Abstract
Aim
Philips recently integrated PlanIQ with Autoplan® in Pinnacle3 TPS (V16.2). The objective of the present work is to quantitatively demonstrate how this integration improves the plan quality.
Background
Pinnacle3 Autoplan® is the tool that generates the treatment plans with clinically acceptable plan quality with less manual intervention. In the recent past, a new tool called PlanIQ (Sun Nuclear Corp.) was introduced for a priori estimation of the best possible sparing of an organ at risk (OAR) for a given patient anatomy. Philips has recently integrated PlanIQ tool with Autoplan® for a seamless and efficient planning workflow.
Materials and methods
We have performed this evaluation in Pinnacle3 TPS (V.16.2) for the VMAT treatment technique. All plans were created using Varian True beam machine with the dual arc technique. Basically, we created two sets of VMAT plans using 6 MV photons. In the first set of VMAT plans (AP_RTOG), we used OAR goals from either RTOG guidelines to perform optimization using Autoplan®. Subsequently, we exported the same dataset to the PlanIQ system to perform feasibility analysis on the OAR goals. These newly obtained OAR goals from PlanIQ were used to generate the other set of plans (AP_PlanIQ plans). We compared the dosimetric results from these two sets of plans in five cases, such as brain, head & neck, lung, abdomen and prostate.
Results
We compared the dosimetric results for AP_RTOG and AP_PlanIQ plans. We used RTOG guidelines to evaluate the plans and observed that while both sets of plans were meeting the RTOG guidelines in terms of OAR sparing, the AP_PlanIQ plans were significantly better in terms of OAR sparing as compared to AP_RTOG plans without any compromise in the target coverage.
Conclusion
The results indicate that, although Autoplan helps achieve the user-defined goals without much manual intervention, the plan quality (OAR sparing) can be significantly improved without taking many iterative steps when PlanIQ suggested clinical goals are used in the Autoplan-based optimization.
Advances in knowledge
At present, there are no published material available about the efficacy of the integration of PlanIQ with Autoplanning®. In the present work, our objective is to evaluate the improvements in plan quality resulting from this integration.
Keywords: IMRT, Autoplan®, PlanIQ, Priori-Estimation of objectives, Optimization, VMAT
1. Background
IMRT has become an established method for treating cancer with ionizing radiation. The process that is central to IMRT is the optimization of beam parameters that yields the best possible treatment plans under given clinical and technical conditions. In the current practice of IMRT planning, there are essentially two main limitations. The first limitation is that IMRT planning requires a considerable manual effort from the planner to drive the optimizer towards an acceptable solution. These efforts mainly include (a) defining the target and OAR goals, (b) tweaking the defined goals and their importance weights and (c) creating “dummy structures” to improve target dose uniformity, OAR sparing and control dose spillage. Due to this limitation, the final plan quality varies according to the expertise of the planners.1 Many researchers have investigated algorithmic methods to drive the optimizer automatically to meet the specified objectives in order to make the IMRT optimization process less dependent on planners.2, 3, 4, 5, 6, 7, 8 In the past, Philips introduced a tool called Autoplan® in Pinnacle TPS with the same intent. Autoplan® is the tool that generates the treatment plans with clinically acceptable plan quality with less manual intervention. In Autoplan®, one can design the treatment technique (generally known as a treatment template) which includes definition of beam parameters and planning goals for OAR(s) and target(s). Autoplan® uses the template definition to create the optimal treatment plan in an iterative manner. The template can be created using standard protocols (RTOG/QUANTEC) or departmental protocols including weights and compromise between target coverage and dose to OAR. However, the quality of the treatment plan created by Autoplan® still depend on user inputs.9 The clinical validation of Autoplan® can be found elsewhere.10, 11
The second limitation in IMRT planning is that the planner may not be sure if the defined clinical objectives could be achieved by the optimizer. In many situations, the defined clinical objective goes unachieved by the optimizer. However, this realization happens only after performing one or many optimizations. This leads to several backtracking steps and, hence, the process becomes ineffective and time consuming. Many researchers have explored ways for predicting achievable dose levels for clinical objectives before invoking the actual optimization.12, 13, 14, 15, 16 Recently a new tool called PlanIQ (Sun Nuclear Corp., Melbourne, FL, USA) was introduced for a priori estimation of the best possible sparing (Feasibility DVH, or FDVH) of an organ at risk (OAR). A priori estimation of the ideal achievable goals based on each patient's unique anatomy can lead to better plan quality without spending much time.17 This prior knowledge about achievable goals could be used as inputs for the optimization to avoid pursuing impossible ones. This approach can help generate plans with superior quality without spending much time in tweaking the goals manually to cater to the anatomy of the given patient. Basically, PlanIQ uses a benchmark 3D dose built outside the target, which is computed using a series of energy-specific dose spread calculations. For the patient, the calculation is performed on the heterogeneous dataset, taking into account the high- (penumbra driven) and low- (PDD and scatter-driven) gradient dose spreading.17 This benchmark dose is used to produce the “best possible sparing” FDVH for an OAR, and based on it, progressively more easily achievable FDVH curves can be estimated.17 The accuracy of PlanIQ tool has been established and validated.1
In addition, as recommended by APEx® and ACR accreditation standards,18, 19 there is a desire to create personalized objectives based on the actual anatomy of the patient. Considering these clinical needs, Philips has recently integrated PlanIQ with Autoplan® for a seamless and efficient workflow. At present, there are no published material available about the efficacy of the integration of PlanIQ with Autoplan®. In the present work, our objective is to evaluate the improvements in plan quality resulting from this integration. We used various anatomic sites in this evaluation such as the prostate, H&N, Lung, abdomen and brain.
2. Aim
Philips recently integrated PlanIQ with Autoplan® in Pinnacle3 TPS (V16.2). The objective of the present work is to quantitatively demonstrate how this integration improves the plan quality.
3. Materials and methods
We performed this evaluation in Pinnacle3 TPS (Version 16.2) for the Volumetric Modulated Arc Therapy (VMAT) treatment technique. All plans were created using Varian True beam machine with the dual arc technique. Basically, we created two sets of VMAT plans using 6 MV photon beams. In the first set of VMAT plans, we used commonly used OAR goals from either RTOG or QUANTEC guidelines and performed the optimization using Autoplan®. Subsequently, we exported the same dataset to PlanIQ system to perform feasibility analysis on the OAR goals. Basically, PlanIQ assumes a 100% target coverage and then computes the feasible DVH lines (FDVH) for each OAR. By using the FDVH lines, planners can determine a minimum achievable dose for each OAR. The details of how PlanIQ generates FDVH can be found elsewhere.17 These newly obtained OAR goals from PlanIQ were used in Autoplan® to generate the second set of VMAT plans (hereafter termed as AP_PlanIQ plans). We performed the study in different anatomic sites (one case per anatomy), such as the brain, head & neck, lung, abdomen and prostate.
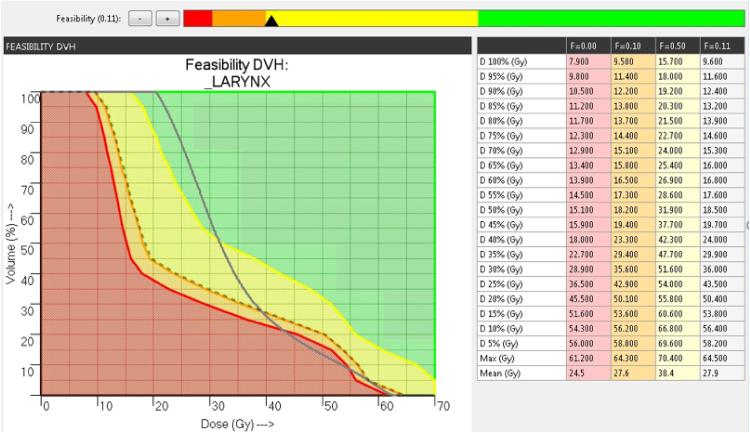
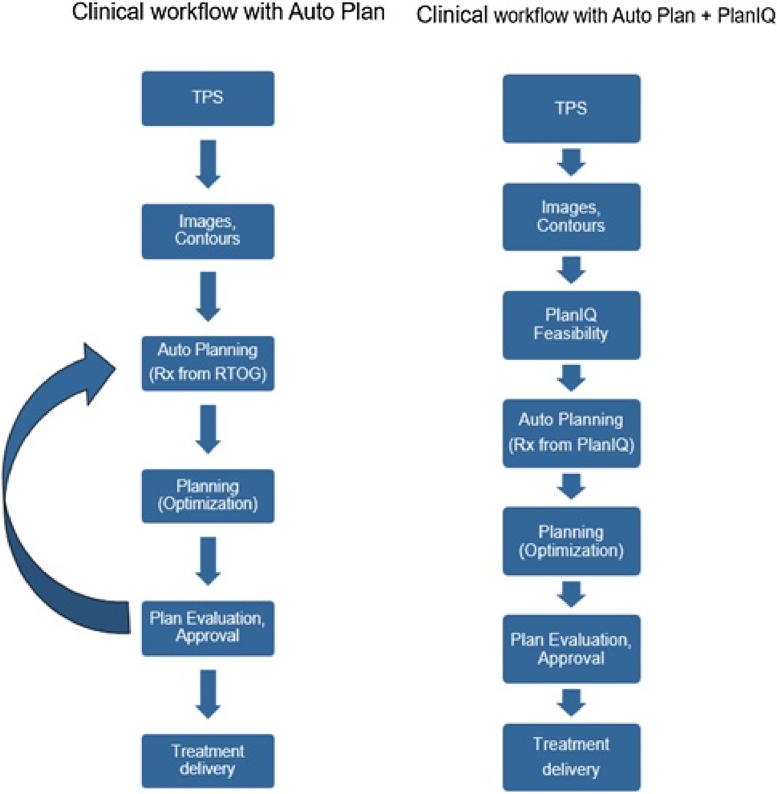
Fig. 1 shows an example FDVH for the larynx in the H&N anatomy computed using PlanIQ tool. The green, yellow, orange and red regions in FDVH indicate that the goals are “achievable”, “challenging to achieve”, “difficult to achieve” and “not achievable”, respectively. By using the slider bar provided on the top, the planner can choose a particular region of FDVH. The dotted lines in Fig. 1 indicate the modified FDVH line corresponding to the cursor in the slider bar. Since there is no protocol available as to which region in the slider bar corresponds to the maximum possible OAR sparing with respect to Autoplan®, we had to rely on our experience with PlanIQ and Autoplan® in order to define the slider bar setting. We selected a region in between “challenging to achieve” and “difficult to achieve”, which, in our experience, provides an “optimal push” to the OAR goals without compromising target coverage. However, in some cases, this setting resulted in sub-optimal plan quality. In such situations, we placed the slider bar in the middle of “challenging to achieve” regions and re-optimized the plan. Table 1 provides the dose–volume objectives for different anatomies specified in AP_RTOG plans and AP_PlanIQ. Fig. 2 illustrates the common clinical workflow and PlanIQ based clinical workflow.
Fig. 1.
Feasibility dose volume histogram (F-DVH) in PlanIQ tool. (For interpretation of the references to color in the text citation, the reader is referred to the web version of the article.)
Table 1.
Dose–volume objectives for different anatomies specified in AP_RTOG plans and AP_PlanIQ.
| Case/Anatomy | OAR | OAR goals used in AP_RTOG | OAR goals used in AP_PlanIQ |
|---|---|---|---|
| Prostate | Rectum | D15% ≤ 70 Gy | D20% ≤ 65 Gy |
| D30% ≤ 65 Gy | D40% ≤ 30 Gy | ||
| D50% ≤ 60 Gy | D50% ≤ 30 Gy | ||
| D60% ≤ 50 Gy | D60% ≤ 50 Gy | ||
| Dmax ≤ 74 Gy | |||
| Dmean ≤ 35 Gy | |||
| Bladder | D5% = 68 Gy | D5% ≤ 70 Gy | |
| D25% = 60 Gy | D25% ≤ 65 Gy | ||
| D50% = 50 Gy | D50% ≤ 27 Gy | ||
| Dmean ≤ 30 Gy | |||
| Left Femur | Dmax ≤ 50 Gy | D60% ≤ 18 Gy | |
| D50% = 10 Gy | |||
| Dmax ≤ 50 Gy | |||
| Right Femur | Dmax ≤ 50 Gy | D50% = 10 Gy | |
| Dmax ≤ 50 Gy | |||
| Head & Neck | Spinal cord | Dmax ≤ 48 Gy | Dmax ≤ 37 Gy |
| D60 ≤ 20 Gy | |||
| Brainstem | Dmax ≤ 54 Gy | Dmax ≤ 54 Gy | |
| Mandible | Dmax ≤ 70 Gy | Dmax ≤ 70 Gy | |
| D65 ≤ 30 Gy | |||
| Right Parotid | Dmean ≤ 26 Gy | Dmean ≤ 30 Gy | |
| D10 ≤ 56 Gy | |||
| D80 ≤ 14 Gy | |||
| Larynx | Dmean ≤ 45 Gy | D50 ≤ 31 Gy | |
| D85 ≤ 20 Gy | |||
| Brain | Left Orbit | Dmean ≤ 5 Gy | Dmean ≤ 1 Gy |
| Optic chiasm | Dmax ≤ 50 Gy | Dmax ≤ 35 Gy | |
| D50% ≤ 10 Gy | |||
| Left Cochlea | Dmax ≤ 50 Gy | Dmax ≤ 27 Gy | |
| Left optic nerve | Dmax ≤ 50 Gy | Dmax ≤ 27 Gy | |
| Brainstem | Dmax ≤ 50.4 Gy | Dmax ≤ 50.4 Gy | |
| Right optic nerve | Dmax ≤ 50 Gy | Dmax ≤ 10 Gy | |
| Right orbit | Dmean ≤ 5 Gy | Dmean ≤ 1 Gy | |
| Abdomen | Left Kidney | Dmean ≤ 18 Gy | Dmean ≤ 6 Gy |
| Right Kidney | Dmean ≤ 18 Gy | Dmean ≤ 6 Gy | |
| Stomach | D100 ≤ 45 Gy | D25 ≤ 10 Gy | |
| Bowel | D5 ≤ 45 Gy | D30 ≤ 10 Gy | |
| Liver | Dmean ≤ 28 Gy | Dmean ≤ 5 Gy | |
| Spinal cord | Dmax ≤ 45 Gy | Dmax ≤ 18 Gy | |
| D15 ≤ 15 Gy | |||
| D35 ≤ 10 Gy | |||
| Lung | Left Lung | D20% ≤ 20 Gy | D25% ≤ 12 Gy |
| D40% ≤ 10 Gy | |||
| Rest Total Lung | Dmean ≤ 20 Gy | Dmean ≤ 20 Gy | |
| D35% ≤ 12 Gy | |||
| Heart | D33% ≤ 60 Gy | D10% ≤ 15 Gy | |
| D67% ≤ 45 Gy | D20% ≤ 12 Gy | ||
| Spinal cord | Dmax ≤ 45 Gy | Dmax ≤ 20 Gy | |
| Esophagus | Dmean = 34 Gy | D30% ≤ 50 Gy | |
| D40% ≤ 30 Gy | |||
| Right Lung | D20% ≤ 30 Gy | D35% ≤ 35 Gy | |
| D20% ≤ 50 Gy | |||
| D50% ≤ 10 Gy | |||
Fig. 2.
Illustration of common clinical workflow and PlanIQ based clinical workflow.
4. Results
We compared the dosimetric results for these two sets of plans (AP_RTOG and AP_PlanIQ) for five cases (brain, head & neck, lung, abdomen and prostate). For serial structures like the spinal cord, optic nerves and brain stem, maximum dose to 0.03 cc volume is used for evaluation whereas for other organs, we used mean dose for comparison. We used RTOG guidelines to evaluate the plans. We observed that while both sets of plans met the RTOG guidelines in terms of OAR sparing, the AP_PlanIQ plans were significantly better in terms of OAR sparing as compared to AP_RTOG plans without any compromise in the target coverage. In addition, we compared the MU performance and low dose spillage (i.e. volume covered by 5 Gy dose) between these two sets of plans.
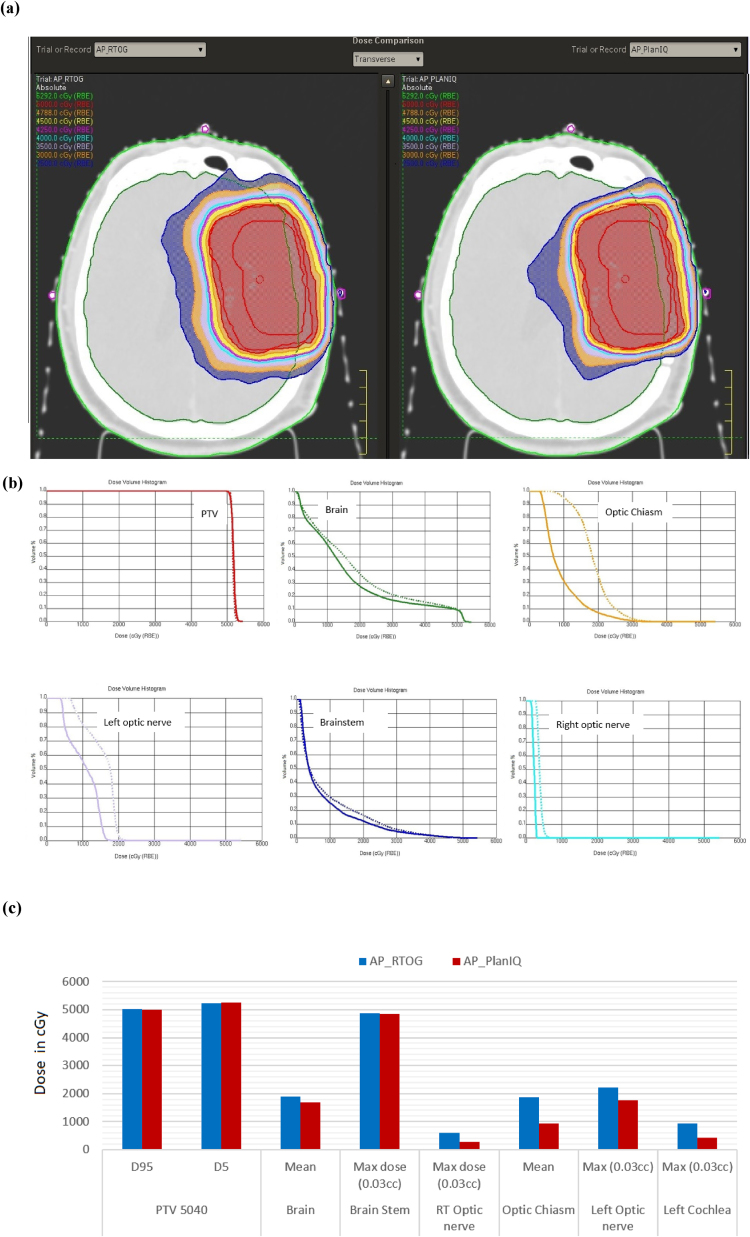
The results for the brain case are shown in Fig. 3: (a) comparison of dose distribution on a transverse slice between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics between AP_RTOG plans and AP_PlanIQ plans.
Fig. 3.
(a) Comparison of dose distribution on a transverse slice for brain case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison for brain case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics for brain case between AP_RTOG plans and AP_PlanIQ plans.
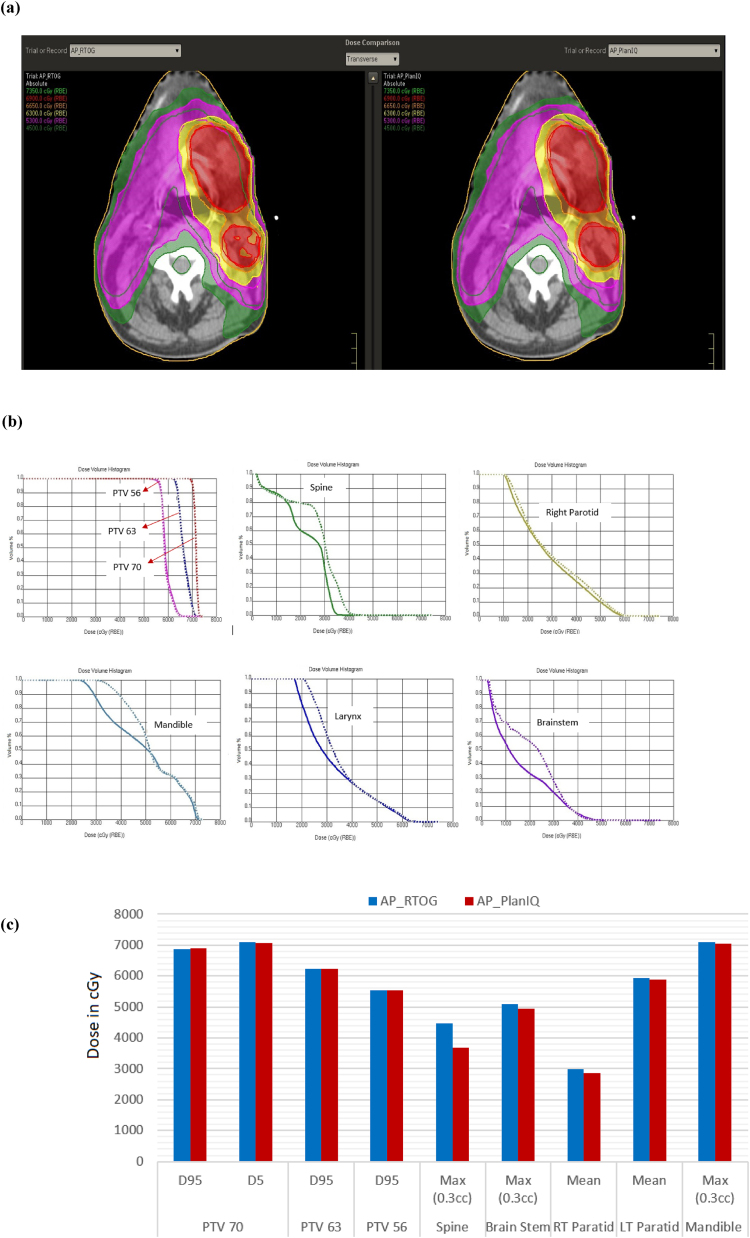
The results for the head & neck case are shown in Fig. 4: (a) comparison of dose distribution on a transverse slice between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics between AP_RTOG plans and AP_PlanIQ plans.
Fig. 4.
(a) Comparison of dose distribution on a transverse slice for head & neck case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison for head & neck case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics for head & neck case between AP_RTOG plans and AP_PlanIQ plans.
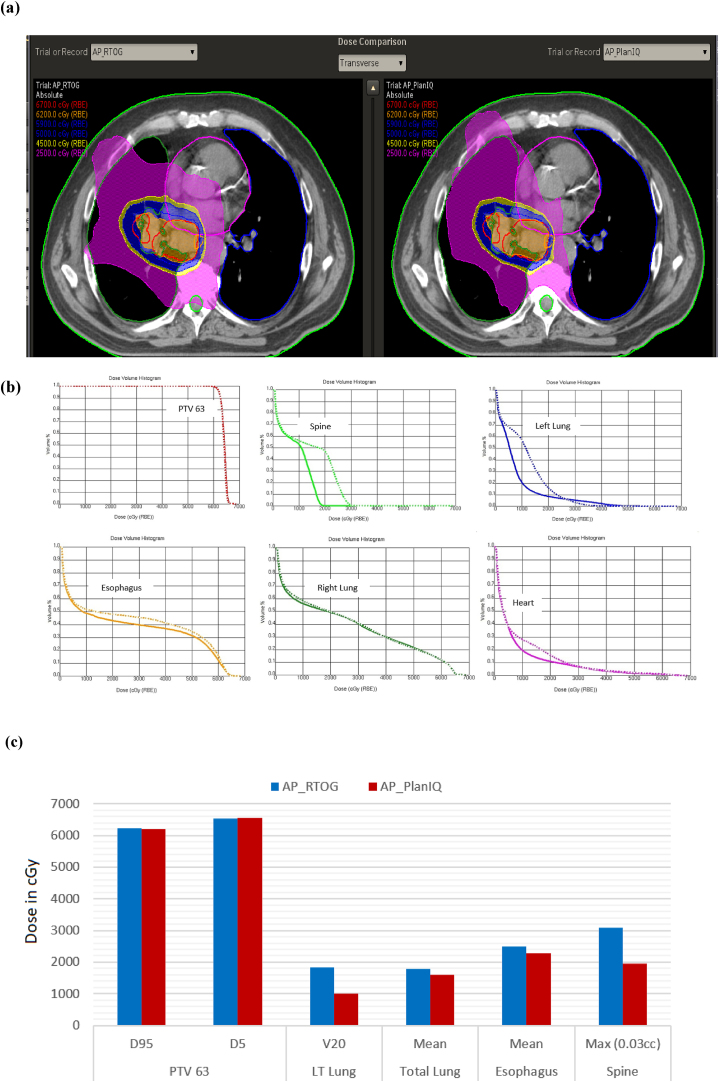
The results for the lung case are shown in Fig. 5: (a) comparison of dose distribution on a transverse slice between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics between AP_RTOG plans and AP_PlanIQ plans.
Fig. 5.
(a) Comparison of dose distribution on a transverse slice for lung case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison for lung case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics for lung case between AP_RTOG plans and AP_PlanIQ plans.
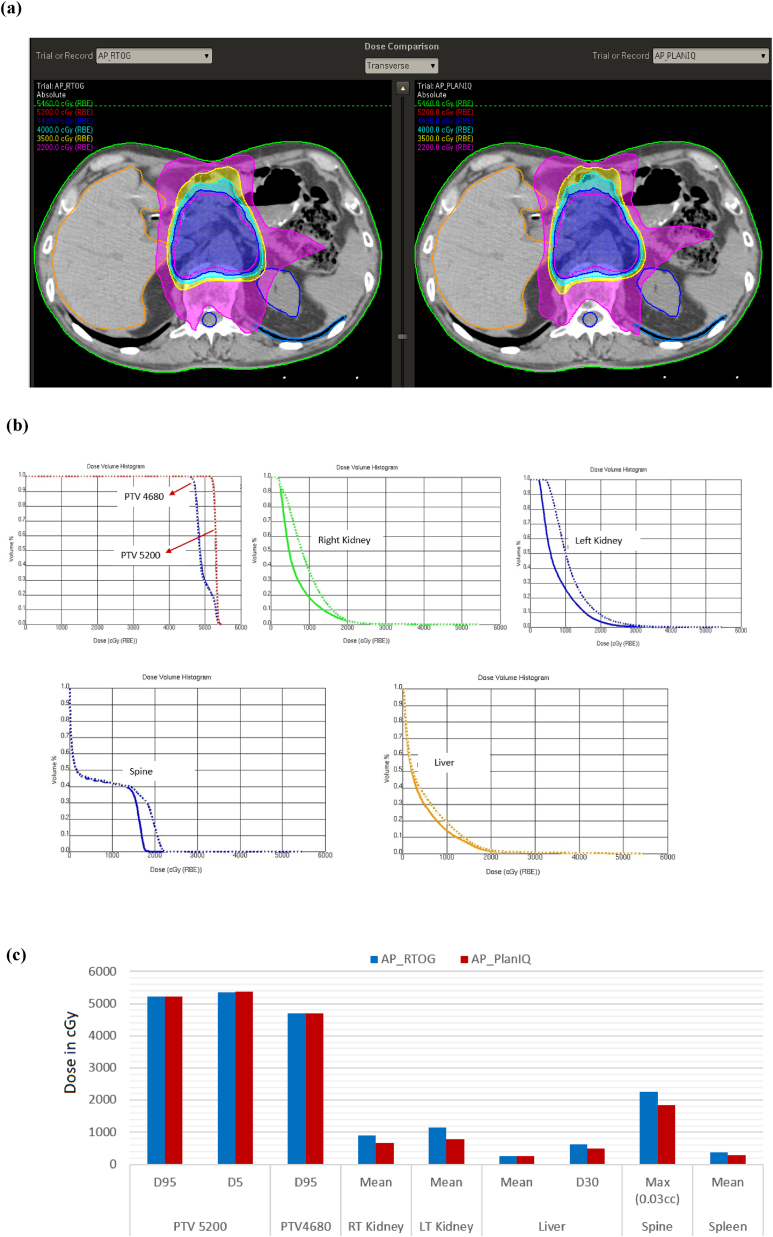
The results for the abdomen case are shown in Fig. 6: (a) comparison of dose distribution on a transverse slice case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics between AP_RTOG plans and AP_PlanIQ plans.
Fig. 6.
(a) Comparison of dose distribution on a transverse slice for abdomen case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison for abdomen case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics for abdomen case between AP_RTOG plans and AP_PlanIQ plans.
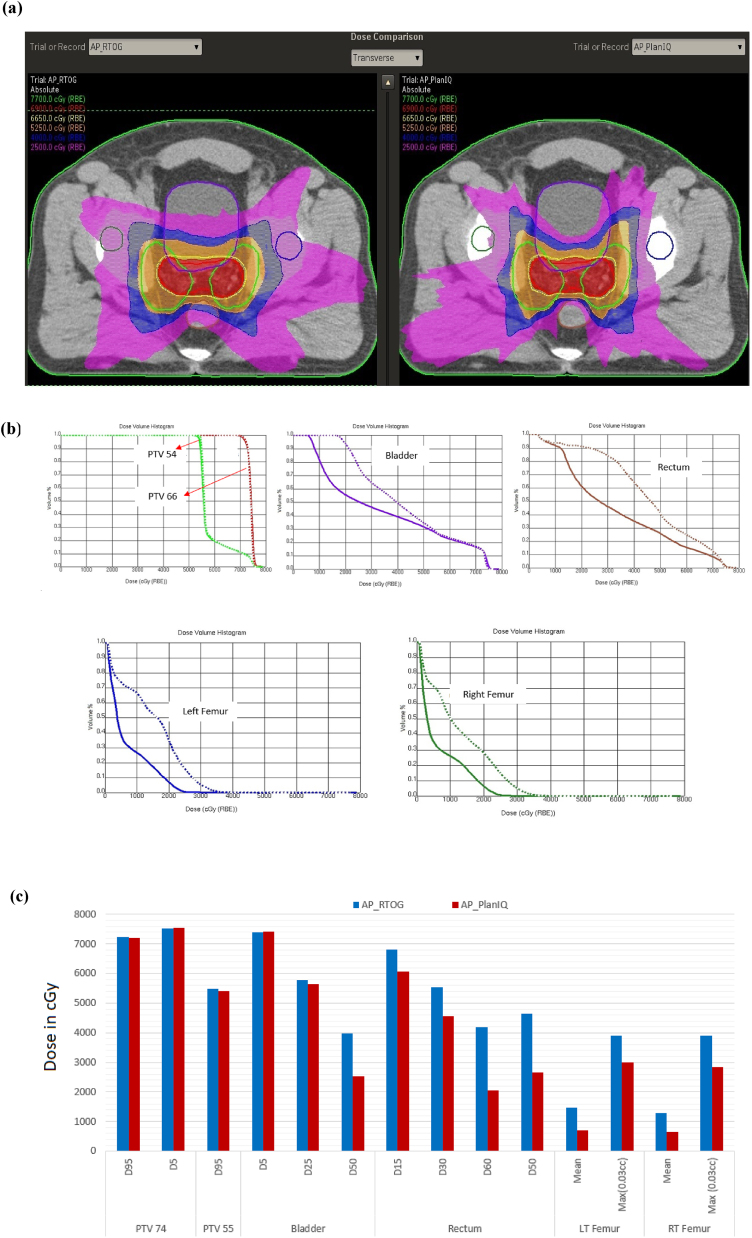
The results for the prostate case are shown in Fig. 7: (a) comparison of dose distribution on a transverse slice between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics between AP_RTOG plans and AP_PlanIQ plans.
Fig. 7.
(a) Comparison of dose distribution on a transverse slice for prostate case between AP_RTOG plans (left) and AP_PlanIQ plans (right), (b) DVH comparison for prostate case between AP_RTOG plans (dotted lines) and AP_PlanIQ plans (solid lines) and (c) comparison of dose statistics for prostate case between AP_RTOG plans and AP_PlanIQ plans.
5. Discussion
This study shows that there is a significant reduction in OAR doses when Autoplan® is guided by PlanIQ. In general, Autoplan® gives clinically acceptable plans meeting RTOG guidelines. However, when PlanIQ is used, user gets an idea about the extent to which the OAR dose can be reduced without compromising target coverage even before invoking the optimization for a given patient. This helps the user define the clinical goals tailored to the anatomy of each patient, which eventually results in a better dosimetric outcome. Apart from that, we observed significant dose reduction in mean dose for the prostate case for both the bladder and rectum of 14.4 Gy and 19.8 Gy, respectively. On the other hand, in the head and neck case, the spinal cord and brain stem maximum doses were lowered by 7.8 Gy and 1.62 Gy, respectively. For the Lung case, the dose reduction for the left lung (V20) is 8.3 Gy and mean dose for the esophagus and total lung is 1.9 Gy to 2.2 Gy respectively, while the maximum dose for the spine is 11.9 Gy lower than that in AP_RTOG plan. In the abdomen case, mean doses for the left and right kidneys are lowered by 3.7 Gy and 2.45, respectively. In the brain case, the maximum doses to the left and right optic nerves were reduced by 4.6 Gy and 3.3 Gy, respectively in AP_PlanIQ plans. The improvement in plan quality can be directly attributed to the higher degree of personalization of treatment goals obtained using PlanIQ. In addition to the significant OAR sparing, the integration has also helped avoid unnecessary optimization iterations in a few instances by enabling the user to wisely define the OAR goals before starting to use Autoplan®.
Table 2, Table 3 show the comparison of low dose spillage (volume covered by 5 Gy dose) and plan MU between AP_RTOG plans and AP_PlanIQ plans, respectively. It is very evident from Table 2 that the low dose volume is significantly reduced in AP_PlanIQ plans compared to that in AP_RTOG plans. Table 3 shows that the calculated plan MUs for AP_PlanIQ plans are higher for Brain, Prostate and Lung cases. This is because the PlanIQ suggested goals were too stringent as compared to RTOG goals which resulted in significant dose reduction in these cases with same target coverage. We observed that there is an additional time and effort involved in using PlanIQ tool with Autoplan®. On the average, it took about 10–15 min to perform the feasibility analysis using the PlanIQ tool.
Table 2.
Comparison of low dose spillage (volume covered by 5 Gy dose) between AP_RTOG and AP_PlanIQ plans.
| Anatomy | AP_RTOG (volume in cc) | AP_PlanIQ (volume in cc) |
|---|---|---|
| Prostate | 8765 | 8034.6 |
| H&N | 7887.69 | 7900 |
| Lung | 13,507.8 | 11,124.4 |
| Abdomen | 4427.54 | 4114.93 |
| Brain | 2108 | 2246.47 |
Table 3.
Comparison of plan MU between AP_RTOG and AP_PlanIQ plans.
| Anatomy | AP_RTOG (MU) | AP_PlanIQ (MU) |
|---|---|---|
| Prostate | 600 | 795 |
| H&N | 833 | 791 |
| Lung | 404 | 533 |
| Abdomen | 507 | 505 |
| Brain | 615 | 688 |
6. Conclusion
Since Autoplan relies on the goals used by the planner, the plan quality resulting from Autoplan is still user-dependent to some extent. By using the goals suggested by PlanIQ, it is possible to use anatomy-specific as well as case-specific clinical goals in the optimization, which in turn allows the planner to use Autoplan in a more effective way. The results indicate that, although Autoplan helps achieve the user-defined goals without much manual intervention, the plan quality (OAR sparing) can be significantly improved without taking many iterative steps when PlanIQ suggested clinical goals are used in the Autoplan-based optimization. Although it takes an additional time to perform the feasibility analysis, the benefit from PlanIQ in improving the plan quality outweighs by far the additional time.
Conflict of interest
Here with we declare that Philips healthcare supported our research titled "Evaluation of plan quality improvements in PlanIQ-guided Autoplanning". We did this study with the supervision and help from Medical physics experts from Bharathiar University Coimbatore and Vellore Institute of Technology, Vellore.
Financial disclosure
None.
Contributor Information
Bojarajan Perumal, Email: bojarajan.perumal@philips.com.
Harikrishna Etti Sundaresan, Email: etti75@gmail.com.
Vaitheeswaran Ranganathan, Email: vaithee1985@gmail.com.
Natarajan Ramar, Email: n.r.natarajan@gmail.com.
Gipson Joe Anto, Email: Gipson.Anto@philips.com.
Samir Ranjan Meher, Email: samirmeher@vit.ac.in.
References
- 1.Fried D.V., Chera B.S., Das S.K. Assessment of PlanIQ Feasibility DVH for head and neck treatment planning. J Appl Clin Med Phys. 2017;18:245–250. doi: 10.1002/acm2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing L., Li J.G., Donaldson S., Le Q.T., Boyer A.L. Optimization of importance factors in inverse planning. Phys Med Biol. 1999;44(10):2525. doi: 10.1088/0031-9155/44/10/311. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Wang X., Dong L., Liu H., Mohan R. A sensitivity-guided algorithm for automated determination of IMRT objective function parameters. Med Phys. 2006;33(8):2935–2944. doi: 10.1118/1.2214171. [DOI] [PubMed] [Google Scholar]
- 4.Wu X., Zhu Y. An optimization method for importance factors and beam weights based on genetic algorithms for radiotherapy treatment planning. Phys Med Biol. 2001;46(4):1085. doi: 10.1088/0031-9155/46/4/313. [DOI] [PubMed] [Google Scholar]
- 5.Kessler M.L., Mcshan D.L., Epelman M.A. Costlets: a generalized approach to cost functions for automated optimization of IMRT treatment plans. Optim Eng. 2005;6(4):421–448. [Google Scholar]
- 6.Stieler F., Yan H., Lohr F., Wenz F., Yin F.F. Development of a neuro-fuzzy technique for automated parameter optimization of inverse treatment planning. Radiat Oncol. 2009;4(1):39. doi: 10.1186/1748-717X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaitheeswaran R., Sathiya Narayanan V.K., Bhangle J., Nirhali A. TU-A-BRA-05: An algorithm for automated determination of IMRT objective function parameters. Med Phys. 2010;37(6 Part 26):3369. [Google Scholar]
- 8.Xhaferllari I., Wong E., Bzdusek K., Lock M., Chen J.Z. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys. 2013;14(1):176–191. doi: 10.1120/jacmp.v14i1.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar P., Bzdusek K.A., Ranganathan V., Palmer M., Kantor M. 2018. Automatic optimal IMRT/VMAT treatment plan generation. U.S. Patent 9,943,702, issued April 17. [Google Scholar]
- 10.Jeong K., Bzdusek K., Kumar P., Tome W. SU-E-T-650: Evaluation of novel IMRT auto-planning tool for nasopharyngeal carcinoma cases. Med Phys. 2013;40(6 Part 21):355. [Google Scholar]
- 11.Hazell I., Bzdusek K., Kumar P. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;1:272–282. doi: 10.1120/jacmp.v17i1.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazhdan M., Simari P., McNutt T. International Conference on Medical Image Computing and Computer-Assisted Intervention. 2009. A shape relationship descriptor for radiation therapy planning; pp. 100–108. [DOI] [PubMed] [Google Scholar]
- 13.Wu B., Ricchetti F., Sanguineti G. Patient geometry-driven information retrieval for IMRT treatment plan quality control. Med Phys. 2009;36(12):5497–5505. doi: 10.1118/1.3253464. [DOI] [PubMed] [Google Scholar]
- 14.Wu B., Ricchetti F., Sanguineti G. Data-driven approach to generating achievable dose–volume histogram objectives in intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;79(4):1241–1247. doi: 10.1016/j.ijrobp.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Wu B., Pang D., Simari P., Taylor R., Sanguineti G., McNutt T. Using overlap volume histogram and IMRT plan data to guide and automate VMAT planning: a head-and-neck case study. Med Phys. 2013;40(2) doi: 10.1118/1.4788671. [DOI] [PubMed] [Google Scholar]
- 16.Vaitheeswaran R., Kumar P., Bzdusek K., Das J.M. SU-G-BRC-01: A data-driven pre-optimization method for prediction of achievability of clinical objectives in IMRT. Med Phys. 2016;43(6 Part 24):3627. [Google Scholar]
- 17.Ahmed S., Nelms B., Gintz D. A method for a priori estimation of best feasible DVH for organs-at-risk: validation for head and neck VMAT planning. Med Phys. 2017;44(10):5486–5497. doi: 10.1002/mp.12500. [DOI] [PubMed] [Google Scholar]
- 18.ASTRO Accreditation Program for Excellence. APEx® Program Standards. https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf.
- 19.Radiation Oncology Practice Accreditation Program Requirements – The American College of Radiology. https://www.acraccreditation.org//media/ACRAccreditation/Documents/ROPA/Requirements.pdf?la=en.