Abstract
Background: L-triiodothyronine (LT3) is a substitute for levothyroxine (LT4) for thyroid cancer (TC) patients during the preparation for nuclear medicine procedures, and it is used in combination with LT4 in patients who do not respond to the standard treatment for hypothyroidism. This therapy is commonly done by using fixed doses, potentially resulting in supraphysiologic levels of triiodothyronine (T3). A good understanding of the LT3 pharmacokinetics (PK) is necessary to design combination treatment schemes that are able to maintain serum T3 levels within the reference range, but data on the PK of LT3 are conflicting. Here, we present a study designed to characterize the PK of LT3 in patients devoid of endogenous thyroid hormone production, and not receiving LT4 therapy.
Methods: We performed an open-label, PK study in patients undergoing thyroid hormone withdrawal in preparation for nuclear medicine procedures for the evaluation and treatment of follicular-derived TC. LT3 was substituted for LT4 at a 1:3 mcg/mcg dosage ratio thrice daily for at least 30 days. PK of the last LT3 dose while at steady state and terminal elimination was assessed over 11 days. Thereafter, a PK study was performed following the nuclear medicine procedure in patients who volunteered for a second study.
Results: Fourteen patients age 48.5 ± 16.0 years completed the last dose study and five completed the second PK study. PK analysis indicates a time to maximum serum concentration of 1.8 ± 0.32 hours and two distinct phases of linear elimination, with a fast distribution phase and slow elimination phases with half-lives of 2.3 ± 0.11 hours and 22.9 ± 7.7 hours, supporting a two-compartment model. PK modeling predicts that a twice-daily administration of low-dose LT3 (0.07 mcg/kg twice daily) in combination with LT4 can predictably increase the serum T3 concentration without significant peaks above the reference range.
Conclusions: The PK of LT3 is well described by a two-compartment model that assumes elimination only from the sampling compartment, with a rapid distribution phase and a slow elimination phase. This information will contribute to design therapeutic strategies for LT3/LT4 combination therapies directed to maintain stable T3 serum levels.
Keywords: L-triiodothyronine, pharmacokinetics, thyroid hormone withdrawal, combination therapy, hypothyroidism
Introduction
Hypothyroidism is a common condition (1,2) associated with an increased cardiovascular risk profile (3–5) and poor quality of life (6,7). The goal of its therapy is the relief of symptoms and restoration of normal thyroid hormone levels by administration of synthetic thyroxine (T4), levothyroxine (LT4), which is then converted in the peripheral tissues into the hormonally active triiodothyronine (T3) (8). During replacement therapy, in the absence of endogenous thyroid hormone production, the entire pool of thyroid hormone derives from the ingested formulations and the peripheral metabolism (9).
Thyroid hormone replacement therapy is based on the use of compounds that are able to provide predictable delivery of the hormonal signal. All the thyroid hormone drugs contain either LT4, thyroid extracts, or synthetic T3, L-triiodothyronine (LT3). Aside from the renewed interest for the use of thyroid extracts or LT3/LT4 combination therapy in hypothyroid patients due to the perceived improvement in symptoms and quality-of-life indices (7,10,11), LT3 is also used in patients with advanced thyroid cancer (TC) for the preparation for nuclear medicine procedures (12) and for the primary ablation or whole-body scan when recombinant thyrotropin (TSH) is not available. We previously demonstrated that substitution of LT3 for LT4 on a 1:3 mcg/mcg ratio on a thrice-daily administration scheme resulted in equivalent responses at the level of thyrotrophs (13) whereas a significant decrease in serum cholesterol and body weight was observed (14). This experimental treatment scheme is not sustainable for long-term therapy because of the multiple daily administration requirements, but it provides empirical data supporting the use of LT3 in combination with LT4 to mitigate symptoms and metabolic effects of hypothyroidism.
Thyroid hormone formulations were developed before the implementation of strict approval requirements, and their therapeutic use has been “grandfathered in” by the Food and Drug Administration (15). Further, because the vast majority of the studies were performed before the development of immune assays for thyroid hormone, the characterization of the pharmacology of these drugs and, in particular, LT3 was technically challenging. Most of the studies were performed in patients or healthy volunteers by measuring the decay of radio-labeled LT3 rather than a direct measurement of T3 (16–19). Since these studies have been performed in subjects with endogenous production of thyroid hormone from the thyroid gland, it is likely that the reuptake and metabolism of iodine in the thyroid caused a spurious increase in the half-life of the drug. In addition, the immunoassays used in some of the studies were not as sensitive as the ones currently available (20–22). Because of the renewed interest in LT3/LT4 combination therapy (23,24), the precise knowledge of the pharmacokinetics (PK) of LT3 at low doses is of paramount importance to design therapeutic regimens and formulations that are able to provide predictable amounts of thyroid hormone while avoiding excursions in the T3 serum concentrations.
Patients who have undergone near-total thyroidectomy for TC are devoid of significant endogenous thyroid hormone production, and the substitution of LT3 for LT4 results in undetectable levels of T4. Thus, these patients represent an ideal experimental model to define the PK of LT3 in the absence of endogenous or exogenous T4. To the best of our knowledge, no such study has been performed in these patients.
Materials and Methods
Study design
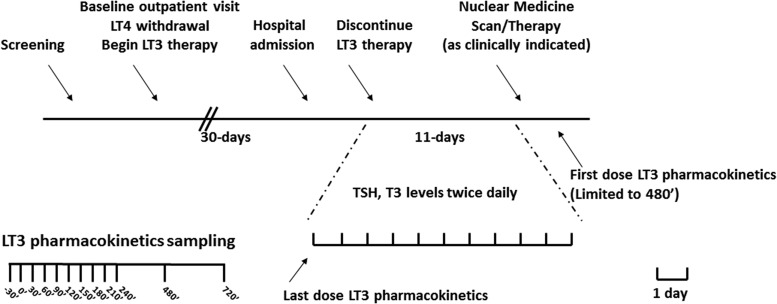
The design was a phase 1 open-label PK study, and it was registered in Clinicaltrials.gov, NCT01441154. The study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board, and it was conducted at the National Institutes of Health (NIH) Clinical Research Center in Bethesda Maryland between September 2011 and October 2013. TC patients with clinical indication for therapy withdrawal (according to the American Thyroid Association guidelines at the time of study design and accrual [25]) in preparation for nuclear medicine procedures were invited to participate in the study. All study participants provided written informed consent. After an initial screening visit and consent, LT4 therapy was adjusted, if needed, to TSH <0.1 mcIU/mL and free thyroxine (fT4) within the reference range. After baseline assessment, an equivalent LT3 dose, prescribed on a thrice-daily regimen (13), was substituted for LT4 for 4 weeks. Study volunteers were instructed to take the LT3 with water after at least a 3-hour fast, and to wait at least 30 minutes before the next meal. Study volunteers were then admitted to the Clinical Center and, after an overnight fast, underwent a last-dose PK study with samples collected at times −30′, 0′, 30′, 60′, 90′, 120′, 150′, 180′, 210′, 240′, 360′, 480′, 600′, 720′, and every 12 hours thereafter for a 10-day period before undergoing nuclear medicine procedures (Fig. 1). Study patients also offered to participate in the first-dose LT3 PK at the same time points (up to 480′) after completion of the nuclear medicine treatment before resuming LT4 therapy.
FIG. 1.
Study design. Top panel: timeline of the study. After enrollment, study volunteers were treated with LT3 for at least 30 days before admission to the Clinical Center for last-dose PK and terminal elimination studies. An abbreviated PK study was offered following the nuclear medicine procedures. Bottom panel: time of PK blood sampling. LT3, L-triiodothyronine; LT4, levothyroxine; PK, pharmacokinetics; T3, triiodothyronine; TSH, thyrotropin.
Therapy formulation
LT3 was substituted for LT4 on a 1:3 mcg:mcg ratio by using generic formulation (5, 25, or 50 mcg) dispensed by the NIH Pharmacy. The therapy was divided in increments of 2.5 mcg (half tablet) in equal doses. Whenever the total dose was not divisible by three, the larger dose was administered in the morning; no adjustment was performed during the study.
Analytes
TSH, total and free triiodothyronine (fT3), and fT4 were measured by immunoassay on a Siemens Immulite 2500 analyzer platform by the Department of Laboratory Medicine of the NIH Clinical Center.
Statistical and PK analyses
Data are represented as mean ± standard deviation, and a comparison between groups was performed by two-tailed paired Student's t-test. Nonparametric tests were performed when appropriate, and an α error of 0.05 was considered the threshold for statistical significance.
The last-dose PK parameters were estimated for each individual by using a two-compartment oral dosing model with first-order absorption, a lag time, and linear elimination from the sampling compartment. The first-dose (following nuclear medicine procedure while hypothyroid) PK parameters were estimated by using a one-compartment oral dosing model utilizing a transit compartment absorption model and linear elimination from the sampling compartment. All PK modeling functions were completed by using Monolix version 2019R1 (Antony, France: Lixoft SAS, 2019).
Combination therapy modeling
Using the average of the individual PK model parameters found to predict the study observed LT3 concentrations, additional plots were generated to predict the expected changes in serum T3 concentrations for hypothetical combination therapy modalities. Plots were also generated to predict percent time in therapeutic window for various administration regimens, including monotherapy LT3 dosing and LT3 in combination with LT4 therapy.
Results
Study population
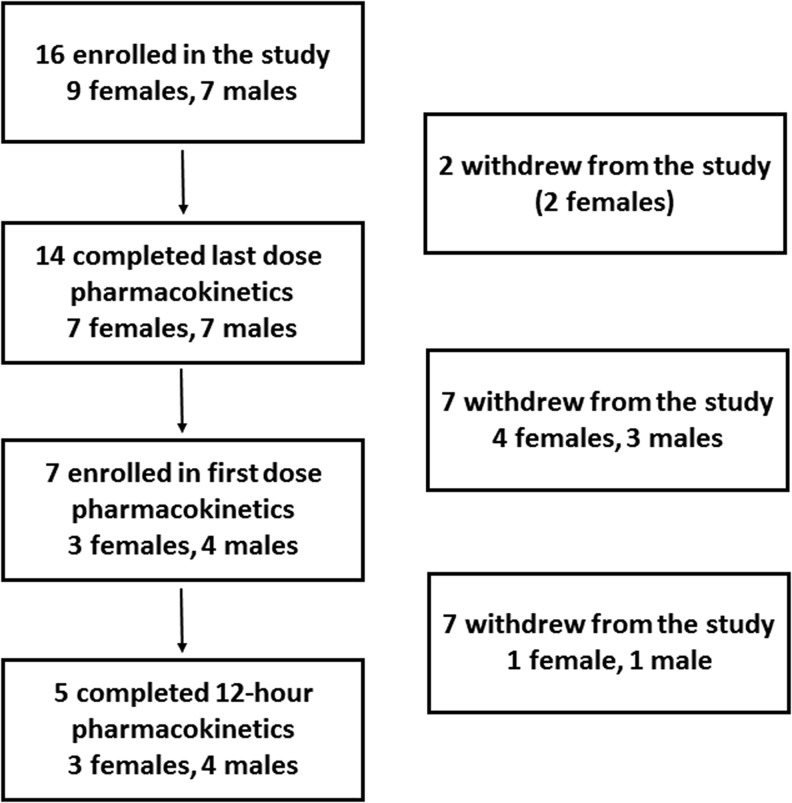
Sixteen patients (seven males) provided informed consent for the participation in the research, but two female patients were unable to complete the inpatient component due to time constraints and withdrew from the study (Fig. 2). The characteristics of the 14 patients who completed the study and their thyroid hormone dose are reported in Table 1. No patient had significant uptake (>3%) on the pretreatment scan with the exception (see “Thyroid hormone levels” section) of a patient who was diagnosed with a glossal residue (5% uptake) at the time of the pretreatment scan. Seven patients performed the first-dose PK study, five of whom completed all sampling time points while profoundly hypothyroid following the radioiodine treatment, with a dose identical to the morning one received while on chronic LT3 therapy before the withdrawal.
FIG. 2.
CONSORT chart.
Table 1.
Study Volunteers' Characteristics
| Age (years) | 48.5 ± 16.0 |
| Sex (male/female) | (7/7) |
| Weight (kg) | 72.3 ± 15.8 |
| BMI (kg/m2) | 25.7 ± 3.6 |
| Daily LT4 dose (mcg) | 164.3 ± 42.4 |
| Daily LT4 dose (mcg/kg) | 2.2 ± 0.3 |
| Daily LT3 dose (mcg) | 53.7 ± 12.3 |
| Daily LT3 dose (mcg/kg) | 0.74 ± 0.1 |
| LT3 AM dose (mcg) | 18.8 ± 4.4 |
BMI, body mass index; LT3, L-triiodothyronine; LT4, levothyroxine.
Thyroid hormone levels
After four weeks of LT3 therapy, the TSH remained suppressed without significant difference when compared with baseline, while on LT4. Conversely total T3 was significantly increased, and fT3 had a similar although nonsignificant trend. fT4 was at or below the limit of detection after LT3 therapy (Table 2). Only one study participant maintained measurable T3 levels during withdrawal of therapy. At the time of the pretreatment scan, this patient was found to have a 1 cm3 glossal thyroid residue anterior to the epiglottis and was treated with low-dose radioactive iodine to prevent a potential airway compromise before undergoing full-dose treatment at a subsequent time. The T3 data of this patient were censored on day 3 when the T3 showed no further decrease from 46 ng/dL. Of note, we observed no significant differences in our analysis by including the entire results of this patient, or by censoring these data altogether. In all the other patients, at the end of the LT3 withdrawal, fT4, total and fT3 were below the limits of detection (30 ng/dL); whereas the TSH was 38.6 ± 20.5 mcIU/mL.
Table 2.
Thyroid Hormone and Thyrotropin Levels at Baseline and After 30 Days of L-Triiodothyronine Therapy
| Analyte | LT4 therapy | LT3 therapy | Significance |
|---|---|---|---|
| TSH (ref. 0.4–4.0 mcIU/mL) | 0.08 ± 0.14 | 0.04 ± 0.07 | p = 0.358 |
| fT4 (ref. 0.8–2.2 ng/dL) | 1.54 ± 0.22 | 0.18 ± 0.07 | p < 0.0001 |
| Total T3 (ref. 90–215 ng/dL) | 115.3 ± 26.4 | 166.1 ± 37.9 | p < 0.0001 |
| fT3 (ref. 230–420 pg/dL) | 350.4 ± 58.0 | 386.5 ± 60.1 | p = 0.08 |
fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyrotropin.
PK data and modeling
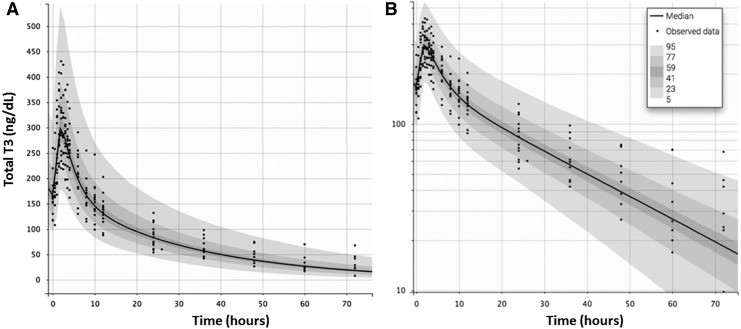
Serial measurements of T3 and fT3 were performed at the time of the last LT3 dose and for the next 10 days, enabling us to determine the PK characteristics of the therapeutic dose of this drug in the absence of endogenous or exogenous T4. For the T3 analysis, our data indicate (Table 3 and Fig. 3) that LT3 is promptly absorbed with a time to maximum serum concentration (Tmax) of 1.8 ± 0.3 hours. The (Cmax) was 320 ± 60 ng/dL, significantly above the upper limit of the reference range. Similarly, for the fT3 analysis, our data indicate (Table 3) a Tmax of 1.9 ± 0.4 hours and a Cmax of 714 ± 99 pg/dL. When concentration data were plotted on a semi-logarithmic scale, two distinct phases of linear elimination were revealed, supporting a two-compartment model for this experimental system. The two-compartment model was utilized with the assumptions of complete, first-order gastrointestinal absorption (without lag time) and linear elimination. This model revealed a fast distribution phase and slow elimination phase with half-lives of 2.3 ± 0.11 hours and 22.9 ± 7.7 hours, respectively. No significant interaction by sex was observed, likely due to the small sample size.
Table 3.
Pharmacokinetics Parameters
| Single dose after 4 weeks of LT3 therapy (two-compartment model with lag time for absorption) | ||||||
|---|---|---|---|---|---|---|
| N = 14 | Tlag (hours) | ka (h−1) | V (dL) | ke (h−1) | k12 (h−1) | k21 (h−1) |
| Total T3 | 0.62 | 1.94 | 86.7 | 0.12 | 0.13 | 0.08 |
| SD | 0.28 | 0.79 | 19 | 0.013 | 0.01 | 0.02 |
| fT3 | 0.66 | 2.40 | 42.4 | 0.10 | 0.10 | 0.05 |
| SD | 0.29 | 0.91 | 9.2 | 0.018 | 0.02 | 0.01 |
| Single dose of LT3 while hypothyroid (transit absorption compartment into a one-compartment model) | |||||
|---|---|---|---|---|---|
| N = 5 | Ktr (h−1) | Mtt (hours) | ka (h−1) | V (dL) | ke (h−1) |
| Total T3 | 11.7 | 2.0 | 3.5 | 149 | 0.24 |
| SD | 10.4 | 1.1 | 1.6 | 42 | 0.14 |
| fT3 | 11.7 | 1.4 | 1.7 | 63,100 | 0.13 |
| SD | 3.8 | 0.9 | 1.2 | 16,300 | 0.003 |
k12, distribution rate constant (from 1 - > 2); k21, distribution rate constant lag time for absorption; ka, absorption rate constant; ke, elimination rate constant; ktr, transit compartment rate constant; Mtt, mean transit time; SD, standard deviation; Tlag, lag time for absorption; V, volume of distribution.
FIG. 3.
PK analysis of last-dose LT3 and terminal elimination (A). Once the data were plotted on a logarithmic scale (B) two distinct phases of linear elimination, a fast distribution phase and a slow elimination phase with half-lives of 1.2 and 29.9 hours, became evident. LOD, limit of detection for total T3.
The observed T3 concentrations for the five patients in hypothyroid conditions after the therapy withdrawal and the release from isolation changed dramatically: the estimated Tmax was 2.9 ± 1.3 hours, and the Cmax was 140 ± 40 ng/dL for total T3, with an apparent distribution volume of 149 ± 42 dL, which is larger than the volume observed for the last dose analysis. In the first dose study, PK was best predicted by a one-compartment model with a transit compartment for absorption and linear elimination. Under these profoundly hypothyroid conditions, the estimated half-life of 3.62 ± 1.6 hours is similar to the rapid distribution phase observed in the last dose study.
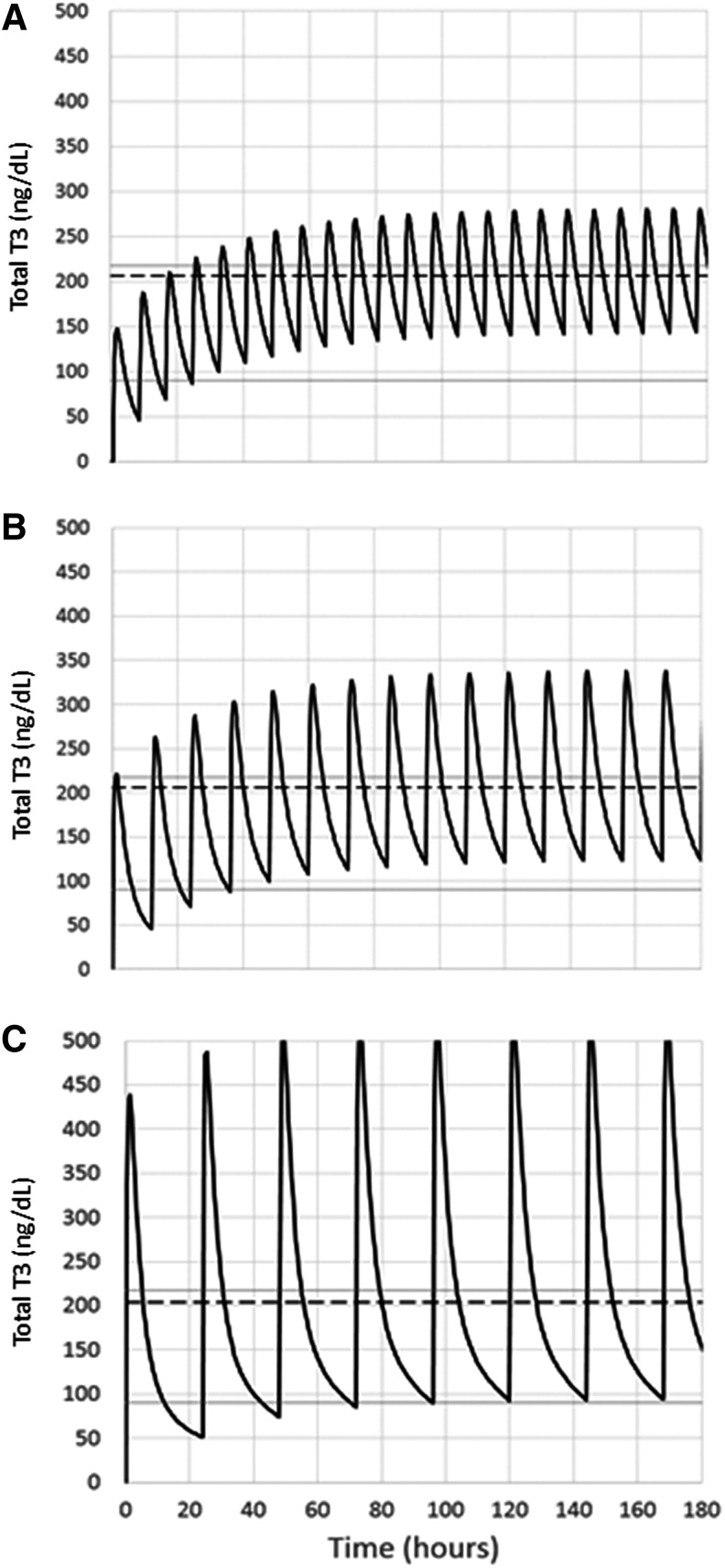
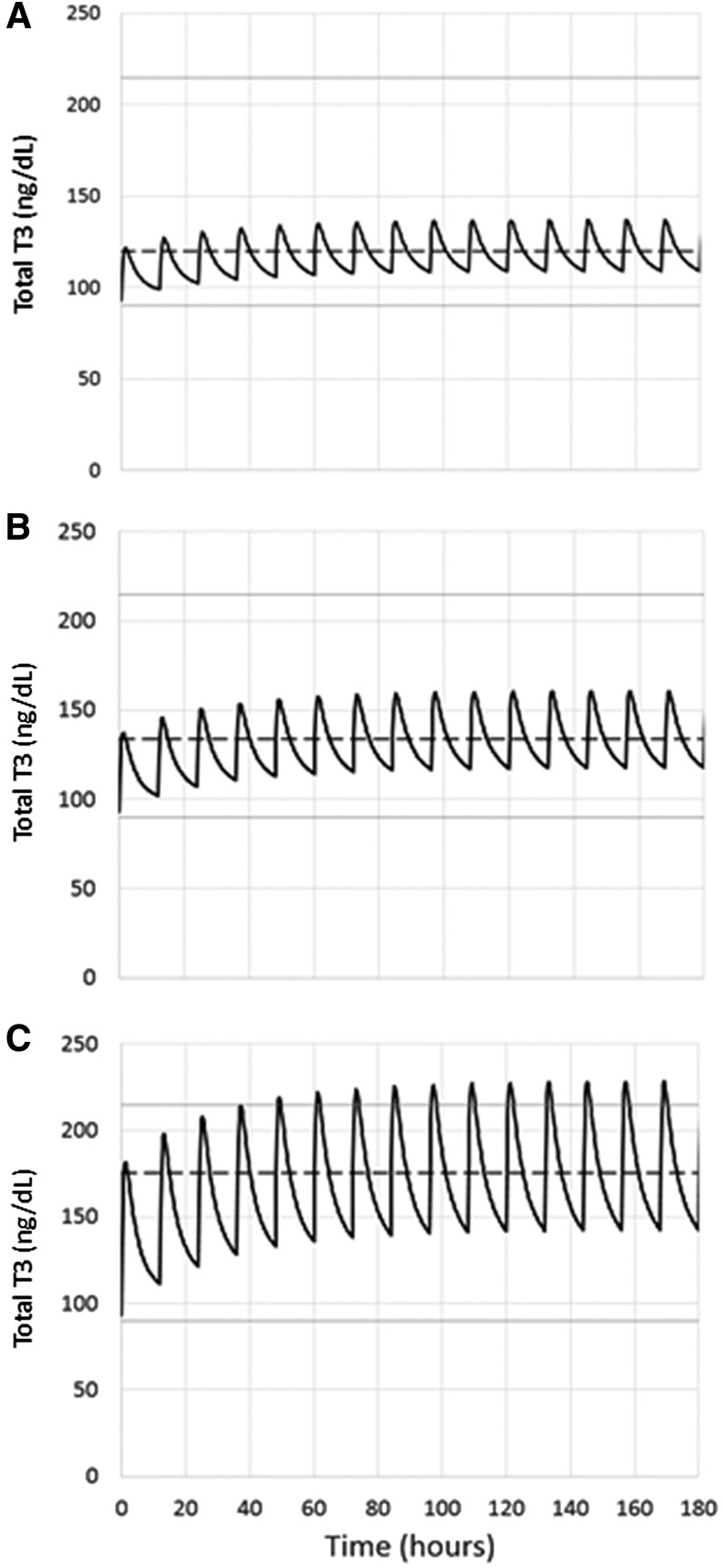
Based on our PK findings, we next generated a model reflecting the excursion of T3 levels at steady state when the total dose of 50 mcg LT3 would be administered and divided into thrice, twice, or single daily doses in a 72.5 kg patient (reflecting the characteristics of our study population). Using these assumptions (Fig. 4), the mean T3 concentration would be almost identical near the upper limit of the reference range, but with dramatically different variances. Thrice-daily LT3 administration at steady state would result in a mean T3 concentration of 206 ± 47 ng/dL, with elevated levels 40.4% of the time; twice-daily administration would result in a mean T3 concentration of 206 ± 69 ng/dL, with elevated levels 40% of the time and without levels below the reference range; and single daily administration would result in a mean T3 concentration of 204 ± 126 ng/dL, with elevated levels 32% of the time and without levels below the reference range.
FIG. 4.
A PK modeling of 50 mcg of LT3 administered on a thrice (A), twice (B), or single (C) daily regimen was generated. Solid lines: reference range of T3. Dashed line: mean concentration of T3. All the proposed treatment schemes result in mean T3 concentrations near the upper limit of reference range, but with dramatic differences in the variance (see Results for details).
A model (Fig. 5) to predict the changes in serum T3 levels in a hypothetical 72.5 kg hypothyroid patient treated with 112 mcg of LT4 alone (∼1.6 mcg/kg) with a baseline T3 level of 93 ng/dL (14) being transitioned to LT4/LT3 therapy at a 16:1 weight/weight combination therapy, as recommended by the European Thyroid Association (ETA) guidelines (26) (i.e., LT3 dose of 3.25 mcg twice daily and LT4 dose of 92.5 mcg daily), was derived from the PK data. Similarly, we also generated a model by decreasing “empirically” the LT4 dose by 25 or 50 mcg and substituting with 5 or 10 mcg LT3 on twice-daily administration, respectively, to approach the 1:3 previously demonstrated pharmacoequivalency (13) (i.e., to an LT3 dose of 5 or 10 mcg twice daily and LT4 dose of 88 or 62 mcg daily, respectively), assuming no significant change in fT4 levels.
FIG. 5.
A PK modeling of LT3/LT4 combination therapy with 3.25 (A), 5 (B), and 10 (C) mcg of LT3 administered on a twice-daily regimen was generated. Solid lines: reference range of T3. Dashed line: mean concentration of T3 (see Results for details).
The data show that the transition from LT4 alone to a 16:1 LT4/LT3 combination treatment with LT3 in twice-daily administration would result in a steady-state increase in serum T3 levels, with an average of 109 ng/dL at trough and 137 ng/dL at peak, with levels well within the laboratory reference range. The average serum T3 level would be 119 ± 9 ng/dL. The substitution of 25 or 50 mcg of LT4 for 5 or 10 mcg LT3 twice daily would result in 117 and 142 ng/dL at trough, and 161 and 228 ng/dL at peak, with an average serum T3 of 134 ± 12 and 175 ± 25 ng/dL, respectively. The substitution of 25 mcg LT4 for 5 mcg LT3 in twice-daily administration would result in serum T3 levels within the reference range, whereas the substitution of 50 mcg LT4 for 10 mcg LT3 in twice-daily administration would result in levels that are above the reference range 14% of the time.
Discussion
LT4 therapy predictably achieves normalization of TSH levels and relief of the symptoms in the majority of hypothyroid patients (8). This modality is associated with a shift in the T4:T3 ratio (27) that is commonly explained by the lack of endogenous production to T3, potentially causing residual hypothyroid symptoms in a significant minority of patients (6). The addition of LT3 to LT4 in the treatment of hypothyroidism has gained renewed interest, even though the guidelines of the major professional organizations do not endorse this modality due to the lack of sufficient evidence. Conversely, the reports of improved quality of life, and to some extent weight loss, after combination therapy or desiccated thyroid extracts described in some studies or in selected patients (28–30) provide some rationale for offering this therapeutic modality on an individual basis. The ETA suggests that “combination therapy should be considered solely as an experimental treatment modality” and provides a guideline to “enhance its safety and to counter its indiscriminate use” (26). Aside from combination therapy, LT3 is also commonly used as a “bridge” therapy to minimize hypothyroid symptoms and signs during LT4 withdrawal in preparation for radioactive iodine therapy in metastatic follicular derived TC (12). When recombinant TSH is not available, this modality is also employed for the primary ablation and diagnostic whole-body scan. Despite the recommendations (8,26), in the majority of cases, LT3 is administered as a single dose, often fixed, when used in combination with LT4.
The common concern associated with the use of LT3 is its short half-life and the risk of developing cardiovascular (31) and mineral metabolism (32) complications of hyperthyroidism, or concern due to overdosing (33,34). This is particularly important in case of prolonged therapy when the perceived benefits could outweigh the risks of potential serious adverse effects. In addition, the evidence that T3 exerts rapid, nongenomic action on the endothelium and cardiovascular system (35–37) further supports the goal of maintaining the serum concentrations of T3 within the reference range, while avoiding or minimizing fluctuations above and below the reference range. To this end, the characterization of the LT3 PK is a necessary first step to design and model combination therapy schemes.
Previous characterization of LT3 PK has been performed by radioactive iodine tracer dilutions, mostly in healthy volunteers or in patients previously treated with LT4 (16,17,20). The models used to predict these data are required to make broad assumptions given the peripheral conversion of T4 and the residual secretion of endogenous T3, which may have affected the accuracy and precision of the model-derived PK parameters. When used, immunoassays were of first generation with limited sensitivity, further decreasing the accuracy and precision of the data. Finally, most of the studies used a pharmacologic dose of LT3. Recently, a PK study on what was believed to be an extended-release T3 formulation was performed by using a modern immunoassay. The study was conducted in healthy volunteers by using a fixed pharmacological dose of T3 (50 mcg) and indicated a Tmax of 2.5 hours with a Cmax of 422 ng/dL (38). Collectively, these limitations and confounders are likely responsible for the wide range of PK data, with a Tmax reported between 1.5 and 6 hours (20,22), and a half-life between 10 and 24 hours (16,20), respectively (Table 4).
Table 4.
L-Triiodothyronine Pharmacokinetics Studies
| Study | Study population | Methods | Half-life hours | Tmax hours | Notes |
|---|---|---|---|---|---|
| Nicoloff et al. (16) | 8 Healthy volunteers | 125I LT3 disappearance rate | 17 | n/a | Intravenous infusion |
| Cavalieri et al. (17) | 5 Healthy volunteers | 131I LT3 disappearance rate | 22 | n/a | Intravenous infusion |
| Woeber et al. (19) | 7 Healthy volunteers | 131I LT3 disappearance rate | 24 | n/a | Intravenous infusion |
| Saberi and Utiger (20) | 8 Hypothyroid | Single daily dose 25 and 50 mcg Immunoassay |
12* | 4–6 | *Estimated from graphic |
| Lieblich and Utiger (21) | 5 Healthy volunteers | Single dose 100 mcg Immunoassay |
16* | 3 | *Estimated from graphic |
| Surks et al. (22) | 4 Hypothyroid (thyroidectomy) | Single dose 75 mcg Immunoassay |
10* | 1.5 | *Estimated from graphic |
| Jonklaas et al. (38) | 12 Healthy volunteers | Single dose 50 mcg Immunoassay |
22 | 2.5 | Compound originally developed as extended release |
| This study | 14 Thyroidectomized patients-on LT3 | Single dose weight-based, terminal elimination | Distribution 2.3 Elimination 22.9 |
1.9 | 11-Day observation Two compartments |
| This study | 5 Thyroidectomized patients-hypothyroid | Single dose weight-based | 3.62 | 2.9 | 12-Hour observation |
131I, radioactive iodine; n/a, not applicable.
Our experimental model is unique because the study volunteers were devoid of endogenous thyroid hormone production and exogenous LT4 administration, and at the time of the PK studies had received a weight-based LT3 dose administered on a thrice-daily regimen (13). The PK studies were performed by using a dose that was close to the one commonly used for combination therapy, although higher than the one suggested by the ETA (26). This, coupled with the prolonged observation after the last dose, allowed us to assess terminal elimination and, ultimately, fully characterize the PK of LT3, which is explained as a two-compartment model with an early rapid distribution phase, and a slow elimination phase. Not surprisingly, the PK characteristics of LT3 as tested after withdrawal in a state of profound hypothyroidism are dramatically different from the ones observed at steady state. This expansion of the apparent volume of distribution is probably due to the overall decrease in metabolism and (possibly to a greater extent) to the net increase in availability of saturable binding sites after complete depletion of thyroid hormone.
The availability of PK data of LT3 at a low dose has also allowed us to generate a model to predict the changes in serum T3 concentrations and its excursions on various combination therapy modalities. The data indicate that a dose of 9.6 mcg on a twice-daily administration (for a hypothetical hypothyroid patient devoid of endogenous thyroid hormone production weighing 72.5 kg) will result in a rise of the average T3 serum concentration from 93 to 175 ng/dL with <15% of the time outside the range. We acknowledge that this modeling is theoretical, and we did not account for the changes in peripheral conversion of T4 into T3 as a result of LT3 administration. The deiodinase 1 (DIO1) gene is positively regulated by T3 (39), and presumably increased T3 levels would prompt T4 to T3 conversion in the liver and kidney. Conversely, the deiodinase type 2 is inhibited by thyroid hormone (40), and the local production of T3 from this enzyme would presumably decrease. Thus, empirical observations are necessary to validate the estimations obtained from this modeling.
Nonetheless, these observations can assist in designing combination therapy regimens targeting serum T3 concentrations, as well as in studying the dose response correlation between serum T3 concentrations and metabolic endpoints.
In conclusion, the data presented in this study demonstrate that the PK of LT3 is best explained by a two-compartment model that is characterized by a rapid distribution phase followed by a prolonged elimination phase. The data provide the rationale for using up to 0.07 mcg/kg twice daily to increase the total T3 levels by 50%, while maintaining the serum concentrations relatively stable with minimal excursions outside the reference range. This, in turn, can provide the basis to design intervention studies that are aimed at characterizing the relationship between modulation of serum T3 levels and end organ effects of thyroid hormone.
Acknowledgments
This study could not have been performed without the selfless dedication of the patients to whom the authors owe their utmost gratitude.
Disclaimer
The views expressed are those of the authors and do not represent the views of the Food and Drug Administration or the U.S. government.
Author Disclosure Statement
Dr. Celi has received consultant fees from Akrimax, IBSA, and Acella. The Division of Endocrinology Diabetes and Metabolism of Virginia Commonwealth University has received an unrestricted grant from IBSA. None of these entities has been involved in the study.
Funding Information
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases program Z01-DK047057-02 National Institutes of Health. The authors gratefully acknowledge the clinical support of Craig Cochran, RN, and James Reynolds, MD.
References
- 1. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 2. Vanderpump MP. 2011. The epidemiology of thyroid disease. Br Med Bull 99:39–51 [DOI] [PubMed] [Google Scholar]
- 3. Duntas LH, Brenta G. 2018. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne) 9:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moon S, Kong SH, Choi HS, Hwangbo Y, Lee MK, Moon JH, Jang HC, Cho NH, Park YJ. 2018. Relation of subclinical hypothyroidism is associated with cardiovascular events and all-cause mortality in adults with high cardiovascular risk. Am J Cardiol 122:571–577 [DOI] [PubMed] [Google Scholar]
- 5. Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. 2018. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid 28:1101–1110 [DOI] [PubMed] [Google Scholar]
- 6. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. 2002. Psychological well-being in patients on “adequate” doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 57:577–585 [DOI] [PubMed] [Google Scholar]
- 7. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, Kopp PA, Ross DS, Samuels MH, Sawka AM, Taylor PN, Jonklaas J, Bianco AC. 2018. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 28:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone Replacement 2014. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. 1990. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol 258:E715–E726 [DOI] [PubMed] [Google Scholar]
- 10. Jonklaas J, Tefera E, Shara N. 2018. Physician choice of hypothyroidism therapy: influence of patient characteristics. Thyroid 28:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jonklaas J, Tefera E, Shara N. 2019. Prescribing therapy for hypothyroidism: influence of physician characteristics. Thyroid 29:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldman JM, Line BR, Aamodt RL, Robbins J. 1980. Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab 50:734–739 [DOI] [PubMed] [Google Scholar]
- 13. Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. 2010. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf) 72:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. 2011. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab 96:3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hennessey JV. 2015. Historical and current perspective in the use of thyroid extracts for the treatment of hypothyroidism. Endocr Pract 21:1161–1170 [DOI] [PubMed] [Google Scholar]
- 16. Nicoloff JT, Low JC, Dussault JH, Fisher DA. 1972. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest 51:473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cavalieri RR, Steinberg M, Searle GL. 1971. Metabolic clearance rate of L-triiodothyronine in man: a comparison of results by single-injection and constant infusion methods. J Clin Endocrinol Metab 33:624–629 [DOI] [PubMed] [Google Scholar]
- 18. Fisher DA, Oddie TH. 1964. Whole-body counting of 131-I-labeled triiodothyronine. J Clin Endocrinol Metab 24:733–739 [DOI] [PubMed] [Google Scholar]
- 19. Woeber KA, Sobel RJ, Ingbar SH, Sterling K. 1970. The peripheral metabolism of triiodothyronine in normal subjects and in patients with hyperthyroidism. J Clin Invest 49:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saberi M, Utiger RD. 1974. Serum thyroid hormone and thyrotropin concentrations during thyroxine and triiodothyronine therapy. J Clin Endocrinol Metab 39:923–927 [DOI] [PubMed] [Google Scholar]
- 21. Lieblich J, Utiger RD. 1972. Triiodothyronine radioimmunoassay. J Clin Invest 51:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Surks MI, Schadlow AR, Oppenheimer JH. 1972. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest 51:3104–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdalla SM, Bianco AC. 2014. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf) 81:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biondi B, Wartofsky L. 2012. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? J Clin Endocrinol Metab 97:2256–2271 [DOI] [PubMed] [Google Scholar]
- 25. American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 26. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J 1:55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. 2011. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 6:e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr. 1999. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 340:424–429 [DOI] [PubMed] [Google Scholar]
- 29. Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. 2009. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 94:1623–1629 [DOI] [PubMed] [Google Scholar]
- 30. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. 2013. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab 98:1982–1990 [DOI] [PubMed] [Google Scholar]
- 31. Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Asvold BO, Sgarbi JA, Volzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N, Thyroid Studies C. 2012. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 172:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, Wirth CD, Peeters RP, Asvold BO, den Elzen WP, Luben RN, Imaizumi M, Bremner AP, Gogakos A, Eastell R, Kearney PM, Strotmeyer ES, Wallace ER, Hoff M, Ceresini G, Rivadeneira F, Uitterlinden AG, Stott DJ, Westendorp RG, Khaw KT, Langhammer A, Ferrucci L, Gussekloo J, Williams GR, Walsh JP, Juni P, Aujesky D, Rodondi N, Thyroid Studies C. 2015. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA 313:2055–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dahlberg PA, Karlsson FA, Wide L. 1979. Triiodothyronine intoxication. Lancet 2:700. [DOI] [PubMed] [Google Scholar]
- 34. Hollingsworth DR, Amatruda TT, Jr., Scheig R. 1970. Quantitative and qualitative effects of L-triiodothyronine in massive obesity. Metabolism 19:934–945 [DOI] [PubMed] [Google Scholar]
- 35. Vicinanza R, Coppotelli G, Malacrino C, Nardo T, Buchetti B, Lenti L, Celi FS, Scarpa S. 2013. Oxidized low-density lipoproteins impair endothelial function by inhibiting non-genomic action of thyroid hormone-mediated nitric oxide production in human endothelial cells. Thyroid 23:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carrillo-Sepulveda MA, Ceravolo GS, Fortes ZB, Carvalho MH, Tostes RC, Laurindo FR, Webb RC, Barreto-Chaves ML. 2010. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res 85:560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis PJ, Davis FB. 2002. Nongenomic actions of thyroid hormone on the heart. Thyroid 12:459–466 [DOI] [PubMed] [Google Scholar]
- 38. Jonklaas J, Burman KD, Wang H, Latham KR. 2015. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit 37:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jakobs TC, Schmutzler C, Meissner J, Kohrle J. 1997. The promoter of the human type I 5′-deiodinase gene—mapping of the transcription start site and identification of a DR +4 thyroid-hormone-responsive element. Eur J Biochem 247:288–297 [DOI] [PubMed] [Google Scholar]
- 40. Leonard JL, Siegrist-Kaiser CA, Zuckerman CJ. 1990. Regulation of type II iodothyronine 5′-deiodinase by thyroid hormone. Inhibition of actin polymerization blocks enzyme inactivation in cAMP-stimulated glial cells. J Biol Chem 265:940–946 [PubMed] [Google Scholar]