Abstract
Objective:
The medial prefrontal cortex (mPFC) is a highly connected cortical region that acts as a hub in major large-scale brain networks. Its dysfunction is associated with a number of psychiatric disorders, such as schizophrenia, autism, depression, substance use disorder (SUD), obsessive-compulsive disorder (OCD), and anxiety disorders. Repetitive transcranial magnetic stimulation (rTMS) studies targeting the mPFC indicate that it may be a useful therapeutic resource in psychiatry due to its selective modulation of this area and connected regions.
Methods:
This review examines six mPFC rTMS trials selected from 697 initial search results. We discuss the main results, technical and methodological details, safety, tolerability, and localization strategies.
Results:
Six different protocols were identified, including inhibitory (1 Hz) and excitatory (5, 10, and 20 Hz) frequencies applied therapeutically to patient populations diagnosed with major depressive disorder, OCD, autistic spectrum disorder, SUD, specific phobia, and post-traumatic stress disorder (PTSD). In the OCD and acrophobia trials, rTMS significantly reduced symptoms compared to placebo.
Conclusion:
These protocols were considered safe and add interesting new evidence to the growing body of mPFC rTMS literature. However, the small number and low methodological quality of the studies indicate the need for further research.
Keywords: Prefrontal cortex, medial prefrontal cortex, noninvasive transcranial stimulation, transcranial magnetic stimulation, depression, PTSD, autism spectrum disorder, substance-related disorders, phobic disorders
Introduction
The medial prefrontal cortex (mPFC) is a heteromodal association area that includes several cortical regions located along the midline of the PFC.1-3 While there is some terminological ambiguity and a lack of precise anatomical delimitation regarding the mPFC, it is generally defined as posteriorly bordering the secondary motor areas, anteriorly extending to the frontopolar cortex and ventrally to include parts of the anterior cingulate cortex (ACC), especially its more dorsal portions.4-6 The mPFC receives association fibers from sensory cortical areas and medial temporal lobe structures, especially the hippocampus and subiculum, as well as projections from the subcortical structures, such as the amygdala.3,7-9 It also connects with the nucleus accumbens (NAcc), the posterior cingulate cortex, the insula, and the hypothalamus.3,4,6,7,10-13 Recently, the mPFC has been considered of central importance for the pathophysiological understanding of mental disorders.13-17
Functionally, the mPFC has been associated with many different neuropsychological processes commonly affected by psychiatric disorders, such as social cognition,18-21 self-referential thinking,22-26 emotion regulation,27-29 behavioral reinforcement,6,10,30,31 implicit associative learning,32-34 decision making,35-39 and episodic memory consolidation and retrieval.8,9,39
Indeed, neuroimaging and lesion studies have identified the mPFC as one of the main structures involved in psychiatric disorders, including schizophrenia,17,40-43 autistic spectrum disorder (ASD),44-46 substance use disorder (SUD),12,30,31,47,48 major depressive disorder (MDD),49-51 obsessive-compulsive disorder (OCD),52-54 and anxiety disorders, such as specific phobia55,56 and post-traumatic stress disorder (PTSD).57-59 In a comparison between neuroimaging studies of various neurological and psychiatric disorders, mental illnesses were more strongly related to mPFC abnormalities.60 Functional neuroimaging of multiple categories of psychiatric disorders have also shown that the mPFC is of transdiagnostic importance, suggesting that mPFC abnormalities may be a common neural substrate for these conditions.61
The associations between the mPFC and different types of mental illness and neuropsychological processes probably stem from its high connectivity and hub function, since it integrates large-scale brain networks, namely the default-mode network (DMN) and the salience network (SN).17,62-64 These two networks, which are associated with emotion, behavior, and the Self, have been identified as central to the pathophysiology of psychiatric disorders, together with the central executive network (CEN), which involves the dorsolateral PFC (dlPFC) and the parietal regions, which are related to cognitive control and working memory.14,17,65,66 Importantly, they seem to influence one another, and functional connectivity data suggest that a mPFC node is a major mediator of this interaction.51,67,68
The SN consists of brain regions usually associated with emotion regulation and reward/motivation.13,62,69 This network is anchored in two subsystems that share a mPFC connection: one based on corticolimbic and fronto-insular connections and noradrenergic amygdala activity, and another based on the frontostriatal circuitry of the dopaminergic reward/motivation system. The DMN is a resting-state brain network that is deactivated during task-related behaviors, essentially becoming silent when external attention is required, although its components do not always follow this pattern.70-73 Anatomically, the DMN mainly consists of midline brain regions, such as the mPFC, the posterior cingulate cortex, the precuneus and the medial temporal lobe. Together, they form a system that involves different aspects of self-related mental processes, which is activated during mental simulation tasks, such as perspective taking or imagining scenes.70 DMN and SN abnormalities have been linked to psychiatric disorders such as SUD, MDD, ASD, and schizophrenia.16,43,70
Transcranial magnetic stimulation of the medial prefrontal cortex
Recent advances in the pathophysiology of psychiatric disorders have brought attention to the mPFC as a promising target for therapeutic intervention, particularly transcranial magnetic stimulation (TMS). Due to its ability to modify brain function in an anatomically selective manner, TMS has been proposed as tool for modulating mPFC activity.14,15,66,74 Interest in the mPFC represents a shift in TMS research, which, over the past decades has been focused on the dlPFC, a region more directly involved in CEN-related functions.14
TMS is achieved through magnetic pulses generated by a coil that receives electrical current at a controlled frequency. The magnetic field can influence electrical activity in conductive media, such as the cerebral cortex, through electromagnetic induction.75 The coil is positioned over predetermined points on the scalp, targeting specific cortical areas at a depth of 4-5 cm, which causes depolarization.76 When magnetic pulse sequences are applied over a period of time, long-lasting effects in cortical plasticity can be achieved.77,78 In this case, the technique is called repetitive TMS (rTMS) and, depending on the frequency of the stimulation, may result in an excitatory (high frequency protocols, usually greater than 5 Hz) or an inhibitory (low frequency protocols, usually 1 Hz or less) effect, although this is disputed.79,80 The geometry of the coil is another relevant factor, since it determines the shape of the magnetic field and, therefore, the depth and focality of stimulation. There is a trade-off between these two characteristics: more depth results in less focus, and vice-versa.81 In psychiatry, rTMS targeting the dlPFC is a well-established treatment for MDD,82 the therapeutic effect of which might be due to DMN and CEN modulation.83,84
The mPFC is readily accessible to TMS, particularly its more dorsal and rostral portions.14,66,85 Some TMS coil designs that generate greater depth of magnetic field can allegedly reach ventral mPFC structures and the ACC, and are usually considered deep TMS (dTMS) techniques.86 Nonetheless, the more usual coils, such as figure-of-eight and circular models, may also modulate deeper regions that are connected to the stimulation site, as has been confirmed by meta-analytic data from functional neuroimaging studies.87 Considering the high connectivity of the mPFC, this rings particularly true, and mPFC TMS studies with functional neuroimaging readings have reported modulated brain activity in cortical and subcortical regions, including the dlPFC, ACC, NAcc, hippocampus, dorsal striatum, and thalamus.31,88-91
Moreover, mPFC TMS studies with healthy subjects have successfully modulated behavioral outcomes related to psychiatric morbidity, such as social cognition,92-97 the processing of self-referential information,98,99 fear conditioning,100 avoidance behavior,101 pain processing,102-104 delayed discounting,105 semantic processing,106 and memory consolidation.107
Some preliminary clinical evidence has been published regarding mPFC rTMS as a treatment for psychiatric disorders: case reports, open-label studies, and chart reviews have demonstrated favorable results in populations with MDD,89,91,108,109 OCD,90 SUD,85,110 and eating disorders,111 reporting good tolerability and feasibility. This has led to the inclusion of mPFC rTMS as a third line alternative for refractory MDD treatment in an influential Canadian guideline for the treatment of mood disorders.74
Thus, mPFC rTMS seems to have a clinical impact on many different psychiatric disorders, possibly due to the high connectivity and hub function of this region and its involvement in the SN and the DMN. Nevertheless, a preliminary search of the literature revealed no existing reviews of clinical trials investigating mPFC rTMS in the area of psychiatry. Therefore, this review systematically searched the literature for randomized, controlled clinical trials on mPFC rTMS in populations diagnosed with psychiatric disorders.
Methods
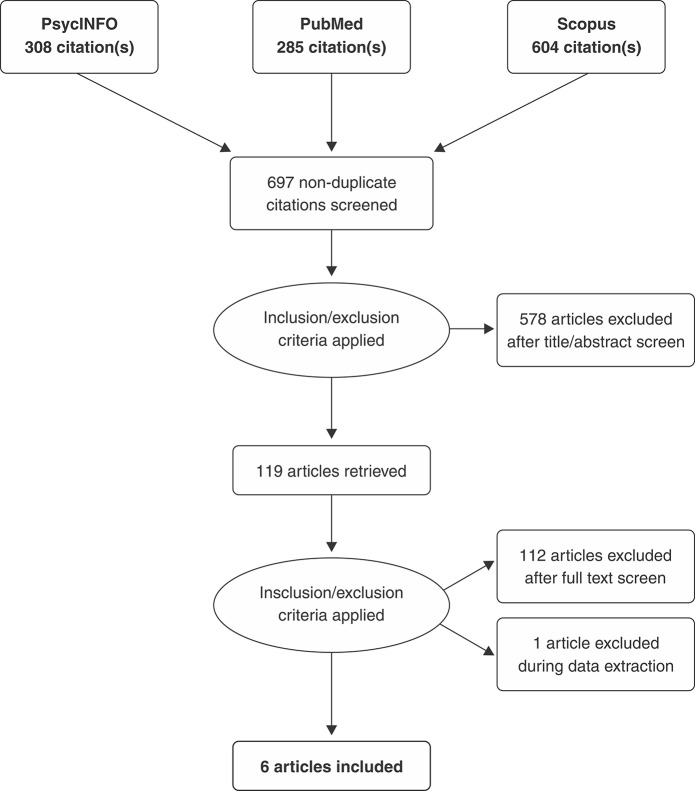
The included studies were randomized, controlled trials (RCT) with clinical outcome measures in populations diagnosed with psychiatric disorders that included a clear description of the mPFC rTMS protocol, including coil types and stimulation parameters. The review protocol was registered in PROSPERO (CRD42018096525). The objectives included reviewing the main results, technical, and methodological details, safety, tolerability, and localization strategies of mPFC rTMS protocols. In May 2018, we searched Medline, PsycINFO, and Scopus using the following string: ((“*medial* prefrontal” OR “medio* prefrontal” OR “cingulate” OR “*ACC” OR DMPFC OR VMPFC) AND (“transcranial magnetic stimulation” OR rTMS OR TMS)). No filters or date and language restrictions were applied. This resulted in a total of 697 articles after excluding duplicates (Figure 1). Study selection was performed independently by two authors (RCM and LV). Exclusion criteria included: not being an original research study (257 articles), not involving mPFC stimulation (195 articles), involving healthy subjects (59 articles), animal studies (16 articles), not involving TMS (121 articles), no clinical outcome (seven articles), no psychiatric disorder (11 articles), and not being a RCT (25 articles). Metanalysis was not performed due to high heterogeneity of selected studies.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) selection process flowchart.
Results and discussion
Overview of selected studies
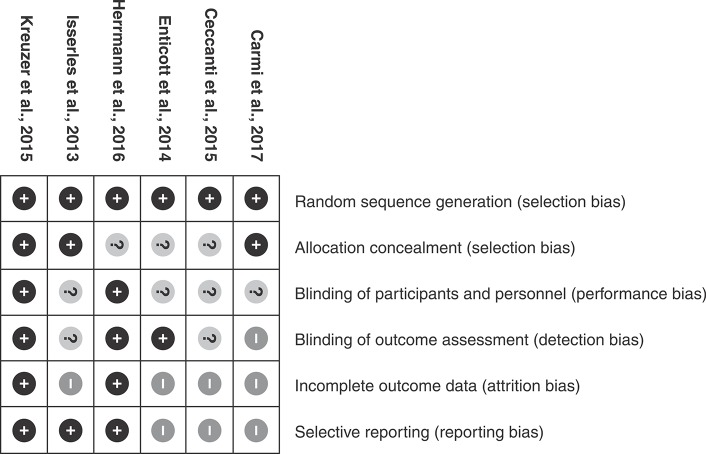
An overview of the main characteristics of the selected studies can be viewed in Table 1. The six selected trials included a total of 211 individuals, of which 98 received active mPFC rTMS. The stimulation frequencies were 1, 5, 10, and 20 Hz. The results of the Cochrane Quality Assessment Tool may be viewed in Figure 2.
Table 1. Main characteristics of the selected studies.
| Article | Participants | Intervention | Outcomes | Notes |
|---|---|---|---|---|
| Carmi112 | 41 OCD patients, 38 completers, 38 reported. | H7 coil, 20 Hz (50,000 total pulses), or 1 Hz (22,500 total pulses), 100-110% RMT.4 cm anterior to the hot spot. | 20 Hz significantly better than sham at completion and 1 week follow up. | Un-blinding and removal of 1 Hz group prior to study completion. |
| Ceccanti113 | 18 male patients with alcohol use disorder, three completers, 18 reported. | H-coil, 20 Hz (15,000 total pulses), 120% RMT.5 cm anterior to the hot spot. | Reduced alcohol intake up to 3 months in active group after completion. | No intergroup difference in clinical outcomes, but significant reduction of prolactinemia and cortisolemia. |
| Enticott114 | 30 high-functioning autistic adults, 19 completers, 18 reported. | H-coil, 5 Hz (15,000 total pulses), 100% RMT.7 cm anterior to the hot spot. | Social relations improved in active group. | Significant differences only in subscales, full clinical measures scores unaffected. |
| Herrmann115 | 47 acrophobic patients, 44 completers, 39 reported. | Round coil, 10 Hz (3,120 total pulses), 100% RMT. Reference point 10% of nasion-inion distance. | Acrophobic symptoms improved in active group. | Results were not sustained at 3 months follow up. |
| Isserles116 | 30 PTSD patients, 25 completers, 26 reported. | H-coil, 20 Hz (20,160 total pulses), 120% RMT.3 cm above nasion. | Improvement in rTMS + traumatic exposure group for up to 2 months follow up. | No intergroup difference with control or rTMS + no exposure groups. Exposure procedure not measured for effectiveness. |
| Kreuzer117 | 45 patients with moderate/severe depression, 40 completers, 40 reported. | Double cone (mPFC) or figure-of-eight (dlPFC), 10 Hz (30,000 total pulses), 110% RMT.1.5 cm anterior to one-third of nasion-inion distance (mPFC stimulation site). | mPFC group responded better than dlPFC group by the end of treatment sessions. | Significant difference in mPFC vs. dlPFC group. Neither group differed from sham. |
dlPFC = dorsolateral prefrontal cortex; mPFC = medial prefrontal cortex; OCD = obsessive-compulsive disorder; PTSD = post-traumatic stress disorder; RMT = resting motor threshold; rTMS = repetitive transcranial magnetic stimulation.
Figure 2. Cochrane Quality Assessment Tool results.
Five studies were dTMS trials with H1,113,114,116 H7,112 or double-cone117 coil models. H1 coils are composed of 14 strips of 7-12 cm long wire encased in a helmet. These configurations produce a summation of the electric field from several coil elements that carry current in the same direction, resulting in a deeper reach for the magnetic pulse.118,119 They are specifically produced for dTMS, and the H7 coil is a more recent version of this model, created with mPFC rTMS in mind. It is hoped that such a coil can stimulate regions such as the NAcc, ACC, and insula. Direct activation of these areas by dTMS may have different therapeutic properties than stimulation with more conventional coil designs.86,118,119
The double-cone coil consists of two large circular coils forming an obtuse angle. At the expense of focus, this type of coil is useful for reaching deeper brain structures of interest, such as the representation of the lower limbs in the primary motor cortex, which is located within the interhemispheric fissure. In particular, such coils may enable stimulation of limbic cortical regions, such as the ACC.120
One study used a round coil,121 a model not used for dTMS, to stimulate a wide region of superficial cerebral tissue (for instance, inducing bilateral effects when placed over the vertex).120
All studies positioned the coil on different points along the midline of the scalp. This positioning promoted bilateral stimulation, allowing the magnetic field to modulate the medial portion of both hemispheres simultaneously. Three protocols used a fixed distance from the motor hot spot to establish coil position (4,112 5,113 7 cm114 anterior to the hot spot). The remainder chose to use the nasion-inion measure to establish the stimulation point (10% of nasion-inion distance115 and 1.5 cm anterior to 30% of the distance117). Although Isserles et al.116 did not report the exact site of coil positioning, in posterior publications, they described the stimulation point as being 3 cm above the nasion.116,122 The Kreuzer et al.116 trial also had a left dlPFC group, with the figure-of-eight coil being positioned 5 cm anterior to the left motor hot spot in sagittal direction.
Safety and tolerability
Although few studies provided detailed tolerability data, overall, the procedure was reported to have good tolerability and safety profiles. Nonetheless, headache and local discomfort were frequent complaints (over 50% of participants in one study117). There were 33 drop-outs among the studies, with 15 coming from sham stimulation groups. One study reported a participant who could not tolerate the proposed stimulation intensity (20 Hz dTMS at 120% motor threshold) and was allowed to complete the protocol at a lower intensity, being excluded from the final analysis.116 This same trial reported a self-limited generalized tonic-clonic seizure that ceased without treatment.116 Although rare, self-limited seizures are a possible complication of TMS and are considered the most severe side effect.123
Sham stimulation
The four studies that used H1 or H7 coils used a sham stimulation method provided by manufacturer (Brainsway, Israel).112-114,116 In this case, sham stimulation involved a helmet containing the same coils used in real stimulation, which produced sounds similar to those heard in active rTMS but did not generate relevant electrical activity. Patient and researcher blinding was accomplished through the use of a randomly assigned card that automatically set the stimulator to real or sham mode, thereby eliminating the need to handle the stimulator equipment. The studies that chose double-cone and round coil used a placebo coil, which also produced acoustic artifacts similar to real stimulation, but with no significant magnetic fields. In this case, the equipment had to be handled by the research team.
Major depressive disorder
Kreuzer et al.117 (n=45) evaluated mPFC rTMS with a double cone coil for MDD, delivering 30,000 total pulses at 10 Hz. This was the only trial with a low risk of bias for each item in the quality assessment tool. The double cone mPFC rTMS group was compared to sham treatment and traditional left dlPFC rTMS with a figure-of-eight coil. After 15 sessions, which were performed over a 3-week period, there was a significant time effect for the primary outcome, reduction of depressive symptoms according to the 21-item Hamilton Depression Rating Scale. There was a trend toward greater MDD severity in the mPFC group, and post-hoc t-tests with baseline corrected values showed a significant difference between double cone and figure-of-eight coils at the end of treatment (p = 0.014), although there were no significant effects when comparing either of the active rTMS groups with placebo (p = 0.216; p = 0.270). Furthermore, the double cone coil’s superiority over the figure-of-eight was lost after 12 weeks of follow up.
Structural and functional neuroimaging evidence indicates that midline PFC structures are intimately involved in MDD, especially the ACC and the mPFC related to the DMN.43,49,51,83,124-126 Open-label and chart review studies have found favorable results and good tolerability for mPFC rTMS treatment in MDD, including a good cognitive safety profile, which sets it apart from brain stimulation treatments such as electroconvulsive therapy.127 Pre-/post-comparisons of functional magnetic resonance imaging (fMRI) readings in MDD patients reveal that mPFC rTMS, even when regular (non-deep) coils are used, may modulate activity in structures such as the amygdala, ventral striatum/NAcc, temporal pole, anterior insula, and left dlPFC, i.e. it mainly affects structures in the SN and DMN.66,89 Some studies have also suggested that certain fMRI patterns may be predictive of a mPFC rTMS response in MDD.91
In the reviewed study, the results of mPFC rTMS were not significantly different from placebo for MDD treatment. Nevertheless, the reduction in depressive symptomatology between pre- and post-intervention and the significant difference between the double cone and figure-of-eight coil types, in addition to the other above-mentioned evidence, make mPFC rTMS a clinically interesting subject for MDD treatment research.
Anxiety disorders
While being clinically distinct syndromes, PTSD and specific phobia share many characteristics, especially the importance of fear learning and memory consolidation mechanisms in the development of both disorders.56,57,100,128 Thus, Herrmann et al.115,121 and Isserles et al.116 will be discussed in the same section. Herrmann et al.121 RCT (n=39) assessed the efficacy of 10 Hz rTMS in accelerating extinction learning in a group of patients with a specific phobia of heights (acrophobia). The rTMS protocol and coil positioning were based on previously reported data, including validating the reference point (Fpz) by near-infrared spectroscopy imaging.100 Stimulation took place immediately before a virtual reality exposure therapy session, and was performed twice over a 2-week period. In the real rTMS group, both the anxiety (p = < 0.05) and avoidance subscales (p = < 0.05) of the main outcome measure (Acrophobia Questionnaire) were significantly reduced compared to sham treatment. Although this effect was detected at the first follow-up assessment 1 week after the therapy sessions, further improvement was noted at the 3-month follow up, with mean scores of 36.3±18.7 in the anxiety subscale (sham = 43.2±19.4) and 27.7±5.2 in the avoidance subscale (sham = 30.0±6.2).
Isserles et al.,116 whose sample included 30 PTSD patients, compared 12 sessions of 20 Hz dTMS with or without script-driven imagery of a traumatic experience prior to the beginning of the session. The control group received sham stimulation combined with the trauma exposure procedure. The Clinician Administered PTSD Scale (CAPS-II) score was the main study outcome. There was a significant pre-/post-treatment reduction in CAPS-II score in the exposure/real stimulation group (p = 0.0003). The reduction was not significant in comparison to the other two groups (no exposure/real stimulation, and exposure/sham stimulation). When the outcome was treated as dichotomous, using a 50% or greater reduction in CAPS-II score as the response criteria, 44% of patients in the exposure/real stimulation group, 12.5% of the no exposure/real stimulation group and 0% of the exposure/sham stimulation qualified as responders. Ten patients crossed over to the exposure/real stimulation group in an open phase of the study, and achieved a mean reduction of 14 points in CAPS-II score, which was significant in a pre-/post-comparison (p = 0.0096). Both the original exposure/real stimulation group and the crossover group retained the clinical response at the 2-month follow up.
The neurocircuitry of anxiety disorders mainly involves the mPFC, ACC, hippocampus, and amygdala (Jin & Maren,9 Zubieta et al.,57 Coutinho et al.,129 Giustino & Maren130). The prefrontal component of this circuit projects a large amount of fibers onto the hypothalamus, thereby exerting an influence over the hypophysis and, thus, over the adrenal glands and the regulation of systemic stress response.7,27,131 These mechanisms, accessed by mPFC rTMS, are pivotal for fear conditioning, the development of pathological anxiety, and the formation of trauma related memories.100,132 Furthermore, modulation of memory reconsolidation by prefrontal-hippocampal circuits is one of the putative mechanisms of action for the clinical effect verified in the reviewed trials, since both used symptom provocation techniques prior to rTMS sessions, which may be interpreted as a method of memory recall followed by an reconsolidation-modifying intervention.34,133-135
Guhn et al.100 investigated the modulation of conditioned fear extinction with mPFC rTMS in 88 healthy volunteers. A 10 Hz protocol successfully increased extinction learning and diminished extinction recall of conditioned fear acquired by aversive auditory stimuli. In particular, the extinction learning phase was associated with significant changes in physiological measures. Memory consolidation has also been targeted by TMS studies,133 and mPFC TMS in particular was tested by Berkers et al.107 in 59 healthy individuals, who found that this form of TMS can modulate memory consolidation, reducing the formation of false memories in a Deese-Roediger-McDermott paradigm.
Taking all this evidence as a whole, it would seem that mPFC rTMS has clinical potential as a treatment for anxiety disorders and as an intervention capable of modulating memory reconsolidation for therapeutic purposes. While the clinical results were only slightly positive and not statistically significant for PTSD treatment, the methodologically sound RCT for acrophobia revealed a significant response rate for mPFC rTMS plus exposure therapy for this specific phobia, thereby encouraging future research in this area.
Autistic spectrum disorder
A trial (n=28) by Enticott et al.114 tested the efficacy of 10 sessions of 5 Hz dTMS over the mPFC for symptom reduction in high-functioning ASD patients. The main outcome measure was the Ritvo Autism-Asperger Diagnostic Scale. Although there was no statistically significant difference in intergroup measures of the full score, there was a significant pre-/post-TMS symptom reduction in the social relatedness subscale (p = 0.004), which remained significant at the 1-month follow up (p = 0.001). There was also a significant reduction in the Interpersonal Reactivity Index score, which broadly measures self-oriented anxiety, comparing the pre-treatment scores with the follow-up measure (p = 0.004). These results are in line with previous evidence from a case report by the same author.136
mPFC dysfunction plays an important role in the neurobiology of ASD.137,138 For example, fetal mPFC is an important nexus for a subset of ASD risk genes, and there is evidence for aberrant mPFC activity and connectivity in both human and animal studies, some of which specifically associate mPFC dysfunction with social cognition impairment in ASD.137-139 In fact, the mPFC has been considered one of the main components of the “social brain,” and evidence from functional neuroimaging meta-analysis indicates that its more dorsal and rostral portions are more directly involved in the processes that contribute to our social behavior, such as theory of mind,4,5,18 a function associated with the DMN.140,141
Part of the evidence that causally links the mPFC with social cognition comes from the TMS literature. TMS studies have shown that mPFC stimulation can modulate several aspects of social cognition, such as facial emotion recognition,95,142 group perception,96,97,143 theory of mind,144,145 empathy,92,146 and the integration of different modalities of social impressions.143
The reviewed trial demonstrates that while some improvement occurred in social functioning, the clinical use of rTMS for ASD did not differ from placebo. Nonetheless, additional evidence from neuroimaging and TMS research adds biological plausibility to the idea that mPFC modulation could contribute to ASD treatment. This, together with very low efficacy rates for traditional therapeutics, make mPFC rTMS an interesting future alternative for ASD patients.
Substance use disorder
In a clinical trial by Ceccanti et al.,113 which had an initial sample of 18 patients with alcohol use disorder, 10 sessions of 20 Hz mPFC stimulation were performed after exposure to a visual and olfactory stimulus (a glass of the participant’s favorite alcoholic drink). After the TMS sessions, follow-up measures were recorded monthly for 6 months and included serum cortisol and prolactin assays. There was a high dropout rate during the follow-up period, with only two patients in the active TMS group completing all proposed measures. Nonetheless, there was a significant time vs. condition reduction in daily alcohol intake until the 3-month follow up (p = 0.046), when statistical significance was lost. The two patients that remained until the 6-month follow up completely ceased alcohol consumption. There was also a significant pre-/post-difference in maximum alcohol intake for the active group (p = 0.013), as well as craving score reductions (p = 0.025). Significant reductions in prolactinemia (p = 0.019) and cortisolemia (p = 0.018) were observed in the active rTMS groups compared to controls.
The mPFC plays a prominent role in reward mechanisms and is one of the main cortical regions that participate in the SN.10,13,16 Structurally, the mPFC has extensive dopaminergic input that extends from the ventral tegmental area and the ventral striatum via the medial forebrain bundle, accounting for the larger part of the cortical destination of these fibers, which are a main component of the SN.13,147 The development of SUD is related to changes in dopaminergic transmission, increased activity of SN regions, including the mPFC, insula and NAcc, and impairment of dlPFC activity, which is more strongly related to the CEN.12,47,65,148,149 The use of provocative stimuli prior do rTMS make this intervention a potential modulator of memory reconsolidation, an approach that has been proposed for SUD treatment.133
Some non-RCT studies have successfully used mPFC rTMS to modulate addiction-related outcomes, especially craving in patients with cocaine85,110 and tobacco use disorder,150 as well as drug self-administration in cocaine use disorder151 and delayed discounting in healthy subjects.105 A study by Hanlon et al.85 with cocaine users also used fMRI to measure NAcc activity, finding a dose-dependent reduction of blood-oxygen-level dependent signal secondary to left mPFC inhibitory rTMS.
The reviewed study presented a high risk of bias and there were no significant differences between groups, although two patients became abstinent after the 6-month follow up. Nonetheless, the biological measures indicated that mPFC rTMS modulated dopaminergic pathways and cortisol release. Given the limited efficacy of traditional treatments for SUD, it would be interesting to conduct bigger and better studies to clarify the real potential of TMS in this area.
Obsessive-compulsive disorder
Carmi et al.112 compared the efficacy of 25 sessions of 20 Hz (2,000 pulses per session), 1 Hz (900 pulses per session), or sham dTMS in reducing Yale-Brown Obsessive Compulsive Scale (YBOCS) scores in 41 OCD patients. Stimulation sessions were performed after a planned provocation of OCD symptoms. Due to the slow recruitment rate, limited resources and a trend demonstrating a lack of clinical benefit (the study was unblinded prior to completion), the 1 Hz group was discontinued and further recruitment was directed to the other two groups. The authors chose to omit the 1 Hz group measures from the final analysis. Compared to sham, 20 Hz dTMS resulted in a significantly greater reduction of YBOCS score beginning at the fourth week of treatment (p = 0.001). Considering a 30% reduction in YBOCS score as a response, 43.75% of the 20 Hz and 7.14% of the sham group were responders at 5 weeks of treatment (p < 0.05). A one-week follow-up visit revealed that the response rate had been maintained, but was no longer significant at the 1-month follow up. As a secondary measure, electroencephalography data collected during a Stroop task revealed higher theta activity in response to a mistake following treatment in the 20 Hz group compared to sham (p = 0.01).
Human and animal studies suggest that abnormalities in frontal-subcortical circuitry may be central to OCD pathophysiology.52,152 When spontaneously active or after provocation, OCD symptoms are associated with higher activity of the OFC, the dorsal ACC, and the thamalus.153 OCD patients have gray matter reductions and lower white matter integrity in the ACC, with gray matter increases in the thalamus and ventral striatum/NAcc. Data from fMRI demonstrate abnormal hyperconnectivity between cortical regions that are part of the mPFC or that maintain a high connectivity pattern with mPFC structures and the ventral striatum/NAcc.90
In an open-label mPFC rTMS trial with fMRI measures, Dunlop et al.90 demonstrated a significant reduction in OCD symptoms and found a significantly different fMRI pattern in the responder group, with increased mPFC connectivity with the bilateral somatosensory cortex and the left precuneus and decreased mPFC connectivity with the bilateral caudate, midbrain, thalamus, superior frontal gyrus, and right hippocampus. Apart from suggesting that mPFC rTMS has positive results in OCD treatment, these findings reaffirm the importance of the mPFC in OCD pathophysiology and the possible use of functional mPFC readings as a biomarker in this population.
Despite methodological difficulties and risk of bias, this study indicates that mPFC rTMS may be a valid therapeutic modality for OCD, with statistically significant symptom reduction compared to placebo. This is in line with additional evidence from non-RCT. Nonetheless, there were important methodological flaws in this RCT, and more studies are needed to confirm these results and establish the appropriate rTMS parameters.
Conclusion
There were significant differences between mPFC rTMS treatment and placebo in OCD and acrophobia patients. The results for SUD, ASD, MDD, and PTSD were also favorable, although they were only significant in within-group analysis. While most reviewed trials used dTMS coil models, results differed from placebo in a study that used a non-deep TMS coil, which suggests that the mPFC may be effectively modulated by more superficial forms of rTMS. Studies not formally included in this review also indicate that mPFC activity is amenable to rTMS, and that this technique may be of therapeutic value in psychiatric disorders. This technique’s potential to modulate large-scale brain networks such as the SN and the DMN, which are prominently involved in the pathophysiology of several psychiatric disorders, lends biological plausibility to the above-mentioned effects.
It seems that mPFC rTMS has a good safety profile, although there was one report of a tonic-clonic seizure with 20 Hz dTMS at an intensity of 120% of the resting motor threshold.116 Another patient could not tolerate the proposed intensity (20 Hz at 120% RMT).116 This would seem to indicate that lower intensities and/or lower frequencies might be safer and more tolerable.
The main limitation of the evidence was the high risk of bias in the majority of studies, with only one study being free of methodological issues according to the quality assessment tool. The reviewed protocols followed different midline scalp reference points for stimulation and only one study guided coil positioning by previously reported neuroimaging data.121 Therefore, it is likely that different divisions of the mPFC were stimulated in each study, which is relevant since the mPFC involves functional diversity and different connectivity patterns over its antero-posterior axis.4-6 In addition, the use of different coil types results in different field geometry and stimulation depths,81 which could extend stimulation to neighboring cortical regions and produce clinical effects not necessarily related to mPFC function.
Despite the growing interest in mPFC rTMS research, only a relatively small and very heterogeneous group of studies have resulted so far, and they have only begun to gather evidence regarding the technique’s safety and efficacy as a treatment for psychiatric disorders. This represents a shift in interest from traditional dlPFC rTMS research and opens new neuroscientific and clinical possibilities, since it may differentially access major large-scale brain networks, particularly the SN and DMN. However, more studies with better methodology are needed to confirm the preliminary clinical findings about mPFC rTMS, providing appropriate comparability and reproducibility for the data and allowing quantitative analysis to be performed.
Disclosure
The authors report no conflicts of interest.
Footnotes
How to cite this article: Marques RC, Vieira L, Marques D, Cantilino A. Transcranial magnetic stimulation of the medial prefrontal cortex for psychiatric disorders: a systematic review. Braz J Psychiatry. 2019;41:447-457. http://dx.doi.org/10.1590/1516-4446-2018-0344
References
- 1.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 3.Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 4.Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, et al. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci. 2013;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional segregation of the human dorsomedial prefrontal cortex. Cereb Cortex. 2016;26:304–21. doi: 10.1093/cercor/bhu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J Neurosci. 2016;36:6553–62. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 8.Genzel L, Dresler M, Cornu M, Jäger E, Konrad B, Adamczyk M, et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–86. doi: 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–9. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- 11.Cole SW, Yoo DJ, Knutson B. Interactivity and reward-related neural activation during a serious videogame. PLoS One. 2012;7:e33909. doi: 10.1371/journal.pone.0033909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon V. Salience network. In: Toga AW, editor. Brain mapping. Vol. 2. Cambridge: Elsevier; 2015. pp. 597–611. [Google Scholar]
- 14.Downar J, Blumberger DM, Daskalakis ZJ. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn Sci. 2016;20:107–20. doi: 10.1016/j.tics.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci. 2017;1394:31–54. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26:251–7. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Molenberghs P, Morrison S. The role of the medial prefrontal cortex in social categorization. Soc Cogn Affect Neurosci. 2014;9:292–6. doi: 10.1093/scan/nss135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bault N, Joffily M, Rustichini A, Coricelli G. Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proc Natl Acad Sci U S A. 2011;108:16044–9. doi: 10.1073/pnas.1100892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- 23.Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2:117–33. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- 24.Lou HC, Nowak M, Kjaer TW. The mental self. Prog Brain Res. 2005;150:197–204. doi: 10.1016/S0079-6123(05)50014-1. [DOI] [PubMed] [Google Scholar]
- 25.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou HC, Luber B, Stanford A, Lisanby SH. Self-specific processing in the default network: a single-pulse TMS study. Exp brain Res. 2010;207:27–38. doi: 10.1007/s00221-010-2425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ 3rd, Thayer JF, Kirschbaum C, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancini-Marie A, Potvin S, Fahim C, Beauregard M, Mensour B, Stip E. Neural correlates of the affect regulation model in schizophrenia patients with substance use history: a functional magnetic resonance imaging study. J Clin Psychiatry. 2006;67:342–50. doi: 10.4088/jcp.v67n0302. [DOI] [PubMed] [Google Scholar]
- 29.Courtin J, Bienvenu TC, Einarsson EO, Herry C. Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience. 2013;240:219–42. doi: 10.1016/j.neuroscience.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanlon CA, Dowdle LT, Moss H, Canterberry M, George MS. Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: an interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology. 2016;41:3032–41. doi: 10.1038/npp.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattavelli G, Zuglian P, Dabroi E, Gaslini G, Clerici M, Papagno C. Transcranial magnetic stimulation of medial prefrontal cortex modulates implicit attitudes towards food. Appetite. 2015;89:70–6. doi: 10.1016/j.appet.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 33.van der Graaf FH, Maguire RP, Leenders KL, de Jong BM. Cerebral activation related to implicit sequence learning in a double serial reaction time task. Brain Res. 2006;1081:179–90. doi: 10.1016/j.brainres.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 34.Abend R, Jalon I, Gurevitch G, Sar-El R, Shechner T, Pine DS, et al. Modulation of fear extinction processes using transcranial electrical stimulation. Transl Psychiatry. 2016;6:e913. doi: 10.1038/tp.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasselmo ME. A model of prefrontal cortical mechanisms for goal-directed behavior. J Cogn Neurosci. 2005;17:1115–29. doi: 10.1162/0898929054475190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fellows LK. Advances in understanding ventromedial prefrontal function: the accountant joins the executive. Neurology. 2007;68:991–5. doi: 10.1212/01.wnl.0000257835.46290.57. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cereb Cortex. 2009;19:1134–43. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 38.Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. 2005;52:336–72. [Google Scholar]
- 39.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr Res. 2011;129:163–8. doi: 10.1016/j.schres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camchong J, MacDonald AW 3rd, Bell C, Mueller BA, Lim KO Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–50. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 46.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–9. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–30. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmari SE, Dougherty DD. Dissecting ocd circuits: from animal models to targeted treatments. Depress Anxiety. 2015;32:550–62. doi: 10.1002/da.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–36. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 2011;26:463–9. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biol Psychol. 2007;75:124–30. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res. 1999;33:259–64. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 58.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29:449–59. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–8. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Crossley NA, Scott J, Ellison-Wright I, Mechelli A. Neuroimaging distinction between neurological and psychiatric disorders. Br J Psychiatry. 2015;207:429–34. doi: 10.1192/bjp.bp.114.154393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koziol LF, Barker LA, Joyce AW, Hrin S. Structure and function of large-scale brain systems. Appl Neuropsychol Child. 2014;3:236–44. doi: 10.1080/21622965.2014.946797. [DOI] [PubMed] [Google Scholar]
- 63.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psychiatry. 2013;4:179. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Downar J, Daskalakis ZJ. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 2013;6:231–40. doi: 10.1016/j.brs.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosch OG, Rihm JS, Scheidegger M, Landolt HP, Stämpfli P, Brakowski J, et al. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc Natl Acad Sci U S A. 2013;110:19597–602. doi: 10.1073/pnas.1317010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 71.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29:14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 73.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 74.Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561–75. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schecklmann M, Landgrebe M, Kleinjung T, Frank E, Sand PG, Rupprecht R, et al. Changes in motor cortex excitability associated with temporal repetitive transcranial magnetic stimulation in tinnitus: hints for cross-modal plasticity? BMC Neurosci. 2014;15:71. doi: 10.1186/1471-2202-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, et al. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: I. Effects of primary motor cortex rTMS. Biol Psychiatry. 2003;54:818–25. doi: 10.1016/s0006-3223(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 80.Loo CK, Sachdev PS, Haindl W, Wen W, Mitchell PB, Croker VM, et al. High (15 Hz) and low (1 Hz) frequency transcranial magnetic stimulation have different acute effects on regional cerebral blood flow in depressed patients. Psychol Med. 2003;33:997–1006. doi: 10.1017/s0033291703007955. [DOI] [PubMed] [Google Scholar]
- 81.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–39. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 83.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taïb S, Arbus C, Sauvaget A, Sporer M, Schmitt L, Yrondi A. How does repetitive transcranial magnetic stimulation influence the brain in depressive disorders? J ECT. 2018;34:79–86. doi: 10.1097/YCT.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 85.Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, et al. What goes up, can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209. doi: 10.1016/j.brainres.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roth Y, Padberg F, Zangen A. Transcranial magnetic stimulation of deep brain regions: principles and methods. Adv Biol Psychiatry. 2007;23:204–24. [Google Scholar]
- 87.Stagg CJ, O’Shea J, Johansen-Berg H. Imaging the effects of rTMS-induced cortical plasticity. Restor Neurol Neurosci. 2010;28:425–36. doi: 10.3233/RNN-2010-0553. [DOI] [PubMed] [Google Scholar]
- 88.Hanlon CA, Canterberry M, Taylor JJ, DeVries W, Li X, Brown TR, et al. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One. 2013;8:e67917. doi: 10.1371/journal.pone.0067917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–85. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 90.Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J. Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology. 2016;41:1395–403. doi: 10.1038/npp.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–98. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christov-Moore L, Sugiyama T, Grigaityte K, Iacoboni M. Increasing generosity by disrupting prefrontal cortex. Soc Neurosci. 2017;12:174–81. doi: 10.1080/17470919.2016.1154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferrari C, Lega C, Vernice M, Tamietto M, Mende-Siedlecki P, Vecchi T, et al. The dorsomedial prefrontal cortex plays a causal role in integrating social impressions from faces and verbal descriptions. Cereb Cortex. 2016;26:156–65. doi: 10.1093/cercor/bhu186. [DOI] [PubMed] [Google Scholar]
- 94.Balconi M, Canavesio Y. High-frequency rTMS improves facial mimicry and detection responses in an empathic emotional task. Neuroscience. 2013;236:12–20. doi: 10.1016/j.neuroscience.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 95.Balconi M, Bortolotti A, Gonzaga L. Emotional face recognition, EMG response, and medial prefrontal activity in empathic behaviour. Neurosci Res. 2011;71:251–9. doi: 10.1016/j.neures.2011.07.1833. [DOI] [PubMed] [Google Scholar]
- 96.Cattaneo Z, Mattavelli G, Platania E, Papagno C. The role of the prefrontal cortex in controlling gender-stereotypical associations: a TMS investigation. Neuroimage. 2011;56:1839–46. doi: 10.1016/j.neuroimage.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 97.Gamond L, Cattaneo Z. The dorsomedial prefrontal cortex plays a causal role in mediating in-group advantage in emotion recognition: a TMS study. Neuropsychologia. 2016;93:312–7. doi: 10.1016/j.neuropsychologia.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 98.De Pisapia N, Barchiesi G, Jovicich J, Cattaneo L. The role of medial prefrontal cortex in processing emotional self-referential information: a combined TMS/fMRI study. Brain Imaging Behav. 2018 May 9 doi: 10.1007/s11682-018-9867-3. doi: http://10.1007/s11682-018-9867-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 99.Amati F, Oh H, Kwan VS, Jordan K, Keenan JP. Overclaiming and the medial prefrontal cortex: a transcranial magnetic stimulation study. Cogn Neurosci. 2010;1:268–76. doi: 10.1080/17588928.2010.493971. [DOI] [PubMed] [Google Scholar]
- 100.Guhn A, Dresler T, Andreatta M, Müller LD, Hahn T, Tupak SV, et al. Medial prefrontal cortex stimulation modulates the processing of conditioned fear. Front Behav Neurosci. 2014;8:44. doi: 10.3389/fnbeh.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hurlemann R, Arndt S, Schlaepfer TE, Reul J, Maier W, Scheele D. Diminished appetitive startle modulation following targeted inhibition of prefrontal cortex. Sci Rep. 2015;5:8954. doi: 10.1038/srep08954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanda M, Mima T, Oga T, Matsuhashi M, Toma K, Hara H, et al. Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin Neurophysiol. 2003;114:860–6. doi: 10.1016/s1388-2457(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 103.Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012;153:1350–63. doi: 10.1016/j.pain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Karmann AJ, Maihöfner C, Lautenbacher S, Sperling W, Kornhuber J, Kunz M. The role of prefrontal inhibition in regulating facial expressions of pain: a repetitive transcranial magnetic stimulation study. J Pain. 2016;17:383–91. doi: 10.1016/j.jpain.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 105.Cho SS, Koshimori Y, Aminian K, Obeso I, Rusjan P, Lang AE, et al. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40:546–53. doi: 10.1038/npp.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brückner S, Kiefer M, Kammer T. Comparing the after-effects of continuous theta burst stimulation and conventional 1Hz rTMS on semantic processing. Neuroscience. 2013;233:64–71. doi: 10.1016/j.neuroscience.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 107.Berkers RM, van der Linden M, de Almeida RF, Müller NC, Bovy L, Dresler M, et al. Transient medial prefrontal perturbation reduces false memory formation. Cortex. 2017;88:42–52. doi: 10.1016/j.cortex.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 108.Downar J, Sankar A, Giacobbe P, Woodside B, Colton P. Unanticipated rapid remission of refractory bulimia nervosa, during high-dose repetitive transcranial magnetic stimulation of the dorsomedial prefrontal cortex: a case report. Front Psychiatry. 2012;3:30. doi: 10.3389/fpsyt.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakker N, Shahab S, Giacobbe P, Blumberger DM, Daskalakis ZJ, Kennedy SH, et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015;8:208–15. doi: 10.1016/j.brs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Hanlon CA, Dowdle LT, Correia B, Mithoefer O, Kearney-Ramos T, Lench D, et al. Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend. 2017;178:310–7. doi: 10.1016/j.drugalcdep.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dunlop K, Woodside B, Lam E, Olmsted M, Colton P, Giacobbe P, et al. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin. 2015;8:611–8. doi: 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158–65. doi: 10.1016/j.brs.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A, et al. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol. 2015;93:283–90. doi: 10.1139/cjpp-2014-0188. [DOI] [PubMed] [Google Scholar]
- 114.Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, et al. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul. 2014;7:206–11. doi: 10.1016/j.brs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 115.Herrmann MJ, Katzorke A, Busch Y, Gromer D, Polak T, Pauli P, et al. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 2017;10:291–7. doi: 10.1016/j.brs.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 116.Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimul. 2013;6:377–83. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 117.Kreuzer PM, Schecklmann M, Lehner A, Wetter TC, Poeppl TB, Rupprecht R, et al. The ACDC pilot trial: targeting the anterior cingulate by double cone coil rTMS for the treatment of depression. Brain Stimul. 2015;8:240–6. doi: 10.1016/j.brs.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 118.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–70. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 119.Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-Coil. Clin Neurophysiol. 2005;116:775–9. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 120.Kreuzer PM, Downar J, de Ridder D, Schwarzbach J, Schecklmann M, Langguth B. A comprehensive review of dorsomedial prefrontal cortex rTMS utilizing a double cone coil. Neuromodulation. 2018 Nov 8 doi: 10.1111/ner.12874. doi: http://10.1111/ner.12874 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 121.Herrmann MJ, Katzorke A, Busch Y, Gromer D, Polak T, Pauli P, et al. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 2017;10:291–7. doi: 10.1016/j.brs.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 122.Tendler A, Roth Y, Barnea-Ygael N, Zangen A. How to use the H1 deep transcranial magnetic stimulation coil for conditions other than depression. J Vis Exp. 2017;119 doi: 10.3791/55100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dobek CE, Blumberger DM, Downar J, Daskalakis ZJ, Fidel Vila-Rodriguez F. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975–87. doi: 10.2147/NDT.S91126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schulze L, Wheeler S, McAndrews MP, Solomon CJ, Giacobbe P, Downar J. Cognitive safety of dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Eur Neuropsychopharmacol. 2016;26:1213–26. doi: 10.1016/j.euroneuro.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–57. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 129.Coutinho JF, Fernandesl SV, Soares JM, Maia L, Gonçalves OF, Sampaio A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016;10:147–57. doi: 10.1007/s11682-015-9375-7. [DOI] [PubMed] [Google Scholar]
- 130.Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98:683–7. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Etkin A. Neurobiology of anxiety: from neural circuits to novel solutions? Depress Anxiety. 2012;29:355–8. doi: 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- 133.Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol Psychiatry. 2014;76:274–80. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 134.Censor N, Dayan E, Cohen LG. Cortico-subcortical neuronal circuitry associated with reconsolidation of human procedural memories. Cortex. 2014;58:281–8. doi: 10.1016/j.cortex.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sandrini M, Cohen LG, Censor N. Modulating reconsolidation: a link to causal systems-level dynamics of human memories. Trends Cogn Sci. 2015;19:475–82. doi: 10.1016/j.tics.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Enticott PG, Kennedy HA, Zangen A, Fitzgerald PB. Deep repetitive transcranial magnetic stimulation associated with improved social functioning in a young woman with an autism spectrum disorder. J ECT. 2011;27:41–3. doi: 10.1097/YCT.0b013e3181f07948. [DOI] [PubMed] [Google Scholar]
- 137.Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multicenter mapping of structural network alterations in autism. Hum Brain Mapp. 2015;36:2364–73. doi: 10.1002/hbm.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gilbert SJ, Bird G, Brindley R, Frith CD, Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–91. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parellada M, Penzol MJ, Pina L, Moreno C, González-Vioque E, Zalsman G, et al. The neurobiology of autism spectrum disorders. Eur Psychiatry. 2014;29:11–9. doi: 10.1016/j.eurpsy.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 140.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–23. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 141.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 142.Balconi M, Bortolotti A. Emotional face recognition, empathic trait (BEES), and cortical contribution in response to positive and negative cues. The effect of rTMS on dorsal medial prefrontal cortex. Cogn Neurodyn. 2013;7:13–21. doi: 10.1007/s11571-012-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferrari C, Vecchi T, Todorov A, Cattaneo Z. Interfering with activity in the dorsomedial prefrontal cortex via TMS affects social impressions updating. Cogn Affect Behav Neurosci. 2016;16:626–34. doi: 10.3758/s13415-016-0419-2. [DOI] [PubMed] [Google Scholar]
- 144.Schuwerk T, Schecklmann M, Langguth B, Dohnel K, Sodian B, Sommer M. Inhibiting the posterior medial prefrontal cortex by rTMS decreases the discrepancy between self and other in theory of mind reasoning. Behav Brain Res. 2014;274:312–8. doi: 10.1016/j.bbr.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 145.Krause L, Enticott PG, Zangen A, Fitzgerald PB. The role of medial prefrontal cortex in theory of mind: a deep rTMS study. Behav Brain Res. 2012;228:87–90. doi: 10.1016/j.bbr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 146.Balconi M, Crivelli D, Bortolotti A. Detection of facial expression of emotion and self-report measures in empathic situations are influenced by ACC inhibition: rTMS evidences. Neuropsychol Trends. 2010;8:95–9. [Google Scholar]
- 147.Coenen VA, Schumacher LV, Kaller C, Schlaepfer TE, Reinacher PC, Egger K, et al. The anatomy of the human medial forebrain bundle: ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin. 2018;18:770–83. doi: 10.1016/j.nicl.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–36. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–9. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 151.Martinez D, Urban N, Grassetti A, Chang D, Hu MC, Zangen A, et al. Transcranial magnetic stimulation of medial prefrontal and cingulate cortices reduces cocaine self-administration: a pilot study. Front Psychiatry. 2018;9:80. doi: 10.3389/fpsyt.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–9. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–32. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]