Abstract
Based on the hollow fiber protected molecularly imprinted polymer, a micro-solid-phase extraction (μ-SPE) method was developed and applied for the analysis of indole-3-butyric acid in mung bean sprouts by high-performance liquid chromatography. The extraction conditions of the μ-SPE method were optimized using L9(34) orthogonal, and optimum conditions were found as follows: pH of sample solution was 2.0, chloroform was the organic solvent for embedding the μ-SPE bars, and acetonitrile was the desorption solvent. In addition, the extraction time was 80 min, desorption time was 5 min, stirring speed was 800 rpm, and concentration of NaCl was 10%. Under the optimum conditions, a standard curve was established for IBA, with a correlation coefficient of 0.9999. After extraction with phosphate buffer solution (pH = 9.0), successful pretreatment of mung bean sprouts was achieved by the μ-SPE method. The limit of detection was 0.075 mg/kg, and the recoveries were found to be in the range of 88.9–106.4%. This method is simple, environmentally friendly, and can be used for the determination of indole auxin contents in green bean sprouts quickly and accurately.
1. Introduction
Plant hormones are present at low concentrations in plant tissues but play an important role in regulating many aspects of plant growth.1 Auxins are the first of the major plant hormones to have been discovered. The auxins including indole-3-acetic acid (IAA), indole-3-propionic acid (IPA) and indole-3-butyric acid (IBA) are involved in many aspects of plant growth and development.2 IBA promotes rooting and is found to be highly effective compared to other auxins. Its concentration level exquisitely controls growing processes of plants. The auxin level has a great value for the study of biological process in plants. The auxins were also applied to vegetables, and high-level residues may cause harm. So, the quantitative determination of the auxin is often very important in modern agriculture.3,4 Various techniques have been used for the analysis of auxins; among them, liquid chromatography is the most commonly used one.5 The quantitative determination of auxins is still a challenging issue6,7 due to their low concentration and complex sample matrix. At this point, sample pretreatment is considered to have vital importance in the whole analytical process.8,9
Although solid-phase extraction (SPE) has been widely applied for the analysis, it consumes a considerable amount of organic solvent as well as more time and causes a blockage of the column bed during the extraction process.10 As an alternative to traditional SPE, membrane-protected micro-solid phase extraction (μ-SPE) was introduced by Lee’s group.11−13 Using μ-SPE, the amounts of sorbent, desorption solvent, and sample volume decreased, resulting in lower cost of the procedure.14−16 Since the solid sorbent is packed inside a membrane, which is often hydrophobic such as polypropylene hollow fibers,17−19 the direct contact of the sorbent and sample matrix is avoided. Hollow fibers are widely used in liquid-phase microextraction (LPME). Its small volume (dozens of microliters) enables concentration of the target analytes. In addition, sample extraction and filtration are achieved simultaneously in a single step owing to the micropores (0.2 μm) on the fiber wall.20,21 Hence, complex samples can be easily treated without filtration.22 Molecularly imprinted polymers (MIPs) are synthetic materials obtained by copolymerization of functional and cross-linking monomers in the presence of a template molecule.23,24 MIPs can selectively adsorb a target molecule in preference to other closely related compounds.25,26 MIPs have superior specific recognition to other sorbents used in SPE. The most advanced application of MIPs was believed to be in SPE.27
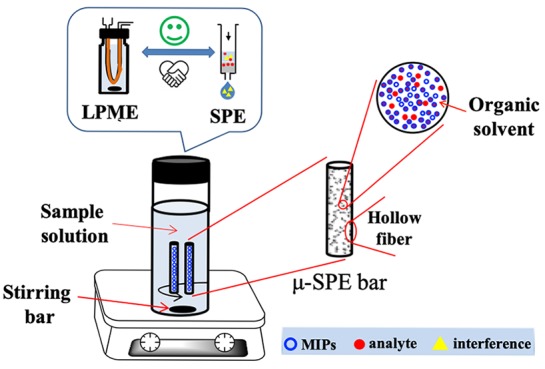
In this study, polypropylene hollow fibers were packed with MIP particles and formed microextraction bars. Tubular hollow fibers were used to protect the MIP rather than a bag made of polymeric membranes. The main purpose is to minimize the volume. A molecular imprinting μ-SPE (MI-μ-SPE) method based on the microextraction bars was proposed. This method takes advantages of both SPE and LPME, as shown in Figure 1. More importantly, it can overcome the poor recognition of MIPs in aqueous solutions. The proposed method integrated the processes of SPE and LPME in a single step and improved extraction efficiency. The microextraction bars were successfully used for the extraction of IBA in the mung bean sprouts and further determined by HPLC.
Figure 1.
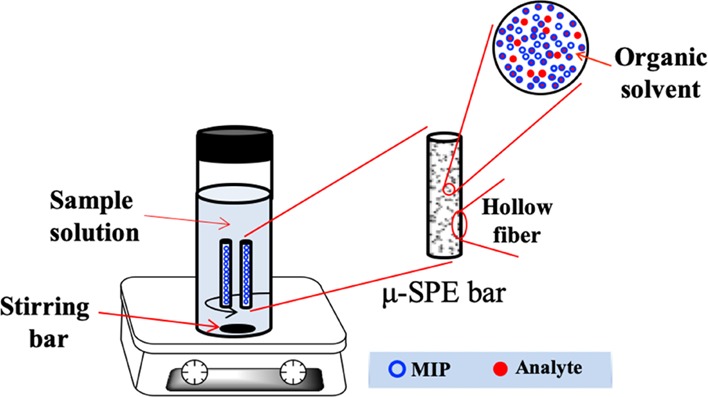
Hollow fiber μ-SPE extraction device packed with MIP.
2. Results and Discussion
2.1. SEM Characterization
Polypropylene hollow fibers, with an inner diameter, wall thickness, and pore size of 600, 200, and 0.2 μm, respectively, were cut into pieces of 2 cm length (Figure 2A,C). Subsequently, one end of the segment was sealed with flame, and the other end allowed packing of polymer particles. The MIP particles of 38–75 μm were sonicated for 5 min in ethanol and formed a slurry. Then the slurry was filled into the lumen of hollow fiber capillary, which was 2 cm in length, with the help of a hypodermic needle. The outer diameter of the needle is about 0.5 mm. The slurry was passed by a syringe attached to the needle and sieved by the tubular membranes. Ethanol passed through the pores of the membrane leaving the polymer particles inside the lumen, and subsequently, they filled up the hollow fibers. The microextraction bars were prepared after sealing other ends of the fibers by flame (Figure 2B,D), and each bar contains about 1.2 mg of polymer particles. Then the extraction bars were dried for 5 min and impregnated with a nonpolar organic solvent before use. Then two pieces of extraction bars were transferred into 4 mL of sample solution and magnetically stirred. After extraction, the bars were immersed in 2 mL of acetonitrile for elution of the analyte. The eluent solution was analyzed by HPLC. Finally, hollow fiber bars were cleaned and conditioned for the next extraction.
Figure 2.
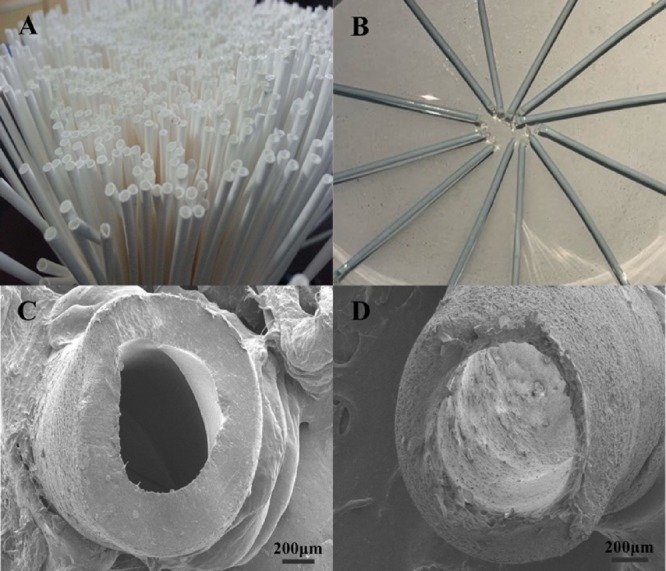
Photos of hollow fibers: empty (A) and packed (B); cross section SEM images of hollow fibers: empty (C) and packed (D).
2.2. Imprinting Effectivity and Selectivity
For the preparation of microextraction bars, MIP for IBA was designed and synthesized. The MIP was prepared using vinylimidazole as the functional monomer and ethylene glycol dimethacrylate as the cross-linker. In our previous work,2 the recipe of the imprinted polymer was optimized concerning the functional monomer, cross-linker, and solvent in view of the molecular recognition of IBA. The MIP and nonimprinted polymer (NIP) obtained were investigated through breakthrough experiments using SPE cartridges, as shown in Figure 3. MIP presented higher binding capacity (1.13 mg/g) to IBA than NIP (0.87 mg/g) did, and the polymer showed an obvious imprinting effect in chloroform. The MIP and NIP particles were packed into an HPLC column (50 × 4.6 mm), and retentions of IBA and its analogues (IAA, IPA and tryptamine) were examined by HPLC. The selectivities of the polymers were also evaluated by comparing the retention times of IBA and analogue compound (Supporting Information, Table S1). It can be observed that the MIP has high selectivity to IBA.
Figure 3.
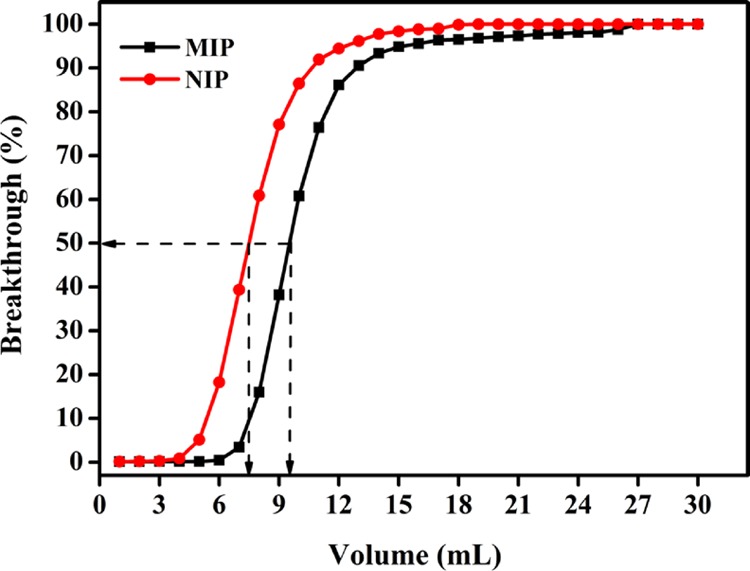
Breakthrough curves of MIP and NIP for 60 mg/L indole-3-butyric acid in chloroform. The binding capacity value is extrapolated from the 50% point of the breakthrough curves.
2.3. Evaluation and Optimization of MI-μ-SPE Bars
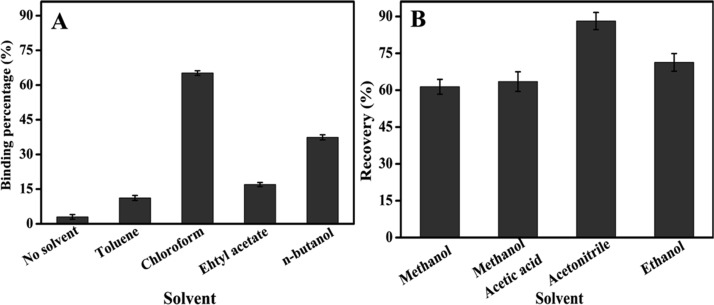
In nonpolar solvents, MIPs usually show good imprinting effects but almost no efficiency in aqueous solutions. MIPs give the best recognition in polymerization solvents such as toluene, chloroform, and acetonitrile. So, microextraction bars prepared with MIP particles were immersed into nonpolar solvents before applying to the aqueous sample. Here, the different organic solvents were investigated for pretreatment of microextraction bars, as shown in Figure 4. The highest binding percentage was achieved with chloroform as shown in Figure 4A. Compared to other hydrophobic solvents, chloroform showed much higher binding to IBA. All these solvents can extract IBA from aqueous solution. Without organic solvents, the binding percentage was the lowest and much lower than that with organic solvents. This indicated that liquid-phase extraction has an important contribution to the overall extraction. MIPs usually have the best binding in the polymerization solvent. Chloroform was used as the solvent for preparation of the MIP against IBA. That is why the best binding was achieved in chloroform. It can be proven that there is the synergic extraction of LPME driven by chloroform and SPE driven by the MIPs.17 So, chloroform was chosen as the extraction solvent for further studies. Compared to liquid–liquid extraction, LPME and SPE, this method consumes much less organic solvent (about 50 μL). That is negligible and still can be regarded as a green extraction process, although chloroform was used here.
Figure 4.
Binding percentages and desorption recoveries of IBA in different organic solvents for pretreatment (A) and elution (B) after extraction of 4 mL of IBA in aqueous solution at 2.0 mg/L.
For quantitative analysis, it was required to desorb IBA from polymer extraction bars with a suitable solvent. Polar solvents such as methanol, methanol/acetic acid (9/1), acetonitrile, and ethanol were investigated, and the highest desorption rate (88%) was achieved with acetonitrile (as shown in Figure 4B). It was also found that 5 min of desorption time was enough to obtain satisfactory results. The extraction bars can be completely recovered with acetonitrile and reused. The results of repeated experiments showed that the extraction bars can be used over 30 times without any obvious loss of extraction efficiency.
2.4. Orthogonal Experiment Analysis
In order to effectively utilize imprinted polymer extraction bars for the clean-up of IBA from green bean sprouts, the extraction conditions were investigated by the L9(34) orthogonal experiment.28,29 The conditions include extraction time, rotation speed, pH, and concentration of salt (NaCl), as listed in Table 1. It can be seen from the analysis results (Supporting Information, Table S2) that contributions of the factors to the extraction are in the following order: extraction time (A) > rotation speed (B) > NaCl concentration (D) > pH (C). It was found that the optimum conditions were as follows: extraction time was 80 min, rotation speed was 800 rpm, sample pH was 2.0, and NaCl concentration was 10%. Originally, we expect to have better mass transfer due to the small diameter of the extraction bar. However, the result indicates that it needs a long time of extraction. That is because of poor mass transfer inside the fiber probably caused by restriction of very small space.
Table 1. Orthogonal Layout Design.
| level | extraction time (A) (min) | rotation speed (B) (rpm) | pH (C) | NaCl concentration (D) (%) |
|---|---|---|---|---|
| 1 | 40 | 600 | 2 | 0 |
| 2 | 60 | 800 | 4 | 5 |
| 3 | 80 | 1000 | 6 | 10 |
2.5. Analytical Performance and Application to Real Sample
Analytical performance and practical application of the proposed method were investigated using mung bean sprouts as a real sample. The auxin samples were collected and extracted using the method described in our previous work.30 A calibration curve was established using HPLC for the quantification of the eluents obtained from extraction bars (Supporting Information, Figure S1). The real samples were spiked within the range of 5–20 mg/L (as shown in Table 2 and Supporting Information, Figure S2). The concentration of IBA in the green bean sprouts was found to be 0.23 mg/L, and recoveries were in the range of 88.9–106.4%. The RSD values were in the range of 1.86–2.22%, which demonstrated that the method has high accuracy and reproducibility.
Table 2. Recoveries of IBA for Green Bean Sprout Samples Spiked at Three Different Concentration Levels.
| added (mg/L) | found (mg/L) | RSD (%) | recovery (%) |
|---|---|---|---|
| 0 | 0.23 | 2.22 | |
| 5 | 5.32 | 1.96 | 106.4 |
| 10 | 10.17 | 2.13 | 101.7 |
| 20 | 17.79 | 1.86 | 88.9 |
Analytical parameters of the proposed method were compared with those of other reported methods (Table 3). It clearly shows that the microextraction bars coupled with HPLC detection exhibited excellent sensitivity, simplicity, and high recovery. This method can be used for the determination of IBA in green bean sprouts. Sorbents, which are used for simultaneous extraction of auxins, are usually not very selective. In such work,31 pollen grains were used as hydrophilic sorbents for simultaneous determination of 16 plant growth regulators. However, the MIP not only has high specific binding to IBA but also has good selectivity to its analogues. Combined with HPLC, it can give high extraction of the auxins. In addition, this material is very useful for the fast measurement of individual IBA combined with the spectrophotometric method, typically where IBA is used alone for promoting plant growth.
Table 3. Comparison of Analytical Performances of Different Methods for the Determination of IBA in Green Bean Sprout Samples.
| method | LOD (mg/kg) | RSD (%) | time (min)d | recovery (%) | ref |
|---|---|---|---|---|---|
| HF-LPMEa | 0.570 | <4.8 | 60 | 88.6–100.7 | (29) |
| HILIC-SPE | 0.010b | <14.4 | 45 | 80.5–119.2 | (31) |
| MI-SPMEc | 0.150 | <7.4 | 260 | 91.5–97.5 | (2) |
| MI-μ-SPE | 0.075 | <2.13 | 80 | 88.9–106.4 | this work |
HF: hollow fiber.
μg/kg.
MI-SPME: molecular imprinting solid-phase microextraction.
Time for extraction.
3. Conclusions
In conclusion, in this work, molecular imprinting μ-SPE was developed that combines the excellent features of both liquid–liquid and solid-phase extractions. The new approach also effectively solved the poor recognition of MIPs in the aqueous phase. This method also allowed selective extraction of the auxin from green bean sprouts and sensitive quantification by HPLC. In addition, it has advantages of being simple, miniature, low consumption of organic solvents, and high reproducibility.
4. Experimental Sections
4.1. Polymer Preparation
The rational design approach was applied for the preparation of MIPs.23 Selection processes of the functional monomer, cross-linker, and solvent were reported in our previous work.2 In brief, MIP and NIP were prepared according to the following procedure: 0.203 g (1 mmol) of template (IBA) and 0.376 g (4 mmol) of vinylimidazole (VI) were dissolved in 3.548 g of N,N-dimethylformamide (DMF) in a screw-capped glass vial. The mixture was sonicated for 5 min followed by the addition of 3.172 g (20 mmol) of ethylene glycol dimethacrylate (EGDMA) as the cross-linker and 0.071 g of initiator (AIBN). The polymer solution was sonicated for 5 min and purged with N2 for 10 min and then sealed. After that, the solution was polymerized at 80 °C for 24 h in an oil bath. The bulk polymer was ground and sieved to yield particles of 38–75 μm. Finally, the polymer particles were washed with methanol/acetic acid (9/1, v/v) and methanol. The same method was used to prepare NIP in the absence of templates (IBA).
4.2. Breakthrough Experiment
For the breakthrough experiments, MIP and NIP particles (100 mg) were packed in a 1 mL SPE column. The SPE column was activated with 5 mL of chloroform. The template IBA chloroform solution (60 mg/L) was loaded into the SPE column with a flow rate of 1 mL/min. Each extract was analyzed by UV–vis spectrophotometers at 280 nm. Finally, the adsorption amount of IBA was calculated by HPLC.
4.3. Preparation of MI-μ-SPE Bars
The hollow fibers were cut into pieces of 2 cm length and ultrasonicated with acetone for 20 min. Subsequently, one of the ends of the segment was sealed with flame, and the other end allowed packing of polymer particles in the form of slurry (carefully weighed, m1). The MIP particles were homogenized by sonication with ethanol for 5 min. Then the slurry was filled into the lumen of hollow fiber capillary, and the excess ethanol leaked through the pores of the membrane leaving the polymer particles inside the lumen. After being packed, the other end was also sealed by flame and dried for 5 min. Finally, hollow fiber bars were carefully weighed (m2), and the packing amount was calculated according to m1 and m2.
4.4. Pretreatment of Mung Bean Samples
One hundred grams of mung bean was put in a 200 mL beaker and washed with distilled water. Under ambient temperature and humidity, water was changed regularly 2–3 times per day. After three days, the grown bean sprouts were collected and stored at −20 °C. 20 mL of NaH2PO4-H3PO4 buffer solution (pH = 9.0) was added into 5 g of mung bean sprouts and kept in the dark overnight at 4 °C. The extraction solution was centrifuged (9000 rpm, 5 min) by high-speed centrifugation. The pH of the supernatant was adjusted to the desired value. Each μ-SPE device was conditioned by ultrasonication in chloroform for 30 s. Then, 4 mL of sample solution (0.1 mg/L NaCl, pH = 2) was added into the glass vial for extraction. Finally, the conditioned hollow fiber bars were transferred to 4 mL of sample solution and stirred for 80 min at 800 rpm. After extraction, the bars were washed with water twice. Then they were immersed in 2 mL of acetonitrile stirred for 5 min at 800 rpm to desorb the analyte. The desorption solution was analyzed by HPLC. Finally, hollow fiber bars were cleaned and conditioned for the next extraction.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21365020, 21565025).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01550.
Table S1: Retention factors (k), imprinting factors (IFs), and selectivity factors (a) of IBA and its analogues on MIP and NIP; Table S2: Results of orthogonal experiment L9(34); Figure S1: HPLC chromatograms of IBA standard solutions λ = 281 nm; concentrations were 1, 2.5, 5, 10, 20, 50 mg/L; (inset) calibration curve: R2 = 0.9999, y = 836.5356x – 5.8531; Figure S2: HPLC chromatograms of real samples were spiked with 5, 10, 20 mg/L IBA (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Du F.; Ruan G.; Liu H. Analytical methods for tracing plant hormones. Anal. Biochem. 2012, 404, 55–74. 10.1007/s00216-011-5623-x. [DOI] [PubMed] [Google Scholar]
- Chang J.; Bahethan B.; Muhammad T.; Yakup B.; Abbas M. A simple and selective fluorescent sensor chip for indole-3-butyric acid in mung bean sprouts based on molecularly imprinted polymer coatings. Sensors 2017, 17, 1954. 10.3390/s17091954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Li Y.; Hu Y.; Li G.; Chen Y. Preparation of magnetic indole-3-acetic acid imprinted polymer beads with 4-vinylpyridine and β-cyclodextrin as binary monomer via microwave heating initiated polymerization and their application to trace analysis of auxins in plant tissues. J. Chromatogr. A 2010, 1217, 7337–7344. 10.1016/j.chroma.2010.09.059. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Lu M.; Xu L.; Xiao R.; Cai Z. Recent advances in the analysis of gibberellins plant hormones. Chin. J. Chromatogr. 2015, 33, 786–791. 10.3724/SP.J.1123.2015.04029. [DOI] [PubMed] [Google Scholar]
- Xi Z.; Zhang Z.; Sun Y.; Shi Z.; Tian W. Determination of indole-3-acetic acid and indole-3-butyric acid in mung bean sprouts using high performance liquid chromatography with immobilized Ru(bpy)32+-KMnO4 chemiluminescence detection. Talanta 2009, 79, 216–221. 10.1016/j.talanta.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tan S. N.; Teo C. H.; Yew Y. R.; Ge L.; Chen X.; Yong J. W. H. Analysis of phytohormones in vermicompost using a novel combinative sample preparation strategy of ultrasound-assisted extraction and solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. Talanta 2015, 139, 189–197. 10.1016/j.talanta.2015.02.052. [DOI] [PubMed] [Google Scholar]
- Zeng Q.; Ruan Y.; Sun L.; Du F.; Guo L.; Cheng Z.; Ruan G.; Li J. Development of graphene oxide functionalized cotton fiber based solid phase extraction combined with liquid chromatography-fluorescence detection for determination of trace auxins in plant samples. Chromatographia 2018, 81, 861–869. 10.1007/s10337-018-3518-0. [DOI] [Google Scholar]
- Yan H.; Wang F.; Han D.; Yang G. Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography. Analyst 2012, 137, 2884–2890. 10.1039/c2an35362h. [DOI] [PubMed] [Google Scholar]
- Wang M.; Chang X.; Wu X.; Yan H.; Qiao F. Water-compatible dummy molecularly imprinted resin prepared in aqueous solution for green miniaturized solid-phase extraction of plant growth regulators. J. Chromatogr. A 2016, 1458, 9–17. 10.1016/j.chroma.2016.06.047. [DOI] [PubMed] [Google Scholar]
- Mukhtar N. H.; Hong H. S. Carbonaceous nanomaterials immobilised mixed matrix membrane microextraction for the determination of polycyclic aromatic hydrocarbons in sewage pond water samples. Anal. Chim. Acta 2016, 931, 57–63. 10.1016/j.aca.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Basheer C.; Chong H.-G.; Hii T. M.; Lee H. K. Application of porous membrane-protected micro-solid-phase extraction combined with HPLC for the analysis of acidic drugs in wastewater. Anal. Chem. 2007, 79, 6845–6850. 10.1021/ac070372r. [DOI] [PubMed] [Google Scholar]
- Lim T. H.; Hu L.; Yang C.; He C.; Lee H. K. Membrane assisted micro-solid phase extraction of pharmaceuticals with amino and urea-grafted silica gel. J. Chromatogr. A. 2013, 1316, 8–14. 10.1016/j.chroma.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Sajid M.; Basheer C.; Narasimhan K.; Choolani M.; Lee H. K. Application of microwave-assisted micro-solid phase extraction for determination of parabens in human ovarian cancer tissues. J. Chromatogr. B 2015, 1000, 192–198. 10.1016/j.jchromb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Sajid M. Porous membrane-protected micro-solid phase extraction: A review of features, advancements and applications. Anal. Chim. Acta 2017, 965, 36–53. 10.1016/j.aca.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Gao G.; Xing Y.; Liu T.; Wang J.; Hou X. UiO-66(Zr) as sorbent for porous membrane-protected micro-solid phase extraction androgens and progestogens in environmental water samples coupled with LC-MS/MS analysis: The application of experimental and molecular simulation method. Microchem. J. 2019, 146, 126–133. 10.1016/j.microc.2018.12.050. [DOI] [Google Scholar]
- Xia L.; Du Y.; Xiao X.; Li G. One-step membrane-protected micro-solid phase extraction and derivatization coupling to high-performance liquid chromatography for selective determination of aliphatic aldehydes in cosmetics and food. Talanta 2019, 202, 580–590. 10.1016/j.talanta.2019.05.035. [DOI] [PubMed] [Google Scholar]
- Zang H.; Yuan J.-P.; Chen X.-L.; Liu C.-A.; Cheng C.-G.; Zhaoa R.-S. Hollow fiber-protected metal-organic framework materials as micro-solid phase extraction adsorbents for the determination of polychlorinated biphenyls in water samples by gas chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 4875–4882. 10.1039/c3ay40305j. [DOI] [Google Scholar]
- Díaz-Álvarez M.; Martín-Esteban A. Hollow fiber membrane-protected molecularly imprinted microspheres for micro-solid phase extraction and clean-up of thiabendazole in citrus samples. J. Chromatogr. A. 2018, 1531, 39–45. 10.1016/j.chroma.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Lashgari M.; Lee H. K. Micro-solid phase extraction of perfluorinated carboxylic acids from human plasma. J. Chromatogr. A 2016, 1432, 7–16. 10.1016/j.chroma.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Yamini Y.; Rezazadeh M.; Seidi S. Liquid-phase microextraction-The different principles and configurations. TrAC, Trends Anal. Chem. 2019, 112, 264–272. 10.1016/j.trac.2018.06.010. [DOI] [Google Scholar]
- Hu S.; Chen X.; Wang R.-q.; Yang L.; Bai X.-h. Natural product applications of liquid-phase microextraction. TrAC, Trends Anal. Chem. 2019, 113, 340–350. 10.1016/j.trac.2018.11.006. [DOI] [Google Scholar]
- Huang Z.; Lee H. K. Micro-solid phase extraction of organochlorine pesticides using porous metal-organic framework MIL-101 as sorbent. J. Chromatogr. A 2015, 1401, 9–16. 10.1016/j.chroma.2015.04.052. [DOI] [PubMed] [Google Scholar]
- Yang W.; Muhammad T.; Yigaimu A.; Muhammad K.; Chen L. Preparation of stoichiometric molecularly imprinted polymer coatings on magnetic particles for the selective extraction of auramine O from water. J. Sep. Sci. 2018, 41, 4185–4193. 10.1002/jssc.201800797. [DOI] [PubMed] [Google Scholar]
- Barahona F.; Díaz-Álvarez M.; Turiel E.; Martín-Esteban A. Molecularly imprinted polymer-coated hollow fiber membrane for the microextraction of triazines directly from environmental waters. J. Chromatogr. A 2016, 1442, 12–18. 10.1016/j.chroma.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Muhammad T.; Cui L.; Wang J.; Piletska E. V.; Guerreiro A. R.; Piletsky S. A. Rational design and synthesis of water-compatible molecularly imprinted polymers for selective solid phase extraction of amiodarone. Anal. Chim. Acta 2012, 709, 98–104. 10.1016/j.aca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Muhammad T.; Yimit O.; Turahun Y.; Muhammad K.; Uludağ Y.; Zhao Z. On-line determination of 4-nitrophenol by combining molecularly imprinted solid-phase extraction and fiber-optic spectrophotometry. J. Sep. Sci. 2014, 37, 1873–1879. 10.1002/jssc.201400211. [DOI] [PubMed] [Google Scholar]
- Figueiredo L.; Erny G. L.; Santos L.; Alves A. Applications of molecularly imprinted polymers to the analysis and removal of personal care products: A review. Talanta 2016, 146, 754–765. 10.1016/j.talanta.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Fang S.; Gu W.; Chen L.; Yu Z.; Dai M.; Lin Y.; Liao Y.; Ma X. Ultrasonic pretreatment effects on the co-pyrolysis of municipal solid waste and paper sludge through orthogonal test. Bioresour. Technol. 2018, 258, 5–11. 10.1016/j.biortech.2018.02.120. [DOI] [PubMed] [Google Scholar]
- Chen L.; Muhammad T.; Abuduani M.; Jappar S. Spectrofluorimetric Determination of Indole Phytohormones in Bean Sprout Using Three Phase Hollow Fiber-Liquid Phase Microextraction. J. Instrum. Anal. 2016, 35, 229–234. [Google Scholar]
- Yang L.; Chen Y.; Zhao S.; Zhang W.; Du H.; Deng Z.; Zhang S. Simultaneous determination of indole-3-acetic acid and indole-3-butyric acid in plant by field-amplified sample stacking open-tubular capillary electrochromatography based on solid-phase extraction with calixarene sorbent. Chromatographia 2016, 79, 243–254. 10.1007/s10337-015-2999-3. [DOI] [Google Scholar]
- Lu Q.; Wu J.-H.; Yu Q.-W.; Feng Y.-Q. Using pollen grains as novel hydrophilic solid-phase extraction sorbents for the simultaneous determination of 16 plant growth regulators. J. Chromatogr. A 2014, 1367, 39–47. 10.1016/j.chroma.2014.09.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.