Abstract
Background
Infective endocarditis (IE) is increasing among persons who inject drugs (PWID) and has high morbidity and mortality. Recurrent IE in PWID is not well described.
Methods
This was a retrospective cohort study conducted between February 2007 and March 2016. It included adult inpatients (≥18) at any of 3 tertiary care centers in London, Ontario, with definite IE based on the Modified Duke's Criteria. The objectives were to characterize recurrent IE in PWID, identify risk factors for recurrent IE, identify the frequency of fungal endocarditis, and establish whether fungal infection was associated with higher mortality.
Results
Three hundred ninety patients had endocarditis, with 212/390 in PWID. Sixty-eight of 212 (32%) PWID had a second episode, with 28/212 (12%) having additional recurrences. Second-episode IE was more common in PWID (11/178 [6.2%] vs 68/212 [32.1%]; P < .001). Peripherally inserted central catheter (PICC) line abuse was associated with increased risk of recurrent endocarditis (odds ratio [OR], 1.97; 95% confidence interval [CI], 1.01–3.87; P = .04). In PWID, fungal IE was more common in second episodes than first episodes (1/212 [0.5%] vs 5/68 [7.4%]; P = .004). Additionally, fungal infections were associated with mortality in second-episode IE in PWID with an adjusted OR of 16.49 (95% CI, 1.12–243.17; P = .041). Despite recurrent infection, likely due to continued drug use, there was a low rate of referral to addiction treatment (14/68 [20.6%]).
Conclusions
PWID have a high risk of recurrent endocarditis, particularly in patients who abuse PICC lines. Fungal endocarditis is more common in second-episode endocarditis and is associated with increased mortality. Consideration of empiric antifungal therapy in PWID with IE history and suspected IE should be considered.
Keywords: infective endocarditis, persons who inject drugs, fungal endocarditis, recurrent endocarditis, opioids
As more data become available to illustrate the severity of the opioid crisis, associated infectious complications are also being identified [1, 2]. Infective endocarditis (IE) represents one of the most common infectious complications of injection drug use, often requiring long-term hospitalization for antimicrobial therapy [3]. PWID represent a demographic of patients with unique barriers to care; they are more likely to leave the hospital against medical advice (AMA) [4], and harm reduction practices are not widely implemented in the inpatient setting [5]. Furthermore, injection drug use has been reported to be the single largest risk factor predisposing patients to recurrent infective endocarditis [2, 6]. Recurrence is defined as repeated IE episodes at least 6 months apart or caused by different microorganisms [7, 8]. In the literature, recurrent IE episodes in PWID affect 5.6%–25% of patients [9, 10] and recurrent episodes were reported in 1 small study to be associated with increased morbidity and mortality [9].
Gram-positive cocci account for 80%–90% of IE cases, with Staphylococcus aureus being the most common causative agent in the industrialized world [11, 12]. Although S. aureus is the most common cause of PWID-associated IE [10, 13, 14], PWID also suffer from IE due to less common organisms such as Pseudomonas spp., the HACEK group, and Candida spp. [8, 14, 18]. Few studies have reported on the microbial etiology and outcomes of recurrent IE episodes [7, 17], likely due to its rarity in most patient populations. Only 1 small study has recently been published on recurrent IE in PWID [9]. Given the current opioid epidemic and its association with IE, there is an urgent need for data on this population. We aimed to characterize recurrent IE among PWID and hypothesized that fungal endocarditis would be more common in recurrent cases due to previously damaged valves, and therefore empiric antifungal therapy may be warranted in these cases.
METHODS
Setting and Population
This was a retrospective cohort analysis of PWID admitted to any of the 3 acute care centers in London, Ontario, Canada. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [18] for cohort studies were fully applied. The study population included all cases with a discharge diagnosis of “definite” IE as per the Modified Duke's Criteria [19] between February 2007 and March 2016, as these criteria can be applied to PWID [20]. Data were obtained by chart review by 2 infectious disease physicians using a standardized form for data abstraction. Classification was based on consensus between reviewers. Only adult (≥18 years old) patients were included, and cases of recurrent endocarditis were excluded if the data from their initial case was not available in the clinical records. Two patients were excluded from analysis because their previous IE information was missing from medical records and we could not verify their time to the outcome (recurrence or mortality). The comprehensive electronic medical record (EMR) used at London Health Sciences contains all clinical data including bloodwork, diagnostic imaging, microbiologic studies, demographic information, medication administration, and clinical notes, which allowed long-term follow-up information to be obtained. Ethical approval for the study was obtained from the Lawson Research Institute's Review Board. Data on the first episodes in PWID patients in this population were recently reported [13]; therefore, the focus herein is on recurrent episodes.
Definitions
Recurrent IE was defined as an episode of “definite” IE occurring >6 months from a previous episode, consistent with previous literature [21]. Additionally, if presenting within 6 months from a previous episode, cases were described as recurrent IE if a new vegetation was identified and a new organism was isolated from blood cultures (a single culture of a commensal organism was excluded). This classification differentiates relapses of IE (likely due to an incompletely treated first episode) from true reinfections or recurrent episodes. If the same species was isolated from blood cultures, the case would be considered recurrent if more than 1 of the antibiotic susceptibilities was different from the previous episode. Right-sided IE was defined as infection only involving right heart structures, and left-sided IE referred to cases in which infection was localized only to the left side of the heart. Bilateral infection included cases where infection occurred on both the right and left heart structures. Fungal endocarditis was defined as IE caused by Candida spp., Aspergillus spp., or Histoplasma capsulatum. Polymicrobial endocarditis cases, which included fungi, were not included in analyses of survival due to difficulties in attributing the organism that led to death. A peripherally inserted central venous catheter (PICC) was used in most patients, and abuse of these lines was defined as nonmedical use or suspected use of the catheter documented by the medical team. All intensive care admissions were before any surgical intervention, such that patients who did receive surgical treatment and required postoperative intensive care were not included. Endocarditis-related mortality was defined as death secondary to sepsis, congestive heart failure, endocardial abscess rupture, endocarditis-related arrhythmia including heart block, or bleeding from a mycotic aneurysm. Injection drug use status was determined through documentation in medical records of current use or use within the last 3 months. Death was documented from electronic medical records and review of obituary records, and patients were considered alive if the medical record demonstrated them to be so. Survival was followed electronically, as any patient presenting to any acute care, rehabilitation, or psychiatric facility in the city was captured. Furthermore, any laboratory testing, radiology, or filling of a prescription in the community was noted on the comprehensive record and demonstrated that the patient remained alive. If there was no further involvement with the health care system, follow-up was considered terminated at the time of the last interaction.
Statistical Analysis
All data were coded dichotomously, with positive outcomes (1) or negative outcomes (0) and additional categorizations where appropriate. The quantity of missing data was recorded for all variables. Categorical variables are presented as frequencies and percentages. Continuous variables are presented as medians and interquartile ranges and were assessed for normality then analyzed with Wilcoxon tests or t tests, as applicable. Comparisons were made between proportions using χ 2 tests or Fisher exact tests. Univariable and multivariable logistic regression was used (including variables of clinical importance [site of infection, prosthetic valve, surgical treatment]) to identify an association between fungal endocarditis and mortality. Patients without mortality data were excluded. Variables were tested for multicollinearity. Odds ratios and 95% confidence intervals were calculated and reported where applicable. Time to outcome data was also used to generate a Kaplan-Meier curve to illustrate mortality among PWID with a fungal IE, with a log-rank test comparing survival among fungal and nonfungal second-episode endocarditis. Follow-up times were assessed using the reverse Kaplan-Meier estimator. An additional multivariable logistic regression model was generated from patients who survived an initial episode of endocarditis (n = 139), with the outcome of interest being a second episode of endocarditis. Variables were selected for inclusion based on clinical significance (surgical treatment, site of infection, referral to addictions treatment, abuse of PICC line, and leaving AMA). Statistical analyses were performed with SAS 9.4 (SAS Institute Inc. Cary, NC) and R statistical software, version 1.1.383 (R Project for Statistical Computing), with P values <.05 considered statistically significant.
RESULTS
At the time of discharge, 1464 episodes of infective endocarditis were identified. Of these, 489 episodes fulfilled the Modified Duke criteria for definite IE. Because there are preexisting differences between IE in PWID and non-PWID and because of the high recurrence rate among PWID in our cohort, comparisons between first-episode IE and subsequent episodes were only assessed in PWID (n = 280 episodes). Among PWID, there were 212 first episodes of endocarditis, 68/212 patients who had a second episode of endocarditis, 22/68 with a third recurrence, and 5/22 with a fourth recurrence. Among non-PWID, there were 178 first episodes of endocarditis, 11/178 patients with a second episode of endocarditis, and only 3/11 patients with a third recurrence. Overall, among PWID the rate of recurrent endocarditis was 32.1% (68/212). As expected, second- episode IE was more common in PWID (11/178 [6.2%] vs 68/212 [32.1%]; P < .001). Demographics of first and second episodes in PWID are shown in Table 1. The median follow-up time for PWID with first and second episodes (interquartile range [IQR]) was 2.87 (1.61–4.95) years.
Table 1.
Demographic Characteristics of PWID Infective Endocarditis Episodes
| First-Episode IE | Second-Episode IE | ||
|---|---|---|---|
| (n = 212) | (n = 68) | ||
| Age, median (IQR), y | 34 (28–42) | 35 (28–42) | |
| Sex | Male | 109 (51.4) | 36 (52.9) |
| Female | 103 (48.6) | 32 (47.1) | |
| Hepatitis C | No | 40 (18.9) | 9 (13.2) |
| Yes | 151 (71.2) | 56 (82.4) | |
| Unknown | 21 | 0 | |
| HIV | No | 149 (70.3) | 40 (58.8) |
| Yes | 16 (7.5) | 7 (10.3) | |
| Unknown | 47 | 21 | |
| Length of stay, median (IQR), d | 22 (12.4–43.3) | 19 (9.7–44.3) | |
| Homeless | No | 173 (81.6) | 55 (80.9) |
| Yes | 37 (17.4) | 13 (19.1) | |
| Unknown | 2 | 0 | |
| Causative organisma | Candida | 1 (0.5) | 5 (7.4) |
| S. aureus | 165 (77.8) | 43 (63.2) | |
| Site of infection | Left | 57 (26.9) | 14 (20.6) |
| Right | 132 (62.2) | 45 (66.2) | |
| Bilateral | 14 (6.6) | 6 (8.8) | |
| Unknown | 9 | 2 | |
| Primary valve | Tricuspid | 133 (62.7) | 46 (67.6) |
| Pulmonic | 2 (0.9) | 0 | |
| Aortic | 33 (15.6) | 8 (11.8) | |
| Mitral | 33 (15.6) | 11 (16.2) | |
| Otherb | 2 (0.9) | 1 (1.5) | |
| Unknownc | 9 | 2 | |
| Prosthetic devicee | 0 | 1 | |
| Prosthetic valve | No | 210 (99.1) | 58 (85.3) |
| Yes | 2 (0.9) | 10 (14.7) | |
| Surgery | No | 172 (81.1) | 56 (82.4) |
| Yes | 40 (18.9) | 12 (17.6) | |
| Substance use | Opiates | 24 (11.3) | 7 (10.3) |
| Stimulants | 6 (2.8) | 0 | |
| Polysubstance | 160 (75.5) | 56 (82.4) | |
| Unknown | 22 | 5 | |
| Referral to infectious diseases | No | 8 (3.8) | 1 (1.5) |
| Yes | 175 (82.5) | 53 (77.9) | |
| Unknown | 29 | 14 | |
| Referral to addiction treatment | No | 168 (79.2) | 53 (77.9) |
| Yes | 44 (20.8) | 14 (20.6) | |
| Unknown | 0 | 1 | |
| Method of treatment | Oral | 38 (17.9) | 11 (16.2) |
| Intramuscular | 2 (0.9) | 0 | |
| Intravenous | 172 (81.1) | 57 (83.8) | |
| PICC line | No | 25 (11.8) | 12 (17.6) |
| Yes | 180 (84.9) | 53 (77.9) | |
| Unknown | 7 | 3 | |
| PICC line abused | No | 174 (78.3) | 44 (73.5) |
| Yes | 37 (20.3) | 23 (25) | |
| Unknown | 1 | 1 | |
| Homeless | No | 173 (81.6) | 55 (80.9) |
| Yes | 37 (17.4) | 13 (19.1) | |
| Unknown | 2 | 0 | |
| Discharge AMA | No | 177 (83.5) | 58 (85.3) |
| Yes | 35 (16.5) | 10 (14.7) | |
| Unknown | 4 | 0 | |
| ICU admission | No | 129 (60.8) | 40 (58.8) |
| Yes | 83 (39.2) | 28 (41.2) | |
| Deathf | No | 139 (65.6) | 41 (60.3) |
| Yes | 72 (34) | 26 (38.2) | |
| Unknown | 1 | 1 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AMA, against medical advice; IE, infective endocarditis; IQR, interquartile range; PICC, peripherally inserted central catheter; PWID, persons who inject drugs.
aSee details in Table 2.
bRight atrial appendage, right atrium, right ventricle.
cFulfilling modified Duke criteria for definite endocarditis in the absence of a vegetation on echocardiography.
dPatient PICC line use for injection of drugs during the respective episode.
eImplantable cardioverter-defibrillator.
fRefers to death within 3 years.
The overall clinical characteristics were highly similar between first and second episodes in PWID. The median time to a second episode in PWID (IQR) was 1.23 (0.33–2.86) years. The majority of both first and second episodes involved the tricuspid valve (131/212 [61.8%] vs 45/68 [66.2%], respectively). Table 2 illustrates the microbiology of initial-episode and second-episode endocarditis cases (with Supplementary Table 1 showing a comparison between initial episodes and all subsequent recurrences). Notably, a significantly higher proportion of fungal endocarditis occurred in the second episodes (5/68 [7.4%] vs 1/212 [0.5%]; P = .004) or in all recurrent episodes when compared with first episodes (7/95 [7.4%] vs 1/212 [0.5%]; P = .0005). All 7 fungal endocarditis cases in recurrent episodes occurred on previously infected valves. In second episodes when polymicrobial cases (in which fungi were present) were included, 6/68 (8.8%) second episodes vs 2/212 (0.94%) first episodes (P = .0007) contained fungi. Full documentation of fungal cases is shown in Table 3. All Candida isolates were fluconazole sensitive, other than a single case with C. glabrata and a single case of C. albicans, which occurred after 3 previous courses of fluconazole for C. albicans azole-sensitive IE. Additionally, we identified a low rate of referral to supportive or substance abuse treatments in both first (44/212 [20.8%]) and second episodes (14/68 [20.6%]) among PWID.
Table 2.
Microbial Etiology of First and Second Infective Endocarditis Episodes in PWID
| First Episode (n = 212) | Second Episode (n = 68) | ||
|---|---|---|---|
| Organism | Staphylococcus aureus | 165 (77.83) | 43 (63.24) |
| MSSA | 119 (56.13) | 28 (41.18) | |
| MRSA | 46 (21.70) | 15 (22.06) | |
| Coagulase-negative staphylococci | 1 (0.47) | 1 (1.47) | |
| Non–viridans group streptococci | 1 (0.47) | 1 (1.47) | |
| Viridans group streptococci | 10 (4.72) | 11 (16.18) | |
| Enterococci | 11 (5.19) | 3 (4.41) | |
| Enterobacteriaceae | 1 (0.47) | 1 (1.47) | |
| HACEK | 0 | 1 (1.47) | |
| Pseudomonas or Actinobacter | 3 (1.42) | 0 | |
| Candida | 1 (0.47) | 5 (7.35) | |
| Polymicrobiala | 13 (6.13) | 2 (2.94) | |
| Culture negativeb | 5 (2.36) | 0 | |
| Other | 1 (0.47) | 0 |
Data are presented as No. (%).
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-resistant Staphylococcus aureus; PWID, people who inject drugs.
aOf the polymicrobial cases, 2 had Candida spp., 1 in the first episode and 1 in the second.
bMet modified Duke's criteria for endocarditis; however, had negative cultures.
Table 3.
Summary of Demographics and Clinical Course of Fungal Endocarditis Cases in PWID
| Patient No./ID | IE Episode | Comorbidities | Site of Infection | Etiology | Substance(s) Abused | Complications | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| #1/35M | 1st | IVDU, Crohn's disease | Native tricuspid valve with right atrial vegetation | MRSA, Candida albicans–sensitive to fluconazole | Hydromorphone–immediate and controlled release forms | Septic PE | Caspofungin IV for 2 weeks and fluconazole PO for 6 weeks after the last negative culture | Recurrence of IE 19 months later with tricuspid and pulmonary valve vegetation (successfully treated) |
| #2/21F | 1st | IVDU, HCV | Native tricuspid valve | Candida albicans–sensitive to fluconazole | Morphine & methamphetamine | Multisystem organ failure | Fluconazole, IV | Death 15 days after admission due to sepsis and large pulmonary emboli with refractory shock |
| #3/46M | 2nd | IVDU, HCV, previous MSSA IE of tricuspid valve; fully treated |
Native tricuspid valve | Candida albicans–sensitive to fluconazole | Methadone | Septic PE and lung infarction | Amphotericin B and fluconazole IV for 4 days | Death 4 days after admission |
| #4/37F | 2nd | IVDU, HCV, previous VGS IE–fully treated with tricuspid valve replacement, complicated by PE, ATN, and secondary C. albicans PICC line infection (June 2011) | Bio-prosthetic tricuspid valve | Candida glabrata–sensitive to amphotericin, resistant to fluconazole | Hydromorphone–controlled release | Renal failure secondary to septic emboli/ATN with hemodialysis | Empiric fluconazole IV then caspofungin IV for 4 weeks and then liposomal amphotericin B until palliative care decision was taken (3x/wk for 3 weeks with dialysis sessions) | Death (palliation at home) |
| #5/35F | 2nd | IVDU, HCV, previous MSSA IE of tricuspid valve with surgical valve repair (Dec 2010); uncertain if patient completed outpatient antibiotic therapy |
Native tricuspid valve | MRSA, Candida dublinensis–sensitive to fluconazole and caspofungin | Hydromorphone–immediate release, benzodiazepines, methamphetamine, methylphenidate & methadone | Septic PE, DIC, and abdominal compartment syndrome | Fluconazole IV started 1 day before death | Death 4 days after admission (withdrawal of care) |
| #6/30M | 2nd | IVDU, HCV, previous MSSA IE of tricuspid valve; treatment incomplete and required surgical repair; subsequent heart failure and acute interstitial nephritis (7 months before current episode) | Native tricuspid valve | Candida dubliniensis–sensitive to fluconazole and amphotericin | Hydromorphone–immediate and controlled release, morphine | Bilateral below-knee amputation secondary to septic emboli, chronic kidney disease secondary to IE, multisystem organ failure | Fluconazole IV for 2 weeks followed by 6 weeks of PO fluconazole, bio-prosthetic tricuspid valve replacement for recurrent fungal & Staph IE (4 months after current episode) | Death from recurrent MSSA PVE (13 months after current episode) |
| #7/26M | 2nd | IVDU, previous MSSA IE with pulmonary and renal emboli (1 year before current episode) | Native tricuspid valve | Candida albicans–sensitive to fluconazole | Hydromorphone–immediate and controlled release, morphine, fentanyl | Mycotic aneurysm (pulmonary artery) | Fluconazole IV for 6 weeks after the 1st negative blood culture | Large septic pulmonary emboli with refractory cardiogenic shock and respiratory failure (6 months after current episode) |
| #8/29F | 2nd | IVDU, HCV, previous enterococcal IE of mitral and tricuspid valves with both valves surgically repaired; uncertain if treatment completed as outpatient | Native mitral valve | Candida albicans–sensitive to fluconazole | Hydromorphone–immediate release, benzodiazepines, methamphetamine, cocaine | Caspofungin IV started and, due to allergy, later changed to IV fluconazole for 6 weeks | Transferred to regional hospital once clinically stable | |
| 3rd | Native mitral valve | Candida albicans–sensitive to fluconazole | Liposomal amphotericin B for 1 week, fluconazole IV for 1 week, followed by fluconazole PO for 6 weeks | Discharged once clinically stable | ||||
| 4th | Native mitral valve | Candida albicans–sensitive to fluconazole | Leg ischemia secondary to septic emboli | AmBisome IV for 1 week followed by liposomal amphotricin B plus PO flucytosine for 1 week, then PO fluconazole lifelong but poor adherence and patient discontinued on her own after 3 months | Alive, recurrent C. albicans–resistant to fluconazole, I7 months later with MV and AV repair; IV caspofungin lifelong thereafter |
Abbreviations: ATN, acute tubular necrosis; AV, aortic valve; DIC, disseminated intravascular coagulation; HCV, hepatitis C virus; IE, infective endocarditis; IV, intravenous; IVDU, intravenous drug use; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-resistant Staphylococcus aureus; MV, mitral valve; PE, pulmonary emboli; PICC, peripherally inserted central catheter; PO, oral; PWID, people who inject drugs; PVE, prosthetic valve endocarditis; VGS, viridans group streptococci.
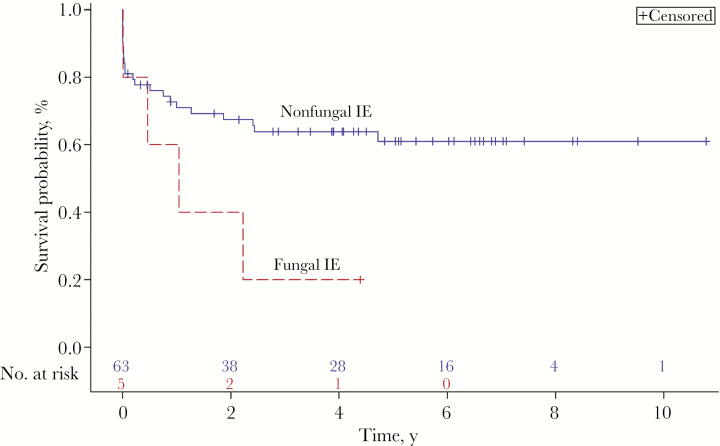
Multivariable analyses showed that the odds of long-term mortality (measured at 3 years) were 16.49 (95% confidence interval [CI], 1.12–243.17; P = .041) times greater for PWID with a fungal infection, relative to other microorganisms, while adjusting for prosthetic valves, site of infection, and surgery. The mortality rate for second-episode endocarditis was 38.2%, with 26 deaths. The most common cause of endocarditis-related mortality was sepsis (21/26 [81%]). The cause of death among patients with fungal IE is also detailed in Table 3. A log-rank test did not identify any significant difference in the probability of death in patients with vs without fungal infections at any time point (χ 2(1) = 3.31; P = .07). This is represented by the Kaplan-Meier curve shown in Figure 1. As seen in Table 4, an association between PICC line abuse and second-episode infective endocarditis was identified. Line use during the first episode increased the odds of recurrent endocarditis with an OR of 1.97 (95% CI, 1.01–3.87; P = .04). Among patients with first-episode endocarditis who were documented to have used their PICC line for nonmedical purposes, 7 experienced recurrent infection (7/37 [19%]). No other variables were associated with recurrence.
Figure 1.
Kaplan-Meier survival analysis of PWID with second-episode infective endocarditis. Abbreviations: IE, infective endocarditis; PWID, persons who inject drugs.
Table 4. .
Logistic Regression Unadjusted and Adjusted OR for Second-Episode Infective Endocarditis in PWID
| Unadjusted OR | 95% CI | P | Adjusted OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Surgery (ref = no surgery) | ||||||
| 1.06 | 0.50–2.22 | .89 | 0.90 | 0.38–2.14 | .81 | |
| Site of infection (ref = right-sided IE) | ||||||
| LSIE | 1.18 | 0.58–2.41 | .65 | 1.31 | 0.58–2.98 | .52 |
| BIE | 1.96 | 0.64–5.95 | .24 | 1.99 | 0.62–6.38 | .24 |
| PICC line abuse (ref = no PICC line abuse) | ||||||
| 1.54 | 0.84–2.85 | .17 | 1.97 | 1.01–3.87 | .04 | |
| Referral to addiction treatment (ref = no referral) | ||||||
| 0.63 | 0.32–1.24 | .18 | 0.54 | 0.26–1.14 | .11 | |
| Leave AMA (ref = did not leave AMA) | ||||||
| 0.56 | 0.24–1.28 | .17 | 0.50 | 0.21–1.20 | .12 | |
Abbreviations: AMA, against medical advice; BIE: bilateral infective endocarditis; CI, confidence interval; LSIE, left-sided infective endocarditis; OR, odds ratio; PICC, peripherally inserted central catheter; PWID, persons who inject drugs.
DISCUSSION
We present a large cohort of injection drug users with recurrent IE. The city of London has been disproportionately affected by the opioid epidemic [22], allowing a large sample of patients, with comprehensive data collection and follow-up enabled by our EMR. Persons who inject drugs experiencing recurrent endocarditis represent a demographic that has emerged with the contemporary opioid crisis [3]. Previous authors have called for the establishment of guidelines outlining an approach to the treatment of recurrent intravenous drug use–associated endocarditis [23]; this is particularly relevant if there is a higher proportion of fungal cases in recurrent endocarditis, as clinical guidelines suggest that the same indications for surgery in the non-PWID population should be applied to PWID in conjunction with addiction treatment [24]. This would include cases with a fungal etiology.
Interestingly, the risk of recurrent endocarditis in PWID has been inconsistent. It has been shown that repeat endocarditis is associated with injection drug use [9, 25]; however, older studies before the recent opioid crisis did not note an association of recurrent endocarditis with injection drug use [17]. We present the largest case series of recurrent endocarditis among PWID to date. The rate of recurrent endocarditis among PWID in our cohort was 32%, with 68 patients experiencing a second episode. This is higher than rates that have previously been reported in non–drug user populations (which range from 2.4% to 10.9%) [26] but is similar to a cohort of PWID from the 1970s where the recurrence rate was 41% [27] and a more contemporary population in North Carolina where the recurrence rate was 25.3% in PWID [9]. It remains unclear whether any clinical factors increase the likelihood of experiencing a second episode of endocarditis, beyond continued injection drug use. We identified that inappropriate use of a PICC line was associated with an increased risk of recurrent endocarditis. This may be a surrogate for the severity of addiction, and those with more severe substance use disorder may be more likely to continue to inject drugs and therefore be at higher risk of recurrent infection. Interestingly, referral for addiction treatment was not associated with a reduced risk of recurrent endocarditis. The low rate of addiction referral may have resulted in the analysis being underpowered to detect a difference (during first-episode endocarditis only, 20.8% of patients were referred).
In PWID, S. aureus is known to be the most common pathogen in first-episode endocarditis [13]. Although the overall rate of fungal IE is low, accounting for 1%–2% of all IE cases and 4% of prosthetic valve IE, it has previously been hypothesized that fungal IE is more common among intravenous drug users [19, 28–30]. We show that this is the case in second-episode IE among PWID, where there was a significantly higher proportion of fungal cases (0.5% in first episode vs 7.4% in first recurrence, 7.4% in all recurrent cases, and 8.8% in first recurrence when including polymicrobial cases containing fungi). Only 2 previous reports that contained a significant number of PWID patients assessed microbial etiology in first episode vs recurrent-episode endocarditis. A lower proportion of S. aureus (21/22 [95%] vs 12/22 [55%]; P = .04) and a numerically higher number of fungal endocarditis cases (0/22 vs 3/22 [14%]; P = .25) were found in the recent report of recurrent endocarditis cases from North Carolina, although the smaller sample size did not afford power to make conclusions on the difference in the incidence of fungal cases. An older literature review that described recurrent endocarditis in both PWID and non-PWID similarly found a rate of 7% of recurrent cases to be due to Candida species, with no cases in the primary episode [7]. The lower prevalence of S. aureus and higher prevalence of other organisms including fungi in recurrent cases may relate to previous valvular damage, which predisposes PWID to infections from organisms that are less able to adhere to a normal valve than S. aureus [31]. In our series, all cases of Candida endocarditis that occurred in a recurrent episode occurred on a valve also involved in a previous episode.
The only fungal species identified in our study population were Candida species, including C. albicans, C. dubliniensis, and C. glabrata (as shown in Table 3). It has been previously shown that up to 50%–80% of fungal IE is caused by Candida spp., and 20%–25% are caused by Aspergillus. Among the Candida spp., Candida albicans is the most common (as was the case in our cohort); however, Candida parapsilosis and Candida glabrata are emerging as causative agents in individuals previously exposed to azoles and PWID [6, 16, 19, 32]. PWID have been shown to have a higher risk of fungal endocarditis, which may relate to fungal contamination of drugs [15, 32]. It has been hypothesized that individuals using lemon juice as the solvent for heroin preparation can acquire Candida species fungemia and endocarditis [29]. The European Society of Cardiology (ESC) recommends that if a PWID uses brown heroin dissolved in lemon juice, Candida spp. should be considered and antifungal treatment added [6, 33]. Notably, heroin use is relatively uncommon in our community, and use of lemon juice is not seen (due to the major local type of heroin being white heroin, which does not require an acidifier, as well as vitamin C being routinely distributed in the local needle exchange program to prevent lemon juice use for heroin or crack cocaine) [34]. An increasing incidence of invasive candidiasis in PWID has also been recently reported in other North American centers [35, 36]. Previously reported mortality rates in fungal IE are around 50% despite aggressive combined medical and surgical intervention [29, 30, 32]. In our cohort, 5/6 (83%) patients with monomicrobial fungal endocarditis died. When including polymicrobial infections containing fungi, 6/8 (75%) patients died. Importantly, second-episode endocarditis caused by fungal organisms was associated with increased odds of death among our cohort, with an adjusted OR of 16.49 (95% CI, 1.12–243.17; P = .041) in multivariate analysis. This suggests that fungal endocarditis may have a higher mortality in PWID.
Fungal infective endocarditis is a “stand-alone indication” for a surgical valve replacement, and studies have reported improved mortality rates among individuals who underwent adjunctive valve surgery [28, 30, 32]. Only 2 patients ultimately underwent valve surgery in our cohort, illustrating the dilemmas faced when considering valvular surgery in PWID. Surgery in patients who are actively injecting drugs is controversial [23, 37], despite evidence that postsurgical outcomes are similar between PWID and non–drug users [17, 25, 38] and that surgery in first-episode endocarditis is associated with lower mortality [13]. Surgery in PWID with recurrent cases is particularly controversial due to a perception of a very high risk of further reinfections in this select group. In our cohort, 68/212 (32.1%) first-episode patients had a second episode, with a similar rate of third endocarditis episodes, 22/68 (32.4%) after an initial reinfection. In addition to surgery in eligible cases, the 2015 IDSA guidelines recommend provision of lifelong suppressive therapy with an oral azole after completion of initial parenteral therapy [19]. Lifelong azoles can be problematic in PWID due to fentanyl levels rising via CYP3A4 inhibition (and thus risk for overdose) and potential QT prolongation, which can complicate methadone maintenance therapy (but not buprenorphine therapy), as well as difficulties with long-term adherence to therapy.
Early administration of antifungal treatment is one of the only management options with the potential to influence outcomes, but despite the known mortality of fungal IE, it is not current practice. Our data suggest that in a recurrent episode of endocarditis in a person who injects drugs, empiric antifungal treatment should be considered. The antifungal chosen would depend on the local antifungal resistance patterns. This finding is particularly relevant in regions such as ours, which are affected by a high prevalence of intravenous drug use and a high rate of recurrent infective endocarditis. It is important to highlight substance use treatment options, as the low rate of referral to addiction services for treatment of patients with recurrent episodes of endocarditis is concerning given that ongoing injection drug use is likely the most significant risk factor for recurrent infections. This is reinforced by current guidelines, where it is suggested that practitioners refer PWID with endocarditis for treatment of their substance use disorder [19, 24]. Hospitalization represents an important opportunity to introduce supportive programs and attempt to engage individuals in substance abuse treatment programs [39].
Our analysis is strengthened by including only definite cases of IE using the modified Duke criteria, as per infectious diseases physician case review, and by being the largest case series of recurrent IE in PWID yet described. However, our analysis is subject to the inherent limitations of a retrospective design. The most significant limitation in our analysis is the small sample size of patients with fungal endocarditis (n = 8; 6/8 with previous endocarditis). However, these findings are consistent with those of previous smaller studies. These results should be interpreted as preliminary and exploratory. Additionally, follow-up data were not obtained for PWID referred to outpatient addiction treatment, which prevented additional analysis looking at the impact of pursuing outpatient addiction treatment on recurrence rates. Although we did see an increase in 3-year mortality in cases with fungal endocarditis, the Kaplan-Meier survival analysis did not show an early increase in mortality (Figure 1). We suspect that this was related to the small sample size of fungal cases.
The long period of this study raises the possibility of temporal changes impacting our findings. Local distribution of harm reduction equipment and opiate substitution therapy were extensive, and these, along with the availability of cardiac surgery, did not change during this time. However, the overall prevalence of PWID-associated IE did increase in Ontario and was associated with increased use of the prescription opioid hydromorphone [22]. Drug excipients within the controlled-release formulation of this opioid preserve S. aureus survival when the drug is contaminated by the complex handling necessary to prepare the drug for injection and thus predispose patients to S. aureus bacteremia and staphylococcal endocarditis [40]. Therefore, we do not feel that temporal changes led to more Candida infections in the recurrent cases.
CONCLUSIONS
Our study demonstrates that the demographics of first-episode IE and subsequent recurrent infections in PWID are similar; however, there is a higher proportion of fungal IE in recurrent episodes. In second-episode IE in PWID, fungal IE is associated with higher long-term mortality. These findings, although preliminary, should be used to prompt further exploration on the inclusion of an empiric antifungal agent in recurrent IE cases among PWID. Treatment according to guidelines is challenging in these patients, and further studies should seek to assess the optimal treatment approach in PWID.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank Dr. Adeel Sherazi for assistance in chart review and Dr. Susana Pearl for editing.
Financial support. An unrestricted grant for this work was obtained from the Ontario HIV Treatment Network and the St Joseph's Hospital Foundation.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jones CM, Christensen A, Gladden RM. Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004–2013. Drug Alcohol Depend 2017;176:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wurcel AG, Anderson JE, Chui KKH, et al. . Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016;3:ofw157. doi:10.1093/ofid/ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fleischauer AT, Ruhl L, Rhea S, Barnes E. Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence — North Carolina, 2010–2015. MMWR Morb Mortal Wkly Rep 2017;66(22):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med 2010; 25:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma M, Lamba W, Cauderella A, Guimond TH, Bayoumi AM. Harm reduction in hospitals. Harm Reduct J 2017;14(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gould FK, Denning DW, Elliott TSJ, et al. . Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012;67(2):269–89. [DOI] [PubMed] [Google Scholar]
- 7. Baddour LM. Twelve-year review of recurrent native-valve infective endocarditis: a disease of the modern antibiotic era. Rev Infect Dis 1998;10(6):1163–70. [DOI] [PubMed] [Google Scholar]
- 8. Lossos IS, Oren R. Recurrent infective endocarditis. Postgrad Med J 1993;69(816):816–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang G, Barnes EW, Peacock JE. Repeat infective endocarditis in persons who inject drugs: “Take Another Little Piece of my Heart”*. Open Forum Infect Dis 2018;X(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortiz-Bautista C, López J, García-Granja PE, et al. . Current profile of infective endocarditis in intravenous drug users: prognostic relevance of the valves involved. Int J Cardiol 2015;187(1):472–4. [DOI] [PubMed] [Google Scholar]
- 11. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387(10021):882–93. [DOI] [PubMed] [Google Scholar]
- 12. Murdoch DR. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med 2009;169(5):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodger L, Glockler-lauf SD, Shojaei E, et al. . Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open 2018;1(7):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thalme A, Westling K, Julander I. In-hospital and long-term mortality in infective endocarditis in injecting drug users compared to non-drug users: a retrospective study of 192 episodes. Scand J Infect Dis 2007; 39:197–204. [DOI] [PubMed] [Google Scholar]
- 15. Colville T, Sharma V, Albouaini K. Infective endocarditis in intravenous drug users: a review article. Postgrad Med J 2016; 92:105–11. [DOI] [PubMed] [Google Scholar]
- 16. Arnold CJ, Johnson M, Bayer AS, et al. . Candida infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother 2015; 59:2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renzulli A, Carozza A, Romano G, et al. . Recurrent infective endocarditis: a multivariate analysis of 21 years of experience. Ann Thorac Surg 2001; 72(1):39–43. [DOI] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 19. Baddour LM, Wilson WR, Bayer AS, et al. . Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 20. Palepu A, Cheung SS, Montessori V, et al. . Factors other than the Duke criteria associated with infective endocarditis among injection drug users. Clin Invest Med 2002; 25:118–25. [PubMed] [Google Scholar]
- 21. Chu VH, Sexton DJ, Cabell CH, et al. . Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis 2005; 41(3):406–9. [DOI] [PubMed] [Google Scholar]
- 22. Weir MA, Slater J, Jandoc R, Koivu S, Garg AX, Silverman M. The risk of infective endocarditis among people who inject drugs: a retrospective, population- based time series analysis. CMAJ 2019; 191(4):93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hull SC, Jadbabaie F. When is enough enough? The dilemma of valve replacement in a recidivist intravenous drug user. Ann Thorac Surg 2014; 97(5):1486–7. [DOI] [PubMed] [Google Scholar]
- 24. Pettersson GB, Coselli JS, Hussain ST, et al. . 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–58.e29. [DOI] [PubMed] [Google Scholar]
- 25. Alagna L, Park LP, Nicholson BP, et al. . Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis - Prospective Cohort Study. Clin Microbiol Infect 2014; 20:566–75. [DOI] [PubMed] [Google Scholar]
- 26. Mansur AJ, Dal Bó CM, Fukushima JT, et al. . Relapses, recurrences, valve replacements, and mortality during the long-term follow-up after infective endocarditis. Am Heart J 2001; 141:78–86. [DOI] [PubMed] [Google Scholar]
- 27. Welton DE, Young JB, Gentry WO, et al. . Recurrent infective endocarditis: analysis of predisposing factors and clinical features. Am J Med 1979; 66(6):932–8. [DOI] [PubMed] [Google Scholar]
- 28. Pierrotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest 2002; 122(1):302–10. [DOI] [PubMed] [Google Scholar]
- 29. Antinori S, Ferraris L, Orlando G, et al. . Fungal endocarditis observed over an 8-year period and a review of the literature. Mycopathologia 2014; 178:37–51. [DOI] [PubMed] [Google Scholar]
- 30. Tattevin P, Revest M, Lefort A, Michelet C, Lortholary O. Fungal endocarditis: current challenges. Int J Antimicrob Agents 2014; 44(4):290–4. [DOI] [PubMed] [Google Scholar]
- 31. Bodén MK, Flock JI. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog 1992; 12:289–98. [DOI] [PubMed] [Google Scholar]
- 32. Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001; 32(1058–4838):50–62. [DOI] [PubMed] [Google Scholar]
- 33. Habib G, Lancellotti P, Antunes MJ, et al. . 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36:3075–123.26320109 [Google Scholar]
- 34. Caldarelli H, Locker A, Warshawsky B. A profile of people who inject drugs in London, Ontario report on the public health agency of Canada. Middlesex-London Heal Unit 2012. [Google Scholar]
- 35. Poowanawittayakom N, Dutta A, Stock S, Touray S, Iii RTE, Levitz SM. Reemergence of intravenous drug use as risk factor for candidemia, Massachusetts, USA. Emerg Infect Dis 2019; 24(4):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hartnett KP, Jackson KA, Felsen C, et al. . Bacterial and fungal infections in persons who inject drugs — Western New York, 2017. Morb Mortal Wkly 2019; 68(26):2017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Østerdal OB, Salminen P-R, Jordal S, Sjursen H, Wendelbo Ø, Haaverstad R. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg 2016; 22(5):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaiser SP, Melby SJ, Zierer A, et al. . Long-term outcomes in valve replacement surgery for infective endocarditis. Ann Thorac Surg 2007; 83(1):30–5. [DOI] [PubMed] [Google Scholar]
- 39. Rosenthal ES, Karchmer AW, Theisen-toupal J, Castillo A. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129(5):481–5. [DOI] [PubMed] [Google Scholar]
- 40. Kasper KJ, Manoharan I, Hallam B, et al. . A controlled-release oral opioid supports S. aureus survival in injection drug preparation equipment and may increase bacteremia and endocarditis risk. PLoS One 2019; 14(8):e0219777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.