Conspectus

In the past decade, research into continuous-flow chemistry has gained a lot of traction among researchers in both academia and industry. Especially, microreactors have received a plethora of attention due to the increased mass and heat transfer characteristics, the possibility to increase process safety, and the potential to implement automation protocols and process analytical technology. Taking advantage of these aspects, chemists and chemical engineers have capitalized on expanding the chemical space available to synthetic organic chemists using this technology.
Electrochemistry has recently witnessed a renaissance in research interests as it provides chemists unique and tunable synthetic opportunities to carry out redox chemistry using electrons as traceless reagents, thus effectively avoiding the use of hazardous and toxic reductants and oxidants. The popularity of electrochemistry stems also from the potential to harvest sustainable electricity, derived from solar and wind energy. Hence, the electrification of the chemical industry offers an opportunity to locally produce commodity chemicals, effectively reducing inefficiencies with regard to transportation and storage of hazardous chemicals.
The combination of flow technology and electrochemistry provides practitioners with great control over the reaction conditions, effectively improving the reproducibility of electrochemistry. However, carrying out electrochemical reactions in flow is more complicated than just pumping the chemicals through a narrow-gap electrolytic cell. Understanding the engineering principles behind the observations can help researchers to exploit the full potential of the technology. Thus, the prime objective of this Account is to provide readers with an overview of the underlying engineering aspects which are associated with continuous-flow electrochemistry. This includes a discussion of relevant mass and heat transport phenomena encountered in electrochemical flow reactors. Next, we discuss the possibility to integrate several reaction steps in a single streamlined process and the potential to carry out challenging multiphase electrochemical transformations in flow. Due to the high control over mass and heat transfer, electrochemical reactions can be carried out with great precision and reproducibility which provide opportunities to enhance and tune the reaction selectivity. Finally, we detail on the scale-up potential of flow electrochemistry and the importance of small interelectrode gaps on pilot and industrial-scale electrochemical processes. Each principle has been illustrated with a relevant organic synthetic example. In general, we have aimed to describe the underlying engineering principles in simple words and with a minimum of equations to attract and engage readers from both a synthetic organic chemistry and a chemical engineering background. Hence, we anticipate that this Account will serve as a useful guide through the fascinating world of flow electrochemistry.
Introduction
The chemical industry consumes more than 20% of all resources to generate energy and end-user products. While the consumption of energy itself is not necessarily a problem, energy consumption is currently strongly correlated with the emission of greenhouse gases. Thus, the chemical industry is responsible for >20% of the global CO2 output and holds great potential to reduce the total carbon footprint on our planet. The Chemistry 4.0 & Industry 4.0 initiative wants to improve the energy efficiency of the chemical industry through use of disruptive technologies, such as artificial intelligence and digitization, and process intensification.1,2 One of the potential strategies that is currently under investigation is to switch to alternative energy sources in the chemical industry, such as the utilization of electrical energy to effect useful synthetic transformations. This so-called “electrification of the chemical industry” not only can aid to reduce the fossil fuel consumption, but also can result in a reduction of the energy consumption and in an increase of reaction efficiency and selectivity.3
Indeed, in the near future, we will witness a massive energy transition from fossil fuels to sustainable energy sources, such as solar and wind energy. It is evident that this drastic change is also needed in the chemical industry to reduce CO2 emissions and minimize the effects of global warming. Solar and wind energy can be transformed into electricity, and this energy form can be directly used to induce chemical transformations. Since often no other reagents are needed, electrochemistry can be regarded as an efficient and green activation mode of organic molecules which avoids additional reagent waste.4 Furthermore, it circumvents the use of expensive and scarce catalysts and ligands to enable chemical transformations which are otherwise elusive.
In times of increasing environmental awareness, sustainable electricity is becoming increasingly available by harvesting sun and wind energy. However, the unsteady generation of electricity by these regenerative resources leads to abundant electric power, which poses significant challenges to the power grid. This transient and sustainable energy supply could be used for electrochemical conversions of organic molecules: for example, by building small-scale chemical installations integrated with windmills or solar panels.5 In addition, local production will also reduce current inefficiencies with regard to transportation, distribution, and storage of hazardous chemicals.
Currently, we witness a complete revival of electrochemistry in the organic synthetic chemistry community.6 However, even though the advantages of electrochemistry appear to be numerous, the renewed interest in electrochemistry has brought some old and tedious technological problems to the surface. Due to the use of solid phase electrodes, electrochemistry can be considered a heterogeneous process and can be quite challenging to those not familiar with mass transfer effects. This means that both mixing effects and interelectrode distances become important parameters to be considered in the development process. Furthermore, the need for specialized equipment and the knowledge gap of most researchers in this—often perceived—esoteric discipline are additional barriers to adopt electrochemistry as viable alternative synthetic routes.
From its start in 2012, our group has taken a particular interest in solving technological problems in synthetic organic chemistry. By taking on such challenges, we hope that we can provide simple solutions which allows the chemist to expand the chemical territory available for synthesis. As described above and by others,7 despite its rich tradition with—among others—initial experiments by Michael Faraday,8 the field of electrochemistry necessitates a technological impetus and thus is definitely worthy of our attention. While both batch and flow electrochemical reactors are on the market, we have experienced in the past that a “Do-it-Yourself” (DIY) approach was really rewarding as it provides a deeper understanding of the technology. Moreover, the DIY approach allows us (i) to reduce the cost price of the required equipment and the concomitant maintenance and reparations, (ii) to tailor the designs to our specific needs, and (iii) to exploit the reactor at its full potential (in contrast to the—often black box—commercial devices). Recently, we developed an electrochemical flow reactor with a flexible reactor volume (Figure 1).9 The reactor can be operated in serial mode (with a volume ranging from 88 μL/channel up to 704 μL when all eight channels are in use) or in parallel mode. The parallel mode allows to scale the chemistry via a so-called numbering-up strategy or to scan different reaction conditions simultaneously. The latter aspect was used to rapidly find optimal reaction conditions for the electrocatalytic reduction of furfural to furfuryl alcohol in flow.10
Figure 1.
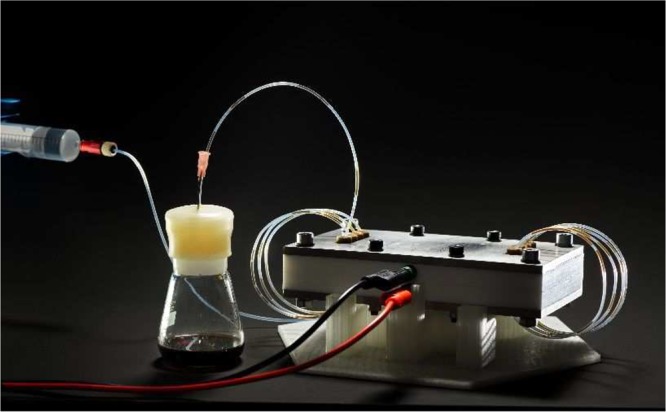
Electrochemical flow reactor developed in our group. (Courtesy of Bart van Overbeeke, TU Eindhoven.)
The primary objective of this Account is to provide the reader with some important guidelines for the use of continuous-flow reactors for electrochemistry. We anticipate that this concise overview will help researchers to gain insight in the basic principles behind this technology and, in addition, it will allow the reader to get the maximum out of their experiments. Further, we aimed to highlight and clarify each fundamental principle with a suitable organic synthetic example. It is, however, not our aim to give an exhaustive overview of the field of continuous-flow electrochemistry, for which we direct the reader to some recent, excellent reviews.11−13
Bringing the Chemicals to the Electrodes—The Importance of Mass Transfer
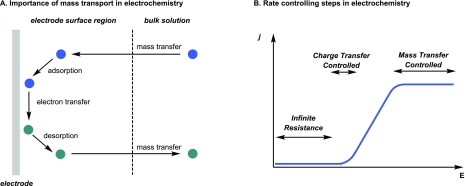
Electrochemical reactions are driven by single electron transfer processes which are initiated at the surface of an electrode. Hence, electrochemical transformations can be regarded as heterogeneous reactions, and thus, substrates or electron mediators need to be transported from the bulk of the solution to the electrode surface. Such mass transport phenomena are, together with electron transfer kinetics, key in the design of an appropriate electrochemical reactor (Figure 2A). Typically, an electrochemical reaction starts with the mass transfer of the substrate from the bulk phase to the heterogeneous electrode surface. Next, the substrate adsorbs onto the electrode and an electron transfer can occur, converting the substrate into product. Finally, the product is desorbed from the electrode and diffuses back to the bulk liquid phase.
Figure 2.
(A) Individual steps in an electrochemical transformation. (B) Simplified rate controlling parameters in electrochemistry with j the current density and E the potential measured versus a reference electrode.
Evidently, the overall reaction rate will depend mainly on the slowest of these steps, i.e. the rate-determining step. Two extreme scenarios can be distinguished: (i) a charge transfer controlled regime and (ii) a mass transfer controlled regime (Figure 2B). With increasing electrode polarization, the reactant concentration at the electrodes becomes zero. In other words, all reactants are immediately converted upon arrival at the electrode surface and, in such a scenario, the rate of mass transport limits the overall reaction rate. Under these conditions, intensified mass transport, e.g., by more vigorous stirring in batch or through use of static mixers in flow, can reduce the overall reaction time.
In microreactors, the flow regime is almost exclusively laminar. This means that convective mass transport is absent. Thus, the mass transport relies on two fundamental phenomena, including diffusion and migration. Diffusion is the movement of molecules due to a concentration gradient which occurs due to consumption of substrate molecules at the electrode. Migration is the movement of charged species in a potential field. However, if the substrate concentration is low in comparison to, e.g., an excess of supporting electrolyte, the contribution of migration to the mass transfer can be neglected. Thus, mass transport is largely dominated by diffusion in electrochemical microreactors. Based on statistics, the distance (d) that a molecule can travel in time (t) is given by eq 1. Using this equation, one can calculate that a molecule in the liquid (with a diffusivity D = 10–5 cm2·s–1) can diffuse 50 μm in 1 s, while it diffuses only 1.3 cm in 1 day. Thus, it can be easily understood that the smaller the reactor channels are, the faster the electrochemical reactions can be due to short diffusion paths to the electrodes.14 In contrast, in batch reactors with larger interelectrode gaps, relying on diffusion will not be sufficient and thus intense stirring is required to assist the mass transfer from the bulk to the electrode surface.
| 1 |
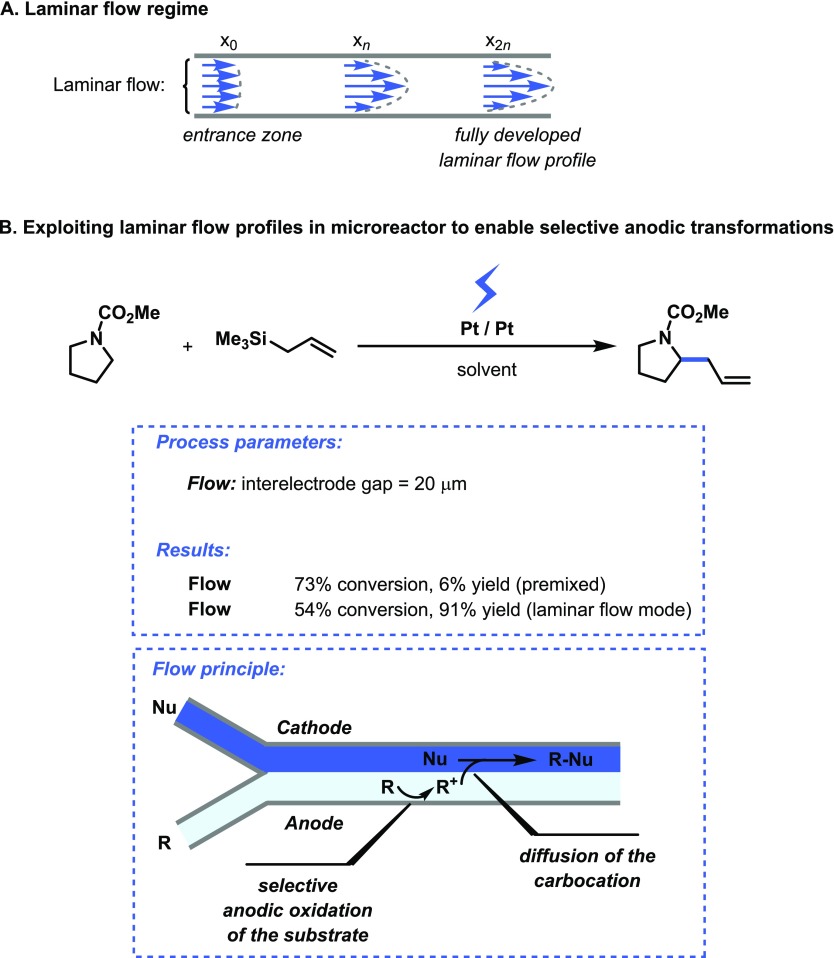
In a laminar flow regime, fluid is flowing in parallel lamellae and mixing between individual segments is governed exclusively by diffusion (Figure 3A). This phenomenon can be exploited to selectively contact one of the reagents with either the anode or the cathode and thus enable selective electrochemical transformations. This principle was demonstrated by Atobe et al. in the selective anodic substitution reaction of N-(methoxycarbonyl)pyrrolidine (Eox = +1.91 V vs Ag/AgCl) with allyltrimethylsilane (Eox = +1.75 V vs Ag/AgCl) (Figure 3B).15 As can be judged from the oxidation potentials, oxidation of the nucleophile is preferred over the formation of the substrate carbocation. However, by using two inlets, the stream containing the substrate can be selectively directed over the anode while the nucleophile is passed over the cathode. This allows for selective formation of the carbocation which can subsequently diffuse to the cathode side and react with the nucleophile. Indeed, when the two solutions were premixed, the target compound was only obtained in low yield (6%). However, by separating the two solutions and by directing only the substrate over the anode, a high yield for the desired product was obtained (91%), albeit at a low conversion of 54%. The use of an ionic liquid as electrolytic solution proved beneficial and allowed to stabilize the formed carbocation long enough to enable its diffusion to the cathodic side.
Figure 3.
(A) Laminar flow regime encountered in microchannels. (B) Selective anodic substitution reaction due selective wetting of the anode and cathode.
The relative distance between two electrodes is also of importance with regard to the so-called ohmic drop. This refers to the voltage drop observed due to the additional resistance, which is encountered by the electric current when traveling through the liquid phase between the two electrodes. According to eq 2, the ohmic drop (Rdrop) is influenced by the conductivity of the solution (κ), the interelectrode gap distance (d), and the magnitude of the current (I):
| 2 |
with ΔU being the potential or ohmic drop between the electrodes, I being the current, and Ae being the electrode surface. In batch, ohmic drops are typically controlled by adding supporting electrolytes, which increase the conductivity of the solution (κ). Large amounts of supporting electrolytes make the overall electrochemical process more complicated, as the supporting electrolyte needs to be separated from the product and preferentially be recycled to minimize the costs. One way to minimize the amount of supporting electrolyte is to keep the distance between the electrodes small. In other words, the shorter the distance between the two electrodes, the lower the ohmic drop will be.16 Consequently, it is easy to understand that if the interelectrode gap (d) is reduced by a factor 100, one can similarly lower the conductivity of the solution (κ) by a factor 100. Interestingly, in microreactors, Nernst diffusion layers are between 10 and 500 μm and can thus overlap which allows for the coupling of the anodic and the cathodic electrode processes.17
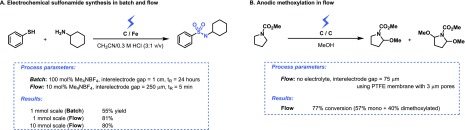
Pioneering work on micro electrochemical flow cells with small interelectrode gap was carried out by Beck and Guthke already in 1969.18 More recently, our group showed that for the electrochemical oxidative coupling of amines and thiols to yield sulfonamides, the amount of supporting electrolyte could be reduced from 100 mol % in batch (interelectrode gap = 1 cm) to 10 mol % in flow (interelectrode gap = 250 μm) (Figure 4A).19 Furthermore, the reaction time could be reduced from 24 h in batch to only 5 min in flow. This acceleration effect in flow can be attributed to the shorter diffusion distances, the large electrode-surface-to-volume ratio and the intensified mass transport due to the formation of hydrogen gas,20 which induces turbulence. In a notable, early example of an electrochemical microreactor, Yoshida et al. showed that in a microreactor with a PTFE membrane (75 μm thick, 3 μm pores) as a spacer, the supporting electrolyte could be even completely avoided for the anodic methoxylation (Figure 4B).21
Figure 4.
Microreactor technology enables the reduction of the total amount of supporting electrolyte by narrowing the interelectrode gap. (A) Electrochemical sulfonamide synthesis through anodic oxidation and coupling of thiols and amines. (B) Anodic methoxylation in a microreactor.
Take the Heat out of the Reactor—Fast Heat Exchange in Microreactors
Due to the electrical current running through the solution, the latter will heat up due to a phenomenon called Joule heating. The ohmic drop plays a crucial role in the heat generation term due the electrochemical process.22 According to Joule’s first law, the power of heating (P) is directly proportional to the ohmic drop (Rdrop) and the current:
| 3 |
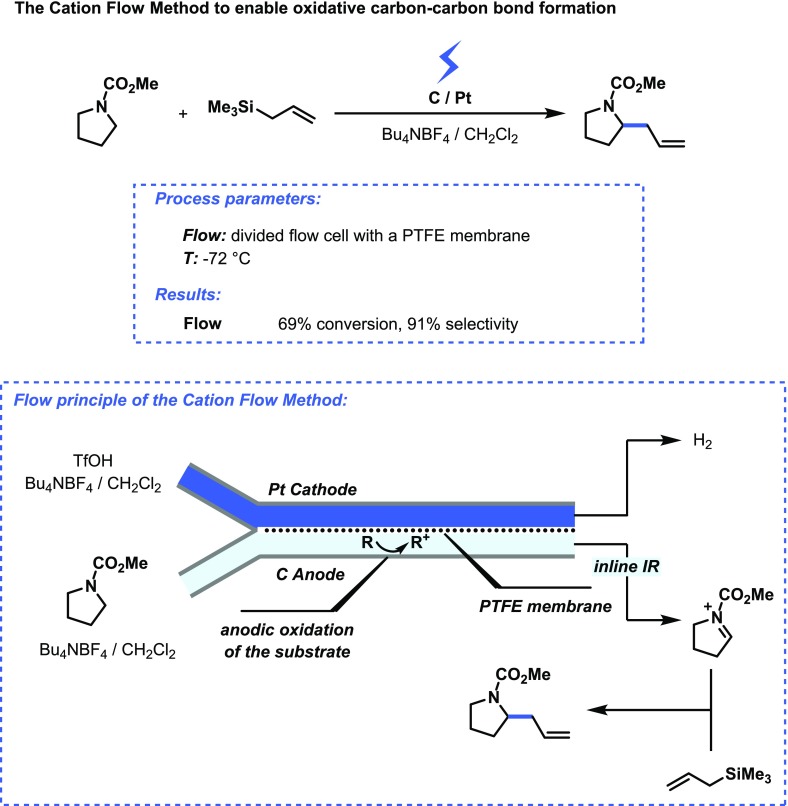
As mentioned before, the ohmic drop can be minimized in microreactors due to the small interelectrode gap. Furthermore, due to the continuous nature of these reactors and the absence of dead zones, the heat is removed and thus not built up in the electrochemical cell. As a consequence of the high surface-to-volume ratio, microreactors are well suited to enable fast heat exchange and in some cases can be even considered as isothermal reactors.23 It should be noted though that an increase in temperature can have a positive impact on the electrochemical reaction due to increases in electrolyte conductivity, mass transfer rate and kinetic rate. However, when a good reaction selectivity is required, careful control over the reaction temperature is crucial. Low temperature processes are important to generate reactive species, as exemplified by the cation flow method developed by Yoshida and co-workers.24 Hereby, unstable carbocations are generated at −72 °C in a divided electrochemical flow reactor and subsequently fed to another flow reactor where they are quenched by a nucleophile (see Figure 5).25 Hence, the incompatibility of using nucleophiles in anodic oxidation processes can be elegantly circumvented. Another notable feature of flow chemistry, i.e., the possible implementation of analytical technology, allowed one to follow the formation of the acyliminium cation in real time using inline infrared spectroscopy.
Figure 5.
Microreactor technology enables low temperature electrochemistry to prepare carbocations in high yield.
Combine it All—Multistep Reaction Sequences in Flow
Synthetic routes toward complex molecules typically comprise many individual synthetic and purification steps. Hence, a total synthesis of such molecules is a time- and labor-intensive undertaking, even for experts. Using continuous-flow technology, many of these steps can be combined in one single, streamlined flow configuration.26,27 This is especially important for the synthesis of short-living species, which can then be subsequently consumed in a follow-up reaction without extensive degradation. It represents also an important strategy to generate and convert hazardous and toxic intermediates. Consequently, the total inventory can be kept small, which enables one to minimize the risks associated with handling such compounds.28−30
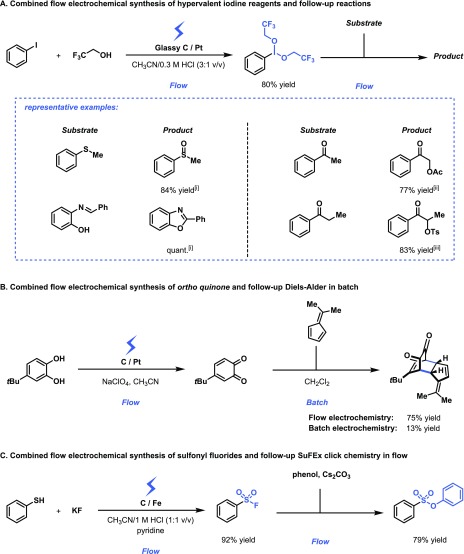
Wirth and co-workers have developed an electrochemical flow method to prepare bench-unstable hypervalent iodine reagents in flow.31−33 Hypervalent iodine reagents are known to be very useful synthetic reagents in organic chemistry, and the researchers combined the electrochemical flow synthesis with a variety of follow-up transformations, including oxidation chemistry, heterocycle synthesis, and α-acetoxylation and tosyloxylation, in a coupled flow protocol (Figure 6A). This strategy allowed the reaction to immediately consume the bench-unstable hypervalent iodine reagents without further decomposition.
Figure 6.
Multistep flow sequences involving an electrochemical step: (A) Synthesis of hypervalent iodine reagents via anodic oxidation in flow and subsequent synthetic applications. [i] CH3CN as solvent; [ii] AcOH/CH3CN (1:4) as solvent, 30 mol % BF3·OEt2; [iii] CH3CN as solvent, 1.2 equiv of TsOH. (B) Synthesis of ortho-quinone via anodic oxidation in flow and subsequent Diels–Alder with 6,6-dimethylfulvene in batch. (C) Electrochemical synthesis of sulfonyl fluorides in flow and follow up sulfur(VI) fluoride exchange “click” reaction in flow with phenol to yield the corresponding phenyl sulfonate derivative.
Another interesting example involves the generation of ortho-quinones via anodic oxidation, which can be subsequently consumed in a Diels–Alder reaction in batch (Figure 6B).34ortho-Quinones are not shelf-stable due to decomposition, isomerization, or polymerization and are often difficult to prepare due to competing oxidation processes with the dienophile. Indeed, Atobe et al. found that the oxidation potentials of 4-tert-butylpyrocatechol and 6,6-dimethylfulvene were relatively close and thus competing oxidations can be expected under such scenarios. The researchers prepared the ortho-quinone by introducing 4-tert-butylpyrocatechol into an electrochemical microreactor. Next, the reaction stream containing ortho-quinone was added dropwise to a batch vessel containing a solution of 6,6-dimethylfulvene to enable the Diels–Alder reaction. The corresponding Diels–Alder product could be obtained in 75% yield, while in batch only 13% could be isolated. Interestingly, the optimal interelectrode gap was 80 μm. Shorter distances resulted in lower yields presumably because of reduction of the anodically formed ortho-quinone at the cathode which is able to diffuse too fast due to the short distances.
Recently, we developed a protocol to prepare sulfonyl fluorides via an anodic oxidation process starting from commodity chemicals like thiols or disulfides and potassium fluoride (Figure 6C).35 Sulfonyl fluorides are important synthetic motifs due to their applicability as stable sulfonyl precursors using sulfur(VI) fluoride exchange “click chemistry” (SuFEx). After the electrochemical step, phenyl sulfonyl fluoride was combined with a stream containing phenol, which enables the SuFEx chemistry in flow and yields the corresponding phenyl sulfonate derivative.36 This strategy enables to produce volatile sulfonyl fluorides and immediately utilize these moieties without intermediate isolation.
Combine the Immiscible—Multiphase Electrochemistry in Flow
Multiphase reactions are quite common in synthetic chemistry and include both gas–liquid and liquid–liquid transformations. In electrochemistry, gas–liquid reactions are often observed, e.g., when hydrogen evolution occurs as a byproduct at the counter electrode. However, such reactions are, from an engineering standpoint, very complex. The gas evolution can lead to a substantial rise in ohmic resistance due to the insulating layer of gas. However, the presence of gases can also substantially increase the mass transport.
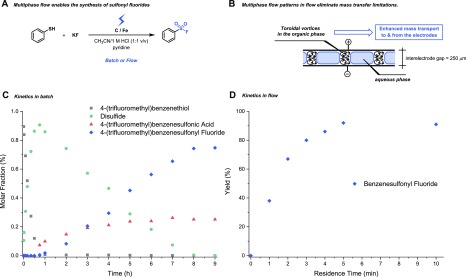
Another important aspect of multiphase electrochemistry is when the reactant is in a second dispersed phase. The reactant needs to either diffuse to the electrode or to the continuous phase prior to reaction. For efficient synthesis, the transport of the reactant to the second phase has to be as fast as possible. In microreactors, several flow regimes can be distinguished depending on the individual flow rates of the two phases, where annular flow and segmented flow (also called slug flow or Taylor flow) constitute the two extremes.37 In annular flow, one of the two phases flows at a higher velocity in the center of the capillary, while the other phase is contacted as a thin film to the electrodes. While this is rather rare in flow electrochemistry, one can anticipate that the intimate contact between the wetting phase and the electrode phase might be beneficial. In contrast, segmented flow is much more common in flow chemistry and is characterized by alternating bubbles and liquid segments. In such segments and bubbles, toroidal vortices are established which ensure a high mixing efficiency inside the segment or bubble (Figure 7B). Moreover, these fluid patterns also increase significantly the mass transfer to the electrode surfaces. Our group took advantage of this flow principle to accelerate the electrochemical sulfonyl fluoride synthesis in flow (Figure 7A).35 The reaction conditions are biphasic and the thiol or disulfide are in the organic phase, while KF is in the aqueous phase. In batch, we observed pseudo-zero-order kinetics which indicate that mass transfer limitations occurred from the bulk to the electrode surface (Figure 7D). However, when the reaction was carried out in an electrochemical microreactor with an interelectrode gap of 250 μm, the reaction time was reduced from 9 h in batch to only 5 min in flow (Figure 7C).
Figure 7.
Multiphase electrochemistry. (A) Electrochemical sulfonyl fluoride formation using a biphasic reaction mixture. (B) Multiphase segmented flow observed in microchannels. The toroidal vortices enhance mass transport form the bulk to the electrodes. (c) Kinetic experiment for the batch electrochemical sulfonyl fluoride synthesis. (d) Kinetic experiment for the flow electrochemical sulfonyl fluoride synthesis.
If Precision Is What You Aim for—Improved Reaction Selectivity and Increased Reproducibility in Flow
Continuous-flow microreactor technology provides a high degree of control over transport phenomena, including mass and heat transfer (vide supra). Furthermore, the impact of human error can be reduced through implementation of process analytical technology38 and automation protocols,39 which allows one to remotely carry out the entire process. Due to the continuous nature of the processes performed in microreactors, flow chemistry practitioners can control reaction times very precisely. For electrochemical transformations, this means that the reaction only occurs when in contact with the electrodes and once the reaction mixture exits the reactor the reactivity is effectively quenched.
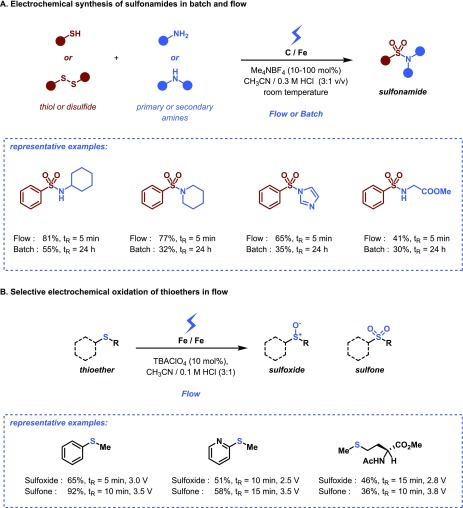
As we have shown above, reaction times can substantially be shortened using microreactor technology, due to the shorter diffusion distances to the electrodes and the large electrode surface-to-volume ratios. Consequently, products require to be only a minimum amount of time in the proximity of the electrodes, which reduces effectively the chances of byproduct formation. For the electrochemical synthesis of sulfonamides, we observed that this feature led consistently to improved yields and less byproduct formation compared to analogous batch experiments (Figure 8A).19
Figure 8.
Selective electrochemistry in flow through (A) reduction of reaction times in the electrochemical synthesis of sulfonamides and (B) control over the cell potential and the residence time for the selective anodic oxidation of thioethers.
Notably, high reaction selectivity can also be established by tuning the potential difference between the electrodes. As an example, our group has shown that the oxidation of thioethers can be selectively steered to either sulfoxides or sulfones in a commercially available electrochemical flow reactor (Figure 8B).40 An attractive feature of this methodology is that the same reagents are used in all scenarios and that the selectivity is solely governed by the applied potential and the residence time spent in the reactor.
Pump up the Volume—Reliable Scale-Up in Flow
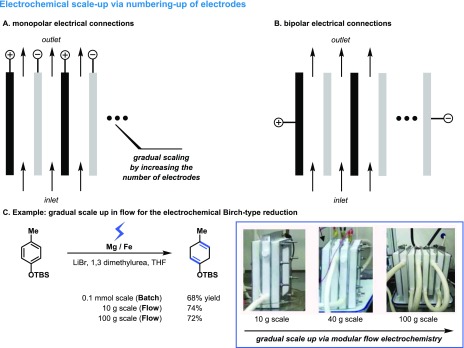
Increasing the productivity from laboratory scale to pilot and production scale is a formidable challenge for any given chemical transformation. However, as electrochemical reactions are surface reactions, classical scale up using a dimension-enlarging strategy is not feasible as the cell voltage and energy costs would dramatically go up when the interelectrode gap increases. Hence, industrial electrochemical reactors are almost exclusively flow cells.41 These flow cells consist of modular units that can be stacked in parallel (numbering-up approach) (Figure 9).42 A narrow interelectrode gap (0.5–10 mm) is kept in combination with a variable electrode area (0.01–16 m2/module), which provides a high specific area and a low cell voltage. The advantage of such a modular reactor concept is that initial results obtained on a smaller scale can be gradually scaled up to pilot and plant scale, requiring only a minimal amount of reoptimization. Electrical current can be fed to different electrodes in a monopolar or a bipolar fashion. In monopolar assemblies, all electrodes are individually connected to the power supply (Figure 9A), while in a bipolar connection only the ends of the stacked electrodes are coupled (Figure 9B). Bipolar connections are cheaper due to fewer electrical connections and improved potential and current distributions. Recently, Baran, Minteer, Neurock, and co-workers have developed a scalable electroreduction of arenes, which represents a safe alternative for the classical Birch reduction (Figure 9C).43 Reaction conditions developed in batch on a 0.1 mmol scale could be readily scaled in flow using a numbering-up strategy by stacking several electrodes in parallel (Figure 9C).
Figure 9.
Scaling of electrochemical transformations in flow using a numbering-up strategy. (A) Monopolar electrical connections. (B) Bipolar electrical connections. (C) Scaling of the electrochemical Birch reduction.
For inorganic electrosynthesis, the conductivity of the reaction solution is usually very high due to presence of highly ionizable species, e.g., NaCl in the Chlor-Alkali industry. In contrast, the conductivity is relatively low for organic electrosynthesis, which makes that process energy costs can become prohibitive.44 Thus, electrochemistry is particularly economically feasible for high value molecules, such as pharmaceuticals and agrochemicals. Another important cost consideration is the number of electron transfers that are required to effect a desired synthetic modification. The higher the electron-to-substrate ratio, the less competitive electrochemistry becomes compared to catalytic routes. The main factors that determine the cell energy consumption are the cell resistance and the operating current density. The cell potential (Ec) can be calculated as follows
| 4 |
and depends on the thermodynamically required potential (Ee), the overpotential (η), and the cell internal resistances (Re). The overpotential is the potential difference observed between the thermodynamically determined potential of the half-reaction and the potential at which the redox event experimentally occurs. The total overpotential comprises different individual contributions, including an electron transfer overpotential, a concentration overpotential and a resistance overpotential. Simply said the presence of overpotentials results in the consumption of more energy by the electrolytic cell than what is thermodynamically expected to drive the reaction forward. Reductions in energy costs can be obtained by (i) using larger amounts of electrolyte, (ii) minimizing the interelectrode gap, and (iii) replacing the counter-electrode reaction by a thermodynamically more favorable reaction which is “spontaneous”. Also the nature of the electrodes is important as these are involved in the electron transfer processes, can adsorb organic compounds, and can even act as a reagent (i.e., sacrificial electrode).45 In general, materials which have a large overpotential for oxygen are used as the anode (e.g., Pt, carbon based electrodes). A large overpotential for hydrogen as the cathode (e.g., carbon-based electrodes) is often preferred, except when hydrogen evolution is a desired reaction at the cathode. In the latter scenario, Pt, Cu, and stainless steel can be used owing to their low hydrogen overpotential. It should be noted that boron doped diamond is currently one of the most versatile electrode materials providing a wide potential window and low background current, and is physically and chemically stable.46
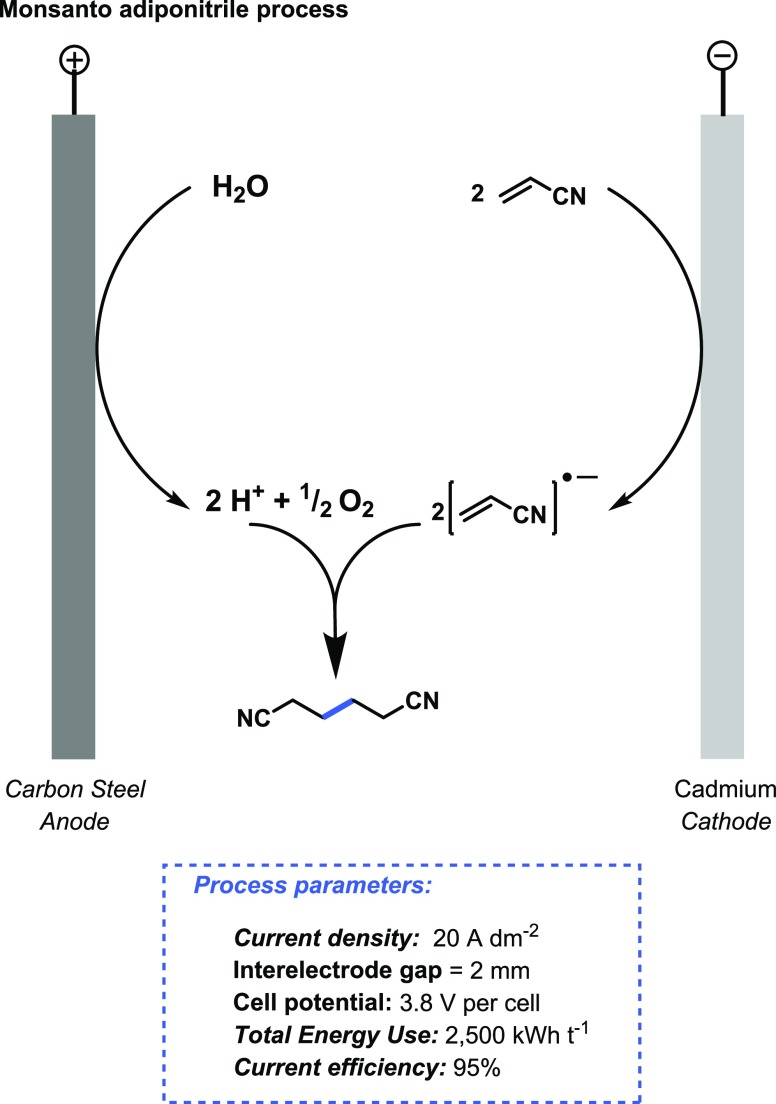
The most successful example of organic electrochemistry is the synthesis of adiponitrile from acrylonitrile, which produced in 2010 about 0.347 million metric tons per year of adiponitrile via a paired electrochemical route (Monsanto adiponitrile process) (Figure 10).47,48 A single, pressurized electrochemical reactor consists of 100–200 undivided cells consisting of a cadmium cathode, which provides the highest adiponitrile selectivity and electrode stability,49 and a carbon steel anode.50 A two-phase mixture of acrylonitrile, adiponitrile, quaternary ammonium salts, and phosphate buffer is introduced in the reactor. The current efficiency is close to 95%, and the cell energy consumption for the adiponitrile synthesis is about 2500 kWh t–1, which is comparable to the chlor-alkali process.
Figure 10.
Monsanto electrochemical process to prepare adiponitrile via a paired electrode process.
Conclusion and Outlook
This Account covers the fundamental principles and the advantages behind the use of continuous-flow microreactors for carrying out synthetic organic electrochemistry. This includes a discussion on the importance of mass and heat transfer, on carrying out multistep reaction sequences and enabling multiphase reaction conditions in a controlled fashion, on how to improve reaction selectivity, and about scale up. It was our aim to compile a document which hopefully will serve as a useful starting point for researchers looking to translate their electrochemistry to flow. Fundamental understanding about these key principles will allow practitioners to get the maximum out of the technology.
While significant progress has been made throughout the past decade, moving forward is not without a challenge. Through use of continuous-flow microreactors, new and uncharted chemical space can be discovered. Hence, the community should focus more on examples that provide decisive advantages over their batch counterparts. In our opinion, multiphase electrochemistry remains largely underrepresented to date.51 Arguably, clogging of the channels continues to be the Achilles heel of microreactor technology, and effective solutions need to be found to accommodate both solid reagents and products in flow.52 Furthermore, implementation of more advanced automation protocols and process analytical technology will be of great added value to minimize human error, to enhance the reproducibility of electrochemical transformations, and to accelerate reaction optimization and discovery.53 Solving these issues undoubtedly requires collaborative efforts between chemical engineers and chemists from both academia and industry.54 We are confident that progress on these aspects will increase the utility of flow reactor technology and will push the boundaries of synthetic organic electrochemistry.
Glossary
Abbreviations
- MeOH
methanol
- TfOH
trifluoromethanesulfonic acid
- THF
tetrahydrofuran.
Biographies
Timothy Noël currently holds a position as associate professor at Eindhoven University of Technology. His research interest ranges from organic chemistry to chemical engineering and encompasses more specifically flow chemistry, organic synthesis, and synthetic catalytic methodology development. His work received several awards, including most recently the VIDI award (2015), the Thieme Chemistry Journal Award (2016) and the DECHEMA prize (2017).
Yiran Cao was born in China in 1991. He completed his undergraduate education at East China University of Science and Technology. Next, he moved to Europe, where he obtained his master degree at the Otto von Guericke University of Magdeburg under the supervision of Professor Eckehard Specht. He is currently pursuing doctoral studies on organic electrochemistry and machine learning under the supervision of Dr. Timothy Noël.
Gabriele Laudadio was born in 1991 near Pescara, Italy. In 2016 he received his M.Sc. degree in Organic Chemistry at the University of Pisa. His master thesis was conducted under the supervision of Professor A. Carpita. He is currently a Ph.D. student at Eindhoven University of Technology in the group of Dr. Timothy Noël. His research interests focus on novel synthetic methodologies combining continuous-flow microreactor technology with electrochemistry and photochemistry.
We acknowledge financial support from the Dutch Science Foundation (NWO) for a VIDI grant for T.N. (SensPhotoFlow, No. 14150). Y.C. received support from the China Scholarship Council (CSC).
The authors declare no competing financial interest.
Dedication
In memory of Prof. Dr. Jun-ichi Yoshida (1952−2019), who has been inspirational in the field of flow electrochemistry.
Special Issue
Published as part of the Accounts of Chemical Research special issue “Electrifying Synthesis”.
References
- Chemistry 4.0: Growth through Innovation in a Changing World. https://www2.deloitte.com/nl/nl/pages/consumer-industrial-products/articles/chemistry-4-0.html (accessed Aug 1, 2019).
- Alcácer V.; Cruz-Machado V. Scanning the Industry 4.0: A Literature Review on Technologies for Manufacturing Systems. Eng. Sci. Technol. Int. J. 2019, 22, 899–919. 10.1016/j.jestch.2019.01.006. [DOI] [Google Scholar]
- Schiffer Z. J.; Manthiram K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. 10.1016/j.joule.2017.07.008. [DOI] [Google Scholar]
- Wiebe A.; Gieshoff T.; Möhle S.; Rodrigo E.; Zirbes M.; Waldvogel S. R. Electrifying Organic Synthesis. Angew. Chem., Int. Ed. 2018, 57, 5594–5619. 10.1002/anie.201711060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo A.; Behnam M.; Chen J.; Jensen K. F.; Monbaliu J.-C. M.; Myerson A. S.; Revalor E. M.; Stelzer T.; Weeranoppanant N.; Wong S. Y.; Beingessner R. L.; Jamison T. F.; Snead D. R.; Zhang P. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- Yan M.; Kawamata Y.; Baran P. S. Synthetic Organic Electrochemical Methods since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M.; Kawamata Y.; Baran P. S. Synthetic Organic Electrochemistry: Calling All Engineers. Angew. Chem., Int. Ed. 2018, 57, 4149–4155. 10.1002/anie.201707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday M. Sechste Reihe von Experimental-Untersuchungen über Elektricität. Ann. Phys. (Berlin, Ger.) 1834, 109, 149–189. 10.1002/andp.18341090803. [DOI] [Google Scholar]
- Laudadio G.; de Smet W.; Struik L.; Cao Y.; Noël T. Design and application of a modular and scalable electrochemical flow microreactor. J. Flow Chem. 2018, 8, 157–165. 10.1007/s41981-018-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Noël T. Efficient Electrocatalytic Reduction of Furfural to Furfuryl Alcohol in a Microchannel Flow Reactor. Org. Process Res. Dev. 2019, 23, 403–408. 10.1021/acs.oprd.8b00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atobe M.; Tateno H.; Matsumura Y. Applications of Flow Microreactors in Electrosynthetic Processes. Chem. Rev. 2018, 118, 4541–4572. 10.1021/acs.chemrev.7b00353. [DOI] [PubMed] [Google Scholar]
- Pletcher D.; Green R. A.; Brown R. C. D. Flow Electrolysis Cells for the Synthetic Organic Chemistry Laboratory. Chem. Rev. 2018, 118, 4573–4591. 10.1021/acs.chemrev.7b00360. [DOI] [PubMed] [Google Scholar]
- Mitsudo K.; Kurimoto Y.; Yoshioka K.; Suga S. Miniaturization and Combinatorial Approach in Organic Electrochemistry. Chem. Rev. 2018, 118, 5985–5999. 10.1021/acs.chemrev.7b00532. [DOI] [PubMed] [Google Scholar]
- Yoshida J.-i.; Kim H.; Nagaki A. Impossible” chemistries based on flow and micro. J. Flow Chem. 2017, 7, 60–64. 10.1556/1846.2017.00017. [DOI] [Google Scholar]
- Horii D.; Fuchigami T.; Atobe M. A new approach to anodic substitution reaction using parallel laminar flow in a micro-flow reactor. J. Am. Chem. Soc. 2007, 129, 11692–11693. 10.1021/ja075180s. [DOI] [PubMed] [Google Scholar]
- Folgueiras-Amador A. A.; Wirth T. Perspectives in flow electrochemistry. J. Flow Chem. 2017, 7, 94–95. 10.1556/1846.2017.00020. [DOI] [Google Scholar]
- Paddon C. A.; Atobe M.; Fuchigami T.; He P.; Watts P.; Haswell S. J.; Pritchard G. J.; Bull S. D.; Marken F. Towards paired and coupled electrode reactions for clean organic microreactor electrosyntheses. J. Appl. Electrochem. 2006, 36, 617–634. 10.1007/s10800-006-9122-2. [DOI] [Google Scholar]
- Beck F.; Guthke H. Entwicklung neuer Zellen für elektro-organische Synthesen. Chem. Ing. Tech. 1969, 41, 943–950. 10.1002/cite.330411702. [DOI] [Google Scholar]
- Laudadio G.; Barmpoutsis E.; Schotten C.; Struik L.; Govaerts S.; Browne D. L.; Noël T. Sulfonamide Synthesis through Electrochemical Oxidative Coupling of Amines and Thiols. J. Am. Chem. Soc. 2019, 141, 5664–5668. 10.1021/jacs.9b02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- While there is a clear enhancement in mass transfer rates due to the disturbance of boundary layers, it should be noted that the presence of gas results in a decrease of the effective electrolyte conductivity since the current is forced to flow around the insulating bubbles.
- Horcajada R.; Okajima M.; Suga S.; Yoshida J.-i. Microflow electroorganic synthesis without supporting electrolyte. Chem. Commun. (Cambridge, U. K.) 2005, 1303–1305. 10.1039/b417388k. [DOI] [PubMed] [Google Scholar]
- Ziogas A.; Kolb G.; O’Connell M.; Attour A.; Lapicque F.; Matlosz M.; Rode S. Electrochemical microstructured reactors: Design and application in organic synthesis. J. Appl. Electrochem. 2009, 39, 2297–2313. 10.1007/s10800-009-9939-6. [DOI] [Google Scholar]
- Noël T.; Su Y.; Hessel V.; Noël T. Beyond Organometallic Flow Chemistry: The Principles Behind the Use of Continuous-Flow Reactors for Synthesis. Top. Organomet. Chem. 2016, 57, 1–41. [Google Scholar]
- Yoshida J.-i.; Suga S. Basic Concepts of “Cation Pool” and “Cation Flow” Methods and Their Applications in Conventional and Combinatorial Organic Synthesis. Chem. - Eur. J. 2002, 8, 2650–2658. . [DOI] [PubMed] [Google Scholar]
- Suga S.; Okajima M.; Fujiwara K.; Yoshida J. I. ″Cation flow″ method: A new approach to conventional and combinatorial organic syntheses using electrochemical microflow systems. J. Am. Chem. Soc. 2001, 123, 7941–7942. 10.1021/ja015823i. [DOI] [PubMed] [Google Scholar]
- Britton J.; Raston C. L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. 10.1039/C6CS00830E. [DOI] [PubMed] [Google Scholar]
- Pieber B.; Gilmore K.; Seeberger P. H. Integrated flow processing - challenges in continuous multistep synthesis. J. Flow Chem. 2017, 7, 129–136. 10.1556/1846.2017.00016. [DOI] [Google Scholar]
- Gutmann B.; Kappe C. O. Forbidden chemistries - paths to a sustainable future engaging continuous processing. J. Flow Chem. 2017, 7, 65–71. 10.1556/1846.2017.00009. [DOI] [Google Scholar]
- Kockmann N.; Thenée P.; Fleischer-Trebes C.; Laudadio G.; Noël T. Safety assessment in development and operation of modular continuous-flow processes. React. Chem. Eng. 2017, 2, 258–280. 10.1039/C7RE00021A. [DOI] [Google Scholar]
- Gutmann B.; Cantillo D.; Kappe C. O. Continuous-Flow Technology-A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem., Int. Ed. 2015, 54, 6688–6728. 10.1002/anie.201409318. [DOI] [PubMed] [Google Scholar]
- Elsherbini M.; Winterson B.; Alharbi H.; Folgueiras-Amador A. A.; Génot C.; Wirth T. Continuous-Flow Electrochemical Generator of Hypervalent Iodine Reagents: Synthetic Applications. Angew. Chem., Int. Ed. 2019, 58, 9811–9815. 10.1002/anie.201904379. [DOI] [PubMed] [Google Scholar]
- Elsherbini M.; Wirth T. Hypervalent Iodine Reagents by Anodic Oxidation: A Powerful Green Synthesis. Chem. - Eur. J. 2018, 24, 13399–13407. 10.1002/chem.201801232. [DOI] [PubMed] [Google Scholar]
- Watts K.; Gattrell W.; Wirth T. A practical microreactor for electrochemistry in flow. Beilstein J. Org. Chem. 2011, 7, 1108–1114. 10.3762/bjoc.7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K.; Yoshizawa H.; Atobe M. A Flow Microreactor Approach to a Highly Efficient Diels - Alder Reaction with an Electrogenerated o-Quinone. Synlett 2019, 30, 1194–1198. 10.1055/s-0037-1611725. [DOI] [Google Scholar]
- Laudadio G.; Bartolomeu A. d. A.; Verwijlen L. M. H. M.; Cao Y.; de Oliveira K. T.; Noël T. Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride. J. Am. Chem. Soc. 2019, 141, 11832–11836. 10.1021/jacs.9b06126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Adriaenssens B.; Bartolomeu A. d. A.; Laudadio G.; de Oliveira K. T.; Noël T.. Accelerating Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride in Flow. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël T.; Hessel V. Membrane microreactors: Gas-liquid reactions made easy. ChemSusChem 2013, 6, 405–407. 10.1002/cssc.201200913. [DOI] [PubMed] [Google Scholar]
- Price G. A.; Mallik D.; Organ M. G. Process analytical tools for flow analysis: A perspective. J. Flow Chem. 2017, 7, 82–86. 10.1556/1846.2017.00032. [DOI] [Google Scholar]
- Reizman B. J.; Jensen K. F. Feedback in Flow for Accelerated Reaction Development. Acc. Chem. Res. 2016, 49, 1786–1796. 10.1021/acs.accounts.6b00261. [DOI] [PubMed] [Google Scholar]
- Laudadio G.; Straathof N. J. W.; Lanting M. D.; Knoops B.; Hessel V.; Noël T. An environmentally benign and selective electrochemical oxidation of sulfides and thiols in a continuous-flow microreactor. Green Chem. 2017, 19, 4061–4066. 10.1039/C7GC01973D. [DOI] [Google Scholar]
- Goodridge F.; Scott K.. Electrolytic Reactor Design, Selection, and Scale-up. In Electrochemical Process Engineering; Springer US: Boston, MA, 1995; pp 177–244. [Google Scholar]
- Zhang J.; Wang K.; Teixeira A. R.; Jensen K. F.; Luo G. Design and Scaling Up of Microchemical Systems: A Review. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 285–305. 10.1146/annurev-chembioeng-060816-101443. [DOI] [PubMed] [Google Scholar]
- Peters B. K.; Rodriguez K. X.; Reisberg S. H.; Beil S. B.; Hickey D. P.; Kawamata Y.; Collins M.; Starr J.; Chen L.; Udyavara S.; Klunder K.; Gorey T. J.; Anderson S. L.; Neurock M.; Minteer S. D.; Baran P. S. Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry. Science 2019, 363, 838–845. 10.1126/science.aav5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. Industrial Electrochemical Synthesis Processes: Recent Developments in Reactor Design. Dev. Chem. Eng. Miner. Process. 1993, 1, 71–117. 10.1002/apj.5500010202. [DOI] [Google Scholar]
- Couper A. M.; Pletcher D.; Walsh F. C. Electrode materials for electrosynthesis. Chem. Rev. 1990, 90, 837–865. 10.1021/cr00103a010. [DOI] [Google Scholar]
- Lips S.; Waldvogel S. R. Use of Boron-Doped Diamond Electrodes in Electro-Organic Synthesis. ChemElectroChem 2019, 6, 1649–1660. 10.1002/celc.201801620. [DOI] [Google Scholar]
- Botte G. G. Electrochemical Manufacturing in the Chemical Industry. Electrochem. Soc. Interface 2014, 23, 49–55. 10.1149/2.F04143if. [DOI] [Google Scholar]
- Sequeira C. A. C.; Santos D. M. F. Electrochemical routes for industrial synthesis. J. Braz. Chem. Soc. 2009, 20, 387–406. 10.1590/S0103-50532009000300002. [DOI] [Google Scholar]
- Danly D. E. Development and Commercialization of the Monsanto Electrochemical Adiponitrile Process. J. Electrochem. Soc. 1984, 131, 435C–442C. 10.1149/1.2115324. [DOI] [Google Scholar]
- Pletcher D.; Walsh F. C.. Organic electrosynthesis. In Industrial Electrochemistry; Springer Netherlands: Dordrect, 1993; pp 294–330. [Google Scholar]
- Mallia C. J.; Baxendale I. R. The Use of Gases in Flow Synthesis. Org. Process Res. Dev. 2016, 20, 327–360. 10.1021/acs.oprd.5b00222. [DOI] [Google Scholar]
- Hartman R. L. Managing solids in microreactors for the upstream continuous processing of fine chemicals. Org. Process Res. Dev. 2012, 16, 870–887. 10.1021/op200348t. [DOI] [Google Scholar]
- Fleming G. S.; Beeler A. B. Integrated drug discovery in continuous flow. J. Flow Chem. 2017, 7, 124–128. 10.1556/1846.2017.00027. [DOI] [Google Scholar]
- May S. A. Flow chemistry, continuous processing, and continuous manufacturing: A pharmaceutical perspective. J. Flow Chem. 2017, 7, 137–145. 10.1556/1846.2017.00029. [DOI] [Google Scholar]