Abstract
Context
Vitamin D2 and vitamin D3 have been hypothesized to exert differential effects on vitamin D metabolism.
Objective
To compare the influence of administering vitamin D2 vs vitamin D3 on metabolism of vitamin D3.
Methods
We measured baseline and 4-month serum concentrations of vitamin D3, 25-hydroxyvitamin D3 [25(OH)D3], 25-hydroxyvitamin D2, 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3], 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], and 4β,25-dihydroxyvitamin D3 [4β,25(OH)2D3] in 52 adults randomized to receive a total of four oral bolus doses of 2.5 mg vitamin D2 (n = 28) or vitamin D3 (n = 24) over four months. Metabolite-to-parent compound ratios were calculated to estimate hydroxylase activity. Pairwise before vs after comparisons were made to evaluate effects of vitamin D2 and vitamin D3 on metabolism of vitamin D. Mean postsupplementation metabolite-to-parent ratios were then compared between groups.
Results
Vitamin D2 was less effective than vitamin D3 in elevating total serum 25(OH)D concentration. Vitamin D2 suppressed mean four-month serum concentrations of 25(OH)D3, 24R,25(OH)2D3, 1α,25(OH)2D3, and 4β,25(OH)2D3 and mean ratios of 25(OH)D3 to D3 and 1α,25(OH)2D3 to 25(OH)D3, while increasing the mean ratio of 24R,25(OH)2D3 to 25(OH)D3. Vitamin D3 increased mean four-month serum concentrations of 25(OH)D3, 24R,25(OH)2D3, 1α,25(OH)2D3, and 4β,25(OH)2D3 and the mean ratio of 24R,25(OH)2D3 to 25(OH)D3. Participants receiving vitamin D2 had lower mean postsupplementation ratios of 25(OH)D3 to vitamin D3 and 1α,25(OH)2D3 to 25(OH)D3 than those receiving vitamin D3. Mean postsupplementation ratios of 24R,25(OH)2D3 to 25(OH)D3 and 4β,25(OH)2D3 to 25(OH)D3 did not differ between groups.
Conclusions
Bolus-dose vitamin D2 is less effective than bolus-dose vitamin D3 in elevating total serum 25(OH)D concentration. Administration of vitamin D2 reduces 25-hydroxylation of vitamin D3 and 1-α hydroxylation of 25(OH)D3, while increasing 24R-hydroxylation of 25(OH)D3.
Data from this randomized controlled trial show that vitamin D2 reduces 25-hydroxylation of vitamin D3 and 1-α hydroxylation of 25(OH)D3, and increases 24R-hydroxylation of 25(OH)D3.
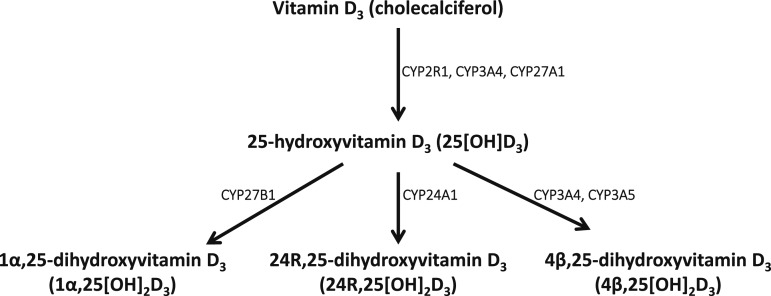
Vitamin D has two forms: ergocalciferol (vitamin D2) is synthesized via UV irradiation of ergosterol, a steroid found in fungi and some plants, whereas cholecalciferol (vitamin D3) is synthesized via UV irradiation of 7-dehydrocholesterol to previtamin D3, followed by a thermal isomerization step. In humans, the source of vitamin D3 may be endogenous (i.e., obtained via cutaneous synthesis) or exogenous (i.e., ingested in foods or supplements), whereas vitamin D2 is only available from exogenous sources. Vitamin D2 and vitamin D3 are structurally distinct: the side chain of vitamin D2 contains a double bond between carbons 22 and 23 and a methyl group on carbon 24, both of which are absent from the side chain of vitamin D3. The two forms also have differing pharmacokinetics: of 14 publications comparing effects of vitamin D2 vs vitamin D3 (1–14), all but three (12–14) reported that vitamin D2 was less effective than vitamin D3 in elevating total 25(OH)D levels. A meta-analysis of data from seven of these studies found that this effect was only statistically significant when vitamin D was administered using intermittent bolus dosing, as opposed to daily administration (15). 25-hydroxyvitamin D3 [25(OH)D3] subsequently undergoes a second hydroxylation step to form the active vitamin D metabolite 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] or the inactive metabolites 24R,25-dihydroxyvitamin D3 [24R,25(OH)2D3] and 4β,25-dihydroxyvitamin D [4β,25(OH)2D3; Fig. 1]. It also undergoes conjugation to circulating inactive sulfate and glucuronide metabolites that may be recycled back to 25(OH)D3 rather than excreted (16, 17).
Figure 1.
Vitamin D3 oxidation pathways. The monohydroxylated and dihydroxylated metabolites investigated in the current study are shown, with the cytochrome P450 enzymes catalyzing each conversion in capitals.
Administration of vitamin D2 has been reported to reduce circulating concentrations of 25(OH)D3 in eight studies (1–7, 11); a ninth study reports a nonstatistically significant trend in the same direction (12). These observations have led investigators to speculate that vitamin D2 may influence metabolism of vitamin D3. In keeping with this hypothesis, a recent study reported that administration of vitamin D2 increases the ratio of 24R,25(OH)2D3 to 25(OH)D3 in the circulation and decreases the ratio of 1α,25[OH]2D3 to 25(OH)D3, findings taken to indicate that vitamin D2 induces 24R-hydroxylation and suppresses 1α-hydroxylation of 25(OH)D3 (18). However, it is not yet known if these effects are vitamin D2-specific, because administration of vitamin D3 also influences the rate of conversion of parent vitamin D3 to its hydroxylated metabolites (19). Moreover, the influence of administering vitamin D2 on circulating concentrations of vitamin D3 and 4β,25(OH)2D3 has yet to be determined.
Studies making a head-to-head comparison of the influence of identical doses of vitamin D2 vs vitamin D3 on circulating concentrations of vitamin D3, 25(OH)D3 and its major dihydroxylated metabolites are needed to resolve these questions. An opportunity to conduct such an investigation recently arose in the context of a randomized controlled trial that we conducted in the United Kingdom to evaluate the effect of administration of four monthly oral doses of 2.5 mg vitamin D2 vs the same dose of vitamin D3 on glycated hemoglobin concentration among people at risk for type 2 diabetes mellitus (11). We therefore determined concentrations of vitamin D3, 25(OH)D3, 24R,25(OH)2D3, 1α,25(OH)2D3, and 4β,25(OH)2D3 in serum samples taken from a subset of trial participants before and after administration of vitamin D2 vs vitamin D3, and calculated the change in postsupplementation metabolite-to-parent ratios to gain insight into the relative effects of vitamin D2 vs vitamin D3 on the activity of enzymes catalyzing 25-hydroxylation of vitamin D3 and 1α-hydroxylation, 24R-hydroxylation, and 4β-hydroxylation of 25(OH)D3. We also measured concentrations of vitamin D2 and 25(OH)D2 in the same samples and compared the influence of vitamin D2 vs vitamin D3 on 25-hydroxylation of vitamin D2.
Methods
Trial design and participants
As previously described, we conducted a double-blind, randomized placebo-controlled trial that enrolled a total of 340 men and women aged 30 to 75 years who had been identified as being at increased risk of developing type 2 diabetes mellitus in London and Cambridge, United Kingdom (11). Full details of inclusion and exclusion criteria are described in the published protocol (20). Eligible participants were randomly allocated to one of three groups on a 1:1:1 basis within four strata defined by age (30 to 50 or 51 to 75 years) and sex, with a block size of six within each stratum. One group received four monthly oral bolus doses of 2.5 mg vitamin D2: each dose was presented as 5 mL Sterogyl solution (Desma Pharma, Paris, France) containing 0.5 mg vitamin D2 per milliliter in ethanol. The second group received four monthly oral bolus doses of 2.5 mg vitamin D3: each dose was presented as 5 mL Vigantol oil (Merck Serono, Darmstadt, Germany) containing 0.5 mg vitamin D3 per milliliter in Miglyol oil (Caesar & Loretz, Hilden, Germany). The third group received four monthly oral doses of placebo (Miglyol oil). The order of treatments within each block was determined by a computer-generated pseudo-random sequence, generated by the study medication manufacturer (Nova Laboratories, Leicester, UK). Neither the participants, the investigators, nor the laboratory staff knew the treatment allocation. Each participant was followed-up for a total of four months from their first visit; serum samples were collected at baseline and at the end of the study. Baseline and four-month serum samples taken from a subset of 28 participants and allocated to vitamin D2 and 25 participants allocated to vitamin D3 were sent for determination of concentrations of vitamin D3 and its metabolites as detailed below. The subset of participants contributing samples to the current study were selected on the basis that they were all recruited in London; that they had each received four directly observed doses of vitamin D2 or vitamin D3; and that they were the 28 samples in each group having the greatest volume of serum available at both baseline and four-month follow-up to be sent for further analysis. For the vitamin D3 group, only 25 samples had sufficient sample volume for analysis. Ethical approval for the trial was provided by the Charing Cross Medical Ethics Committee (ref 09/H0711/85) and the Cambridge Local Research Ethics Committee (ref 04/Q0108/19), and written informed consent was obtained from all participants. The trial was registered under the numbers EudraCT 2009-011264-11 and ISRCTN86515510 on 23 October 2009.
Laboratory assays
Serum concentrations of vitamin D3, vitamin D2, 25(OH)D3, 25(OH)D2, 24R,25(OH)2D3, 1α,25(OH)2D3, 1α,25(OH)2D2, and 4β,25(OH)2D3 were determined by liquid chromatography-tandem mass spectrometry in the Thummel Laboratory, Department of Pharmaceutics, University of Washington, Seattle, Washington, as previously described (21). Lower limits of quantitation (LLOQ) were 0.23 nmol/L for vitamin D3, 0.15 nmol/L for vitamin D2, 0.50 nmol/L for 25(OH)D3, 0.24 nmol/L for 25(OH)D2, 0.14 nmol/L for 24R,25(OH)2D3, and 7.7 pmol/L for 1α,25(OH)2D3, 1α,25(OH)2D2 and 4β,25(OH)2D3. Where concentrations of a given analyte were less than the LLOQ, a value equal to the LLOQ divided by the √2 was imputed, as performed elsewhere (22). Intraday and interday coefficients of variation were <15% for all analytes, as previously reported (21).
Sample size and statistical analyses
We estimated that paired before and after serum samples from 21 participants would need to be evaluated to have 90% power to detect a 15 nmol/L difference in 25(OH)D3 concentration preadministration vs postadministration of vitamin D2 with α = 0.05, based on a standard deviation for postsupplementation serum 25(OH)D3 concentration of 20 nmol/L (11). This sample size was inflated to 28 to allow for potential assay failure. Serum samples from a similar number of participants allocated to vitamin D3 were also evaluated.
Statistical analyses were conducted using GraphPad Prism version 6.04 (GraphPad Software Inc., La Jolla, CA) and STATA IC version 12 (StataCorp, College Station, TX). Intragroup differences in absolute concentrations of vitamin D3 and its metabolites before vs after supplementation with vitamin D2 or vitamin D3 were evaluated using paired Student t tests. Intergroup differences in end-study values of these parameters were evaluated with linear regression, adjusting for baseline values. Mean differences are presented with 95% CI and P values, with statistical significance inferred where P values are less than 0.05.
Results
Participant enrollment and baseline characteristics
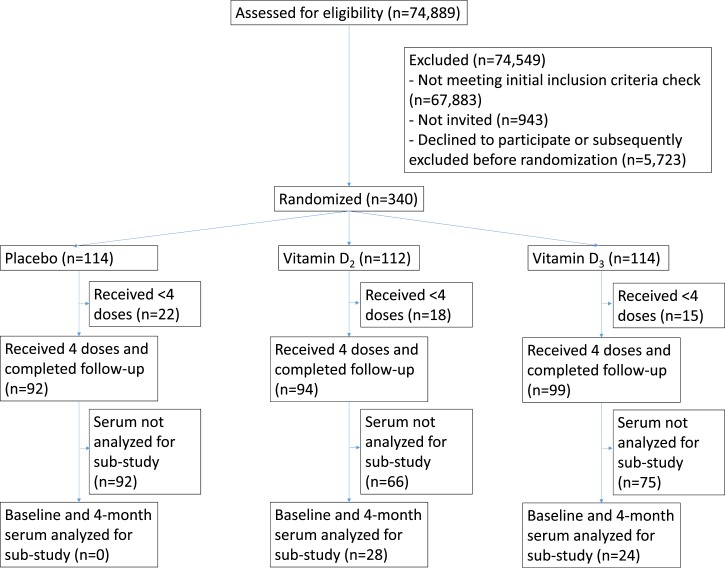
A total of 340 adults were randomly assigned to receive supplementation with vitamin D2 (n = 112) vs vitamin D3 (n = 114) vs placebo (n = 114) between 2010 and 2012, of whom 285 (94 randomized to vitamin D2 vs 99 randomized to vitamin D3 vs 92 randomized to placebo) took all four doses of study medication and completed follow-up. For the current study, baseline and four-month serum samples collected from a subset of participants recruited in London who took four doses of vitamin D2 (n = 28) or vitamin D3 (n = 25) were sent for determination of concentrations of vitamin D and its metabolites (Fig. 2). Effects of the intervention on the primary outcome of the main trial, and on safety, are reported elsewhere (11). The trial ended on the date of the final study visit of the last participant to be randomized. One participant selected for the substudy and randomly assigned to vitamin D3 was found to have a high outlying baseline 25(OH)D2 concentration (51.8 nmol/L) and was excluded from statistical analyses at a reviewer’s request. Baseline characteristics of participants whose serum samples contributed to the current study are presented in Table 1. Overall, mean age was 55.6 years (SD 10.0 years) and 21 of 52 (40.4%) participants were female. Baseline demographic and clinical characteristics, serum concentrations of vitamin D3, vitamin D2 and their metabolites and metabolite-to-parent ratios were similar for those randomized to receive vitamin D2 vs vitamin D3 where measurable; concentrations of 1α,25(OH)2D2 were undetectable (<7.7 pmol/L) in all samples.
Figure 2.
Trial profile.
Table 1.
Participants’ Baseline Characteristics by Allocation
| Characteristics | Vitamin D2 (n=28) | Vitamin D3 (n = 24) | |
|---|---|---|---|
| Sex | Female, n (%) | 10 (35.7) | 11 (45.8) |
| Male, n (%) | 18 (64.3) | 13 (54.2) | |
| Mean age, y (SD) | 56.0 (10.8) | 55.0 (9.1) | |
| Ethnic origin | White, n (%) | 18 (64.3) | 18 (75.0) |
| Other, n (%) | 10a (35.7) | 6 (25.0)b | |
| Total serum concentration of vitamin D and its metabolitesc | Mean total vitamin D, nmol/L (SD)d | 6.5 (6.7) | 8.6 (8.5) |
| Mean total 25(OH)D, nmol/L (SD)e | 49.7 (32.3) | 45.5 (25.0) | |
| Mean total 25(OH)D-to-total vitamin D molar ratio (SD) | 19.3 (27.5) | 18.6 (22.4) | |
| Serum concentration of vitamin D3 and its metabolites | Mean vitamin D3, nmol/L (SD) | 2.9 (3.3) | 3.5 (4.2) |
| Mean 25(OH)D3, nmol/L (SD) | 46.1 (32.4) | 42.0 (24.7) | |
| Mean 1α,25(OH)2D3, pmol/L (SD) | 77.2 (46.0) | 96.9 (55.1) | |
| Mean 24R,25(OH)2D3, nmol/L (SD) | 3.2 (2.7) | 2.9 (1.8) | |
| Mean 4β,25(OH)2D3, pmol/L (SD) | 126.4 (133.8) | 97.1 (104.9) | |
| Mean 25(OH)D3-to-vitamin D3 molar ratio (SD) | 39.0 (47.2) | 34.1 (44.2) | |
| Mean 24R,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.07 (0.03) | 0.07 (0.04) | |
| Mean 1α,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.0023 (0.0018) | 0.0029 (0.0022) | |
| Mean 4β,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.0024 (0.0016) | 0.0020 (0.0014) | |
| Serum concentration of vitamin D2 and its metabolites | Mean vitamin D2, nmol/L (SD) | 3.6 (5.3) | 5.1 (7.7) |
| Mean 25(OH)D2, nmol/L (SD) | 3.5 (1.3) | 3.5 (1.5) | |
| Mean 1α,25(OH)2D2, pmol/L, (SD) | <7.7f | <7.7f | |
| Mean 25(OH)D2-to-vitamin D2 molar ratio (SD) | 18.5 (17.2) | 16.2 (19.5) | |
Of whom 6 were of black or black British ethnic origin and 4 were of Asian or Asian British ethnic origin.
Of whom 5 were of Asian or Asian British ethnic origin and 1 was of black or black British ethnic origin.
Not calculated for 1,25-dihydroxyvitamin D (1,25-dihydroxyvitamin D2 was undetectable in all) or 24,R,25-dihydroxyvitamin D/4β-dihydroxyvitamin D (neither 24,R,25-dihydroxyvitamin D2 nor 4β-dihydroxyvitamin D2 were measured).
Calculated by summing values for vitamin D2 and vitamin D3.
Calculated by summing values for 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3.
1α,25(OH)2D2 undetectable in all; lower limit of quantification for this metabolite was 7.7 pmol/L.
Influence of vitamin D2 vs vitamin D3 on total 25(OH)D concentrations
Both vitamin D2 and vitamin D3 elevated total 25(OH)D concentrations at follow-up: the mean increase in total 25(OH)D concentrations after administration of vitamin D2 was 31.4 nmol/L (95% CI 21.5 to 41.2 nmol/L, P < 0.001), and the corresponding increase in total 25(OH)D concentrations after administration of vitamin D3 was 46.4 nmol/L (95% CI 33.7 to 59.0 nmol/L, P < 0.001). The difference in the mean change in total 25(OH)D concentration at follow-up for participants randomized to vitamin D3 vs vitamin D2 was 13.0 nmol/L (95% CI -0.5 to 26.6 nmol/L, P = 0.06; Table 2;Fig. 3). No difference in the ratio of total 25(OH)D to total parent vitamin D at follow-up was seen between participants randomized to vitamin D2 vs vitamin D3 (P = 0.32).
Table 2.
Serum Concentrations of Vitamin D3, Vitamin D2 and Their Metabolites After Administration of Vitamin D2 vs Vitamin D3
| Post-Vitamin D2 (n = 28) | Post-Vitamin D3 (n = 24) | Paired Analyses | Unpaired Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Difference, Post-Vitamin vs Pre-Vitamin D2 (95% CI) | P | Mean Difference, Post-Vitamin vs Pre-Vitamin D3 (95% CI) | P | Mean Difference, Post-Vitamin D3 vs Post-Vitamin D2 (95% CI)a | P | |||
| Total vitamin D and its metabolitesb | ||||||||
| Mean total vitamin D, nmol/L (SD)c | 7.4 (7.8) | 6.8 (5.7) | 0.9 (−3.1 to 5.0) | 0.65 | −1.7 (−5.8 to 2.3) | 0.39 | −0.6 (−4.6 to 3.3) | 0.75 |
| Mean total 25(OH)D, nmol/L (SD) d | 81.0 (21.9) | 91.9 (34.5) | 31.4 (21.5 to 41.2) | <0.001 | 46.4 (33.7 to 59.0) | <0.001 | 13.0 (−0.5 to 26.6) | 0.06 |
| Mean total 25(OH)D-to-total vitamin D molar ratio (SD) | 27.8 (49.1) | 17.6 (7.9) | 8.5 (−13.9 to 31.0) | 0.44 | −1.1 (−10.2 to 8.1) | 0.81 | −10.3 (−30.9 to 10.3) | 0.32 |
| Vitamin D3 and its metabolites | ||||||||
| Mean vitamin D3, nmol/L (SD) | 5.6 (7.3) | 6.3 (5.5) | 2.7 (−0.3 to 5.8) | 0.07 | 2.8 (0.3 to 5.3) | 0.03 | 0.5 (−3.2 to 4.1) | 0.80 |
| Mean 25(OH)D3, nmol/L (SD) | 26.2 (19.1) | 88.2 (34.1) | −19.9 (−29.3 to −10.5) | <0.001 | 46.2 (33.3 to 59.2) | <0.001 | 64.0 (51.0 to 77.1) | <0.001 |
| Mean 24R,25(OH)2D3, nmol/L | 2.0 (1.5) | 7.7 (3.0) | −1.2 (−2.0 to −0.3) | 0.007 | 4.8 (3.7 to 6.0) | <0.001 | 5.8 (4.7 to 7.0) | <0.001 |
| Mean 1α,25(OH)2D3, pmol/L | 36.0 (50.5) | 161.4 (95.4) | −41.1 (−63.8 to 18.5) | <0.001 | 64.5 (21.4 to 107.6) | 0.005 | 119.7 (77.6 to 161.8) | <0.001 |
| Mean 4β,25(OH)2D3, pmol/L | 73.2 (81.9) | 305.4 (438.6) | −53.2 (−99.2 to −7.2) | 0.03 | 208.3 (40.7 to 375.9) | 0.02 | 258.8 (98.0 to 419.7) | 0.002 |
| Mean 25(OH)D3-to-vitamin D3 molar ratio (SD) | 9.3 (11.4) | 18.2 (7.7) | −29.7 (−48.5 to −10.9) | 0.003 | −15.9 (−34.5 to 2.7) | 0.09 | 9.0 (3.4 to 14.6) | 0.002 |
| Mean 24R,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.0822 (0.0435) | 0.0928 (0.0297) | 0.0163 (0.0010 to 0.0315) | 0.04 | 0.0214 (0.0069 to 0.0360) | 0.006 | 0.0078 (−0.0114 to 0.0270) | 0.42 |
| Mean 1α,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.0011 (0.0015) | 0.0020 (0.0011) | −0.0012 (−0.0022 to −0.0002) | 0.02 | −0.0009 (−0.0020 to 0.0001) | 0.06 | 0.0009 (0.0001 to 0.0017) | 0.03 |
| Mean 4β,25(OH)2D3-to-25(OH)D3 molar ratio (SD) | 0.0024 (0.0022) | 0.0030 (0.0027) | 0.0000 (−0.0009 to 0.0009) | 0.97 | 0.0010 (−0.0001 to 0.0021) | 0.06 | 0.0008 (−0.0006 to 0.0021) | 0.25 |
| Vitamin D2 and its metabolites | ||||||||
| Mean vitamin D2, nmol/L (SD) | 1.8 (1.1) | 0.6 (0.8) | −1.8 (−4.0 to 0.3) | 0.09 | −4.5 (−7.7 to −1.3) | 0.008 | −1.2 (−1.8 to −0.6) | <0.001 |
| Mean 25(OH)D2, nmol/L (SD) | 54.8 (12.9) | 3.6 (1.5) | 51.2 (46.2 to 56.3) | <0.001 | 0.1 (−0.5–0.7) | 0.64 | −51.1 (-56.5 to −45.7) | <0.001 |
| Mean 1α,25(OH)2D2, pmol/L | <7.7e | <7.7e | — | — | — | — | — | |
| Mean 25(OH)D2-to-vitamin D2 molar ratio, (SD) | 111.0 (185.2) | 24.6 (18.5) | 92.5 (21.8 to 163.3) | 0.01 | 8.4 (−0.8 to 17.7) | 0.07 | −83.7 (−160.0 to −7.4) | 0.03 |
Adjusted for baseline value.
Not calculated for 1,25-dihydroxyvitamin D (1,25-dihydroxyvitamin D2 was undetectable in all) or 24,R,25-dihydroxyvitamin D/4β-dihydroxyvitamin D (neither 24,R,25-dihydroxyvitamin D2 nor 4β-dihydroxyvitamin D2 were measured).
Calculated by summing values for vitamin D2 and vitamin D3.
Calculated by summing values for 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3.
1α,25(OH)2D2 undetectable in all; LLOQ for this metabolite was 7.7 pmol/L.
Figure 3.
Influence of oral administration of vitamin D3 and vitamin D2 on serum concentrations of (A) total 25(OH)D, (B) 25(OH)D3, (C) 25(OH)D2, and (D) 1α,25(OH)2D3. Baseline and 4-mo data are presented for 24 adults receiving four bolus doses of 2.5 mg vitamin D3 at 0, 1, 2, and 3 mo postrandomization and 28 adults receiving an equivalent regimen of vitamin D2. Lines link data points from the same individual; P values for within-group comparisons before vs after supplementation are from paired Student t tests. P values for intergroup comparisons of postsupplementation values in participants randomized to vitamin D3 vs vitamin D2 are from linear regression with adjustment for baseline values.
Influence of vitamin D2 on metabolism of vitamin D3
To characterize effects of vitamin D2 on metabolism of D3, we conducted pairwise statistical analyses comparing circulating concentrations of vitamin D3 and its metabolites in 28 individuals before vs after oral administration of four monthly doses of 2.5 mg vitamin D2. Results are presented in Table 2. Administration of vitamin D2 had no statistically significant effect on serum concentrations of vitamin D3 (P = 0.07), but it did reduce mean serum concentrations of 25(OH)D3 (43.2% decrease, P < 0.001; Fig. 3), 24R,25(OH)2D3 (37.5% decrease, P = 0.007), 1α,25(OH)2D3 (53.4% decrease, P < 0.001; Fig. 3) and 4β,25(OH)2D3 (42.0% decrease, P = 0.03). Administration of vitamin D2 reduced molar ratios of 25(OH)D3 to vitamin D3 (76.2% decrease, P = 0.003) and 1α,25(OH)2D3 to 25(OH)D3 (52.2% decrease, P = 0.02), but increased the molar ratio of 24R,25(OH)2D3 to 25(OH)D3 (24.5% increase, P = 0.04). No statistically significant effect of vitamin D2 on the molar ratio of 4β,25(OH)2D3 to 25(OH)D3 was seen (P = 0.97).
Influence of vitamin D3 on its own metabolism
Having characterized the effects of vitamin D2 on metabolism of vitamin D3, we proceeded to conduct a second set of pairwise before and after statistical analyses to evaluate the effects of vitamin D3 on the same biochemical parameters in a separate group of 24 individuals who received four monthly doses of 2.5 mg vitamin D3. Results of these analyses (Table 2) show that administration of vitamin D3 elevated serum concentrations of vitamin D3 (80.0% increase, P = 0.03), 25(OH)D3 (110.0% increase, P < 0.001; Fig. 3), 24R,25(OH)2D3 (165.5% increase, P < 0.001), 1α,25(OH)2D3 (66.6% increase, P = 0.005), and 4β,25(OH)2D3 (214.5% increase, P = 0.02). Administration of vitamin D3 also increased the ratio of 24R,25(OH)2D3 to 25(OH)D3 (32.6% increase, P = 0.006) but had no statistically significant effect on ratios of 25(OH)D3 to vitamin D3 (P = 0.09), 1α,25(OH)2D3 to 25(OH)D3 (P = 0.06), or 4β,25(OH)2D3 to 25(OH)D3 (P = 0.06).
Comparing effects of vitamin D2 vs vitamin D3 on metabolism of vitamin D3
To determine whether the two forms of vitamin D exerted different effects on metabolism of vitamin D3, we undertook unpaired statistical analyses comparing postsupplementation values of vitamin D3 metabolite-to-parent ratios between individuals supplemented with vitamin D2 vs vitamin D3, using linear regression with adjustment for baseline values. Results of these analyses (Table 2) reveal that participants receiving vitamin D2 had lower mean postsupplementation ratios of 25(OH)D3 to vitamin D3 (P = 0.002) and 1α,25(OH)2D3 to 25(OH)D3 (P = 0.03) than those who received vitamin D3. No statistically significant difference in mean postsupplementation ratios of 24R,25(OH)2D3 to 25(OH)D3 (P = 0.42) or 4β,25(OH)2D3 to 25(OH)D3 (P = 0.25) was seen between participants receiving vitamin D2 vs vitamin D3.
Influence of vitamin D3 vs vitamin D2 on metabolism of vitamin D2
Although the primary focus of our study was to compare the effects of vitamin D2 vs vitamin D3 on metabolism of vitamin D3, limited data were also available to evaluate relative effects of the two forms of vitamin D on metabolism of vitamin D2 (Table 2). Pairwise analyses revealed that administration of vitamin D2 increased mean serum 25(OH)D2 concentration (P < 0.001; Fig. 3) and mean 25(OH)D2-to-D2 molar ratio (P = 0.01), but had no statistically significant effect on mean serum concentration of vitamin D2 itself (P = 0.09). By contrast, administration of vitamin D3 reduced vitamin D2 concentrations over time (P = 0.008) but did not influence 25(OH)D2 concentrations (P = 0.64; Fig. 3) or 25(OH)D2-to-D2 ratio (P = 0.07). Unpaired analysis comparing postsupplementation 25(OH)D2-to-D2 ratios between individuals supplemented with vitamin D2 vs vitamin D3, with adjustment for baseline values, showed that the mean postsupplementation ratio of 25(OH)D2 to vitamin D2 among participants receiving vitamin D2 was higher than that of participants who received vitamin D3 (P = 0.03).
Discussion
To our knowledge, this is the first investigation to evaluate the influence of vitamin D2 on circulating concentrations of parent vitamin D3 and its dihydroxylated metabolite 4β,25(OH)2D3 in addition to serum concentrations of 25(OH)D2 and 25(OH)D3. We found that administration of vitamin D2 exerted a greater inhibitory effect than administration of vitamin D3 on mean ratios of 25(OH)D3 to D3 and 1α,25(OH)2D3 to 25(OH)D3 in the circulation. We also observed that vitamin D2 and vitamin D3 increased the mean 24R,25(OH)2D3-to-25(OH)D3 ratio to a similar extent, and that neither form of vitamin D had a statistically significant effect on the mean serum 4β,25(OH)2D3-to-25(OH)D3 ratio. By contrast with findings of a recently published study (6), we found that administration of vitamin D3 did not suppress serum concentrations of 25(OH)D2, nor did it influence mean serum 25(OH)D2-to-vitamin D2 ratio. Administration of vitamin D2 resulted in an increase in the mean 25(OH)D2-to-D2 ratio.
Our findings are consistent with reports that administration of vitamin D2 reduces serum concentrations of 25(OH)D3 (1–4), and that this phenomenon is associated with an increase in the ratio of 24R,25(OH)2D3 to 25(OH)D3 and a decrease in the ratio of 1α,25(OH)2D3 to 25(OH)D3 in the circulation. Interestingly, serum 25(OH)D2 concentrations were markedly elevated in all study participants receiving vitamin D2, consistent with induction of the vitamin D2 25-hydroxylation pathway, although there was an opposite effect on 25(OH)D3 and rate of formation in the same treated individuals (Fig. 3), raising the possibility that different enzymes might catalyze 25-hydroxylation of the two forms of vitamin D. Alternatively, the decline of 25(OH)D3 following administration of vitamin D2 may reflect competition of vitamin D2 for the same 25-hydroxylation pathway as vitamin D3. Although changes in metabolite-to-parent ratios may reflect alteration in rates of conversion of one metabolite to another, they could also be explained by removal of vitamin D and its metabolites from the circulation (e.g., via direct excretion or disposition into depots such as adipose tissue and muscle). Further investigations to compare the effects of different forms of vitamin D on expression and activity of the enzymes responsible for metabolizing vitamin D3 are needed to resolve the question of whether changes in metabolite-to-parent ratios truly reflect changes in activity of cytochrome P450 enzymes. Such studies would potentially require liver and renal biopsies: both are invasive procedures and their inclusion in a study protocol could raise issues relating to ethics and acceptability to participants.
One aspect in which our findings differ from those of other investigators relates to the lack of detectable 1,25-dihydroxyvitamin D2 at follow-up among participants receiving vitamin D2 in our study. By contrast, Biancuzzo et al. reported that daily administration of 1,000 IU vitamin D2 for 11 weeks induced a mean increase in serum 1,25(OH)2D2 concentration of 5.2 pg/mL (13.5 pmol/L) (14). This difference may reflect use of intermittent bolus dosing in the current study, which contrasts with the daily dosing regimen used by Biancuzzo et al.
Our findings provide insights into the differential effects of ergocalciferol and cholecalciferol on vitamin D metabolism, however, from the clinician’s perspective, a key question relates to relative effects of the two forms of vitamin D on total 25(OH)D levels, which reflect vitamin D status. Among substudy participants, the mean increase in total 25(OH)D for participants randomized to vitamin D2 (n = 28) vs vitamin D3 (n = 25) was 31.4 nmol/L vs 46.4 nmol/L, respectively (P for intergroup comparison = 0.06). This trend is in keeping with findings from the main trial, in which the difference in increase in total 25(OH)D for participants randomized to vitamin D2 (n = 112) vs D3 (n= 114; 31.2 vs 38.3 nmol/L increase, respectively) attained statistical significance (P = 0.03).
Our study has several strengths. Participants randomized to vitamin D2 vs D3 were well matched with regard to baseline characteristics, and directly observed administration of vitamin D2 and vitamin D3 at identical doses via the same route allowed for a head-to-head comparison of their effects. Determination of concentrations of parent vitamin D2 and D3, 25(OH)D2, 25(OH)D3, and its major dihydroxylated metabolites allowed us to compare effects of vitamin D2 vs vitamin D3 on both the synthesis and the catabolism of 25(OH)D3. Moreover, we utilized the gold standard method (liquid chromatography-tandem mass spectrometry) to measure concentrations of vitamin D and its metabolites with high degrees of accuracy and sensitivity, avoiding issues of cross-reactivity between metabolites of vitamin D2 and vitamin D3 that may arise with immunoassays (23).
Our study also has some limitations. We measured concentrations of vitamin D metabolites at a single time point, one month after the fourth bolus dose was given; thus, we do not capture the pharmacokinetics of vitamin D metabolism at multiple points over the period of the dosing interval. In particular, conclusions relating to concentrations of parent vitamin D2 and vitamin D3 in the circulation should be guarded, because of their short half-life. Participants all had an elevated risk of type 2 diabetes mellitus: thus, our findings cannot necessarily be generalized to other groups. However, we have no specific reason to believe that effects of vitamin D2 are likely to be different in this group compared with the general population. We did not measure concentrations of 1,24,25-trihydroxyvitamin D3 or 24R,25(OH)2D2: this could have provided insights into the effects of vitamin D2 vs vitamin D3 on 24-hydroxylation of 1α,25(OH)2D3 and 25(OH)D2, respectively. Preparations of vitamin D2 and vitamin D3 were presented in different vehicles (alcohol vs oily solution, respectively), which could theoretically have impacted differently on absorption and/or metabolism. However, a study in schoolchildren comparing ethanol vs oil as a vehicle for a weekly oral dose of 14,000 IU vitamin D3 showed no difference in the 25(OH)D response to supplementation between groups over eight weeks (24), rendering this explanation for the findings in the current study unlikely.
In conclusion, the current study confirms reports that vitamin D2 is less effective than vitamin D3 in elevating total 25(OH)D levels, and extends prior findings by showing that administration of vitamin D2 reduces 25-hydroxylation of vitamin D3 and 1-α hydroxylation of 25(OH)D3 and increases 24R-hydroxylation of 25(OH)D3.
Acknowledgments
We acknowledge the contribution of all participants and general practices, members of the Trial Steering Committee [Prof. Andrew Morris (Chair), Dr. Ann Millward and Mrs. Yvonne Hunt), and members of the Data Monitoring Committee [Prof. Desmond Johnston (Chair), Prof. Garry John and Dr. Ian White]. The trial was jointly sponsored by Queen Mary University of London and the Medical Research Council Epidemiology Unit at Cambridge.
Financial Support: Tower Hamlets Primary Care National Health Service Trust; East London Comprehensive Local Research Network; United Kingdom Medical Research Council (MC_UP_A100_1003, MC_U106179474, MC_UU_12015/5 and MC_UU_12015/4); United Kingdom National Institute for Health Research (IS-BRC-1215-20014); US National Institutes of Health (R01 GM63666).
Clinical Trial Registration: EudraCT 2009-011264-11 and ISRCTN86515510 (registered 23 October 2009).
Author Contributions: G.A.H., N.G.F., B.J.B., S.J.G., and A.R.M. contributed to design of the main trial, for which G.A.H. was Chief Investigator and N.G.F. was lead investigator. A.R.M., D.A.J., and K.E.T. had the idea for the substudy presented in this manuscript. Z.W. and K.E.T. developed and ran laboratory assays. A.R.M. analyzed the data and wrote the first draft of the manuscript; all other authors critically reviewed it and approved the final version.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Glossary
Abbreviations:
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 4β,25(OH)2D3
4β,25-dihydroxyvitamin D3
- 24R,25(OH)2D3
24R,25-dihydroxyvitamin D3
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D3
25-hydroxyvitamin D3
- LLOQ
lower limit of quantitation
References and Notes
- 1. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387–5391. [DOI] [PubMed] [Google Scholar]
- 2. Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, Vasikaran SD, Taranto M, Musk AA, Fraser WD. Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone. 2009;45(5):870–875. [DOI] [PubMed] [Google Scholar]
- 4. Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98(11):4339–4345. [DOI] [PubMed] [Google Scholar]
- 5. Tjellesen L, Hummer L, Christiansen C, Rødbro P. Serum concentration of vitamin D metabolites during treatment with vitamin D2 and D3 in normal premenopausal women. Bone Miner. 1986;1(5):407–413. [PubMed] [Google Scholar]
- 6. Hammami MM, Abuhdeeb K, Hammami S, Yusuf A. Vitamin-D2 treatment-associated decrease in 25(OH)D3 level is a reciprocal phenomenon: a randomized controlled trial. BMC Endocr Disord. 2019;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr. 2013;109(6):1082–1088. [DOI] [PubMed] [Google Scholar]
- 8. Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–858. [DOI] [PubMed] [Google Scholar]
- 9. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447–E452. [DOI] [PubMed] [Google Scholar]
- 10. Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D’Erasmo E, Carnevale V, Scillitani A, Minisola S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. [DOI] [PubMed] [Google Scholar]
- 11. Forouhi NG, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, Rickard AP, Boucher BJ, Chowdhury TA, Griffiths CJ, Greenwald SE, Griffin SJ, Hitman GA. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2016;18(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, Ameri A, Reitz R, Salameh W, Chen TC, Holick MF. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010;91(6):1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98(3):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, Goff JP, Horst RL, Chaudhry AS, Schuetz EG, Thummel KE. Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility, and interindividual variability. Endocrinology. 2014;155(6):2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong T, Wang Z, Chapron BD, Suzuki M, Claw KG, Gao C, Foti RS, Prasad B, Chapron A, Calamia J, Chaudhry A, Schuetz EG, Horst RL, Mao Q, de Boer IH, Thornton TA, Thummel KE. Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans. Drug Metab Dispos. 2018;46(4):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Batacchi Z, Robinson-Cohen C, Hoofnagle AN, Isakova T, Kestenbaum B, Martin KJ, Wolf MS, de Boer IH. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12(9):1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–1742. [DOI] [PubMed] [Google Scholar]
- 20. Menon RK, Rickard AP, Mannan N, Timms PM, Sharp SJ, Martineau A, Boucher BJ, Chowdhury TA, Griffiths CJ, Griffin SJ, Hitman GA, Forouhi NG. The effects of vitamin D2 or D3 supplementation on glycaemic control and related metabolic parameters in people at risk of type 2 diabetes: protocol of a randomised double-blind placebo-controlled trial. BMC Public Health. 2013;13:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Senn T, Kalhorn T, Zheng XE, Zheng S, Davis CL, Hebert MF, Lin YS, Thummel KE. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4β,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganmaa D, Khudyakov P, Buyanjargal U, Jargalsaikhan B, Baigal D, Munkhjargal O, Yansan N, Bolormaa S, Lkhagvasuren E, Sempos CT, Bromage S, Wu Z, Ochirbat B, Gunchin B, Martineau AR. Prevalence and determinants of QuantiFERON-diagnosed tuberculosis infection in 9810 Mongolian schoolchildren. Clin Infect Dis. 2019;69(5):813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Goff C, Peeters S, Crine Y, Lukas P, Souberbielle JC, Cavalier E. Evaluation of the cross-reactivity of 25-hydroxyvitamin D2 on seven commercial immunoassays on native samples. Clin Chem Lab Med. 2012;50(11):2031–2032. [DOI] [PubMed] [Google Scholar]
- 24. Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, El-Hajj Fuleihan G. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]