Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To assess benefit and safety of cannabis‐based medicines, including synthetic, or herbal and plant‐derived cannabinoids, for people with MS.
Background
Description of the condition
Multiple sclerosis (MS) is an immune‐mediated disease of the central nervous system (CNS) that leads to a progressive functional decline. The worldwide prevalence of MS is reported to be 50 to 300 per 100,000 people. About 2.3 million people are estimated to live with MS globally, although this number may be underestimated because data are lacking from large populations, such as populations in India and China (Thompson 2018a). Although the aetiology of MS remains unknown, associations with genetic, environmental, and lifestyle factors have been reported (Thompson 2018a). MS is commonly classified into different forms: relapsing‐remitting (RRMS), secondary progressive (SPMS), primary progressive (PPMS), and progressive‐relapsing (PRMS). Symptoms vary widely from person to person, and include fatigue, muscle painful spasms and stiffness, weakness, chronic neuropathic and musculoskeletal pain, mobility restrictions, visual impairment, depression, anxiety, and bladder and bowel dysfunction (Newsome 2017; Rommer 2018).
People with MS have multiple symptoms; for example, people with spasticity may also have chronic pain resulting from their spasticity. Therefore it is necessary to consider the overlap of indications people have when use a symptomatic treatment. Spasticity (muscle stiffness) is a common and serious feature of MS that increases with disease progression and leads to disability worsening, weakness, and fatigability. Adaptive features may develop including contractures in muscle, tendons, and joints which can further worsen limb positioning, movement, and function. Spasticity causes also pain, bed sore, fatigue, instability, and difficulties in maintaining hygiene. Treatment with anti‐spasticity medication is made for different reasons in people with MS. People with severe mobility disability are treated for symptomatic relief (pain and spasms) and are treated in order to make nursing care and seating easier. Those who are able to walk are treated with the additional aim of improving or preserving mobility (Amatya 2013; Shakespeare 2003). Chronic neuropathic pain occurs in more than half of people with MS and is directly related to MS pathology (Newsome 2017).
Description of the intervention
Cannabis is a plant (Cannabis sativa) that contains over 120 phytocannabinoids. The most well‐known cannabinoids are: delta‐9‐tetrahydrocannabinol (THC), which produces a variety of effects including altered cognition and motor function, analgesia, psychotropic effects; and cannabidiol (CBD), a non‐psychoactive molecule (Hazekamp 2018; Izzo 2009; Morales 2017). Several standardized medicinal cannabis‐based products are currently manufactured. Nabiximols (Sativex) is made from extracts of Cannabis sativa plant and contains an equal mix of the cannabinoids THC and CBD. It is taken as an oral spray. Bedrocan and Bedrobinol are standardized preparations of cannabis flowers containing a CBD‐level below 1% (in both preparation) and 22% and 13.5% THC, respectively. Bediol (6.3% THC and 8% CBD) and Bedrolite (less than 1% THC and 9% CBD) are standardized cannabis flowers both available in granular form. Bedica, featuring 14% THC and less than 1% CBD, is a standardized preparation, available in granular form, obtained from the variety indica of Cannabis flowers. Dronabinol (Marinol or Syndros) is a plant‐derived cannabinoid containing synthetic delta‐9‐THC. It is administered as oral capsules or oral solution. Nabilone (Cesamet or Canemes) is a synthetic THC analogue and it is administered as oral capsules. Both Dronabinol and Dabilone and other synthetic compounds, which are identical in structure to naturally occurring cannabinoids such as THC, have been evaluated in many studies that have investigated medicinal cannabis.
A titration period is required to reach optimal dose of nabiximols. The number and timing of sprays vary between patients. The dose is gradually increased by one spray per day, up to a maximum of 12 sprays per day, until optimum symptom relief is achieved. The median dose in clinical trials for people with MS is eight sprays per day. After oral administration of nabiximols, plasma levels of THC and other cannabinoids are lower compared with the levels achieved following smoking or inhalation of cannabinoids at a similar dose. According to the literature, elimination of oral cannabinoids from plasma is biphasic with an initial half‐life of approximately four hours, and the terminal elimination half‐lives are of the order of 24 to 36 hours or longer due to its slow release from fatty tissue (MHRA 2014).
An international survey found that MS was one of the five medical conditions for which cannabinoids were most often used; with back pain, sleep disorders, depression, and post‐injury pain being the other four conditions (Hazekamp 2013). The UK MS Society conducted a survey of 3994 people with MS from across the UK in September 2014, requesting their attitudes and experiences of cannabis and Sativex. The survey was conducted anonymously through various channels to capture the range of experiences and views that people with MS hold. More than 1 in 5 people (22%) reported they had used cannabis to try to manage their MS symptoms and 7% of those surveyed were still using cannabis. Most people (56%) currently using cannabis for medicinal purposes felt that the benefits outweighed the side effects. Of those currently using cannabis, 40% were doing so because they were unable to obtain a prescription for a licensed alternative. Medical cannabis use was associated with recreational cannabis use. The symptoms reported by medical cannabis users to be most effectively relieved were stress, sleep, mood, stiffness/spasm, and pain (MS Society 2014). A recent internet‐based survey in the USA found that 66% of people with MS used cannabis for symptom treatment (Kindred 2017), and a study from Canada reported that about 50% of people with MS would consider the legal use of cannabis if evidence of benefit is available (Banwell 2016).
How the intervention might work
Plant‐derived and synthetic cannabinoids exert their biological effects primarily via interaction with the endocannabinoid system which includes cannabinoid receptors (CB1 and CB2), endogenous cannabinoids (endocannabinoids, chiefly anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG)), and the enzymes responsible for the synthesis and degradation of the endocannabinoids (Di Marzo 2018; Kaur 2016; Papaseit 2018). Transient receptor potential (TRP) channels, peroxisome proliferator activated receptors (PPARs), glycine receptors, and the orphan G protein‐coupled receptors (GPR55 and GPR18) are also engaged by cannabinoids (Morales 2017). The psychoactive effects of cannabis are mainly due to the presence of THC. THC binds to cannabinoid receptors CB1 and CB2, acting as a partial agonist. CB1 receptors are located in the CNS (cerebral cortex, hippocampus, basal ganglia, and cerebellum) and are involved in memory processing, motor function, appetite, and sensory perception. CB2 receptors are essentially expressed in immune cells and they have been attributed a role modulating the immune response. Cannabinoids have been considered to have the potential to affect both pathogenic mechanisms and symptoms of MS due to their ability to suppress neuroinflammation (via CB2 activation) (Mestre 2018), and to exert neuroprotective effects in the CNS (via CB1 activation) (Constantinescu 2018; Gowran 2011; Kaur 2016; Mecha 2019). The effect of cannabinoids on the immune system may also play a role, in the light of the autoimmune hypothesis of MS etiology (Fitzpatrick 2017; Mestre 2018; Oláh 2017).
Why it is important to do this review
Results of available surveys show that the demand of people with MS for symptomatic treatment with cannabis‐based medicines is high, even though these medicines are unavailable in the usual way (Banwell 2016; Hazekamp 2013; Kindred 2017; MS Society 2014). Many people with MS have a combination of pain and spasticity and would benefit from a symptomatic treatment. Available therapies that relieve the disabling symptoms of MS include botulinum toxin injections, baclofen or tizanidine for spasticity, anticonvulsants, antidepressant or analgesic medications for neuropathic pain, and anticholinergic drugs for bladder dysfunction. However, these symptomatic therapies are of limited efficacy or are often poorly tolerated (Mücke 2018; Newsome 2017). Moreover, many patients with MS have a combination of symptoms, e.g. pain and spasticity, and would benefit from a cannabis‐based medicine that could have an overlap of indications.
Recent systematic reviews on the use of cannabis‐based medicines in people with MS reported different conclusions on safety and benefit of these medicines in spasticity, chronic neuropathic pain, bladder dysfunction, and other symptoms (Amato 2017; Davies 2018; HPRA 2017; Koppel 2014; Lynch 2015; Meza 2017; Mücke 2018; NASEM 2017; Nielsen 2018; Whiting 2015; WHO 2018). Conclusive or substantial evidence that oral cannabinoids are effective for improving patient‐reported MS spasticity or pain symptoms was reported by several studies (Amato 2017; HPRA 2017; NASEM 2017; moderate‐quality evidence was reported by Whiting 2015; low‐ to moderate‐quality evidence by the Australian Government 2017, whose results were based on an overview of 11 systematic reviews by Nielsen 2018). The overview by Nielsen 2018 reported modest effects in MS for pain or spasticity. Meza 2017 concluded that cannabinoids did not reduce spasticity or pain in MS and the certainty of the evidence was high. Mücke 2018 concluded that the potential benefits of cannabis‐based medicine in chronic neuropathic pain might be outweighed by their potential harms. Discrepancies between the results and conclusions of these reviews are expected since they used different eligibility criteria of study design, participants, and outcomes measures and different analytic methods. Moreover, the search strategy of these reviews was updated to the end of 2016 and new studies are available for inclusion in our review (Table 1).
Table 1.
Published systematic reviews on the use of cannabis‐based medicines in people with MS
| Author year country | SR (search) | Included studies | Interventions | Primary outcomes | RoB/quality | Meta‐analysis | Conclusion |
|
Amato 2017 Italy |
SR: yes (updated September 2016) | RCTs (n 15) Parallel and cross‐over |
• Cannabis in any dose, used either as monotherapy or adjunct to conventional drugs • Placebo |
• Spasticity ‐ Ashworth scale* ‐ NRS scale • Pain • Quality of sleep |
RoB GRADE | Yes | Concerning the efficacy of cannabis (compared with placebo) in patients with MS. Quality or confidence in the estimate was high in favour of cannabis for spasticity (NRS and VAS scales but not the Ashworth scale) and pain but not for sleep (confidence in estimate moderate). |
|
HPRA 2017 Ireland |
SR: no Source: Barnes 2016 commissioned by the UK All‐Party Parliamentary Group (APPG) and other reports |
− | − | − | Criteria of the American Academy of Neurology | No | The scientific evidence, and the availability of an authorized medicine, support the use of cannabis in the treatment of spasticity associated with MS, where other treatments have failed. |
| NASEM 2017 USA | SR: yes (January 1999 to August 2016) Source: Koppel 2014; Whiting 2015 Updated search to 2016 |
• RCTs parallel (Koppel 2014; Whiting 2015) and cross‐over (Koppel 2014) • Non randomised studies |
• All types of plant derived and synthetic cannabis • Placebo |
• Spasticity ‐ Ashworth scale ‐ NRS scale • Pain |
RoB Newcastle‐Ontario scale. Five weight‐of‐evidence categories |
No (reported results of Whiting |
Conclusion 4‐1. There is substantial evidence that cannabis is an effective treatment for chronic pain in adults. Conclusion 4‐7. There is substantial evidence that oral cannabinoids are an effective treatment for improving patient‐reported MS spasticity symptoms (NRS), but limited evidence for an effect on clinician‐measured spasticity (Ashworth scale).* |
|
Whiting 2015 UK |
S: yes (up to April 2015) | RCTs parallel and cross‐over Pain: 1 RCT Spasticity: 11 RCTs (2138 participants) |
• Cannabinoids • Usual care, placebo, or no treatment |
• Spasticity ‐ Ashworth scale ‐ NRS scale • Pain |
RoB GRADE | Yes | • Cannabinoids (nabilone and nabiximols) were associated with a greater average improvement in spasticity assessed using numerical rating scales (MD −0.76 (95% CI −1.38 to −0.14; 3 trials). There was no evidence of a difference in association according to type of cannabinoid for either analysis. • Cannabinoids (nabiximols, dronabinol, and THC/CBD) were associated with a greater average improvement on the Ashworth scale* for spasticity compared with placebo, although this did not reach statistical significance (WMD, −0.12, 95% CI, −0.24 to 0.01; 5 trials). • The average number of patients who reported an improvement on a global impression of change score was also greater with nabiximols than placebo (OR 1.44, 95% CI 1.07 to 1.94; 3 trials). This was supported by a further crossover trial of dronabinol and oral THC/CBD that provided continuous data for this outcome (Killestein 2002). • Sensitivity analyses that included crossover trials showed results consistent with those based on parallel group trials alone. Conclusion: there was moderate quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity. |
| Australian Government 2017 | Based on overview of Nielsen 2018 and qualitative reviews done by 5 working groups | − | − | − | − | − | • Overall, there is low to moderate quality evidence that suggests pharmaceutical‐grade THC (dronabinol or THC extract) is effective for treating symptoms of pain. • THC:CBD (nabiximols, Sativex) may be effective for treating symptoms of pain and spasticity in MS, in certain patient populations. • Findings were mixed as to whether cannabinoids assisted in improving bladder function, sleep, patient quality of life, ataxia or tremor, and disability/disease progression. • No studies included active alternatives (non‐cannabinoid medicines) as comparators, which is an important limitation. |
|
Nielsen 2018 Australia |
Yes, overview (1980 up to 30 November 2016) |
SR (n = 11) (AMSTAR criteria 3 and 6). Included studies RCTs and non randomised studies |
• Plant‐based and pharmaceutical cannabinoids | • Disability and disability progression • Pain • Spasticity • Bladder function • Ataxia and tremor • Sleep • Quality of life • Adverse effects |
SIGN (for the reviews) GRADE |
No | Recent high‐quality reviews find cannabinoids may have modest effects in MS for pain or spasticity. |
|
Meza 2017 Chile |
Epistemonikos database | SRs (n = 25) Spasticity: 4 RCTs (1247 participants) Pain: 3 RCTs (327 participants) |
− | • Pain: evaluated according to VAS or NRS • Bladder dysfunction: evaluated according to NRS or irritative symptoms •Spasticity: evaluated according to Ashworth scale* or NRS • Adverse effects: such as sedation, dizziness, headache, euphoria, among others • Quality of life: according to subjective evaluation by participants • Coordination: according to subjective evaluation by participants • Mobility: according to subjective evaluation by participants • Others: sleep quality, tremor, posture and balance, dependence |
GRADE | Yes | • Cannabinoids do not reduce spasticity in MS. The certainty of the evidence is high. • Cannabinoids do not reduce pain in MS. The certainty of the evidence is high. • Cannabinoids are associated to adverse effects, which are probably frequent in MS. The certainty of the evidence is moderate. |
|
Mücke 2018 Germany |
Cochrane Review | RCTs. Parallel, cross‐over, and enriched enrolment randomized withdrawal design with at least 10 participants per treatment arm. Participants: different types of participants including central neuropathic pain (e.g. MS) |
• Cannabis‐based medicines, either herbal cannabis (hashish, marijuana), plant‐based cannabinoids (dronabinol: nabiximols), or pharmacological (synthetic) cannabinoids (e.g. levonantradol, nabilone) | • Pain | RoB GRADE | Yes | The potential benefits of cannabis‐based medicine in chronic neuropathic pain might be outweighed by their potential harms. |
Abbreviations: SR systematic review; RCTs randomised controlled trials; RoB risk of bias; NRS Numeric Rating Scale; VAS Visual Analogue Scale; WMD weighted mean difference; CI confidence interval.
* The Ashworth scale (Ashworth 1964) has been criticized as unreliable, insensitive to therapeutic benefit, and reflective only of passive resistance to movement and not of other features of spasticity (Pandyan 1999; Wade 2010).
International guidelines have reached different recommendations on the use of cannabis‐based medicines in people with MS. The NICE guidelines did not recommend nabiximols for MS on cost‐effectiveness grounds for the NHS in England, Scotland, and Northern Ireland (NICE 2014). However, nabiximols is considered cost‐effective in Wales. A new review and a guideline scoping document on cannabis‐based medicines is in development (NICE 2019). The Association of British Neurologists on the use of cannabis‐based products in neurology advised clinicians to use nabiximols only in people with MS who have had an unsatisfactory response to conventional spasticity drugs (ABN 2018; RCP 2018). The American Academy of Neurology does not support the legalization or prescribing of medical marijuana for use in MS, but supports scientific research to investigate the safety and potential benefits (AAN 2018). The Food and Drug Administration (FDA) has not approved any marketing application for cannabis‐based medicine for MS, but was recently asked to place cannabis‐based therapy for progressive MS on the fast track (Reston 2019). The European Medicines Agency (EMA) authorized in 2014 the use of nabiximols for the management of moderate to severe spasticity in adults with MS who have not responded to conventional treatment, and who show clear clinical improvement in the initial period with this therapy (EMA 2014). The guidance released in 2018 by the Australian Government Department of Health recommended to use cannabis‐based medicines in people with MS who have not responded adequately to other anti‐spasticity medication (Australian Government 2017).
There are differences between countries in the legal authorization and use of medical cannabis for MS. Nabiximols is approved and available for MS related spasticity in Canada, the USA, Israel, and 21 European countries and it is reimbursed by health insurance companies or state social security systems in 11 European countries (Austria, Belgium, Germany, Israel, Italy, Portugal, San Marino, Spain, Turkey, UK, and Norway) (Abuhasira 2018; Krcevski‐Skvarc 2018). Approval of cannabis‐based medicines (i.e. the cannabis flowers Bedrocan, Bediol, Bedica, Bedrobinol, Bedrolite) for treatment of chronic neuropathic pain that is refractory to conventional treatment is available in Canada, Croatia, Czech Republic, Denmark, Germany, Israel, Italy, the Netherlands, Norway, Serbia, Slovenia, and Switzerland, but with striking differences in legal and reimbursement rules (Krcevski‐Skvarc 2018). Medical cannabis can be prescribed to people with MS under strict controlled conditions, but there are differences between countries on who can and cannot prescribe cannabis‐based medicines, e.g. in the UK nabiximols can be prescribed only by specialist doctors with expertise in treating MS.
The legalization of cannabis has allowed new studies to be carried out and therefore new clinical data are available. The evidence that will come from these studies (if they are positive) might encourage the legalization of medicinal cannabis in countries where cannabis is not yet legal.
There is a growing interest into the therapeutic benefit of cannabis‐based medicines in the treatment of illness including MS. Following the review of the Chief Medical Advisor to the UK Government, on 1 November 2018, unlicensed cannabis based products were moved from Schedule 1 to Schedule 2 in the UK. This decision would allow cannabis medicines to be prescribed under controlled conditions by registered practitioners for medical benefit. In addition, moving the whole class of cannabis based medicinal products out of Schedule 1, will allow the evidence base on the therapeutic benefits associated with using this class of drugs to be improved through research, maximising benefits to patients. enabling them to be prescribed for the first time (Davies 2018). Moreover, the FDA recently asked to place cannabis‐based therapy for progressive MS on fast track (Reston 2019).
Due to the conflicting conclusions of recent systematic reviews on the benefit and safety of cannabis‐based medicines for symptomatic treatment of MS, as well as different recommendations in international guidelines, we see the need for a Cochrane Review undertaken according to rigorous standards.
Objectives
To assess benefit and safety of cannabis‐based medicines, including synthetic, or herbal and plant‐derived cannabinoids, for people with MS.
Methods
Criteria for considering studies for this review
Types of studies
We will include randomized parallel or cross‐over trials (RCTs). We will include cross‐over trials irrespective of the length of the washout period.
Types of participants
We will include adults, males and females (18 years or older), diagnosed with MS, according to the Poser (Poser 1983) or McDonald criteria and its revisions (McDonald 2001; Polman 2005; Polman 2011; Thompson 2018b), and all types of MS such as RRMS, SPMS, PPMS, and PRMS. We will include participants regardless of disease duration and disability degree.
Types of interventions
Any cannabinoid‐based medicine including herbal cannabis (e.g. marijuana), cannabis flowers (Bedrocan, Bedrobinol, Bediol, Bedrolite, Bedica), plant‐based cannabinoids (Nabiximols, Cannabidiol), or synthetic cannabinoids (Dronabinol, Nabilone), irrespective of dose, route, frequency, or duration of use. We will include as a comparison intervention placebo or any active comparator. We will include concomitant interventions if they were used in all the comparison groups.
Types of outcome measures
We will include patient‐reported outcomes as critical or important outcomes, because the primary scope and aim of this Cochrane Review is to assess the effects of the intervention on symptoms such as chronic pain and functional limitations due to spasticity. These symptoms are better known to the patients themselves than to clinicians, and the patients' perspective on treatment benefit is a priority. We will include short‐ and long‐term outcomes reported in the included trials.
1. Critical outcomes
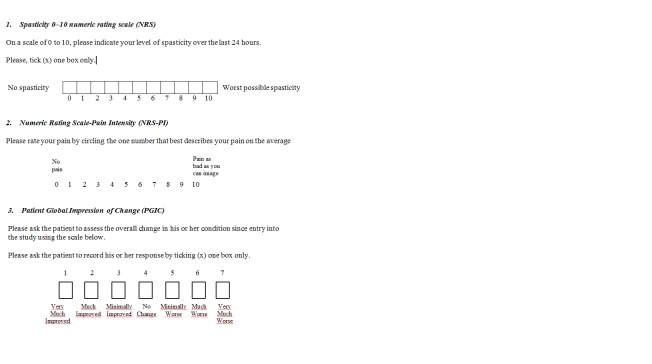
Spasticity: number of participants reporting reduction of 30% in the spasticity Numeric Rating Scale (NRS), over baseline. This reduction has been identified as a change that represented a minimum clinically important difference (MCID) in participants with MS–related spasticity (Farrar 2008). NRS is a patient‐rated measure of the perceived severity of spasticity. Scores range from 0 (no spasticity) to 10 (worst possible spasticity) (Figure 1).
Chronic neuropathic pain: number of participants reporting pain relief of 50% or greater, over baseline. According to Cochrane Pain, Palliative and Supportive Care (Moore 2010), we will prefer composite neuropathic pain scores (e.g. pain intensity and physical function) over single‐scale generic pain scores if both measures were used by studies, or the Numeric Rating Scale‐Pain Intensity (NRS‐PI), a 0 to 10 rating scale with scores ranging from 0 ‘no pain' to 10 ‘worst possible pain' (Farrar 2010; Figure 1).
Figure 1.
Spasticity and Pain scales; Patient Global Impression of Change
Where studies measure these outcomes as continuous data only, we will include them as separate analyses as important outcomes.
Number of participants withdrawn due to adverse events (tolerability).
2. Important outcomes
Patient Global Impression of Change (PGIC): number of participants reporting much or very much improvement in the PGIC. PGIC provides a patient reported assessment of overall change in health status on a seven point categorical scale with scores ranging from 1 (very much improved) to 7 (very much worse) (Guy 1976; Farrar 2008; Dworkin 2008) (Figure 1).
Quality of life, e.g. Multiple Sclerosis Quality of Life‐54 (MSQOL‐54) (Vickrey 1995) or other QOL validated measures reported in the included studies. MSQOL‐54 is a multidimensional health‐related quality of life measure.The questionnaire includes the generic Short‐Form 36‐item QoL instrument, supplemented with 18 MS‐specific items that were based on expert opinion and literature review. There is no single overall score for MSQOL‐54. Two summary scores — physical health and mental health — can be derived from a weighted combination of scale scores (scale scores range from 0 to 100 and a higher scale score indicates improved quality of life). No MCIDs were identified for the summary scores.
Where studies measure these outcomes as continuous data only, we will include them as separate analyses as outcomes of limited importance.
The total number of serious adverse events (SAEs). If an insufficient number of studies reported the total number of SAEs and person‐years, we plan to use the number of participants with at least one SAE as defined in the study.
Number of participants reporting specific adverse events, including nervous system (e.g. cognitive dysfunction, dizziness, somnolence, headache), psychiatric disorders (e.g. confusion state; paranoia, psychosis), and physical dependence effects (e.g. withdrawal and tolerance) according to the Medical Dictionary for Regulatory Activities (MedDRA) (ICH 2019), or as reported in the included studies.
3. Outcomes of limited importance
Reduction in spasticity measured by clinical reported measure, e.g. the Ashworth scale (Ashworth 1964) or Modified Ashworth (MAS) (Ansari 2009), or the Tardieu or Modified Tardieu scale (Ansari 2008).
Participant‐reported pain relief of 30% or greater in a composite neuropathic pain scale or in a single generic pain scale, e.g. the NRS‐PI (0‐10 NRS‐PI).
Improvement of bladder symptoms measured by patient reported outcome, e.g. the Overactive Bladder questionnaire (OAB‐q) (Coyne 2005).
Participant‐reported frequency and severity of spasms, e.g. Penn Spasm Frequency Scale (Penn 1989).
Fatigue, e.g. questionnaire Modified‐Fatigue Impact Scale (M‐FIS) (Multiple Sclerosis Council 1998). M‐FIS is a 21‐item multidimensional questionnaire that measures the physical, cognitive, and psychosocial impact of fatigue using a five‐point ordinal scale (range 0 to 84). Higher scores indicate greater impact or severity of fatigue symptoms. A difference of four points on the M‐FIS as been identified as a clinically significant difference in fatigue (Rooney 2019).
Sleep problems, e.g. the NRS (0‐10 NRS).
Improvement of mobility, balance, and daily functioning, specifically the activities of daily living (ADL), e.g. Barthel index (BI) (Mahoney 1965) or timed 10‐metre walk test (Kempen 2011).
Depression and anxiety measured by validated scales, e.g. the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983).
Caregiver’s global impression of change (CGIC), rating ease of transfer, dressing, and perineal hygiene (Collin 2010).
Reduced use of other symptomatic treatments (e.g. for spasticity or pain).
Search methods for identification of studies
Electronic searches
We will search the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library) (latest issue).
MEDLINE (PubMed) (1966 to date).
Embase (1974 to date).
CINAHL (EBSCO host) (1981 to date).
LILACS (Bireme) (1982 to date).
Physiotherapy Evidence Database (PEDro) (1990 to date).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch).
US National Institutes of Health clinical trial register (www.ClinicalTrials.gov).
European Union Clinical Trials Register (www.clinicaltrialsregister.eu).
International Association for Cannabinoid Medicines (IACM) databank (www.cannabis‐med.org/studies/study.php).
Information on the Group’s Trials Register and details of search strategies used to identify trials can be found in the Specialized Register section on the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group’s website (https://msrdcns.cochrane.org/). We have listed the keywords that we will use for the electronic search in Appendix 1.
Searching other resources
We will review the references of any RCTs identified, review articles, and textbooks. We will contact study investigators to request missing data.
Data collection and analysis
Selection of studies
We will use the search strategy described in the ‘Search methods for identification of studies' section to obtain titles and abstracts of studies. Two review authors (FB and GF) will independently screen the titles and abstracts and discard studies that are not applicable; however, they will initially retain studies and reviews that might include relevant data or information on trials. Two review authors (FB and AAI) will independently assess the retrieved abstracts and, when necessary, the full‐text articles to determine which studies satisfy the inclusion criteria. The two review authors will compare multiple reports of the same study and use the most comprehensive report. They will link together multiple publications as companion reports, but exclude true duplicates. FB and AAI will resolve discrepancies in judgement by discussion with a third review author (GF), and will report excluded studies and their reasons for exclusion in the ‘Characteristics of excluded studies’ table. We will create a PRISMA flow chart reporting the selection process (Moher 2009).
Data extraction and management
Two review authors (FB and AAI) will independently extract data using a predefined data extraction form in an Excel spreadsheet. They will resolve any disagreements by discussion with a third review author (GF). When necessary data are unavailable from the study report, we will try to obtain them through correspondence with the study authors.
Outcome data
We will extract from each included study the number of participants who:
had reduction of 30% in the spasticity NRS, or the PGIC much or very much improved;
had pain relief of 50% or greater in a composite neuropathic pain score, or in the NRS‐PI, or PGIC much or very much improved;
withdrew due to any adverse event;
had at least one SAE;
measures and results of the secondary outcomes (Secondary outcomes) that were reported in the included studies.
For the spasticity and pain relief outcomes, we will extract from cross‐over trials the number of participants who:
improved with both treatments;
improved with experimental treatment, deteriorated with control treatment;
improved with control treatment, deteriorated with experimental treatment;
deteriorated with both treatments.
For the adverse event outcomes, we will extract from cross‐over trials the number of withdrawals due to any AE, and the number of SAEs on each treatment in each treatment period (if possible).
For continuous outcomes we will extract mean and standard deviation of the comparison groups, where possible, and between‐period correlation in cross‐over studies. To analyse carry‐over, where possible, we will extract also mean and standard deviation by sequence in period I and period II.
We will extract the authors’ definition of spasticity, neuropathic pain, and secondary outcomes included in the review. We will extract the measure used in the trial to assess each reported outcome. We will extract arm‐level data when possible. When arm‐level data are not available we will extract effect sizes. We will extract data at the authors' defined timing points.
Data on potential effect modifiers
We will extract data on the following potential effect modifiers from each included study:
population: types of MS (RRMS, SPMS, PPMS, and PRMS), disability, spasticity, and pain score baseline; prior or actual or both treatment with anti‐spasticity or analgesic or both; prior cannabis use; duration of spasticity or pain or both;
study design: placebo or active control; co‐therapies allowed; rescue medication; study duration (less than four weeks; 4 to 12 weeks; 13 to 26 weeks; more than 26 weeks);
intervention: drug, dose, frequency, or duration of treatment.
Other data
From each included study we will extract data on the following:
study: first author or acronym; number of centres; year of publication; years that the study was conducted (recruitment and follow‐up); publication (full‐text publication, abstract publication, unpublished data);
study design (parallel or cross‐over); inclusion and exclusion criteria; number of randomized participants; early termination of trial;
conflict of interests of study authors;
funding of the study.
We will extract length of the washout period in cross‐over trials.
Assessment of risk of bias in included studies
For the scope of this review, we will assess the effect of the assignment to the intervention (“Intention to treat effect”) for critical and important outcomes. For the total number of SAEs and specific adverse events we will assess the effect of adhering to the intervention (‘per protocol effect').
Three review authors (SM, GF, and TL) will independently assess the risk of bias of each included study using version 2 of the Cochrane ‘Risk of bias' tool (RoB2) for both parallel and cross‐over trials (Higgins 2019). We will assess RoB2 for the critical and important outcomes reported in the ‘Summary of findings' table. RoB2 assesses:
bias arising from the randomization process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
Additional considerations for cross‐over trials include (Higgins 2016):
period effect;
carryover effect;
selection of the reported results, i.e. selective reporting of first period data on the basis of a test for carry‐over (Freeman 1989).
To implement RoB2 assessment, we will use the Excel tool available at: drive.google.com/file/d/18ilz6dx9voaTGH1mb_UN8WFTaic9‐p9z/view?usp=drive_open.
We will judge each domain as being at low risk of bias, some concerns, or high risk of bias. We will reach an overall risk of bias of each included study according to the following criteria:
low risk of bias: low risk of bias for all domains;
some concerns: some concerns in at least one domain, but not at high risk of bias for any domain;
high risk of bias: high risk of bias in at least one domain or some concerns for multiple domains in a way that substantially lowers confidence in the result.
We will assess characteristics associated with the monitoring and reporting of adverse events considering specific factors that may have a large influence on adverse event data. We will evaluate methods of monitoring and detecting adverse events in each primary study:
did the researchers actively monitor for adverse events, or did they simply provide spontaneous reporting of adverse events that arose?
did the authors define adverse events according to an accepted international classification and report the number of SAEs?
We will report this information in an additional table called ‘Assessment of adverse events monitoring’.
We will resolve any disagreement by discussion to reach consensus and, if needed, by discussion with a fourth review author (RD).
Measures of treatment effect
We will calculate dichotomous outcomes as odds ratios (OR) and 95% confidence intervals (CIs) for parallel and cross‐over trials. We will attempt to analyse paired data from cross‐over trials given that spasticity and chronic neuropathic pain do not resolve over time in people with MS. For continuous outcomes, we will calculate mean difference (MD) or standardized mean difference (SMD) for the same continuous outcome measured with different metric. We will back calculate any results that we generate with a SMD based on scales that most closely reflect the outcome measure of interest to the review as listed under secondary outcomes.
Unit of analysis issues
Studies with multiple treatment groups
For multi‐arm trials, the intervention groups of relevance will be all those that could be included in a pairwise comparison of intervention groups which, if investigated alone, would meet the review inclusion criteria. For example, if we identify a study comparing ‘Nabiximols versus tizanidine versus nabiximols plus tizanidine', only one comparison (‘Nabiximols versus tizanidine') would be used since it addresses the review objective. Thus, data from the ‘Nabiximols plus tizanidine' treatment group is not relevant to the review. However, if the study compares ‘Nabiximols versus tizanidine versus baclofen', all three pairwise comparisons of interventions are relevant to the review. In this case we will treat the multi‐arm studies as multiple independent two‐arm studies. We will convert multi‐arm trials involving the same agent at different doses compared to a control treatment into a single arm by merging of doses and summing the number of participants who had the event and the sample size. For continuous outcomes, we will combine means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over studies
We will enter MD and standard errors from paired data for cross‐over studies with the generic inverse variance (GIV) function in Review Manager 5 (RevMan 5) (Review Manager 2014). We will assume that participants unavailable for primary outcome assessment had not improved, as that is probably a conservative estimate effect. We will conduct sensitivity analyses to explore this assumption.
Dealing with missing data
We will use data that reflect the intention‐to‐treat (ITT) analysis for each included outcome with the exception of safety outcomes where assessment of risk of bias will be in relation to the effect of assignment. We will attempt to retrieve missing data from study authors. In order to assess the effect of missing outcome data where not reported or provided, we will assume that treated and control group participants who are missing both had an unfavourable outcome. For continuous outcomes, where standard deviations are missing, we will calculate them according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
Assessment of clinical heterogeneity within treatment comparisons
To evaluate the presence of heterogeneity deriving from different characteristics of study participants, where possible we will assess differences in types of MS; disability; spasticity and pain score at baseline; duration of spasticity, or pain, or both; prior, or actual, or both treatment with anti‐spasticity, or analgesic, or both; prior cannabis use; type of interventions across the trials, using information reported in the ‘Characteristics of included studies' table. We will assess differences in design and duration of included studies.
Assessment of statistical heterogeneity
We will assess the presence of statistical heterogeneity using the I2 statistic. When the I2 statistic value is greater than 50% (substantial heterogeneity), we will consider possible reasons for this by performing subgroup and sensitivity analyses.
Assessment of reporting biases
We will evaluate the possibility of reporting bias by means of contour‐enhanced funnel plots (Peters 2008). Contour‐enhanced funnel plots show areas of statistical significance, and can help in distinguishing reporting bias from other possible reasons for asymmetry. Note that any asymmetry in the plot indicates the presence of small study effects and not necessarily reporting bias.
Data synthesis
We will combine data from parallel‐group and cross‐over trials. For dichotomous outcomes, we will combine ORs with 95% CIs from parallel and cross‐over trials according to the method of Becker 1993 that combines lnORs from parallel trials with marginal cross‐over lnORs, which are estimators independent from the correlation, where these data are available (Becker 1993; Curtin 2002). We will report adverse event outcomes narratively if a quantitative analysis is not possible.
For continuous outcomes, we will calculate MD or SMD, if the outcome is measured on different assessment scales (such as pain), with 95% CIs. We will use a random‐effects model because we assume that the studies are not all estimating the same intervention effect, and are estimating intervention effects that follow a distribution across studies (DerSimonian 1986). We will conduct analyses using RevMan 5 and Stata (Review Manager 2014; Stata).
Subgroup analysis and investigation of heterogeneity
We will perform subgroup analyses for efficacy outcomes by using the following effect modifiers as possible sources of heterogeneity:
population: types of MS, disability, spasticity, and pain score baseline; prior or actual or both treatment with anti‐spasticity or analgesic or both; prior cannabis use;
study design: parallel or cross‐over; co‐therapies allowed; rescue medication;
study duration: very short‐term (less than four weeks), short‐term (4 to 12 weeks), intermediate‐term (13 to 26 weeks), and long‐term (more than 26 weeks);
intervention: different cannabis‐based medicine; CBD only product versus THC only product, versus THC‐CBD combination; herbal product versus pharmaceutical products;
intervention: different dose, frequency, or duration of treatment.
We will restrict subgroup analyses to outcomes that have a sufficient number of studies available. We will consider the relevance of subgroups where at least 10 studies for a subgroup analysis are available. We will interpret the results with caution.
Sensitivity analysis
We will assess the impact of studies that have results for critical and important outcomes that we judge to be at high risk of bias or to raise some concerns in at least one domain of RoB2, by removing them from the analysis. We will use the sensitivity analyses to inform the downgrading decisions relating to risk of bias.
We will consider different assumptions relating to missing outcome as the basis for sensitivity analyses.
Assessing the certainty of evidence and ‘Summary of findings’ tables
We will present the main results of the review as ‘Summary of findings’ tables, according to Cochrane guidance (Schünemann 2011). We will provide estimates based on the methodology developed from the GRADE Working Group (Atkins 2004).
In the ‘Summary of findings’ tables we will include comparison of cannabinoids with placebo and an overall assessment of the evidence for critical and important outcomes :
number of participants reporting reduction of 30% in the spasticity NRS;
number of participants reporting pain relief of 50% or greater in the NRS‐PI;
number of participants reporting much or very much improvement in the Patient Global Impression of Change (PGIC);
number of participant reporting improvement in quality of life;
number of participants withdrawn due to adverse events (tolerability);
total number of SAEs;
number of participants reporting specific adverse events including nervous system disorders, psychiatric disorders, or physical dependence.
In the SoF, we will prioritise long‐term outcomes if they will be available, otherwise we will include short term outcomes.
We will assess the certainty of evidence for each outcome considering risk of bias, indirectness, inconsistency, imprecision of effect estimates, and risk of publication bias. Using GRADEpro GDT software, GRADEpro GDT, we will assign one of four levels of certainty of evidence: high, moderate, low, or very low.
Acknowledgements
We thank Sarah J Nevitt and Bernard Le Foll for helpful comments and suggestions.
Appendices
Appendix 1. Draft search strategy
CENTRAL
#1 MESH DESCRIPTOR Cannabis
#2 ((cannabi* or hash* or hemp or marijuana or marihuana or ganka or bhang)):TI,AB,KY
#3 MESH DESCRIPTOR Dronabinol
#4 ((dronabinol or marinol or nabilone or cesamet or dexanabinol or tetrahydrocannabinol or sativex or “HU 211”)):TI,AB,KY
#5 #1 OR #2 OR #3 OR #4
#6 MESH DESCRIPTOR Multiple sclerosis EXPLODE ALL TREES
#7 #5 AND #6
MEDLINE (PubMed) search strategy
((("Cannabis"[Mesh]) OR ("cannabi*"[Text Word]) OR ("hash*"[Text Word]) OR (hemp[Text Word]) OR (marijuana[Text Word]) OR (marihuana[Text Word]) OR (ganka[Text Word]) OR (bhang[Text Word])) OR (("Dronabinol"[Mesh]) OR (dronabinol[Text Word]) OR (marinol[Text Word]) OR (nabilone[Text Word]) OR (cesamet[Text Word]) OR (cannabidiol[Text Word]) OR (nabiximols[Text Word]) OR (dexanabinol[Text Word]) OR (tetrahydrocannabinol[Text Word]) OR (sativex[Text Word])))) AND (((("Multiple Sclerosis"[mh]) OR ("Myelitis, Transverse"[mh:noexp]) OR ("Demyelinating Diseases"[mh:noexp]) OR ("Encephalomyelitis, Acute Disseminated"[mh:noexp]) OR ("Optic Neuritis"[mh])) OR ((("multiple sclerosis") OR ("neuromyelitis optica") OR ("transverse myelitis") OR (encephalomyelitis) OR (devic) OR ("optic neuritis")) OR ("demyelinating disease*") OR ("acute disseminated encephalomyelitis"))) AND (((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT ((animals[mh]) NOT ((animals[mh]) AND (human[mh])))))
Embase
#1 'encephalomyelitis'/exp OR 'demyelinating disease'/exp OR 'multiple sclerosis'/exp OR 'myelooptic neuropathy'/exp OR 'multiple sclerosis':ab,ti OR 'neuromyelitis optica':ab,ti OR encephalomyelitis:ab,ti OR devic:ab,ti
#2 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'randomized controlled trial'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR placebo*:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
#3 'cannabis'/exp OR hash* OR 'hemp'/exp OR cannabis:ab,ti OR hash*:ab,ti OR hemp:ab,ti OR marijuana:ab,ti OR 'marijuana'/exp OR marihuana:ab,ti OR 'marihuana'/exp OR ganka:ab,ti OR bhang:ab,ti OR 'dronabinol'/exp OR dronabinol:ab,ti OR marinol:ab,ti OR nabilone:ab,ti OR cesamet:ab,ti OR cannabidiol:ab,ti OR nabiximols:ab,ti OR dexanabinol:ab,ti OR tetrahydrocannabinol:ab,ti OR sativex:ab,ti
#4 #1 AND #2 AND #3
CINAHL (EBSCO host)
S1 (encephalomyelitis) OR (demyelinating disease) OR (multiple sclerosis) OR (AB multiple sclerosis) OR (AB neuromyelitis optica) OR (AB encephalomyelitis) OR (devic)
S2 (crossover procedure) OR (double blind procedure) OR (single blind procedure) OR (randomized controlled trial) OR (random*) OR (factorial*) (OR crossover) OR (cross AND over) OR (placebo) OR (double blind) OR (single blind) OR (assign*) OR (allocat*) OR (volunteer*) OR (AB crossover ) OR (AB cross AND AB over ) or (AB placebo* ) OR (AB double blind) OR (AB single blind ) OR (AB assign*) OR (AB allocat*) OR (AB volunteer*)
S3 (cannabis) OR (hash*) OR (hemp) OR (marijuana) OR (marihuana) OR (AB cannabis) OR (AB hash*) OR (AB hemp) OR (AB marijuana) OR (AB marihuana) OR (AB ganka) OR (AB bhang) OR (dronabinol) OR (AB dronabinol) OR (AB marinol) OR (AB nabilone) OR (AB cesamet) OR (AB cannabidiol) OR (AB nabiximols) OR (AB dexanabinol) OR (AB tetrahydrocannabinol) OR (AB sativex)
S4 S1 AND S2 AND S3
LILACS (Bireme)
multiple sclerosis or encephalomyelitis or demyelinating disease or devic [Words] AND cannabis OR hemp OR marijuana OR marihuana OR dronabinol OR marinol OR nabilone OR cesamet OR cannabidiol OR nabiximols OR dexanabinol OR tetrahydrocannabinol OR sativex [Words]
Contributions of authors
All protocol authors drafted the protocol, provided input into the protocol development, and agreed the final protocol version. GF and SM are the guarantors of the review.
Sources of support
Internal sources
Fondazione Istituto Neurologico Carlo Besta ‐ Milan, Italy.
External sources
No sources of support supplied
Declarations of interest
GF: none SM: none TJL: He is employed by Cochrane RDA: none KD: She is employed as statistical editor by Cochrane AAI: He received research grants from GW pharmaceuticals (Cambridge, UK) to perform preclinical studies on phytocannabinoids and intestinal diseases, and patents on phytocannabinoids and colorectal cancer or inflammatory bowel diseases. FB: She received research grants from GW pharmaceuticals (Cambridge, UK) to perform preclinical studies on phytocannabinoids and intestinal diseases, and patents on phytocannabinoids and colorectal cancer or inflammatory bowel diseases.
New
References
Additional references
- American Academy of Neurology. Position statement: use of medical marijuana for neurologic disorders. February 2018. Available at www.aan.com/policy‐and‐guidelines/policy/position‐statements/medical‐marijuana/ (accessed 15 May 2019).
- Association of British Neurologists. ABN interim guidelines: use of cannabis‐based products in neurology. December 2018. Available at https://cdn.ymaws.com/www.theabn.org/resource/collection/6750BAE6‐4CBC‐4DDB‐A684‐116E03BFE634/ABN_2018_Guidelines_‐_Use_of_cannabis‐based_pr.pdf (accessed 18 May 2019).
- Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products ‐ Regulations in Europe and North America. European Journal of Internal Medicine 2018;49:2‐6. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Mitrova Z, Parmelli E, Saulle R, Cruciani F, et al. Systematic review of safeness and therapeutic efficacy of cannabis in patients with multiple sclerosis, neuropathic pain, and in oncological patients treated with chemotherapy [Revisione sistematica sull’efficacia terapeutica e la sicurezza della cannabis per i pazienti affetti da sclerosi multipla, dolore neuropatico cronico e pazienti oncologici che assumono chemioterapie]. Epidemiologia & Prevenzione 2017;41(5‐6):279‐93. [DOI] [PubMed] [Google Scholar]
- Amatya B, Khan F, Mantia L, Demetrios M, Wade DT. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009974.pub2] [DOI] [PubMed] [Google Scholar]
- Ansari NN, Naghdi S, Hasson S, Azarsa MH, Azarnia S. The modified Tardieu scale for the measurement of elbow flexor spasticity in adult patients with hemiplegia. Brain Injury 2008;22(13‐14):1007‐12. [DOI] [PubMed] [Google Scholar]
- Ansari NN, Naghdi S, Hasson S, Fakhari Z, Mashayekhi M, Herasi M. Assessing the reliability of the Modified AshworthScale between two physiotherapists in adult patients with hemiplegia. NeuroRehabilitation 2009;25(4):235‐40. [DOI] [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964;192:540‐2. [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government, Department of Health, Therapeutic Goods Administration. Guidance for the use of medicinal cannabis in the treatment of multiple sclerosis in Australia. Version 1, December 2017. Available at https://www.tga.gov.au/publication/guidance‐use‐medicinal‐cannabis‐treatment‐multiple‐sclerosis‐australia (accessed 15 May 2019).
- Banwell E, Pavisian B, Lee L, Feinstein A. Attitudes to cannabis and patterns of use among Canadians with multiple sclerosis. Multiple Sclerosis and Related Disorders 2016;10:123‐6. [DOI] [PubMed] [Google Scholar]
- Barnes MP, Barnes JC. Review of the evidence of the effectiveness of medicinal cannabis. All Party Parliamentary Group for Drug Policy Reform. Available at: https://www.drugpolicyreform.net/2016.
- Becker MP, Balagtas CC. Marginal modeling of binary cross‐over data. Biometrics 1993;49(4):997‐1009. [PubMed] [Google Scholar]
- Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurological Research 2010;32(5):451‐9. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Gershkovich P. Therapeutic cannabinoids in multiple sclerosis: immunomodulation revisited. European Journal of Neurology 2018;25(7):905‐6. [DOI] [PubMed] [Google Scholar]
- Coyne KS, Matza LS, Thompson CL. The responsiveness of the Overactive Bladder Questionnaire (OAB‐q). Quality of Life Research 2005;14(3):849‐55. [DOI] [PubMed] [Google Scholar]
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
- Davies SC. Cannabis scheduling review: part 1. The therapeutic and medicinal benefits of cannabis based products – a review of recent evidence. Published 3 July 2018. Available at www.gov.uk/government/publications/cannabis‐scheduling‐review‐part‐1 (accessed prior to 11 September 2019).
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nature Reviews. Drug Discovery 2018;17(9):623‐39. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. EMEA‐000181‐PIP02‐13. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA‐000181‐PIP02‐13/pip_001257.jsp&mid=WC0b01ac058001d129 (accessed 15 May 2019).
- Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0‐10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double‐blind, placebo‐controlled trial. Clinical Therapeutics 2008;30(5):974‐85. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. Journal of Pain 2010;11(2):109‐18. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JK, Downer EJ. Toll‐like receptor signalling as a cannabinoid target in multiple sclerosis. Neuropharmacology 2017;113(Pt B):618‐26. [DOI] [PubMed] [Google Scholar]
- Freeman PR. The performance of the two‐stage analysis of two‐treatment, two‐period cross‐over trials. Statistics in Medicine 1989;8(12):1421‐32. [DOI] [PubMed] [Google Scholar]
- Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids:implications for treating neurodegeneration. CNS Neuroscience & Therapeutics 2011;17(6):637‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 June 2019.
- Guy W. ECDEU assessment manual for psychopharmacology (DHEW Publication No. ADM 76‐338). https://ia600306.us.archive.org/35/items/ecdeuassessmentm1933guyw/ecdeuassessmentm1933guyw.pdf (accessed prior to 11 September 2019).
- Hazekamp A, Ware MA, Muller‐Vahl KR, Abrams D, Grotenhermen F. The medicinal use of cannabis and cannabinoids‐‐an international cross‐sectional survey on administration forms. Journal of Psychoactive Drugs 2013;45(3):199‐210. [DOI] [PubMed] [Google Scholar]
- Hazekamp A. The trouble with CBD oil. Medical Cannabis and Cannabinoids 2018;1:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, (updated March 2011). [Google Scholar]
- Edited by Julian PT Higgins on behalf of the RoB 2 working group on crossover trials. Revised Cochrane risk of bias tool for randomized trials (RoB 2). Additional considerations for crossover trials. Available at: https://sites.google.com/site/riskofbiastool/welcome/rob‐2‐0‐tool/archive‐rob‐2‐0‐cross‐over‐trials‐2016 (accessed 15 June 2019).
- Julian PT Higgins, Jelena Savović, Matthew J Page, Jonathan AC Sterne, on behalf of the ROB2 Development Group. Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: The Cochrane Collaboration, 22 August 2019. Available at: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view (accessed 26 August 2019). [Google Scholar]
- Health Products Regulatory Authority. Cannabis for medical use ‐ a scientific review. 2017. Available at www.hpra.ie/docs/default‐source/publicationsforms/newsletters/cannabis‐for‐medical‐use‐‐‐a‐scientific‐review.pdf?sfvrsn=7 (accessed 15 May 2019).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. MedDRA. Medical Dictionary for Regulatory Activities. www.meddra.org (accessed prior to 11 September 2019).
- Izzo AA, Borrelli F, Capasso R, Marzo V, Mechoulam R. Non‐psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends in Pharmacological Sciences 2009;30(10):512‐27. [DOI] [PubMed] [Google Scholar]
- Kaur R, Ambwani SR, Singh S. Endocannabinoid system: a multi‐facet therapeutic target. Current Clinical Pharmacology 2016;11(2):110‐7. [DOI] [PubMed] [Google Scholar]
- Kempen JC, Groot V, Knol DL, Polman CH, Lankhorst GJ, Beckerman H. Community walking can be assessed using a 10‐metre timed walk test. Multiple Sclerosis 2011;17(8):980‐90. [DOI] [PubMed] [Google Scholar]
- Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Loenen AC, Staats PG, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002;58(9):1404–7. [DOI] [PubMed] [Google Scholar]
- Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with Parkinson's disease and Multiple Sclerosis: a web‐based investigation. Complementary Therapies in Medicine 2017;33:99‐104. [DOI] [PubMed] [Google Scholar]
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014;82(17):1556‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krcevski‐Skvarc N, Wells C, Häuser W. Availability and approval of cannabis‐based medicines for chronic pain management and palliative/supportive care in Europe: a survey of the status in the chapters of the European Pain Federation. European Journal of Pain 2018;22(3):440‐54. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non‐cancer pain: an updated systematic review of randomized controlled trials. Journal of Neuroimmune Pharmacology 2015;10(2):293‐301. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [DOI] [PubMed] [Google Scholar]
- Mecha M, Yanguas‐Casás N, Feliú A, Mestre L, Carrillo‐Salinas F, Azcoitia I, et al. The endocannabinoid 2‐AG enhances spontaneous remyelination by targeting microglia. Brain, Behaviour, and Immunity 2019;77:110‐26. [DOI: 10.1016/j.bbi.2018.12.013] [DOI] [PubMed] [Google Scholar]
- Mestre L, Carrillo‐Salinas FJ, Mecha M, Feliú A, Guaza C. Gut microbiota, cannabinoid system and neuroimmune interactions: new perspectives in multiple sclerosis. Biochemical Pharmacology 2018;157:51‐66. [DOI] [PubMed] [Google Scholar]
- Meza R, Peña J, García K, Corsi O, Rada G. Are cannabinoids effective in multiple sclerosis?. Medwave 2017;17(Suppl 1):e6865. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Agency. Sativex oromucosal spray. UK/H/2462/001/DC. www.mhra.gov.uk/home/groups/par/documents/websiteresources/con084961.pdf (accessed prior to 11 September 2019).
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. “Evidence” in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386–9. [DOI] [PubMed] [Google Scholar]
- Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Progress in the Chemistry of Organic Natural Products 2017;103:103‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MS Society. Cannabis and MS. Available at www.mssociety.org.uk/about‐ms/treatments‐and‐therapies/complementary‐and‐alternative‐therapies/cannabis (accessed 23 April 2019).
- Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and multiple sclerosis: evidence‐based management strategies for fatigue in multiple sclerosis. Washington (DC): Paralyzed Veterans of America, 1998:pp.1–33. [Google Scholar]
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis‐based medicines for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012182.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The Health Effects of Cannabis and Cannabinoids. The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press, 2017. [PubMed] [Google Scholar]
- Newsome SD, Aliotta PJ, Bainbridge J, Bennett SE, Cutter G, Fenton K, et al. A framework of care in multiple sclerosis, part 2: symptomatic care and beyond. International Journal of MS Care 2017;19(1):42‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Clinical guideline [CG186]. Published date: October 2014. Last updated: July 2019. Available at www.nice.org.uk/guidance/cg186 (accessed 10 August 2019).
- National Institute for Health and Care Excellence. Cannabis‐based medicinal products. In development [GID‐NG10124]. Available at www.nice.org.uk/guidance/indevelopment/gid‐ng10124 (accessed 19 May 2019).
- Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L, Hall W, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Current Neurology and Neuroscience Reports 2018;18(2):8. [DOI] [PubMed] [Google Scholar]
- Oláh A, Szekanecz Z, Bíró T. Targeting cannabinoid signaling in the immune system: "high"‐ly exciting questions, possibilities, and challenges. Frontiers in Immunology 2017;8:1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandyan AD, Johnson GR, Priec CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clinical Rehabilitation 1999;13:373‐83. [DOI] [PubMed] [Google Scholar]
- Papaseit E, Pérez‐Mañá C, Pérez‐Acevedo AP, Hladun O, Torres‐Moreno MC, Muga R, et al. Cannabinoids: from pot to lab. International Journal of Medical Sciences 2018;15(12):1286‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spasticity. New England Journal of Medicine 1989;320(23):1517‐21. [DOI] [PubMed] [Google Scholar]
- Peters J, Sutton A, Jones D, Abrams K, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians (RCP). Recommendations on cannabis‐based products for medicinal use. October 2018. Available at www.rcplondon.ac.uk/medicinal‐use‐cannabis‐based‐products (accessed 16 May 2019).
- Reston VA. MMJ files FDA fast track approval application for cannabis multiple sclerosis drug [press release]. February 2019. Available at markets.businessinsider.com/news/stocks/mmj‐files‐fda‐fast‐track‐approval‐application‐for‐cannabis‐multiple‐sclerosis‐drug‐1027948379 (accessed 13 March 2019).
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rommer PS, Eichstädt K, Ellenberger D, Flachenecker P, Friede T, Haas J, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Multiple Sclerosis 2018 Sep 19 [Epub ahead of print]. [DOI: 10.1177/1352458518799580] [DOI] [PubMed]
- Rooney S, McFadyen DA, Wood DL, Moffat DF, Paul PL. Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Multiple Sclerosis and Related Disorders 2019;35:158‐63. [DOI] [PubMed] [Google Scholar]
- Schünemann H, Oxman A, Higgins J, Vist G, Glasziou P, Guyatt G. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Shakespeare D, Boggild M, Young CA. Anti‐spasticity agents for multiple sclerosis. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001332] [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata. Version 15. College Station, TX, USA: StataCorp, 2017.
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet 2018;391(10130):1622–36. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 2018;17(2):162‐73. [DOI] [PubMed] [Google Scholar]
- Vickrey B, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Qualiity of Life Research 1995;4(3):187–206. [DOI] [PubMed] [Google Scholar]
- Wade DT, Collin C, Stott C, Duncombe P. Meta‐analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Multiple Sclerosis 2010;16(6):707‐14. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA 2015;313(24):2456‐73. [DOI] [PubMed] [Google Scholar]
- World Health Organization Expert Committee on Drug Dependence. Cannabidiol (CBD) critical review report. Available at: https://www.who.int/medicines/access/controlled‐substances/CannabidiolCriticalReview.pdf (accessed 7 May 2019).
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]