Abstract
A 13-year-old female with a novel THRB gene mutation (c.1033G>T, p.G345C) presented with 3- to 6-fold higher serum iodothyronine levels and more severe clinical manifestation than 2 other family members carrying the same mutation. The leukocytes of the proband expressed both wild-type and mutant THRB mRNAs, excluding the possibility of a partial deletion of the allele not carrying the mutation. The proband's fibroblasts showed reduced responsiveness to triiodothyronine compared with those of another affected family member. The more severe clinical and biochemical phenotype suggest a modifier-mediated worsening of the resistance to thyroid hormone.
Keywords: resistance to thyroid hormone, thyroid hormone receptor, mutation, cofactor, non-TR-RTH
Introduction
Resistance to thyroid hormone (RTH) β is a syndrome of reduced tissue responsiveness to thyroid hormone (TH). Most individuals are heterozygous for mutations in the THRB gene, which produce receptors that interfere with the function of the normal (wild-type[WT]) receptor. Patients homozygous for a mutant or deleted THRB gene have more severe phenotype (1). RTHβ without a THRB gene mutation referred as non-TR-RTH is clinically and biochemically undistinguishable of heterozygotes with THRB gene mutations (2). However, its mechanism remains elusive.
In this study, we describe a family in which one of three affected members harboring a novel mutation in the THRB gene manifested more severe clinical and biochemical findings than the other two affected family members.
Subjects
The proband was a 13-year-old female, the first born to nonconsanguineous French Canadian parents. She presented with tachycardia, exercise intolerance, heat intolerance, and tremor. Her history was unremarkable with normal growth, puberty, and regular menses. On physical examination, she had a large goiter, a fine tremor, and nonpitting leg edema (Supplementary Fig. S1). Thyroid function tests revealed markedly elevated total thyroxine, total triiodothyronine (T3), total reverse triiodothyronine, free thyroxine index, and a slightly high thyrotropin (TSH) (Fig. 1A). Antibodies against the TSH receptor, thyroperoxidase (TPO), and thyroglobulin (TG) were negative. Thyroid ultrasonography showed a large homogenous goiter (Supplementary Fig. S1). Her pituitary magnetic resonance imaging was normal.
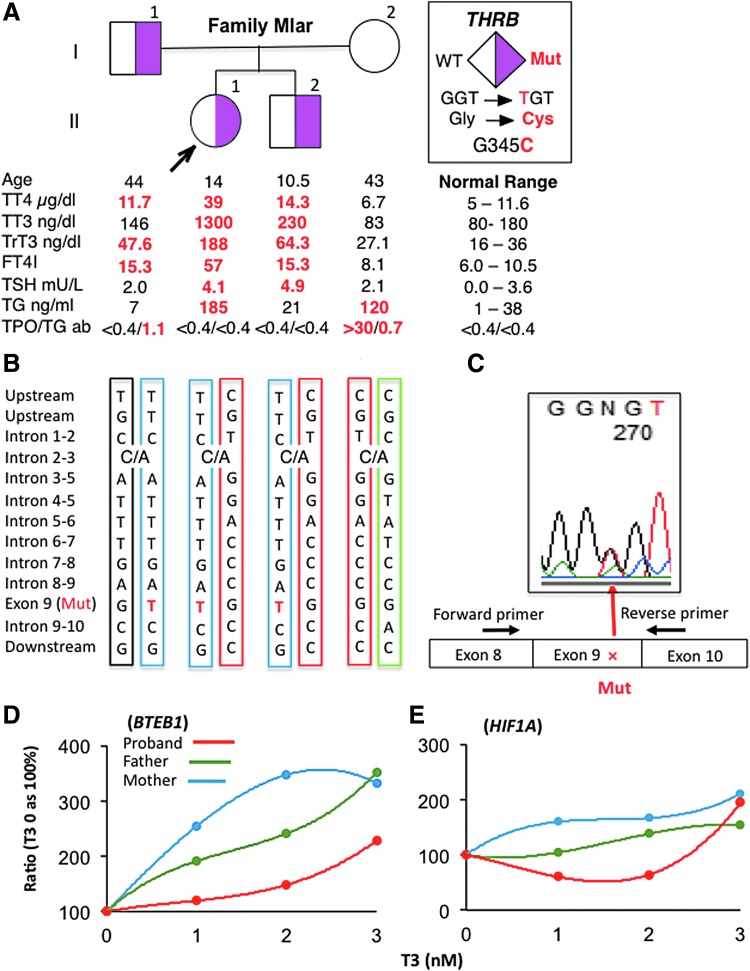
FIG. 1.
(A) Pedigree of the family indicating the genotype of each individual and the results of thyroid function tests, aligned with the symbol representing each family member. (B) Results of THRB locus haplotyping show that the proband and brother inherited the same maternal allele. (C) Sequence of cDNA derived from white blood cells mRNA of the proband, covering the region of the THRB mutation. The location of the primers used in the cDNA amplification is indicated. (D, E) TH responsiveness of the subjects' skin fibroblasts. After culture for 48 hours in medium depleted of TH, fibroblasts were treated with three different amounts of T3, 0.5, 2, and 5 nM, for additional 24 hours. The relative amount of mRNAs, compared with that in fibroblasts cultured in the absence of T3, is given as percentage increase. TH, thyroid hormone; T3, triiodothyronine.
The family history included a father with no complaints and a younger brother with exercise intolerance. Both had elevated iodothyronine levels but of distinctly lesser magnitude than those of the proband (Fig. 1A), as well as smaller thyroid glands (Supplementary Table S1). No family member had a heart murmur, and liver or spleen enlargement. Vital signs are summarized in the Supplementary Table S1. The mother had normal thyroid function tests with the exception of a high TG and positive TG and TPO antibodies (Fig. 1A).
Results
The investigation was approved by the Institutional Review Board. Sanger sequencing of the THRB gene demonstrated a novel heterozygous missense mutation (c.1033G>T) resulting in the substitution of the normal glycine 345 with a cysteine (p.G345C) in the proband, her father and brother, but not the mother. No mutations were found in the sequence coding for THRB2. The mother had a WT sequence. The presence of an albumin gene gain-of-function mutation to explain the higher iodothyronine levels of the proband was also excluded by sequencing.
As the phenotype of the proband was reminiscent of patients with RTHβ expressing only a mutated THRB allele (1), the possibility that the proband expresses only the mutant allele through a partial deletion of the maternal allele was considered. Haplotyping revealed that both the proband and her brother shared the same maternal and paternal alleles (Fig. 1B). Furthermore, THRB cDNA from the proband's white blood cell, sequenced across exons junctions to prevent amplification of genomic DNA, showed that both the WT and mutant alleles were equally expressed (Fig. 1C), thus excluding the possibility of decreased expression of the normal maternal THRB allele.
Finally the tissue sensitivity to TH was assessed in vitro using the subjects' cultured skin fibroblasts. The expression of two genes, BTEB1 and HIF1A, positively regulated by TH, showed the largest response to the addition of small increments of T3 in fibroblasts from the unaffected mother, the smallest response in those of the proband, with an intermediate dose response in fibroblasts from the heterozygous father (Fig. 1D, E). These data confirm the increased severity of RTHβ in the proband compared with an affected family member carrying the same heterozygous THRB mutation.
Discussion
We report a novel THRB gene mutation in an individual with clinical and biochemical manifestations more severe than in the affected sibling and the father harboring the same mutation. The more pronounced resistance to TH was confirmed in cultured skin fibroblasts. The resistance was not caused by reduced expression of the normal allele inherited from the mother by both the proband and affected brother. While the magnitude of iodothyronine elevation in the proband was comparable to that of homozygotes for THRB gene mutations (1), her TSH was only minimally elevated and she showed no delay in growth, bone, and cognitive development, nor hearing loss. An increase in the bioactivity of TSH, previously reported in RTHβ (3), cannot explain the large goiter in the proband, a finding that was not present in her affected brother with a similar TSH concentration. Sequence abnormalities in the 5 known cofactors, 2 corepressors, and 3 coactivators were not identified (data not shown). However, a putative defect in one of the multiple proteins involved in regulation of nuclear receptor-mediated transcription (4) remains a distinct possibility to explain the observed greater severity of RTHβ.
Supplementary Material
Acknowledgments
We are grateful to the family who consented to participate in this study.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported, in part, by grants DK 15070 to SR and DK110322 to AMD from the National Institutes of Health, United States.
Supplementary Material
References
- 1. Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S. 2012. Homozygous thyroid hormone receptor beta gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J Clin Endocrinol Matab 97:1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sadow P, Reutrakul S, Weiss RE, Refetoff S. 2000. Resistance to thyroid hormone in the absence of mutations in the thyroid hormone receptor genes. Curr Opin Endocrinol Diabetes 7:253–259 [Google Scholar]
- 3. Persani L, Asteria C, Tonacchera M, Vitti P, Chatterjee VKK, Beck-Peccoz P. 1994. Evidence for secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J Clin Endocrinol Metab 78:1034–1039 [DOI] [PubMed] [Google Scholar]
- 4. Lonard DM, Lanz RB, O'Malley BW. 2007. Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.