Dear Editor,
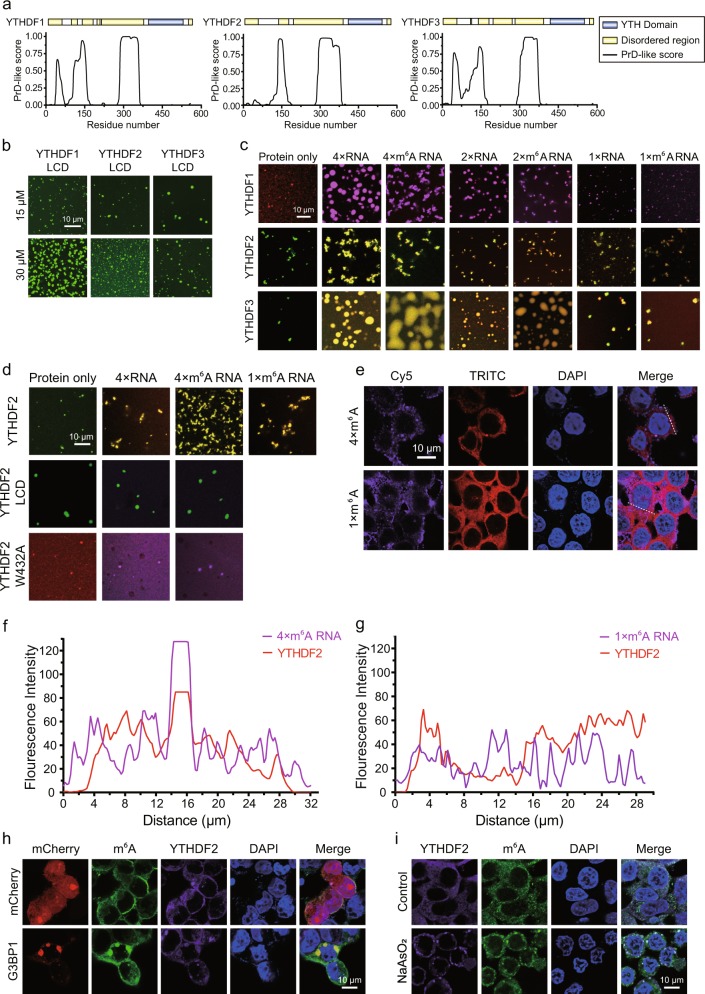
Posttranscriptional modifications of coding and non-coding RNAs are prevalent in cells. N6-methyladenosine (m6A) is the most abundant type of modification on eukaryotic messenger RNAs (mRNAs).1 This modification plays important roles in multiple fundamental biological processes, for example, cell differentiation, tissue development and tumorigenesis.2,3 Like many other chemical modifications on biological macromolecules (proteins, DNAs, and RNAs), m6A can also be recognized by specific reader domains. The best-studied m6A reader domain is the YT521-B homology (YTH) domain, which is conserved from yeast to human and preferentially binds to a RR(m6A)CU (R = G or A) consensus motif.4–7 Humans have five proteins containing a YTH domain, of which three, YTHDF1-3, belong to the same protein family, the DF family.7 In addition to the well-folded YTH domain at their C-termini, all three proteins also have a low complexity domain (LCD) at their N-termini (Fig. 1a). Their LCD regions are predicted to be Prion-like domains (Fig. 1a). Prion-like domain-containing proteins often have the potential to undergo phase separation.8 Phase separation is often found to contribute to biomolecular condensation.9 Indeed, the LCDs of all three YTHDF proteins (Fig. S1) underwent concentration-dependent phase separation in the absence of RNA (Fig. 1b and S2). The resulting condensates exhibited liquid properties as two or more droplets can grow into a much larger droplet (Fig. S3a). Full-length YTHDF1/2/3 proteins (Fig. S1) also underwent phase separation under physiological conditions in the absence of RNA, although their capacity for phase separation was decreased in comparison with their LCDs (Fig. 1b and S3b).
Fig. 1.
Multivalent N6-methylated RNAs promote phase separation of YTHDFs. a Prediction of the disordered regions and Prion-like domains (PrD) of YTHDF1/2/3; b The LCDs of YTHDFs undergo phase separation at 15 μM and 30 μM in 150 mM NaCl. YTHDF1/2/3-LCDs were labeled with Alexa–488 (depicted in green); c Quadruple m6A, double m6A and unmodified YTH recognition RNA motifs enhance phase separation of YTHDFs in 150 mM NaCl. All RNA motif concentrations were 15 μM and all protein concentrations were 10 μM. YTHDF1 was labeled with Alexa–568 (depicted in red), YTHDF2/3 were labeled with Alexa–488 (depicted in green), and all the RNAs were labeled with Cy5 (represented by the pseudocolor purple for images with YTHDF1 and the pseudocolor red for images with YTHDF2/3). All the images shown here are merged; d Enhancement of YTHDF2 phase separation by the quadruple m6A motif in 300 mM NaCl depends on the YTH domain. All the RNA motifs were 15 μM and all the proteins were 10 μM; YTHDF2 and YTHDF2-LCD were labeled with Alexa–488 (represented by green), YTHDF2-W432A was labeled with Alexa–568 (depicted in red), and all the RNAs were labeled with Cy5 (represented by the pseudocolor red in the first row, and the pseudocolor purple in the second and third rows). All images shown are merged. e The quadruple m6A motif drives the formation of YTHDF2 puncta in HEK293 cells. All the synthetic RNA motifs were labeled with Cy5 (represented by the pseudocolor purple), YTHDF2 was shown by TRITC channel. f, g Co-localization analysis of quadruple m6A and single m6A with YTHDF2 in cells along the dotted lines shown in the merged images in (e); h Endogenous YTHDF2 and m6A RNA are recruited into stress granules induced by overexpression of G3BP1 in HEK293 cells; i Endogenous YTHDF2 and m6A RNA are recruited into stress granules in HEK293 cells after NaAsO2 treatment
The molecular mechanism by which these YTHDF1/2/3 proteins carry out their functions is largely unknown. It is also unclear whether the phase separation properties of the proteins contribute to their functions and, if so, how they are regulated. Recent studies using nucleotide-resolution sequencing techniques in human or mouse cell lines identified tens of thousands of m6A modification sites,10,11 which preferentially localize around the stop codons or the beginning of the last exon of mRNAs.5,11 For instance, 19 and 17 m6A modification sites are detected within an 820-bp and a 1040-bp region of EEF2 mRNA and JUN mRNA, respectively (Fig. S4). Some long noncoding RNAs also contain clusters of m6A modification sites. For example, 19 m6A modification sites are detected within an 820-bp region of XIST (Fig. S4). In a polyA-containing dataset published by Jaffery and colleagues,12 we identified nearly 200 m6A clusters (Table S1), which, by definition, contain at least 5 m6A sites. These observations led us to wonder whether multiple co-existing m6A motifs can promote phase separation of YTHDFs by simultaneously binding multiple copies of YTHDF proteins.
To this end, we tested whether RNA oligos containing different numbers of unmodified RNA motifs (AUGGACUCC) or m6A RNA motifs (AUGG(m6A)CUCC) (Table S2) could modulate phase separation of YTHDF proteins. Quadruple m6A RNA motifs, but not single m6A RNA motifs, dramatically enhanced phase separation of YTHDF proteins (Figs. 1c, S6a–d), while the RNA oligos alone cannot undergo phase separation (Fig. S5). Under mild conditions, phase separation enhancement is modification-independent, as quadruple unmodified RNA motifs also enhanced phase separation of all three YTHDF proteins (Figs. 1c, S6a–d). In cells, the molecular environment is highly complex and there is constant competition from an enormous assortment of non-modified RNAs. To better mimic this scenario, we titrated increasing amounts of the single unmodified RNA motif into phase separation reaction systems containing the quadruple unmodified or m6A RNA motifs with YTHDF2 or YTHDF3. The single unmodified RNA motif efficiently diminished punctum formation by YTHDFs with the quadruple unmodified RNA oligo, but not by YTHDFs with the quadruple m6A RNA oligo (Fig. S7a–c). Fusion was observed between droplets formed by YTHDF3 protein mixed with quadruple unmodified or m6A RNA motifs (Fig. S8a–b), whereas no fusion was detected for droplets formed by YTHDF3 protein only (data not shown). This indicates that RNA contributes to the dynamics of YTHDF3 liquid droplets. FRAP data demonstrated that the droplets are dynamic, with properties that enable both YTHDF3 protein and RNA molecules to exchange between the droplets and the surrounding solution (Fig. S8c, d).
Under stringent conditions such as high salt, the quadruple m6A RNA oligo, but not the quadruple unmodified RNA oligo, promoted phase separation of YTHDF2 (Figs. 1d and S9a–d). The enhancement is m6A valency-dependent, as the quadruple m6A RNA motif enhance phase separation of YTHDF2 much more than single m6A motif (Figs. 1d and S9a–d). The enhancement is also YTH domain-dependent, as the quadruple m6A RNA oligo failed to enhance phase separation of the LCD of YTHDF2 (Figs. 1d and S9d). Furthermore, the enhancement is YTH domain binding-dependent, as the quadruple m6A RNA oligo failed to enhance phase separation of YTHDF2 (W432A) (Figs. 1d and S9d), a mutant YTHDF2 with compromised m6A binding.13 These in vitro experiments indicated that multivalent m6A motifs enhance phase separation of YTHDFs in vitro.
To study whether multivalent m6A regulates phase separation of YTHDFs in cells, we transfected RNA oligos with varying number of m6A RNA motifs into HEK293 cells and detected endogenous YTHDF2 distribution using immunofluorescence staining. Consistent with the in vitro experimental results mentioned above, the quadruple m6A RNA oligo, but not the single m6A RNA oligo or unmodified RNA oligo (marked as 0 × m6A RNA), promoted formation of YTHDF2-positive puncta (Figs. 1e–g and S10a–c). In order to assess the identity of the YTHDF2-positive puncta, we performed co-transfection experiments in HEK293 cells. Ectopically expressed mCherry-G3BP1, a stress granule marker, co-localizes with GFP-YTHDF2 in punctum-like structures (Fig. S11a). FRAP experiments indicated that G3BP1 exhibited fluidity within the puncta and also underwent fast exchange between puncta and the surrounding solution (Fig. S11b), consistent with the notion that stress granules are phase-separated compartments.14 Importantly, similar to G3BP1, YTHDF2 exhibited fluidity within puncta and underwent fast exchange between puncta and the surrounding solution as shown in FRAP experiments (Fig. S11b). Next, we used two approaches to induce the formation of stress granules and detected the distribution patterns of endogenous YTHDF2 and m6A by immunofluorescence staining. First, we promoted stress granule formation by overexpressing mCherry-G3BP1 in HEK293 cells. The endogenous YTHDF2 and m6A co-localized within stress granules as judged by the enrichment of mCherry-G3BP1 (Figs. 1h and S12a, b). Furthermore, we used sodium arsenite (NaAsO2), a well-established stress granule inducer, to treat HEK293 cells. Endogenous YTHDF2 and m6A accumulated and co-localized in stress granule-like puncta (Fig. 1i and S13b). In contrast, no granules formed without NaAsO2 treatment (Figs. 1i and S13a). These results indicated that YTHDF2 and multivalent m6A localize to stress granules, consistent with a recent study by Parker and colleagues.15
In this study, we revealed that multivalent m6A-containing RNAs can enhance the phase separation potential of YTHDF proteins in vitro and in cells (Fig. S14). These data suggest that phase separation of m6A and YTHDF2 is associated with the cellular response to stress. The functions of m6A modification are diverse, and many of them are performed through recognition of m6A by its reader proteins. Multivalent m6A-driven phase separation of YTHDFs is likely important for many of the aforementioned functions of m6A.
Supplementary information
Acknowledgements
This work was supported by NSFC Grant No. 31871443 to P.L.
Author contributions
Y.G. and P.L. conceived the project and designed experiments. Y.G., G.P., D.L. and R.L. performed experiments and analysis the data. S.Q. and Q.Z. performed the bio-information analysis. Y.G. and P.L. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yifei Gao, Gaofeng Pei, Dongxue Li
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41422-019-0210-3.
References
- 1.Nachtergaele S, He C. Annu. Rev. Genet. 2018;52:23.1–23.24. doi: 10.1146/annurev-genet-120417-031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng X, et al. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixit D, et al. Cancer Cell. 2017;31:474–475. doi: 10.1016/j.ccell.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil DP, Pickering BF, Jaffrey SR. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominissini D, et al. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, et al. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao S, Sun H, Xu C. Genom. Proteom. Bioinforma. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, et al. Nature. 2013;495:467–476. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banani SF, et al. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke S, et al. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer KD, et al. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder B, et al. Nat. Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, et al. Cell Res. 2014;24:1490–1492. doi: 10.1038/cr.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler JR, et al. Elife. 2016;5:1–25. [Google Scholar]
- 15.Jain S, et al. Cell. 2016;164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.