHINITIS | Mild | 301 | 39.8 | – |
| HCGR_001 | Female | 36 | RHINITIS | Mild | 493 | – | 73.2 |
| HCGR_002 | Male | 44 | RHINITIS | Mild | 14 | – | 1.23 |
| HCGR_003 | Male | 28 | ASTHMA + RHINITIS | Mild | 324 | – | 51.8 |
| HCGR_004 | Female | 34 | RHINITIS | Mild | 1237 | – | 15.9 |
| HCGR_005 | Male | 28 | RHINITIS | Mild | 924 | – | 28.4 |
| HCGR_006 | Female | 37 | RHINITIS | Mild | Nd | – | Nd |
| HCGR_007 | Male | 31 | RHINITIS | Mild | 17,3 | – | 5.35 |
| HCGR_008 | Male | 43 | RHINITIS | Mild | 148 | – | 2.54 |
| HCGR_009 | Female | 30 | ASTHMA + RHINITIS | Mild | 228 | – | 12.5 |
| HCGR_010 | Female | 56 | ASTHMA + RHINITIS | Mild | 11,01 | – | 2.1 |
| HCGR_011 | Female | 11 | RHINITIS | Mild | 140 | – | 26.8 |
| HCGR_012 | Female | 24 | ASTHMA + RHINITIS | Mild | 988 | – | 89.6 |
| HCGR_013 | Male | 46 | RHINITIS | Mild | 242 | – | 29 |
| HCGR_014 | Female | 36 | RHINITIS | Mild | 374 | – | 23.6 |
| HCGR_015 | Male | 48 | RHINITIS | Mild | 107 | – | 21.2 |
| HCGR_016 | Female | 54 | RHINITIS | Mild | 44,6 | – | 7.32 |
| HCGR_017 | Male | 44 | RHINITIS | Mild | 34,7 | – | 6.04 |
| HCGR_018 | Male | 25 | RHINITIS | Mild | 136 | – | 16.3 |
| HCGR_020 | Male | 57 | RHINITIS | Mild | 409 | – | 57.3 |
| HCGR_021 | Female | 16 | RHINITIS | Mild | 436 | – | >100 |
| HCGR_022 | Male | 33 | RHINITIS | Mild | 194 | – | 72.2 |
Dr p: Dermatophagoides pteronyssinus; Phl p: Phleum pratense
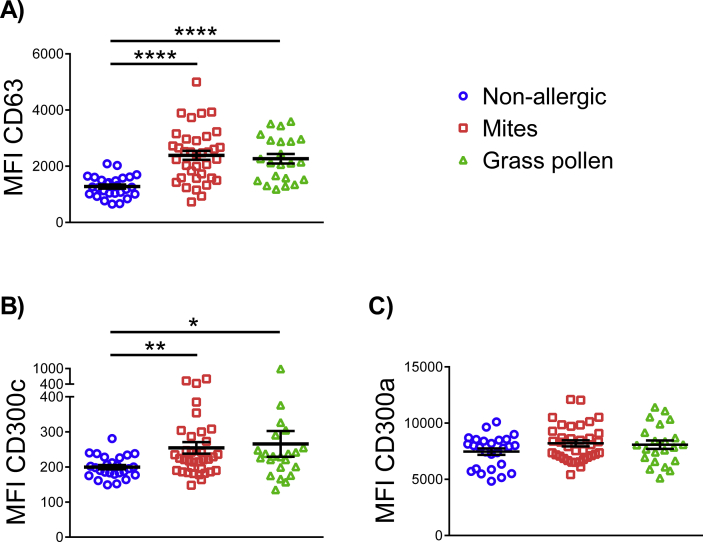
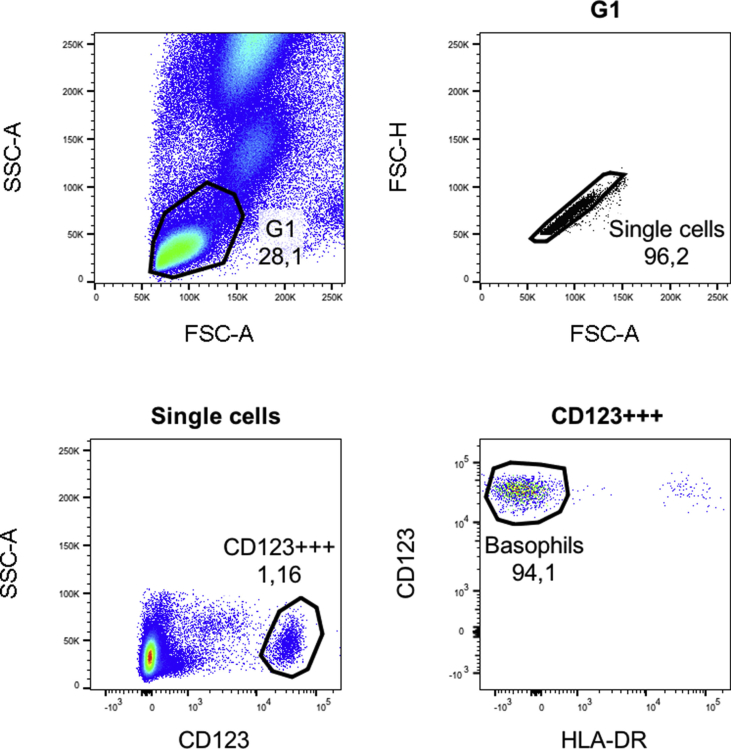
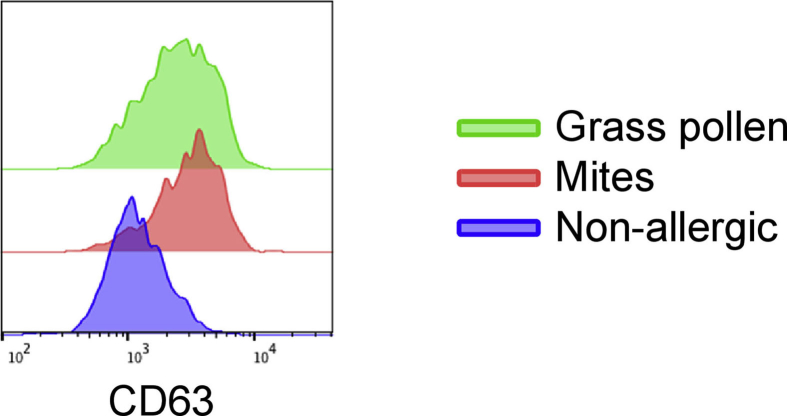
To identify basophils among the peripheral blood mononuclear cells (PBMCs), we have used a staining strategy based on the expression of the surface receptor CD123 and the absence of human leukocyte antigen - DR isotype (HLA-DR) (shown in the article's online supplementary Figure S1). First, we analyzed the expression of CD63, a basophil activation marker which is rapidly mobilized to the cell surface by polyclonal anti-IgE and allergens, as well as other degranulation stimuli.6 In agreement with previous publications,7 we observed that subjects with dust mites and/or grass pollen allergy show a significant increase in the median fluorescence intensity (MFI) of CD63 compared with basophils from non-allergic subjects, indicating that they are activated in vivo (Fig. 1A and online supplementary Figure S2). We cannot confirm that the increased expression on CD63 is exclusively due to a higher expression on basophils, and that other possibilities, as for example a greater adhesion of platelets, have some role in it. However, in several clinical studies it has been demonstrated that the presence of platelets in the cluster of CD63-positive basophils is minor.8, 9, 10
Fig. 1.
Basophils from allergic individuals exhibit greater expression of CD63 and CD300c. Dot graph showing basal median fluorescence intensity (MFI) of CD63 (A), CD300c (B) and CD300a (C) on basophils from non-allergic (blue), allergic to dust mites (red) and allergic to grass pollen (green) individuals. Each dot represents a donor, means ± SEMs are shown. *P < .05, **P < .01, ****P < .0001
Next, we analyzed cell surface expression levels of the inhibitory and activating CD300a and CD300c receptors, respectively. We observed that surface CD300c expression levels are significantly higher in basophils from individuals with an IgE-dependent allergy (dust mites and grass pollen) than in those from non-allergic individuals (Fig. 1B). This increased expression of CD300c could diminish basophils FcεRI-mediated activation threshold.5 It has been previously described that the basal expression of the CD300a inhibitory receptor is lower in basophils from patients with birch pollen allergy than in basophils from healthy control subjects.11 However, we have not seen significant differences between healthy and allergic individuals related to CD300a expression (Fig. 1C), which is in agreement with previous results in cow's milk allergic children.5
We performed correlation analysis between the intensity of CD300c and CD63 expression, but we did not find any significant result (data not shown). As it has been mentioned before, an association between the severity of the hypersensitivity symptoms and the levels of CD300c expression on basophils in cow's milk allergic children has been described.5 However, in this study the vast majority of the recruited patients (88%) were classified to have mild symptomatology (Table 1), and therefore it was not adequate to perform this analysis.
Unlike mite allergy, grass pollen allergy is a seasonal affection, and the exposure of patients to the allergen varies throughout the year. Although the great majority of samples have been collected during the grass season, we have two samples of the same individual collected at different times of the year, one in grass season and the other not. We observed that the expression of CD300c is higher during the grass pollen season than out of the season (MFI 492 vs 376, respectively). This may suggest that the exposure of allergic patients to higher amounts of allergen could induce an up-regulation of basophil activating receptors such as CD300c. The specific mechanism regulating CD300c expression in these allergic patients is still unknown and deserves further studies.
To date, the only tested stimulus able to upregulate CD300c expression is IL-3.5 This is a very important cytokine for the development, maturation, and survival of basophils.12 This cytokine is known to markedly increase the activation and release of mediators from basophils in IgE-dependent responses,12 and the autocrine priming with IL-3 has been described as an important mechanism behind the hyper-reactive nature of basophils in the allergic disease.13 We analyzed IL-3 in plasma from allergic subjects, but the levels of this cytokine were mostly undetectable. It is possible that the mild symptoms exhibited by the majority of patients may be related to the observed results. Based on our data, we propose that baseline expression levels of CD300c, together with CD63 expression, on human basophils could be helpful for the diagnosis of IgE-dependent allergies. Furthermore, considering that CD300c is capable of modulating the threshold of IgE-mediated activation in human basophils,5 we believe that, as previously demonstrated,5 an increased expression of CD300c decreases the IgE-dependent activation threshold of basophils in allergic individuals.
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication and ethics approval
Blood samples from healthy donors and allergic patients were collected through the Basque Biobank (http://www.biobancovasco.org). The Basque Biobank complies with the quality management, traceability, and biosecurity set out in the Spanish Law 14/2007 of Biomedical Research and the Royal Decree 1716/2011. All subjects provided written and signed informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Basque Ethics Committee for Clinical Research (PI2015182; 15–56; version 3; March 23, 2017).
Acknowledgements
The authors thank all of the patients and healthy donors who participated in the study and the staff of the “Hospital Universitario Cruces”, in Barakaldo and the “Centro Vasco de Transfusión y Tejidos Humanos”, in Galdakao, who cared for the patients and donors. The authors also thank the “Biobanco Vasco” for samples processing and collection. This study was supported by a grant from “Instituto de Salud Carlos III” through the project PI16/01223 (co-funded by European Regional Development Fund “A way to make Europe”). Joana Vitallé is recipient of a predoctoral contract funded by the Department of Education, Basque Government (PRE_2018_2_0211). Iñigo Terrén is recipient of two fellowships from the Jesús de Gangoiti Barrera Foundation (FJGB17/003 and FJGB18/002) and a predoctoral contract funded by the Department of Education, Basque Government (PRE_2018_1_0032). Olatz Zenarruzabeitia is recipient of a postdoctoral contract funded by “Instituto de Salud Carlos III-Contratos Sara Borrell 2017 (CD17/00128)” and the European Social Fund (ESF)-The ESF invests in your future. Francisco Borrego is an Ikerbasque Research Professor, Ikerbasque, Basque Foundation for Science.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100060.
Contributor Information
Joana Vitallé, Email: joana.vitalleandrade@osakidetza.eus.
Iñigo Terrén, Email: inigo.terrenmartinez@osakidetza.eus.
Ane Orrantia, Email: ane.orrantiarobles@osakidetza.eus.
Aritza Segurola, Email: aritza.segurolaazkarate@osakidetza.eus.
Yolanda Seras, Email: yolanda.serasmiera@osakidetza.eus.
Pedro M. Gamboa, Email: pedromanuel.gamboasetien@osakidetza.eus.
Francisco Borrego, Email: francisco.borregorabasco@osakidetza.eus.
Olatz Zenarruzabeitia, Email: olatz.zenarruzabeitiabelaustegui@osakidetza.eus.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Fig. S2.
References
- 1.Lieberman J.A., Chehade M. Use of omalizumab in the treatment of food allergy and anaphylaxis. Curr Allergy Asthma Rep. 2013;13:78–84. doi: 10.1007/s11882-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 2.Frischmeyer-Guerrerio P.A., Masilamani M., Gu W. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2017;140:1043–1053. doi: 10.1016/j.jaci.2017.03.028. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvima I.T., Levi-Schaffer F., Draber P. Molecular targets on mast cells and basophils for novel therapies. J Allergy Clin Immunol. 2014;134:530–544. doi: 10.1016/j.jaci.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood. 2013;121:1951–1960. doi: 10.1182/blood-2012-09-435057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zenarruzabeitia O., Vitallé J., Terrén I. CD300c costimulates IgE-mediated basophil activation, and its expression is increased in patients with cow's milk allergy. J Allergy Clin Immunol. 2019;143:700–711. doi: 10.1016/j.jaci.2018.05.022. e5. [DOI] [PubMed] [Google Scholar]
- 6.Gober L.M., Eckman J.A., Sterba P.M. Expression of activation markers on basophils in a controlled model of anaphylaxis. J Allergy Clin Immunol. 2007;119:1181–1188. doi: 10.1016/j.jaci.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Vasagar K., Vonakis B.M., Gober L.M., Viksman A., Gibbons S.P., Saini S.S. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin Exp Allergy. 2006;36:770–776. doi: 10.1111/j.1365-2222.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- 8.Knol E.F., Kuijpers T.W., Mul F.P., Roos D. Stimulation of human basophils results in homotypic aggregation. A response independent of degranulation. J Immunol. 1993;151:4926–4933. [PubMed] [Google Scholar]
- 9.Shreffler W.G. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006;6:226–233. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 10.Sainte-Laudy J. [Application of flow cytometry to the analysis of activation of human basophils. Immunologic validation of the method] Allerg Immunol (Paris) 1998;30:41–43. [PubMed] [Google Scholar]
- 11.Sabato V., Verweij M.M., Bridts C.H. CD300a is expressed on human basophils and seems to inhibit IgE/FcεRI-dependent anaphylactic degranulation. Cytom B Clin Cytom. 2012;82B:132–138. doi: 10.1002/cyto.b.21003. [DOI] [PubMed] [Google Scholar]
- 12.Valent P., Dahinden C.A. Role of interleukins in the regulation of basophil development and secretion. Curr Opin Hematol. 2010;17:60–66. doi: 10.1097/MOH.0b013e328331fae9. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder J.T., Chichester K.L., Bieneman A.P. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–2438. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.