Abstract
Olive (Olea europaea L.) is a major fruit crop in the Mediterranean Basin. Ex-situ olive management is essential to ensure optimal use of genetic resources in breeding programs. The Worldwide Olive Germplasm Bank of Córdoba (WOGBC), Spain, and Marrakech (WOGBM), Morocco, are currently the largest existing olive germplasm collections. Characterization, identification, comparison and authentication of all accessions in both collections could thus provide useful information for managing olive germplasm for its preservation, exchange within the scientific community and use in breeding programs. Here we applied 20 microsatellite markers (SSR) and 11 endocarp morphological traits to discriminate and authenticate 1091 olive accessions belonging to WOGBM and WOGBC (554 and 537, respectively). Of all the analyzed accessions, 672 distinct SSR profiles considered as unique genotypes were identified, but only 130 were present in both collections. Combining SSR markers and endocarp traits led to the identification of 535 cultivars (126 in common) and 120 authenticated cultivars. No significant differences were observed between collections regarding the allelic richness and diversity index. We concluded that the genetic diversity level was stable despite marked contrasts in varietal composition between collections, which could be explained by their different collection establishment conditions. This highlights the extent of cultivar variability within WOGBs. Moreover, we detected 192 mislabeling errors, 72 of which were found in WOGBM. A total of 228 genotypes as molecular variants of 74 cultivars, 79 synonyms and 39 homonyms as new cases were identified. Both collections were combined to define the nested core collections of 55, 121 and 150 sample sizes proposed for further studies. This study was a preliminary step towards managing and mining the genetic diversity in both collections while developing collaborations between olive research teams to conduct association mapping studies by exchanging and phenotyping accessions in contrasted environmental sites.
Introduction
Olive (Olea europaea, ssp. europaea, var. europaea [1]) is widely cultivated for oil and canned fruit. It represents a commercially important fruit crop in the Mediterranean Basin, where about 95% of the world’s olives are produced. More than 3,300,000 t of olive oil are produced annually throughout the world [2] on an area of over 10.8 million ha [3], ranking 7th among all vegetable oils produced worldwide, while olive ranks 25th among the 160 most cultivated crops in the world [3]. Of the 47 olive growing countries, Spain, Italy and Greece are the top three countries, accounting for around 38%, 13% and 10% of total olive oil production, respectively [2]. Cultivated olive was domesticated from the wild type (Olea europaea subsp. Europaea var. sylvestris) in the Middle East 6,000 years ago [4,5]. Early domesticated forms were probably disseminated during successive human migrations from east to west and introgressed with local wild olives, in turn giving rise to local cultivated forms through selection by farmers [6–10]. Olive spread from Mediterranean areas throughout the world. This crop is now of increasing commercial interest beyond the Mediterranean Basin, including countries such as Australia, Chile and USA [2].
Over the long history of olive domestication, cultivated forms have been selected, propagated and disseminated by farmers. An international initiative was thus conducted to pool olive germplasm information in a single database. The 2008 web-based edition (http://www.oleadb.it/) is currently the largest database with information extracted from almost 1520 publications [11]. This database nevertheless represents an underestimated level of domesticated olive diversity since it overlooks many minor local cultivars specific to olive growing areas such as Morocco, Cyprus and Syria. There are currently more than 1,200 cultivars with over 3,000 synonyms reported in 54 different countries and maintained in almost 100 separate collections at international, national and regional levels for conservation and evaluation purposes [11–13], this includes: in the Mediterranean Basin, at Cosenza (Italy, 500 cultivars [14]), Izmir (Turkey, 96 cultivars [15]) and Chania (Greece, 47 cultivars [16]), in addition to new olive-growing regions of the world such as at Davis (USA, [17]) and Mendoza (Argentina, [18]). However, few of these cultivars have been fully characterized using molecular markers and morphological traits. Two worldwide olive germplasm banks (WOGB) exist in the Mediterranean Basin, the first was set up in the 1970s at Cordoba (Spain, WOGBC), with about 500 accessions from 21 countries [19–21]. In 2003, in the framework of the ResGen-T96/97 Project funded by the EU and IOOC and including 16 partners, a second WOGB was created at the INRA Research Station of Tassaoute, Marrakech (Morocco, WOGBM). This worldwide collection presently includes almost 560 accessions originating from 14 Mediterranean countries [22–23]. To optimize olive germplasm sampling, local genetic resources had been characterized by different partners using standardized morphological and/or molecular descriptors. WOGBM was thus established while including previously characterized olive genetic resources from each Mediterranean country.
Recently, socioeconomic changes in most olive producing countries have led to significant improvements in olive growing, including the establishment of modern orchards based exclusively on a few high-yielding and low-vigor cultivars, such as cv. Arbequina. These trends may potentially lead to the erosion of local olive germplasm because several minor traditional cultivars are being replaced by a few cultivars. In the current setting of climate change [24] and the emergence of new diseases, such as Xylella fastidiosa [25], olive cropping systems based solely on a few cultivars may be less resilient than conventional systems. Therefore, the preservation of genetic diversity in ex-situ collections could provide material to substantially enhance modern orchards and breeding programs. The identification and authentication of accessions in collections is therefore considered essential prior to any use of olive germplasm.
As a clonally propagated fruit crop, olive trees have been widely disseminated throughout the Mediterranean Basin and in new olive-growing regions worldwide, leading in many cases to synonymy (different names used for the same cultivar) [26], homonymy (the same name used for different cultivars) [27] and molecular variants, i.e. intra-cultivar variation [28–30]. Moreover, mislabeling errors within collections, which may occur at any step during the plant material processing, is another serious problem in germplasm collection management [21]. Such constraints have also been identified in other clonally propagated fruit crops such as grape [31], fig [32] and apple [33]. This issue could be overcome via cultivar characterization and identification, thus avoiding varietal confusion and providing authenticated cultivars.
Olive cultivar identification was first based solely on morphological and agronomical traits. Descriptors were then used for the characterization of olive flowers, pollen, leaves, fruit and endocarp, oil content, vigor, yield and flowering phenology [34–40]. Although these descriptors are very useful for field surveys and germplasm bank characterization [18,21,41,42], they were found to be only partially informative due to their limited number and the fact that they were impacted by environment conditions. Molecular markers were then applied to better characterize and clearly identify olive cultivars. Molecular studies were initially conducted using isoenzyme markers [43,44] and then DNA-based markers, including restriction fragment length polymorphism (RFLP) [45], random amplified polymorphic DNA (RAPD) [46,47], inter-simple sequence repeat (ISSR) [48–49], amplified fragment length polymorphism (AFLP) [50], simple sequence repeat (SSR) [17,21,22,30,51–55], and single nucleotide polymorphism (SNP) [19,56–58]. Recent available information on olive genome assembly [59,60] will broaden the prospects for varietal identification using DNA-based fingerprinting with EST-SSR and SNP markers [61–63].
Most olive cultivar characterization and identification studies have been carried out independently [17,21–23,51,64]. Although the same panels of SSR loci have been used, attempts to align databases of different collections have led to discrepancies among accessions, which could be mainly explained by the nature of the SSR loci studied as most of them have di-nucleotide repeats (AG/CT) [65]. As reported by Weeks et al. [66], 83% of between-laboratory discrepancies in scoring di-nucleotide microsatellites have been due to erroneous length attributions during the binning process.
With the aim of generating a complete database of identified and authenticated cultivars from the Mediterranean Basin by analyzing the world’s largest collections, here for both WOGBC and WOGBM, we applied an approach for the characterization, identification and authentication similar to that previously described by Trujillo et al. [21] on WOGBC. Using 33 selected SSR markers and 11 endocarp traits on 824 olive trees, representing 499 accessions from 21 countries, the latter authors identified 332 cultivars in which 200 were considered as authenticated cultivars, and a total of 537 profiles (based on SSR loci and endocarp descriptors) were revealed.
Here we sought answers to two main questions: (i) could pooling the two WOGBs increase the level of variability contained in the conserved genetic resources? and (ii) would pooling of the two WOGB collections have an impact on defining a core collection suitable for association mapping? We thus applied 20 SSR loci and 11 endocarp traits in order: (i) to characterize, identify and authenticate olive cultivars within both WOGBs, (ii) to establish one consensus olive dataset for both WOGBs using SSR markers and morphological traits to manage, use and exchange plant material, (iii) to propose a subset of cultivars encompassing all of the genetic diversity in both collections, and (iv) to release information and methodologies that could be used for characterizing other national and international ex-situ olive collections [14–17,47,49,67].
Material & methods
Plant material
The study was carried out using 554 accessions from WOGBM, identified by single codes, corresponding to 486 denominations from 14 countries (Table 1 and S1 Table). For WOGBC, a total of 537 olive trees with different profiles, as identified by Trujillo et al. [21], corresponding to 499 accessions and 405 denominations from 21 countries, was used (Table 1 and S1 Table). These 537 profiles were identified based on a total of 824 olive trees using 33 SSR markers and 11 endocarp traits [21].
Table 1. Number of olive accessions compared per country in both collections and the number of genotypes including variants and cultivars that were identified and authenticated.
WOGBM (M), WOGBC (C), Total (T), shared between both collections (Both).
| Origin | No. of trees | No. of accessions | No. of genotypes1 | No. of identified cultivars2 | No. of authentic cultivars3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | C | T | M | C | T | Botha()b | M | C | T | Both | M | C | T | Both | M | C | T | Both | ||
| 1 | Albania | 13 | 13 | 12 | 12 | 1 | 11 | 11 | 1 | 10 | 10 | 3 | 3 | |||||||
| 2 | Argentina | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |||||||||||
| 3 | Algeria | 43 | 3 | 46 | 43 | 2 | 45 | 2(4) | 27 | 1 | 27 | 1 | 26 | 1 | 26 | 1 | 1 | 1 | 1 | 1 |
| 4 | Chile | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||
| 5 | Cyprus | 31 | 3 | 34 | 31 | 3 | 34 | 3(9) | 4 | 2 | 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 6 | Croatia (HRV) | 16 | 7 | 23 | 16 | 7 | 23 | 4(9) | 10 | 7 | 14 | 4 | 9 | 7 | 13 | 3 | 3 | 5 | 5 | 3 |
| 7 | Egypt | 19 | 5 | 24 | 19 | 5 | 24 | 4(8) | 17 | 3 | 20 | 17 | 3 | 20 | 1 | 1 | ||||
| 8 | France | 13 | 13 | 26 | 13 | 10 | 23 | 8(18) | 9 | 10 | 13 | 6 | 8 | 8 | 11 | 5 | 5 | 6 | 6 | 5 |
| 9 | Greece | 17 | 20 | 37 | 17 | 18 | 35 | 7(15) | 14 | 17 | 26 | 5 | 13 | 15 | 22 | 6 | 6 | 11 | 11 | 6 |
| 10 | Iran | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||||||
| 11 | Israel | 9 | 9 | 9 | 9 | 3 | 3 | 3 | 3 | 2 | 2 | |||||||||
| 12 | Italy | 163 | 40 | 203 | 163 | 36 | 199 | 16(47) | 128 | 30 | 146 | 12 | 92 | 20 | 100 | 12 | 12 | 17 | 17 | 12 |
| 13 | Lebanon | 16 | 2 | 18 | 16 | 2 | 18 | 2(13) | 11 | 2 | 13 | 3 | 4 | 1 | 4 | 1 | 1 | 1 | 1 | 1 |
| 14 | Mexico | 7 | 7 | 7 | 7 | 2 | 2 | 2 | 2 | |||||||||||
| 15 | Morocco | 27 | 4 | 31 | 27 | 4 | 31 | 3(8) | 11 | 3 | 12 | 2 | 10 | 1 | 10 | 1 | 1 | 1 | 1 | 1 |
| 16 | Portugal | 15 | 11 | 26 | 15 | 10 | 25 | 7(14) | 10 | 8 | 14 | 5 | 10 | 6 | 12 | 4 | 4 | 4 | 4 | 4 |
| 17 | Slovenia | 10 | 10 | 10 | 10 | 1(3) | 3 | 1 | 3 | 1 | 3 | 3 | ||||||||
| 18 | Spain | 89 | 298 | 387 | 89 | 279 | 368 | 91(220) | 100 | 232 | 247 | 85 | 86 | 186 | 191 | 81 | 81 | 136 | 136 | 81 |
| 19 | Syria | 70 | 61 | 131 | 70 | 56 | 126 | 24(67) | 42 | 42 | 80 | 5 | 35 | 37 | 64 | 8 | 2 | 2 | 2 | 2 |
| 20 | Tunisia | 25 | 7 | 32 | 25 | 7 | 32 | 6(13) | 16 | 6 | 19 | 3 | 14 | 6 | 18 | 2 | 2 | 5 | 6 | 2 |
| 21 | Turkey | 20 | 20 | 19 | 19 | 2 | 17 | 17 | 2 | 1 | 15 | 15 | 1 | 1 | 8 | 8 | 1 | |||
| 22 | USA | 4 | 4 | 4 | 4 | 2 | 2 | 2 | 2 | |||||||||||
| 23 | Unkown | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| Total | 554 | 537 | 1091 | 554 | 499 | 1053 | 178(448) | 402 | 400 | 672 | 130 | 329 | 332 | 535 | 126 | 120 | 210 | 211 | 120 | |
1based on 20 SSR loci.
2based on both SSR loci and endocarp traits.
3 based on comparison with WOGBC.
aNumber of similar denominations in both collections.
bNumber of accessions with similar names in both collections.
Characterization of WOGBM and WOGBC
SSR genotyping of WOGBM
For the 554 WOGBM accessions, total DNA was extracted from 1 g young leaves, as described in Khadari et al. [30]. DNA quality was checked on 0.8% agarose gel, whereas the DNA concentration was estimated using spectrofluorometry (GENios Plus, TECAN, Grödig, Austria).
Twenty SSR loci were used in this study: DCA01, DCA03, DCA04, DCA05, DCA08, DCA09, DCA10, DCA11, DCA15, DCA16 and DCA18 [68]; GAPU59, GAPU71A, GAPU71B, GAPU101 and GAPU103A [69]; UDO99-11, UDO99-17 and UDO99-43 [28] and EMO90 [70]. These markers were selected based on their clear amplification, high polymorphism and reproducibility, as observed by Trujillo et al. [21] and El Bakkali et al. [23]. PCR amplification was carried out in a total volume of 20 μl containing 20 ng of genomic DNA, 1x PCR buffer, 1.5 mM MgCl2, 0.2 M of each dNTP, 0.1 U of Taq DNA polymerase, and 2 pmol of forward (fluorescent labelled) and reverse primers. PCR reactions were performed in a thermal cycler (Mastercycler ep gradient S) at 94°C for 5 min, followed by 35 denaturation cycles at 94°C for 30 s, 50, 55 or 57°C for 1 min for annealing, depending on the locus, and 72°C extension for 1 min. A post-thermocycling 10 min extension at 72°C was carried out. PCR products were separated using an automatic capillary sequencer (ABI prism 3130XL Genetic Analyzer Applied Biosystems, Foster City, CA, USA), using GeneScan 400 HD-Rox as internal standard, and chromatograms were then visualized and analyzed with GeneMapper 3.7 software (Applied Biosystems).
Aligning SSR alleles of WOGBM and WOGBC databases
To align alleles between the two collections, 47 accessions from WOGBC already analyzed by Trujillo et al. [21] were re-genotyped with the 20 SSR loci in laboratory conditions similar to those used for genotyping and visualizing WOGBM accessions (S2 Table). A total of 407 alleles among a set of 466 alleles (87.3%) were observed within this panel of 47 accessions using 33 SSR markers from the previous study of Trujillo et al. [21]. Once the 47 accessions were genotyped, the sizes of alleles observed in WOGBM were adjusted to match those recorded by Trujillo et al. [21] in WOGBC (S2 Table).
Morphological characterization
Morphological characterization was independently carried out by two observers using a representative sample of 40 endocarps/tree for 518 olive trees among the 554 analyzed with SSR markers. Each tree was analyzed twice during 2015 and 2016. Based on the protocol described by Trujillo et al. [21], we used eleven endocarp traits to characterize WOGBM and to compare the morphological datasets of both collections: weight, shape in position A, symmetry in positions A and B, position of maximum transverse diameter in position B, shape of apex in position A, shape of base in position A, surface roughness, number of grooves on the basal end, distribution of the grooves on the basal end and presence of mucro.
Data analysis
Total genotypes detected within the whole dataset (1091 olive trees) were discriminated by pairwise comparison of their SSR profiles using the Excel Microsatellite Toolkit [71]. Genetic diversity in each collection separately and in the whole dataset (both collections) was estimated by calculating different parameters for each microsatellite locus using the Excel Microsatellite Toolkit, including: allele size (bp), number of alleles (Na), number of alleles observed once (Nu), observed (Ho) and expected heterozygosity (He; [72]) and polymorphism information content (PIC; [73]). Otherwise, pairwise comparisons between samples based on endocarp traits were conducted to identify similar morphological profiles using a binary matrix of different morphological states.
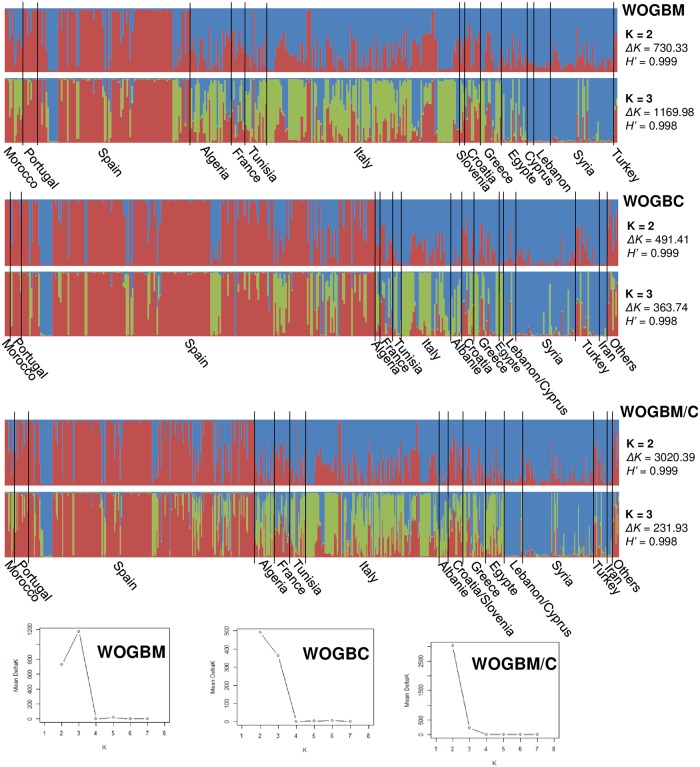
Genetic structure in the whole dataset and for each collection was determined using a model-based clustering method implemented in Structure v.2.3.4 [74]. Bayesian analysis was run under the admixture model for a burn-in period of 200,000 iterations and a post-burning simulation length of 1,000,000 while assuming a correlation among allele frequencies. Analyses were run for K clusters from 1 to 8 with 10 replicates per K value. The most likely number of clusters was determined using the ad-hoc ΔK measure [75] with the R program [76], whereas the similarity index between the 10 replicates for the same K clusters (H′) was calculated with the Clumpp v1.1.2 program (Greedy algorithm [77]).
To describe the spatial distribution of genotypes, a principal coordinate analysis (PCoA), as implemented in the DARwin v.5.0.137 program [78], was constructed based on SSR data with the simple matching coefficient [79]. For genotypes showing molecular variants in two collections, SSR data were converted into binary matrix (0 and 1) and the dendrogram was generated using the Dice similarity index [80] and UPGMA method with NTSys-PC v2.02 software [81].
Comparisons between the two collections were carried out based on: (1) the accession denomination, (2) number of genotypes and cultivars in common, (3) genetic parameters such as number of alleles (Na) and Nei diversity index (He), (4) the genetic distance distribution between shared genotypes and those specific to each collection using the Smouse and Peakall index [82] in Genalex 6.5 [83], (5) the allelic richness (Ar [84]); and (6) the genetic structure within both collections using the model-based Bayesian clustering approach implemented in Structure. The allelic richness (Ar) was computed according to a generalized rarefaction approach at the standardized G value using the ADZE program [85]. Significant differences in rarefied Ar and He were determined using the Mann-Whitney comparison test (p≤0.05) with the Past program [86].
Core collections representative of the genetic diversity in the whole dataset were constructed based on the two-step method, as described by El Bakkali et al. [23], by combining approaches implemented in Mstrat [87] and Core Hunter [88] programs, and using Maximization [89] and ‘Sh’ strategies, respectively. Fifty final core collections were generated independently and one core collection was arbitrary selected and described.
Results
Characterization of WOGBM
SSR polymorphism
Using the 20 SSR loci, a total of 370 alleles were observed within the WOGBM collection among which 49 alleles were just observed once. These alleles were checked by re-amplification to determine their correct SSR profiles. The number of alleles ranged from 6 for DCA15 to 35 for DCA10, with a mean of 18.5 alleles per locus (Table 2). Allele frequencies ranged from 0.09% to 70.5%, while 203 alleles (54.8%) showed a frequency of less than 1%.
Table 2. Summary of genetic diversity parameters of 20 SSR markers observed in the WOGBM and WOGBC collections.
Number of alleles (Na), number of alleles observed once (Nu), allelic richness (Ar), expected (He) and observed (Ho) heterozygosity, and polymorphic information content (PIC).
| WOGBM | WOGBC | Whole dataset | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (bp) | Na | Nu | Ar1 | Ho | He | PIC | Size (bp) | Na | Nu | Ar1 | Ho | He | PIC | Size (bp) | Na | Na2 | Nu | Nu2 | Ho | He | PIC | |
| DCA01 | 204–274 | 21 | 7 | 15.7 | 0.734 | 0.622 | 0.574 | 204–274 | 14 | 3 | 11.1 | 0.787 | 0.624 | 0.565 | 204–274 | 21 | 14 | 3 | 6 | 0.760 | 0.624 | 0.571 |
| DCA03 | 227–255 | 13 | 2 | 11.6 | 0.910 | 0.854 | 0.836 | 227–255 | 15 | 1 | 13.7 | 0.937 | 0.843 | 0.823 | 227–255 | 15 | 13 | 2 | 0.923 | 0.850 | 0.832 | |
| DCA04 | 116–198 | 32 | 2 | 28.6 | 0.621 | 0.848 | 0.831 | 116–198 | 28 | 1 | 24.5 | 0.630 | 0.801 | 0.782 | 116–198 | 35 | 25 | 3 | 0.625 | 0.829 | 0.811 | |
| DCA05 | 191–213 | 12 | 11.3 | 0.498 | 0.489 | 0.473 | 191–211 | 10 | 9.9 | 0.378 | 0.387 | 0.376 | 191–213 | 12 | 10 | 0.439 | 0.440 | 0.428 | ||||
| DCA08 | 123–163 | 21 | 3 | 18.3 | 0.764 | 0.840 | 0.819 | 123–168 | 18 | 2 | 16.0 | 0.597 | 0.801 | 0.773 | 123–168 | 23 | 16 | 3 | 1 | 0.684 | 0.826 | 0.804 |
| DCA09 | 160–218 | 25 | 2 | 23.1 | 0.946 | 0.878 | 0.865 | 160–214 | 22 | 2 | 20.4 | 0.955 | 0.863 | 0.847 | 160–218 | 26 | 21 | 2 | 1 | 0.951 | 0.877 | 0.860 |
| DCA10 | 138–263 | 35 | 3 | 31.0 | 0.309 | 0.857 | 0.845 | 138–260 | 36 | 3 | 31.4 | 0.229 | 0.800 | 0.783 | 138–263 | 41 | 30 | 3 | 3 | 0.271 | 0.833 | 0.820 |
| DCA11 | 126–182 | 23 | 21.0 | 0.914 | 0.827 | 0.804 | 126–185 | 26 | 4 | 22.1 | 0.922 | 0.810 | 0.783 | 126–185 | 26 | 23 | 2 | 2 | 0.918 | 0.819 | 0.795 | |
| DCA15 | 243–267 | 6 | 5.5 | 0.432 | 0.569 | 0.518 | 243–267 | 7 | 1 | 6.2 | 0.261 | 0.524 | 0.469 | 243–267 | 7 | 6 | 1 | 0.348 | 0.550 | 0.499 | ||
| DCA16 | 122–230 | 34 | 9 | 26.9 | 0.960 | 0.867 | 0.852 | 122–228 | 32 | 10 | 24.3 | 0.965 | 0.850 | 0.832 | 122–230 | 39 | 27 | 6 | 10 | 0.962 | 0.861 | 0.845 |
| DCA18 | 154–189 | 17 | 2 | 15.6 | 0.865 | 0.817 | 0.797 | 158–193 | 16 | 1 | 15.0 | 0.927 | 0.820 | 0.796 | 154–193 | 19 | 14 | 3 | 0.896 | 0.823 | 0.801 | |
| EMO90 | 181–208 | 9 | 8.6 | 0.739 | 0.675 | 0.639 | 181–208 | 10 | 2 | 9.0 | 0.684 | 0.637 | 0.600 | 181–208 | 10 | 9 | 1 | 1 | 0.712 | 0.659 | 0.624 | |
| GAPU59 | 206–239 | 11 | 3 | 9.3 | 0.626 | 0.621 | 0.579 | 194–226 | 10 | 2 | 8.8 | 0.637 | 0.608 | 0.565 | 194–239 | 13 | 8 | 4 | 1 | 0.631 | 0.616 | 0.574 |
| GAPU71A | 206–256 | 16 | 6 | 11.4 | 0.571 | 0.473 | 0.422 | 206–246 | 10 | 3 | 7.8 | 0.471 | 0.430 | 0.372 | 206–256 | 16 | 10 | 3 | 4 | 0.522 | 0.452 | 0.398 |
| GAPU 71B | 118–147 | 9 | 8.4 | 0.899 | 0.803 | 0.772 | 118–147 | 9 | 8.4 | 0.940 | 0.801 | 0.770 | 118–147 | 10 | 8 | 0.919 | 0.803 | 0.773 | ||||
| GAPU101 | 183–219 | 13 | 1 | 11.8 | 0.948 | 0.852 | 0.834 | 183–219 | 13 | 1 | 12.0 | 0.979 | 0.833 | 0.810 | 183–219 | 14 | 12 | 1 | 1 | 0.963 | 0.845 | 0.826 |
| GAPU103A | 133–194 | 26 | 5 | 21.3 | 0.820 | 0.849 | 0.832 | 133–208 | 26 | 4 | 22.3 | 0.734 | 0.814 | 0.789 | 133–208 | 30 | 22 | 5 | 3 | 0.777 | 0.836 | 0.817 |
| UDO99-11 | 103–140 | 14 | 1 | 12.6 | 0.973 | 0.849 | 0.831 | 103–142 | 14 | 3 | 12.0 | 0.952 | 0.830 | 0.809 | 103–142 | 16 | 12 | 2 | 1 | 0.962 | 0.842 | 0.823 |
| UDO99-17 | 152–173 | 7 | 7.0 | 0.775 | 0.782 | 0.749 | 152–173 | 6 | 6.0 | 0.775 | 0.785 | 0.751 | 152–173 | 7 | 6 | 0.775 | 0.784 | 0.751 | ||||
| UDO99-43 | 166–225 | 26 | 3 | 23.0 | 0.870 | 0.876 | 0.863 | 162–225 | 24 | 1 | 21.9 | 0.898 | 0.868 | 0.854 | 162–225 | 27 | 23 | 1 | 2 | 0.883 | 0.875 | 0.862 |
| Mean | 18.50 | 2.45 | 16.1a | 0.758 | 0.762b | 0.737 | 17.30 | 2.20 | 15.1a | 0.732 | 0.736 b | 0.707 | 20.35 | 15.45 | 2.15 | 0.746 | 0.752 | 0.725 | ||||
| Total | 370 | 49 | 321 | 346 | 44 | 302 | 407 | 309 | 43 | 38 | ||||||||||||
1Computed at G value of 400. No significant difference between both collections (Mann-Whitney test, p-value>0.05).
2Shared alleles between both collections.
a,bIndex of significance at p-value < 0.05.
The observed heterozygosity (Ho) ranged from 0.309 (DCA10) to 0.973 (UDO-11), with a mean of 0.758. He ranged from 0.473 (GAPU-71A) to 0.878 (DCA09) with a mean of 0.762. Eighteen markers among the 20 had a PIC of above 0.5 (Table 2).
Cultivar identification using SSR markers and morphological traits
The 20 SSR markers identified 402 unique genotypes among the 554 WOGM accessions with at least one dissimilar allele (Table 1). In line with the findings of Trujillo et al. [21], each genotype was coded with an ordinal number (S1 and S3 Tables). The 554 accessions were classified as follows: (i) 323 accessions were identified as unique SSR profiles (not duplicated in WOGBM), and (ii) 231 accessions had SSR profiles in common with other accessions in the collection, resulting in the identification of 79 different SSR profiles.
Three SSR markers (DCA09, UDO099-043 and DCA16) were able to identify 84% of the accessions. Six markers (DCA09, UDO099-043, DCA16, DCA10, DCA04 and GAPU103) were used to discriminate 95% of the accessions, whereas eight additional markers (DCA03, DCA08, DCA18, GAPU71B, DCA11, UDO099-011, DCA01 and DCA15) were applied to distinguish all WOGBM accessions.
Based on endocarp traits, a total of 251 different morphological profiles were identified that were coded with an ordinal number (S1 and S4 Tables). The accessions were classified as follows: (i) 164 accessions had unique morphological profiles (not duplicated in WOGBM), and (ii) 354 accessions had morphological profiles in common with other accessions in the collection, resulting in the identification of 87 different morphological profiles.
Then 329 different olive cultivars were identified using 20 SSR markers and 11 endocarp traits (Table 1; S1 and S4 Tables). SSR markers were used as the main criteria for cultivar identification for the 36 accessions with no endocarp trait data (S1 Table). Among all genotype pairs in WOGBM, only 5 were observed which had identical SSR profiles but distinct morphological profiles: “Fouji vert” (MAR00291)/“Besbessi” (MAR00464), “Lechin de Sevilla” (MAR00243)/“Zarza” (MAR00280), “Beladi” (MAR00572)/“Beladi Aitaroun” (MAR00577), “Olivastra di Montalcino” (MAR00375)/“Mortellino” (MAR00373) and “Beldi” (MAR00288)/“Giarfara” (MAR00106; S1 Table).
Characterization of WOGBC
SSR polymorphism
A total of 346 alleles were observed in WOGBC, with a mean of 17.3 alleles per locus, while 189 of these alleles (54.62%) showed a frequency of less than 1%, and 44 alleles were just observed once (Table 2). The allele number ranged from 6 for UDO99-17 to 36 for DCA10 loci.
Ho ranged from 0.229 (DCA10) to 0.979 (GAPU101), with a mean of 0.732. He ranged from 0.387 (DCA05) to 0.868 (UDO099-43), with a mean of 0.736. Seventeen among the 20 markers had a PIC of above 0.5 (Table 2).
Cultivar identification using SSR markers and morphological traits
Using 20 SSR markers, the 537 WOGBC profiles were classified in 400 different genotypes coded with an ordinal number, as reported by Trujillo et al. [21] (S1 and S3 Tables). Only 15 SSR profiles were switched using the current set of markers compared to 33 analyzed by Trujillo et al. [21] (S1 Table). For instance, Trujillo et al. [21] identified 239 different genotypes originating from Spain whereas the 20 SSR markers only revealed 232 genotypes in the current analysis. Furthermore, the “Alameño de Marchena” (COR000254) and “Zarza” (COR000038) accessions were identified by Trujillo et al. [21] as being different from “Picholine Marocaine” and “Lechín de Sevilla”, respectively. They had two dissimilar alleles at GAPU82 and UDO-42 loci that were not taken into account in the current set of markers for the first accession, and at UDO-05 for the second. Otherwise, among the 400 different genotypes, 320 were identified as unique SSR profiles (not duplicated) whereas 217 accessions had SSR profiles in common, resulting in the identification of 80 different SSR profiles.
Based only on endocarp traits, a total of 245 different morphological profiles were identified in WOGBC (S1 and S4 Tables). No morphological data were available for 31 accessions. The 506 olive trees were classified as follows: (i) 147 olive trees had unique morphological profiles (not duplicated in WOGBC), and (ii) 359 had morphological profiles in common with other accessions in the collection, resulting in the identification of 98 different morphological profiles. Each of the following eight pairs of accessions showed similar or nearly identical SSR profiles but presented morphological differences: “Zarza” (COR000038)/“Lechin de Sevilla” (COR000005), “Menya” (COR000669)/“Manzanilla Picua” (COR000377)/“Menya de Reus” (COR001071), “Chemlali” (COR000744)/“Chetoui” (COR000113), “Gatuno” (COR000380)/“Abbadi Abou Gabra” (COR001033), “Pulazeqin” (COR001085)/“Itrana” (COR000068), “Azulejo” (COR000959)/“Negrinha” (COR000123), “Dulzal de Carmona” (COR000031)/“Imperial de Jaén” (COR000030) and “Jabaluna” (COR000392)/“Escarabajuelo de Úbeda” (COR000353). By combining both SSR and morphological data, a total of 332 cultivars were identified in WOGBC (Table 1).
Comparisons between the two WOGBs
Based on accession denominations
When focusing on the accession denominations, 713 accession names in both collections were listed, corresponding to 1037 accessions, i.e. 495 in WOGBC and 542 in WOGBM, while 16 accessions had no denominations and were assigned codes such as “Unkown-OMDZ” (MAR00536) and “Klon-14” (COR001081). Among the 713 denominations, 178 that were found to be common in both collections corresponded to a total of 448 accessions, i.e. 207 observed only in WOGBM and 241 in WOGBC (Table 1). A total of 308 denominations (335 accessions) and 227 (254 accessions) were specific to WOGBM and WOGBC, respectively. The highest number of shared denominations was observed in Spanish germplasm (91; 51%). The other accessions were classified as follows: 1 from Slovenia, 2 (Algeria and Lebanon), 3 (Cyprus and Morocco), 4 (Egypt and Croatia), 6 (Tunisia), 7 (Greece and Portugal), 8 (France), 16 (Italy) and 24 (Syria; Table 1).
Based on SSR markers and morphological traits
In the combined WOGBM and WOGBC dataset, 407 alleles were revealed using 20 SSR markers, with a mean of 20.35 alleles per locus. Among the 407 alleles, 309 alleles were in common, whereas 61 and 37 alleles were specific to WOGBM and WOGBC, respectively (Table 2). A total of 43 unique alleles (observed once) were detected in 54 genotypes in both collections (27 in WOGBM, 21 in WOGBC and 6 in common). No significant difference was observed between the two collections regarding the allelic richness, computed at a G value of 400, and the diversity index (He; Mann-Whitney test p-value > 0.05; Table 2).
Similar pairwise genotype patterns were observed in both WOGBs (S1 Fig). In both collections, only 1054 (0.68%) and 610 (0.42%) pairwise comparisons, for WOGBM and WOGBC, respectively, represented closely related genotypes that differed by one to four dissimilar alleles, whereas the remaining pairwise genotypes were distinguished by 5 to 39 dissimilar alleles. The highest SSR dissimilarity (39 distinct SSR alleles) was observed in only two genotype pairs in WOGBM and six pairs in WOGBC.
The analysis of both datasets (1091 olive trees) revealed 672 different SSR profiles. Three SSR markers (DCA09, DCA16 and UDO099-043) were able to distinguish 77% of the identified genotypes, whereas six markers (DCA04, DCA09, DCA10, DCA16, UDO099-043 and GAPU103) could be applied to discriminate 94% of the total identified genotypes. The 672 genotypes were classified as: (i) 130 SSR profiles observed in common between the two collections, with a total of 436 accessions (213 and 223 for WOGBM and WOGBC, respectively), and (ii) 542 genotypes were specific to WOGBC (270) or WOGBM (272), corresponding to 655 accessions (341 and 314 for WOGBM and WOGBC, respectively; Tables 1 and 3, S1 and S3 Tables). The highest number of genotypes in common was identified within Spanish germplasm with 85 genotypes, followed by 12 within Italian germplasm (Table 1).
Table 3. Comparison of genotypes shared between the two collections and those specific to each one.
The number of alleles (Na), allelic richness (Ar) and index of diversity (He).
| Group of genotypes | Size of genotypes | N. olive trees | Na (%)1 | Ar2 | He3 | |
|---|---|---|---|---|---|---|
| WOGBM | WOGBC | |||||
| Shared between collections | 130 | 213 | 223 | 233 (57.2%) | 9.7a | 0.724a |
| Specific to WOGBM | 272 | 341 | 358 (87.9%) | 12.7a | 0.774b | |
| Specific to WOGBC | 270 | 314 | 329 (80.8%) | 11.5a | 0.746ab | |
| Mean | 20.35 | 0.758 | ||||
| Total | 672 | 554 | 537 | 407 | ||
1 Compared to total number of alleles in both collections (407 alleles).
2 Computed at G value of 130, no significant difference between both collections (Mann-Whitney comparison test, p-value >0.05).
3 Significant difference between genotypes in common and those specific to WOGBM (Mann-Whitney comparison test, p-value <0.05).
a, b Index of significance at p-value < 0.05.
We identified two genotypes originating from Turkey and one from Albania which were not represented in WOGBM (Table 1). Genotypes related to the “Gemlik” (COR000092) and “Samsun Tuzlamalik” (COR000684) accessions from Turkey in WOGBC were actually identified as being similar to “Safrawi” (MAR00608) and “Ayrouni” (MAR00633)/“Kfar Zita” (MAR00609), respectively. Similarly, some accessions in WOGBM were found to be close or similar to some accessions from different countries in WOGBC. For instance, “Dahbia” (MAR00391-Morocco)/“Callosina” (COR000040, COR000060-Spain), “Bouchouk Rkike” (MAR00396-Morocco)/“Ocal” (COR000282-Spain), “Stanboli” (MAR00624-Syria)/“Gordal de Granada” (COR000761-Spain), “Throumbolia” (MAR00184-Greece)/“Grossolana” (MAR0044-Italy)/“Cirujal” (COR000963-Spain) and “Verdial Alentejana” (MAR00213-Portugal)/“Verdial de Huévar” (COR000155-Spain; S1 Table).
In the whole dataset of 672 genotypes, we identified 8 genotypes from different countries which were duplicated. When focusing on these 8 genotypes, the identified discrepancy included: (i) four cases observed only in WOGBC, e.g. “Abbadi Abou Gabra” (COR001033-Syria)/“Gatuno” (COR000380-Spain), “Frantoio A. Corsini” (COR000081-Italy)/“Kokerrmadh Berati” (COR001080-Albania), “Ayrouni” (COR000134-Lebanon)/“Verdial de Huévar” (COR000006-Spain) and “Adramitini” (COR000102-Greece)/“Ayvalik” (COR001474-Turkey); and (ii) four genotypes were shared between the two collections, including “Cordovil de Castelo Branço” (COR000886-Portugal)/“Llorón de Iznalloz” (MAR00342, COR000400-Spain), “Itrana” (MAR0017, COR000068-Italy)/“Pulazeqin” (COR001085-Albania), “Crnica” (COR000734-Croatia)/“Crnica” (MAR00399-Slovenia) and “Analiontas” (MAR00306-Cyprus)/“Sourani Red” (COR001478-Syria; S1 Table).
A total of 233 alleles were observed within the common 130 genotypes, with a mean of 11.65 alleles per locus, whereas 358 and 329 alleles were observed within genotypes specific to WOGBM (272) and WOGBC (270), respectively (Table 3 and S5 Table). A similar genetic distance distribution was observed within the three groups, while being shared and specific to each collection (S2 Fig). The PCoA plot revealed an even distribution of the shared genotypes along both axes accounting for 12.34% of the total genetic variation, whereas those originating from Spain were clustered (S3 Fig). No significant difference in allele number was observed between common genotypes and those specific to each collection regarding allelic richness (Mann-Whitney comparison test; p>0.05). However, a significant difference was observed for He (diversity index) between common genotypes and those specific to WOGBM (p<0.05; Table 3 and S5 Table), while no significant difference was revealed for the He diversity index regarding genotypes specific to each collection.
Based only on endocarp traits, a total of 371 different morphological profiles were identified in both collections (S1 and S4 Tables). Among these accessions: (i) 158 accessions were classified as 120 unique morphological profiles in WOGBC; (ii) 204 accessions as 126 unique morphological profiles in WOGBM; and (iii) 662 accessions, including 348 in WOGBC and 314 in WOGBM, as shared profiles with other accessions in both collections, resulting in the identification of 125 different morphological profiles.
Based on genetic structure
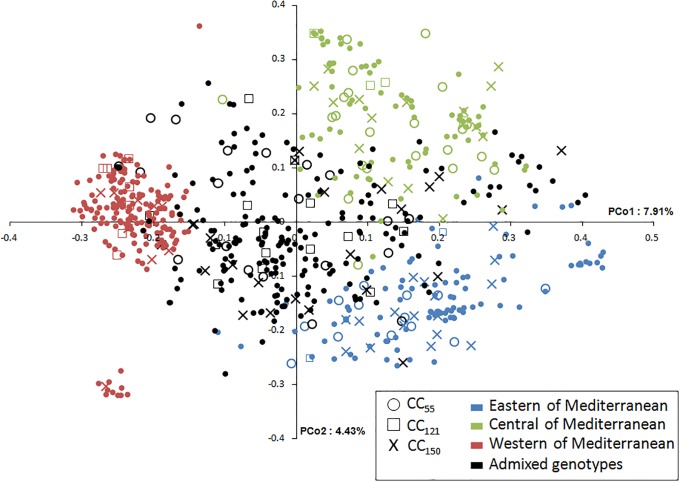
Based on the whole dataset (672) and data for each collection; 402 for WOGBM and 400 for WOGBC, the genetic structure was examined under the models with K = 2 to 8 clusters. According to ΔK and H’, K = 3 were revealed as being the most probable genetic structure model for WOGBM, as previously reported by El Bakkali et al. [23] (ΔK = 1169.98 and H’ = 0.998), whereas for WOGBC, K = 2 followed by K = 3 were revealed as being the most probable genetic structure models (Fig 1). With all 672 genotypes, the K = 2 model was revealed as being the most probable genetic structure model (ΔK = 3020.39 and H’ = 0.999) followed by K = 3 clusters (ΔK = 231.93 and H’ = 0.999; Fig 1). Most olive genotypes from Morocco, Portugal and Spain were distinguished from other Mediterranean olives, whereas genotypes from France, Algeria, Albania, Tunisia, Italy, Slovenia, Croatia, Greece and Egypt were mostly assigned as a second cluster (named central Mediterranean) distinguished from the third cluster, which included genotypes from the eastern Mediterranean region. A clear distinction between the three clusters was observed when plotting the 672 genotypes, with membership probabilities of Q< 0.8, using PCoA (Fig 2).
Fig 1. Inferred structure for K = 2 and 3 within the unique genotypes of the WOGBM (402 genotypes) and WOGBC (400 genotypes) collections and total datasets (672 genotypes; WOGBM/C).
H’ represents the similarity coefficient between runs, whereas ΔK represents the ad-hoc measure of Evanno et al. [75].
Fig 2. Two-dimensional distribution of the principal coordinate analysis (PCoA) for both collections and nested core collection of 55, 121 and 150 sample sizes based on SSR markers.
Colors indicated the three gene pools with membership probabilities of Q> 0.8 (eastern, western and central Mediterranean Basin). Nested core collections span the range of all the genotypes among the three gene pools.
Cultivar identification process in both WOGBs
Cultivar identification in WOGBM was carried out using nuclear markers and morphological traits as a complement. For accessions with no endocarp trait data, SSR markers were used as the main criteria to identify cultivars and therefore the cultivar identification process was suspended, as mentioned in S1 Table (pending identification). We defined individual cultivars as having accessions with the same molecular and morphological profiles. Molecular variants detected for any identified cultivar were considered in case of minimal differences in SSR profiles between accessions (1 to 4 mismatched alleles) despite similar morphological profiles. We confirmed the varieties authenticated by Trujillo et al. [21] and propose a new set of authenticated varieties on the basis of their matching molecular and morphological profiles with varieties within WOGBC. Detected errors in WOGBM (or mislabeling errors) were considered if the accession did not match its putative cultivar identified in WOGBC and by considering the cultivar name and area of cultivation. Synonymous cases were considered if the identified cultivars displayed similar (or close) SSR and morphological profiles while having the same or close area of cultivation within and/or between collections. Similarly, homonymy cases were considered if the identified cultivars displayed different SSR and morphological profiles while also considering the area of cultivation within and/or between collections.
Cultivar identification and authentication processes
Using endocarp traits as a complement to SSR markers, a total of 535 cultivars were identified in both collections in which 126 cultivars were shared (Table 1 and S1 Table). In addition to the four cultivar pairs identified by Trujillo et al. [21] in WOGBC which showed an identical or nearly identical SSR profile but presented a distinct morphological profile, i.e. “Chemlali-744”/“Chetoui”, “Azulejo”/“Manzanilla Cacereña”, “Cordovil Castelo Blanco”/“Verdial de Badajoz” and “Zarza”/“Lechin de Sevilla”, we identified 8 other cultivar pairs showing similar patterns, i.e. “Crnica-399”/“Crnica”, “Ronde de la Ménara”/“Picholine Marocaine”, “Jabaluna”/“Escarabajuelo de Úbeda”, “Meski”/“Fouji vert”/“Besbessi”, “Oblica”/“Lumbardeska”, “Beldi”/“Giarfara”, “Gremigno di Fauglia”/“Caninese” and “Sourani Red”/“Beladi”/“Beladi-577” (S1 and S4 Tables). Otherwise, between-collection discrepancies were noted regarding the accession names and cultivar identification process. For instance, “Meski” cultivars from Tunisia, “Crnica” from Croatia and “Lentisca” from Spain in WOGBC were found to differ from those in WOGBM (S1 Table).
Finally, we were able to authenticate a total of 120 cultivars in WOGBM. Regarding WOGBC, 110 were previously authenticated from the panel of 200 cultivars described by Trujillo et al. [21], including “Picholine marocaine” from Morocco, “Amargoso” from Spain and “Zaity” from Syria (S1 and S4 Tables). Moreover, 10 new cultivars in WOGBM that were not previously authenticated by Trujillo et al. [21] were found to be authentic as they displayed SSR and morphological profiles similar to those in WOGBC. For instance, “Chemlal de Kabilye” cultivars from Algeria and “Chalkidikis” and “Kolybada” from Greece were authenticated in the current study as they showed similar SSR and morphological profiles in both collections (S1 and S4 Tables).
Plantation mislabeling and molecular variants
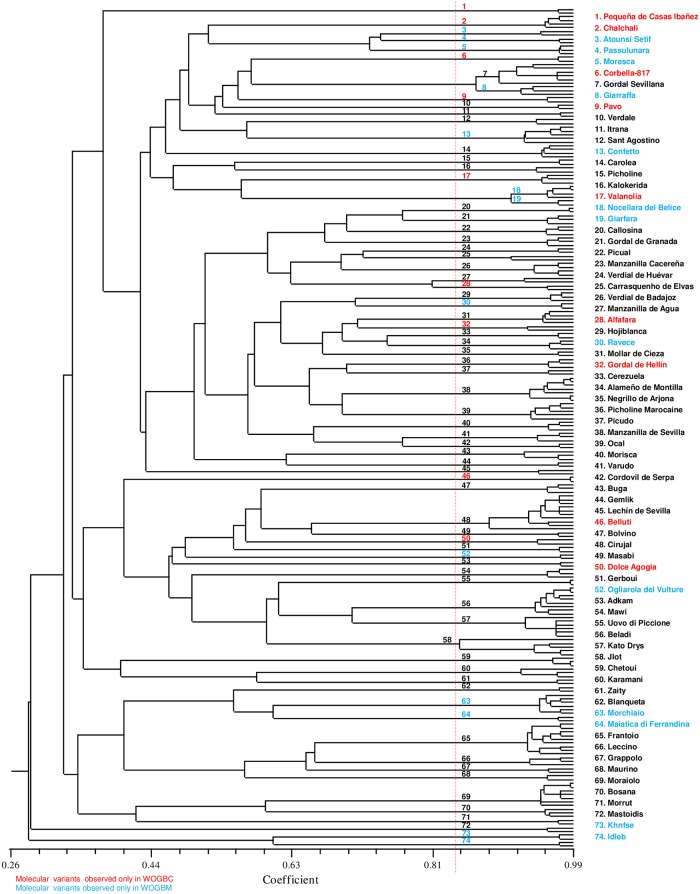
The identification process allowed identification of 79 cases of mislabeled plantations in WOGBM compared to 120 in WOGBC (S1 Table). For instance, “Chemlali” accessions from Tunisia (MAR00301 and MAR00296) were detected as plantation errors as they showed profiles similar to those of well-known “Koroneiki” and “Arbequina” cultivars, respectively, and different from the profile of “Chemlali” (COR000744) in WOGBC (S1 Table). Similarly, the “Varudo” (MAR00275) accession was identified as differing from its putative cultivar in WOGBC. Moreover, 228 genotypes were considered to be molecular variants of 74 cultivars (1 to 4 dissimilar alleles) because no endocarp morphological differences were observed between them (Fig 3, S6 Table). The highest number of cultivars showing molecular variants was observed for Spanish cultivars with 29 (92 SSR profiles) followed by Italian and Syrian cultivars with 20 (66 SSR profiles) and 9 cultivars (25 SSR profiles), respectively. These 74 cultivars with molecular variants were classified as follows: (i) 13 cultivars observed only in WOGBM with 37 SSR profiles, (ii) 9 cultivars observed only in WOGBC with 21 SSR profiles and (iii) 52 cultivars shared between the two collections with a total of 170 SSR profiles (Fig 3, S6 Table).
Fig 3. Dendrogram based on the UPGMA method and Dice similarity index of 74 different cultivars showing molecular variants.
Numbers indicate groups of molecular variants of each cultivar as indicated on the right. Colors indicate the collections in which molecular variants were observed; red color in WOGBC, blue color in WOGBM and black color sharing between the two collections.
Synonymy and homonymy cases
A total of 175 possible cases of synonyms among 78 cultivars were identified in this study. We counted 79 cases considered as newly observed in WOGBM and 96 previously described (S7 Table). For instance, a new synonymous group was identified that included four names for the “Picholine marocaine” cultivar formed by the “Hamrani” (MAR00534) accessions from Morocco, “Aghenfas” (MAR00523) and “Limli” (MAR00442) from Algeria and “Sinawy” (MAR00498) from Egypt, and a second group of four names for the “Frantoio” cultivar formed by the “Arancino” (MAR0028), “Puntino” (MAR0054) and “San Lazzaro” (MAR0065) accessions from Italy and “Cailletier” (MAR00190) from France (S7 Table). Moreover, 39 cases of homonyms in WOGBM and 36 in WOGBC were identified among a total of 60 in both collections, thus accounting for 179 identified cultivars, e.g. the “Lentisca” denomination, which included three different cultivars, including “Lentisca” and “Lentisca-244” from Spain and “Lentisca-206” from Portugal. Similarly, the “Corsicana” denomination included two cultivars from Italy, i.e. “Corsicana da Mensa” and “Corsicana da Olio” (S8 Table).
Core collection sampling
According to the two-step method proposed by El Bakkali et al. [23], 110 individuals (16.3%) were necessary to capture the 407 alleles in the whole dataset using the Mstrat program (672 genotypes). The Core Hunter program was run at 8.2% sample size (half of the initial sample size) using the ‘Sh strategy’ to sample a primary core collection (CC55; S9 Table). The 55 entries thus allowed capture of 328 alleles (80.6%), and only 7 genotypes in common between both collections, while the others were from either WOGBM (33 genotypes) or WOGBC (17). No genotype assigned to western gene pools was selected in the CC55 core collection (Table 4).
Table 4. Summary of the nested core collections at different sample sizes compared to the whole dataset.
Number of alleles (Na), Marrakech (M), Cordoba (C).
| N. genotypes | WOGB | Na | N. of SSR profiles | N. of morph. Profiles | N. of countries | Genetic clusters | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | C | M & C | Western1 | Central1 | Eastern1 | Admixture2 | |||||
| 55 | 33 | 15 | 7 | 328 | 55 | 49 | 11 | 22 | 13 | 20 | |
| 121* | 67 | 40 | 14 | 407 | 121 | 95 | 16 | 9 | 38 | 30 | 44 |
| 150 | 69 | 42 | 39 | 407 | 150 | 120 | 16 | 16 | 42 | 31 | 61 |
| 672 | 272 | 270 | 130 | 407 | 672 | 371 | 23 | 183 | 118 | 128 | 243 |
*One core collection was arbitrary selected among the 50 generated using Mstrat (S9 Table).
1as identified by Structure program with a membership probability of Q ≥ 0.8
2as identified by Structure program with a membership probability of Q < 0.8
The primary CC55 was used as a kernel with Mstrat to capture the remaining alleles. Hence, a total of 121 entries (CC121; 18%) were sufficient to capture the total diversity and 50 core collections with 121 entries were generated using Mstrat (S9 Table, S4 Fig). No differences in the Nei diversity index (He) were observed in 50 independent runs. In addition to the 55 genotypes used as a kernel, in all 50 independent runs 32 genotypes were found to be carrying unique alleles, while a combination of 34 complement genotypes could be selected among a panel of 131 genotypes to capture the total diversity (S9 Table). Moreover, 29 varieties which were not selected in the 50 repetitions generated were added, including the most cultivated ones, to account for the morphological trait variability. These 29 varieties could be added to CC121 as supplementary genotypes to propose a final core collection of 150 entries (CC150; S9 Table).
One core collection CC121 was arbitrarily selected in addition to the 29 cultivars added. The 150 genotypes sampled were found to belong to 16 different countries among the 23 analyzed herein (Table 4). Only 39 genotypes shared between the two WOGBs among the 150 genotypes of CC150 were selected while the others were either from WOGBM (69 genotypes) or WOGBC (42) and only 16 genotypes were assigned to western genetic clusters compared to 42 and 31 that were assigned to central and eastern genetic clusters, respectively. The selected 150 entries were plotted according to genetic cluster assignations using PCoA. The results revealed that the genotypes sampled spanned the entire range of genotypes in the whole dataset among the three genetic clusters (Fig 2).
Discussion
The main purpose of this study was to develop a consensus database for Mediterranean olive cultivars based jointly on molecular markers and endocarp traits to serve as an efficient tool for scientists conducting research on breeding and adaptation, as well as other users of local genetic resources. Germplasm banks are crucial for breeding programs as they offer variability in target agronomic traits and allelic variation in genes linked to these traits. Development of reference standards and a well-defined nomenclature system free of homonymy, synonymy and molecular variants is essential for making effective use of olive genetic resources. Here we discuss our results based on: (i) the efficiency of the joint use of molecular markers and endocarp descriptors for olive cultivar characterization; (ii) the relevance of available genetic resources in worldwide collections; and (iii) the importance of this information and of establishing a core collection for the scientific community and other users of olive genetic resources.
Combining SSR markers and endocarp descriptors to efficiently characterize olive cultivars
Many studies have revealed the efficiency of microsatellites markers for characterizing olive cultivars [14,17,21,22,51,52,63,64]. The 20 SSR markers used here were selected based on their high polymorphism, clear amplification and reproducible patterns, as reported by many authors [21–23,51,52]. Among the 33 SSR loci used by Trujillo et al. [21] for WOGBC genotyping, only 17 were able to discriminate the 411 identified genotypes, while 14 loci were in common with the 20 loci used in the current study. Moreover, eight of the 20 loci were used by Sarri et al. [51] to discriminate between 118 cultivars, whereas 11 were selected among 37 SSR loci by Baldoni et al. [52] as a consensus list of microsatellites recommended for genotyping 77 olive cultivars.
The 20 SSR markers used in the present study were able to discriminate 400 genotypes among the 411 genotypes identified in WOGBC using 33 SSR markers. The 11 genotypes not identified here corresponded to 15 olive accessions, most of which Trujillo et al. [21] considered were molecular variants and/or synonyms of well-known cultivars, e.g.“Alameño de Marchena” (COR000254) for “Picholine Marocaine”. Only two exceptions were noted for four different cultivars displaying distinct morphological traits: “Zarza” (COR000038)/“Lechin de Sevilla” (COR000005) and “Pulazeqin” (COR001085)/“Itrana” (COR000068), which were revealed to be similar based on 20 instead of 33 loci. We therefore noted that using a lower number of loci did not diminish the discrimination power as only 15 out of 537 profiles were grouped.
Characterization of large collections with a high number of loci is costly. Generating a subset of SSR markers able to discriminate all cultivars is thus essential to enable olive researchers to mine local olive diversity relative to known cultivars. The first step would be to eliminate duplicate samples, which could be done using a minimum of SSR loci. Here we aimed to develop a minimum SSR locus subset by exploring both collections. Among the 20 SSR loci used, we identified 3 and 6 markers that could discriminate 77% and 94% of 672 genotypes present in both WOGBs, respectively. Surprisingly, Trujillo et al. [21] proposed a set of 5 and 10 markers to distinguish 79% and 93% of cultivars present in WOGBC, respectively. By focusing on the 6 markers proposed here, five (UDO-43, DCA04, DCA09, DCA16, and GAPU103) were in common with the 10 proposed by Trujillo et al. [21], which was sufficient to distinguish 91.5% of genotypes (615 among 672). This set of five markers has been widely used in molecular characterization, including parentage analysis [53].
Molecular characterization was complemented by using 11 morphological descriptors related to endocarp traits in both collections. Endocarp traits are still considered to be the most discriminating and stable olive morphological traits as they are not highly influenced by environmental conditions, while being easily and quickly evaluated, even in the field. Moreover, the endocarp could be conserved in the long term and used as a reference. Endocarp descriptions have thus been widely used for olive cultivar identification and for clarifying domestication and diversification processes involving stones at archeological sites [26,27,90–93]. However, we used SSR genotyping for a first classification of the different genotypes complemented by considering the endocarp traits: (i) the discrimination power of SSR (672 molecular profiles) compared to endocarp traits (371 morphological profiles); (ii) scoring discrepancies regarding endocarp traits due to their qualitative features, which can lead to confusion and misclassification of cultivars between observers; and (iii) phenotypic changes due to genetic differences might be expressed in organs other than endocarp (fruit, leaves, etc.). However, endocarp traits are still very useful when considering genetically similar/close cultivars revealed by SSR markers, e.g. “Zara”/“Lechin de Sevilla”, “Azulejo”/“Manzanilla Cacereña”, “Fouji vert”/“Besbassi”, “Olivastra di Montalcino”/“Mortellino” and “Pulazeqin”/“Itrana”.
Molecular variants have been regularly reported in olive cultivars [21,28–31], relict wild olive trees in the Hoggar Mountains [94] and even in other fruit crops such as grapevine [31,95] and fig [32]. This slight allelic variation has been noted in olive cultivars that are widely grown in several olive growing areas, e.g. “Frantoio” (Italy), “Picholine Marocaine” (Morocco) and “Picual” (Spain). This has also been observed for ancient cultivars grown during several periods throughout history, e.g. “Picholine Marocaine” [30,96], “Cobrançosa” (Portugal) [29], “Manzanilla de Sevilla” (Spain) [97], “Gemlik” (Turkey) [98] and “Carolea” (Italy) [99]. These cultivars have undergone massive clonal propagation on a spatial or temporal scale, or both. It can thus be assumed that the massive clonal propagation process can lead to slight allelic variations, especially in the case of SSR markers. Indeed, these markers are considered to be mutational hotspots due to the presence of short repetitive units [100], and most variations occur at loci with dinucleotides and abundant GA repeat units which are more susceptible to mutations and slippage [101]. Hence, 10 amongst the 17 loci showing intra-varietal molecular variations were noted for SSR loci with abundant GA repeat units and in cultivars which are massively clonally propagated (Fig 3, S10 Table).
Identifying a single SSR profile as a molecular variant of a known cultivar is still challenging as no consensus approach has been proposed to clarify this issue. Here we proposed 228 genotypes as molecular variants of 74 cultivars showing light allelic variations (Fig 3, S6 Table). We considered that a threshold of less than 4 mismatched alleles among the set of 20 SSR loci would be strong enough to classify close accessions displaying similar endocarp traits within a single cultivar. As the allelic variation could be extended to up to six mismatched alleles (S1 Fig), we adopted a conservative but robust approach to identify these molecular variants. Several observations confirmed the relevance of our approach: (i) pairwise comparisons between the two WOGBs revealed that for WOGBM and WOGBC only 0.68% and 0.42% pairs, respectively, represented closely related genotypes with 1 to 4 distinct alleles, whereas the remaining pairs were mostly distinguished by 8 to 39 distinct alleles (S1 Fig); (ii) for all genotypes showing less than 4 mismatched alleles, similar endocarp profiles were observed, whereas significant differences were revealed at 5 mismatched alleles, e.g. “Morchiaio”/“Razzaio” and “Mantonica”/“Cirujal”; (iii) most genotypes showing less than 4 dissimilar alleles were identified within the same country (e.g. “Kato Drys” cultivar from Cyprus) or among closely located countries (e.g. “Morchiaio” cultivar from Italy and Slovenia), indicating that massive clonal propagation has been under way in large growing areas, thus leading to slight molecular variation; and (iv) Trujillo et al. [21], based on 33 SSR markers, considered that all accessions with a similarity index of 0.99 to 0.91, corresponding to 1 to 5 mismatched alleles, were molecular variants.
Identifying olive cultivars
With more than 1,200 olive varieties from around the Mediterranean Basin found in almost 100 ex-situ collections [13], two major goals have to be fulfilled: (i) characterization to eliminate mislabeling cases and identify synonymy and homonymy cases, and (ii) authentication of cultivars to ensure that accessions in one collection match true-to-type cultivars. However, most studies have been focused on characterization of ex-situ collections despite the fact that cultivar authentication should be a pre-requisite for exchanging material among scientists and nurseries. Trujillo et al. [21] defined authenticated cultivars based on their identity with respect to endocarp control samples from their original growing areas and DNA control samples. Hence, they were able to authenticate 200 cultivars by comparing endocarps with those of the same cultivar from the countries of origin (172 cultivars) and both endocarp and control DNA samples (28 cultivars). Even though the combination of molecular and morphological characterization was applied only to 28 out of 200 cultivars, the authentication approach used by Trujillo et al. [21] could be considered efficient since it targeted known cultivars from Spanish germplasm which represented 66% of the authenticated cultivars. However, for local and minor cultivars that are less known but especially present in limited geographic areas, the authentication process should systematically include both molecular and morphological characterization, as previously proposed for French olive germplasm [47]. Indeed, these authors defined a reference genotype for one cultivar when at least three olive trees grouped under the same denomination while presenting similar morphological traits and originating from different collections, nurseries and/or orchards display the same molecular pattern. Here, regarding the limits noted in the authentication process, we adopted a conservative strategy by focusing on the panel of 200 cultivars previously authenticated by Trujillo et al. [21]. We were able to authenticate 120 cultivars among the 329 identified in WOGBM, and most of them originated from Spain (81 cultivars; 67.5%). All of these 120 cultivars were previously authenticated in WOGBC by Trujillo et al. [21], except for 10 cultivars with matching molecular and morphological profiles between the two WOGBs.
Within and among accession mislabeling cases could occur at any step during plant establishment in the collection, as reported by many authors with regard to different fruit species [33,47]. Here we identified 79 cases of plantation mislabeling in WOGBM compared to 120 in WOGBC (S1 Table). Using the same approach as Trujillo et al. [21], mislabeling error was assumed when the profile of a known cultivar did not match its putative profile. Hence, when considering identified cultivars based on both molecular markers and morphological traits, Trujillo et al. [21] highlighted that the “Chemlal de Kabylie” (COR000118-Algeria), “Zaity” (COR000788-Syria), “Adkam” (COR001038-Syria) and “Aggezi Shami” (COR000723-Egypt) accessions were “Frantoio” cultivar mislabeling errors. Similarly, here for the same cultivar, i.e. “Frantoio”, we highlighted three accessions as mislabeling errors: “Fakhfoukha” (MAR00533-Morocco), “Jlot” (MAR00587-Lebanon) and “Beladi” (MAR00583-Lebanon). This highlights the mislabeling issues that may arise as a result of broad diffusion of clonally propagated known cultivars such as “Frantoio”.
The cultivar identification process was helpful for describing homonymy and synonymy cases in both collections. A total of 39 homonymy cases in WOGBM and 36 in WOGBC were identified among a total of 60 in the two collections involving a total of 179 identified cultivars, e.g. the Lentisca denomination which encompassed three distinct genotypes. Many authors have reported homonymy cases in olive [14,21,22,27,102,103]. Here we noted that homonymy cases mostly involved well known and widely cropped cultivars such as “Azeradj” (Algeria), “Cornicabra”, “Manzanilla” and “Picual” (Spain), “Toffahi” (Egypt and Syria). This reflected a farming strategy of known cultivar appropriation whereby distinct genotypes were pooled under a single denomination.
Otherwise, synonymy is likely the result of plant material dissemination and of traditional olive vegetative propagation practices. Such practices led to complex relationships among cultivars. We identified 175 synonymy cases among 78 cultivars in both collections (e.g. the “Gordal Sevillana” cultivar from Spain was synonymous with “Santa Caterina” from Italy). We highlighted 88 new synonyms in WOGBM or shared between the two collections including not previously described cultivars such as “Kiti”, “Meniko” and “Peristerona” from Cyprus as synonyms of “Kato Drys”, whereas 87 had already been described by other authors (e.g. “Olivastra di Montalcino” as a synonym of “Olivastra Seggianese” described by Cimato et al. [104] (S7 Table). Many synonym cases identified in WOGBM were in agreement with those obtained by Trujillo et al. [21] in WOGBC as they showed similar morphological and molecular profiles in both collections, e.g. “Sigoise”/“Alameño de Marchena”/“Haouzia” for “Picholine marocaine”, thus showcasing the power and efficiency of tools used to characterize and compare the two collections. The high number of synonymy cases observed in WOGBM compared to WOGBC could be explained by two factors. First, accessions received from many countries during the establishment of WOGBM were characterized in-situ using only morphological traits (fruit, endocarp, leaves, etc.), which could generate confusion compared to characterization based on the combination of morphological descriptors and molecular markers. For instance, accessions from Cyprus, Syria and Slovenia in which 31, 70 and 10 accessions, respectively, were analyzed and only 4 (13%), 42 (60%) and 3 (30%) genotypes were identified, respectively (Table 1). Conversely, analyzed accessions from Spain were found to be distinct due to previous analyses using molecular markers prior to WOGBM establishment [46]. Second, the varietal composition in the two collections contrasted with at least 50% of germplasm in WOGBC originated from Spain. This could be explained by the fact that most of the synonymy cases identified by Trujillo et al. [21] concerned Spanish cultivars (17 cultivars among 30 cultivars). Here, the highest number of synonyms was revealed for three cultivars that are widely cultivated throughout the Mediterranean Basin, with 13 cases for each one: “Beladi”, “Frantoio” and “Picholine marocaine” (S7 Table). Most synonymy cases were observed within one country or among closely located countries.
Identifying synonymy cases is essential for managing ex-situ collections by eliminating denomination redundancy. The synonymy cases identified in the present study could be a focus of interest for the olive research community, and we are aware that further investigations with large samples and comparisons of several control samples are required using both endocarp and DNA control samples.
In perennial clonally-propagated fruit species, a cultivar is defined as a group of similar plants that have been selected for one or more interesting characters that are distinct, uniform and stable. It is thus not surprising to observe cases of molecular variants, synonymy and homonymy within cultivars resulting from vegetative propagation and long-term diffusion of domesticated olive via farming practices. Here, within the two collections, we identified a total of 539 cultivars among 672 genotypes, 210 of which are considered as authenticated cultivars. We also defined a set of six SSR makers as efficient tools for discriminating almost all diversity present in both collections. The database generated and the approach used in our study could be applied to compare and harmonize more collections at national (Italy-Cosenza, [14]; Turkey-Izmir, [15]; Greece-Chania, [16]; France-Porquerolles, [47]) and international (USA-Davis, [17]; Argentina-Mendoza, [18]) levels. Setting up an international consortium for the identification, authentication and cataloguing of more than 1,200 cultivars across the Mediterranean Basin, under a common protocol with the six SSR loci selected in the present study and 11 endocarp traits is a necessary step. The expected results would consolidate partnership cooperation between communities of scientists conducting research on olive breeding and other users of olive genetic resources. Our study will enhance the establishment of the third worldwide collection in the eastern Mediterranean Basin (Izmir-Turkey) and will help to update and extend the Olea database [11], which is the most comprehensive global olive science portal to date.
The identification and authentication of cultivars in the two largest worldwide olive germplasm collections will ensure sustainable use of local genetic resources. In fact, studies on the resilience of true-to-type cultivars in different environments using germplasm collections across the IOOC network will guarantee the future of the crop under different climate change scenarii. The use of true-to-type and healthy plant material by commercial nurseries and by farmers for selected local cultivars will promote the use of authentic cultivars through protected designations of origin (PDO).
The importance of pooling the two collections
The two WOGB collections have two distinct histories. WOGBC, as the top olive germplasm bank in the world, was established in 1970 and contains 499 accessions from 21 countries, most of which originated from Spain (56%) [21]. WOGBM, as the second ranking olive germplasm bank, was established 33 years later and contains accessions from 14 countries. Compared to WOGBC, WOGBM was set up in a scientific setting with more knowledge available about the plant material and previously characterized genetic resources were introduced from each Mediterranean country [22]. Therefore, the Marrakech collection has a more balanced representation of accessions from Mediterranean olive growing countries such as Morocco, Algeria, Tunisia, Egypt and Cyprus, whereas the Cordoba collection has accessions from Albania, Turkey and Iran that are not included in the Marrakech collection. Hence, the two collections are complementary and not duplicated, as supported by the following observations. First, only 178 accession names among 713 are shared between the two collections. Only 130 genotypes (20%) out of 672 are in common, with a dominance of Spanish olive germplasm. Forty-eight discrepancy cases were identified, including Meski, Lentisca and Varudo, which could mainly be explained by mislabeling errors during germplasm propagation and/or subsequent planting. Second, the high proportion of accessions and therefore genotypes and cultivars specific to each collection was noted due to the contrasted varietal composition of each collection. Third, the genetic structure analysis revealed that the most probable genetic structure models are K = 2 and K = 3 for the Cordoba and Marrakech collections, respectively, while with all of the 672 genotypes, the K = 2 model was found to be the most relevant genetic structure model (ΔK = 3020.39 and H’ = 0.999). The presence of a high proportion of Spanish germplasm in WOGBC has certainly contributed to the genetic structure pattern observed when pooling the two datasets. However, a high proportion of alleles were shared between the two collections, i.e. 309 alleles (75.9%), despite the contrasted varietal composition between the two collections. Moreover, genetic diversity in terms of allelic richness (Ar) and diversity index (He) were similar and no significant differences were observed between the two collections, as previously reported by Trujillo et al. [21] using 11 SSR loci in common. Hence, the level of genetic diversity remained stable despite the considerable contrasted varietal composition between collections.
As more than 1,200 olive cultivars have been reported in the Mediterranean Basin [11,37], the current set of cultivars preserved and identified in both collections represented almost 45% of the described cultivars (535 cultivars). Further studies are required to encompass all olive germplasm from the Mediterranean Basin through the inclusion of new local accessions from different countries that are less represented in the two collections, such as French germplasm (more than 150 cultivars [47]), Cosenza-Italy (500 cultivars [14]), Turkey-Izmir (96 cultivars [15]) and Greece-Chania (47 cultivars [16]).
Here we propose a nested core collection based on the two WOGB collections, at three different levels, i.e. 55, 121 and 150 sample sizes. Core collections facilitate the exchange and efficient use of germplasm by providing a set of cultivars representing the overall genetic diversity available in two germplasm banks. We adopted the two-step methodology proposed by El Bakkali et al. [23], which has been found to be the most efficient approach for selecting subsets suitable for genetic association mapping. Our approach was based exclusively on genetic criteria but we believe that the diversity sampled in core collections based on SSR markers only are correlated with other criteria such as morphological and agronomic traits. Compared to WOGBM alone, a high number of cultivars were sampled that captured the total diversity in the two collections, as reported by El Bakkali et al. [23]; 121 vs 94, which could be explained by the contribution of specific alleles present in WOGBC (37 alleles). At the 55 sample size, only 7 genotypes (12.7%) were in common between the two collections, whereas 33 (60%) were revealed to be from WOGBM. Similar proportions were observed at the 121 sample size where 55.3% were from WOGBM and 11.6% were shared between the two collections (Table 4). These observations underline the importance of cultivar variability within WOGBM.
Defining a set of cultivars to serve as a core collection for the two WOGBs and its field assessment will certainly enhance knowledge on the genetic basis of target agronomical traits which is still at early stage. Genetic association mapping using unrelated cultivars is a powerful tool and has been successfully used to identify the genetic basis of many complex traits in plants [105,106]. The proposed core collection will boost insight into the genetic basis of most agronomic and adaptive traits in olive by taking advantage of advances achieved in next generation sequencing (NGS) with the development of high-throughput SNP markers through genotyping by sequencing approaches (GBS) [107,108], while also exploiting the recently released olive reference genome (http://olivegenome.org/) [59,60].
Conclusion
Intensive olive cropping systems with mechanical harvesting are leading to a reduction in the number of cultivars used in orchards, therefore increasing the risk of genetic erosion. Genetic resources included in germplasm collections represent a major pillar for sustainable use of olive genetic resources and for breeding programs geared towards selecting new resilient cultivars that are better adapted to climate change. Cultivar identification and authentication should thus be compulsory before using olive plant material. Here we first harmonized allele sizes between the two worldwide collections for a set of 20 SSR markers and used 11 endocarp traits as complementary tools for the characterization and identification of olive cultivars. We thus conducted the first in-depth analysis on olive cultivar germplasm. The information and database generated from this study will help manage olive cultivars from the Mediterranean Basin through the launch of a consortium under IOOC supervision. They also represent valuable tools for conducting further studies such as genetic association mapping.
Supporting information
Codes in collections, names of accessions and their origins, number of trees analyzed, number of SSR profiles, SSR code, morphological code, name of the identified cultivar and main cultivation area are specified. Identified and authenticated cultivars are mentioned.
(XLSX)
Each allele size for each locus and each accession in two different genotyping conditions were superimposed.
(DOCX)
Shared genotypes and those specific to each collection are indicated in the WOGB column.
(DOCX)
Shared cultivars and specific to each collection are indicated.
(DOCX)
Number of alleles (Na), expected (He) and observed (Ho) heterozygosity, allelic richness (Ar).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Cultivar names, origins, SSRs and morphological codes are indicated.
(XLSX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We would like to thank Luis Rallo, Conception M Diez, Isabel Trujillo, Diego Barranco for providing us DNA of 47 accessions from WOGB Cordoba and for their kind remarks on an earlier version of the manuscript; Claire Lanaud and the reviewers for comments on the final version of the manuscript. Molecular analysis was conducted at UMR AGAP Montpellier and we acknowledge the staff of the Genotyping platform. We also acknowledge the International Olive Oil Council and INRA Morocco for their contribution towards the management of WOGB Marrakech.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by BeFOre project N. 645595 (Bioresources for Oliviculture)/H2020-MSCA-RISE-2014 Marie Skłodowska-Curie Research and Innovation Staff Exchange Agropolis Fondation OliveMed N. 1202-066 through the “Investissements d’avenir”/Labex Agro ANR-10-Labex-0001-01 program. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Green PS. A revision of Olea L. (Oleaceae). Kew Bull. 2002;57: 91–140. [Google Scholar]
- 2.IOOC. International Olive Oil Council, 2018. http://www.internationaloliveoi.org/.
- 3.FAO. The Statistical Database (FAOSTAT). Food and Agriculture Organization of the United Nations (FAO), Rome. 2017. http://www.fao.org/faostat/en/#data.
- 4.Kaniewski D, Van Campo E, Boiy T, Terral JF, Khadari B, Besnard G. Primary domestication and early uses of the emblematic olive tree: palaeobotanical, historical and molecular evidences from the middle east. Biol Rev Camb Philos Soc. 2012;87: 885–899. 10.1111/j.1469-185X.2012.00229.x [DOI] [PubMed] [Google Scholar]
- 5.Zohary D, Hopf M, Weiss E. Domestication of plants in the Old World: The origin and spread of cultivated plants in Southwest Asia, Europe, and the Mediterranean basin. Oxford, UK: Oxford University Press; 2012. 10.1093/acprof:osobl/9780199549061.001.0001 [DOI] [Google Scholar]
- 6.Terral JF.La domestication de l’olivier (Olea europaea L.) en Méditerranée nord-occidentale: approche morphométrique et implications paléoclimatiques”, Ph.D. thesis, Université Montpellier II, Montpellier, France; 1997.
- 7.Besnard G, Breton C, Baradat P, Khadari B, Bervillé A. Cultivar identification in the olive (Olea europaea L.) based on RAPDs. J Am Soc Horti Sci. 2001;126: 668–675. [Google Scholar]
- 8.Breton CM, Terral JF, Pinatel C, Médail F, Bonhomme F, Bervillé A. The origins of the domestication of the olive tree. C R Biol. 2009;332(12): 1059–64. 10.1016/j.crvi.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Besnard G, Khadari B, Navascués M, Fernández-Mazuecos M, El Bakkali A, Arrigo N, et al. The complex history of the olive tree: from Late Quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proc R Soc B Biol Sci. 2013; 280(1756). 10.1098/rspb.2012.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diez CM, Trujillo I, Martinez-Urdiroz N, Barranco D, Rallo L, Marfil P, et al. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015;206: 436–447. 10.1111/nph.13181 [DOI] [PubMed] [Google Scholar]
- 11.Bartolini G. Olive Germplasm (Olea europaea L.) (Cultivars, synonyms, cultivation area, collections, descriptors); 2008.
- 12.Bartolini G, Messeri C, Prevost G. Olive tree germplasm: descriptor lists of cultivated varieties in the world. Acta Hortic. 1994;356: 116–118. [Google Scholar]
- 13.Bartolini G, Prevost G, Messeri C, Carignani C. Olive germplasm: cultivars and world-wide collections. In: FAO SaPGRSo (ed) FAO; 2005.
- 14.Muzzalupo I, Vendramin GG, Chiappetta A. Genetic Biodiversity of Italian Olives (Olea europaea) Germplasm Analyzed by SSR Markers. Hindawi, Sci. World J. 2014. Article ID 296590, 12 pages. 10.1155/2014/296590. [DOI] [PMC free article] [PubMed]
- 15.Kaya HB, Cetin O, Kaya H, Sahin M, Sefer F, Kahraman A, et al. SNP Discovery by Illumina-Based Transcriptome Sequencing of the Olive and the Genetic Characterization of Turkish Olive Genotypes Revealed by AFLP, SSR and SNP Markers. PLoS One. 2013;8(9): e73674 10.1371/journal.pone.0073674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xanthopoulou A, Ganopoulos I, Koubouris G, Tsaftaris A, Sergendani C, Kalivas A, et al. Microsatellite high-resolution melting (SSR-HRM) analysis for genotyping and molecular characterization of an Olea europaea germplasm collection. Plant Genet Resour. 2014;12: 273–277. [Google Scholar]
- 17.Koehmstedt AM, Aradhya MK, Soleri D, Smith JL, Polito VS. Molecular characterization of genetic diversity, structure, and differentiation in the olive (Olea europaea L.) germplasm collection of the United States Department of Agriculture. Genet. Resour. Crop Evol. 2011;58(4): 519–531. [Google Scholar]
- 18.Trentacoste ER, Puertas CM. Preliminary characterization and morpho-agronomic evaluation of the olive germplasm collection of the Mendoza province (Argentina). Euphytica. 2011;177: 99–109. [Google Scholar]
- 19.Belaj A, Dominguez-García MC, Atienza SG, Martín Urdíroz N, De la Rosa R, Satovic Z, et al. Developing a core collection of olive (Olea europaea L) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet Genomes. 2012;8: 365–378. [Google Scholar]
- 20.Díez CM, Imperato A, Rallo L, Barranco D, Trujillo I. Worldwide core collection of olive cultivars based on simple sequence repeat and morphological markers. Crop Sci. 2012;52: 211–221. [Google Scholar]
- 21.Trujillo I, Ojeda MA, Urdiroz NM, Potter D, Barranco D, Rallo L, et al. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes. 2014;10(1): 141–155. [Google Scholar]
- 22.Haouane H, El Bakkali A, Moukhli A, Tollon C, Santoni S, Oukabli A, et al. Genetic structure and core collection of the World Olive Germplasm Bank of Marrakech: towards the optimised management and use of Mediterranean olive genetic resources. Genetica. 2011;139(9): 1083–94. 10.1007/s10709-011-9608-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Bakkali A, Haouane H, Moukhli A, Costes E, Van Damme P, Khadari B. Construction of Core Collections Suitable for Association Mapping to Optimize Use of Mediterranean Olive (Olea europaea L.) Genetic Resources. PLoS ONE 2013;8(5): e61265 10.1371/journal.pone.0061265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponti L, Gutierrez AP, Ruti PM, Dell’Aquila A. Fine-scale ecological and economic assessment of climate change on olive in the Mediterranean Basin reveals winners and losers. PNAS. 2014;111(15): 5598–5603. 10.1073/pnas.1314437111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giampetruzzi A, Morelli M, Saponari M, Loconsole G, Chiumenti M, Boscia D, et al. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. Pauca. BMC Genomics. 2016;17(475). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barranco ND, Cimato A, Fiorino P, Rallo RL, Touzani A, Castañeda C, et al. World catalogue of olive varieties. IOC. 2000; Pages: 360.
- 27.Barranco D, Trujillo I, Rallo L. Elaiografía Hispanica In: Rallo L, Barranco D, Caballero JM, Del Rio C, Martin A, Tous J, Trujillo I (eds) Variedades de olivo en España. Mundi-Prensa, Madrid; 2005. [Google Scholar]
- 28.Cipriani G, Marrazzo MT, Marconi R, Cimato A. Microsatellite markers isolated in olive (Olea europaea L.) are suitable for individual fingerprinting and reveal polymorphism within ancient cultivars. Theor Appl Genet. 2002;104: 223–228. 10.1007/s001220100685 [DOI] [PubMed] [Google Scholar]
- 29.Lopes MS, Mendoca D, Sefc KM, Sabino Gil F, Da Camara Machado A. Genetic evidence of intra-cultivar variability within Iberian olive cultivars. Hortic Sci. 2004;39: 1562–1565. [Google Scholar]
- 30.Khadari B, Charafi J, Moukhli A, Ater M. Substantial genetic diversity in cultivated Moroccan olive despite a single major variety: a paradoxical situation evidenced by the use of SSR loci. Tree Genet Genomes. 2008;4: 213–221. [Google Scholar]
- 31.Cipriani G, Spadotto A, Jurman I, Di Gaspero G, Crespan M, Meneghetti S, et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages, and reveals a large admixture amongst varieties of different geographic origin. Theor Appl Genet. 2010;121(8): 1569–1585. 10.1007/s00122-010-1411-9 [DOI] [PubMed] [Google Scholar]
- 32.Achtak H, Ater M, Oukabli A, Santoni S, Kjellberg F and Khadari B. Traditional agroecosystems as conservatories and incubators of cultivated plant varietal diversity: the case of Fig (Ficus carica L.) in Morocco. BMC Plant Biol. 2010;10: 28 10.1186/1471-2229-10-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans KM, Patocchi A, Rezzonico F, Mathis F, Durel CE, Fernández-Fernández F, et al. Genotyping of pedigreed apple breeding material with a genome-covering set of SSRs: trueness-to-type of cultivars and their parentages. Mol. Breed. 2011;28: 535–554. [Google Scholar]
- 34.Del Río C, Caballero JM. Preliminary agronomical characterization of 131 cultivars introduced in the olive germplasm bank of Córdoba in March 1987. Acta Hort. 1994;356: 110–115. [Google Scholar]
- 35.Bartolini S, Minnocci A, Vitagliano C. Morphological studies on pollen in some clones of olive cv. "Leccino". Agricoltura Mediterranea. 1992;122: 282–286. [Google Scholar]
- 36.Cantini C, Cimato A, Sani G. Morphological evaluation of olive germplasm present in Tuscany region. Euphytica.1999;109(3): 173–181. [Google Scholar]
- 37.Bartolini G, Petruccelli R. Classification, origin, diffusion and history of the olive. FAO press, Rome; 2002. [Google Scholar]
- 38.Blazakis KN, Kosma M, Kostelenos G, Baldoni L, Bufacchi M, Kalaitzis P. Description of olive morphological parameters by using open access software. Plant Methods. 2017;13: 111 10.1186/s13007-017-0261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barone E, Di Marco L, Motisi A, Caruso T. The Sicilian olive germplasm and its characterisation by using statistical methods. Acta Hort. 1994;356: 66–69. [Google Scholar]
- 40.Taamalli W, Geuna F, Banfi R, Bassi D, Daoud D, Zarrouk M (2006) Agronomic and molecular analyses for the characterisation of accessions in Tunisian olive germplasm collections. Electronic J Biotechnol. 2006;9(5): 468–481. 10.2225/vol9-issue5-fulltext-12 [DOI] [Google Scholar]
- 41.Barranco D, Trujillo I, Rallo L. Are ‘Oblonga’ and ‘Frantoio’ the same cultivar? HortScience. 2000;35: 1323–1325. [Google Scholar]
- 42.Caballero JM, Del Rıo C, Barranco D, Trujillo I. The olive world germplasm of Cordoba, Spain. Olea. 2006;25: 14–19. [Google Scholar]
- 43.Ouazzani N, Lumaret R, Villemur P, Di Giusto F (1993) Leaf Allozyme Variation in Cultivated and Wild Olive Trees (Olea europaea L.). J Hered. 1993;84 10.1093/oxfordjournals.jhered.a111274. [DOI] [PubMed] [Google Scholar]
- 44.Trujillo I, Rallo L, Arus P. Identifying olive cultivars by isozyme analysis. J Amer Soc Hort Sci. 1995;120: 318–324. [Google Scholar]
- 45.Besnard G, Bervillé A (2002) On chloroplast DNA variations in the olive (Olea europaea L.) complex: comparison of RFLP and PCR polymorphisms. Theor Appl Genet. 2002;104(6–7): 1157–1163. 10.1007/s00122-001-0834-8 [DOI] [PubMed] [Google Scholar]
- 46.Belaj A, Satovic Z, Cipriani G, Baldoni L, Testolin R, Rallo L, et al. Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effectiveness in establishing genetic relationships in olive. Theor Appl Genet. 2003;107: 736–744. 10.1007/s00122-003-1301-5 [DOI] [PubMed] [Google Scholar]
- 47.Khadari B, Breton C, Moutier N, Roger JP, Besnard G, et al. The use of molecular markers for germplasm management in French olive collection. Theor Appl Genet. 2003;106: 521–529. 10.1007/s00122-002-1079-x [DOI] [PubMed] [Google Scholar]
- 48.Gemas VJV, Almadanim MC, Tenreiro R, Martins A, Fevereiro P. Genetic diversity in the Olive tree (Olea europaea L. subsp. europaea) cultivated in Portugal revealed by RAPD and ISSR markers. Genet Resour Crop Evol. 2004;51: 501–511. [Google Scholar]
- 49.Brake M, Migdadi H, Al-Gharaibeh M, Ayoub S, Haddad N, El Oqlah A. Characterization of Jordanian olive cultivars (Olea europaea L.) using RAPD and ISSR molecular markers. Sci Hortic. 2014;176: 282–289. [Google Scholar]
- 50.Angiolillo A, Mencuccini L, Baldoni L. Olive genetic diversity assessed using Amplified Fragment Lenght Polymorphism. Theor Appl Genet. 1999;98: 411–421. [Google Scholar]
- 51.Sarri V, Baldoni L, Porceddu A, Cultrera NGM, Contento A, Frediani M, et al. Microsatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically defined populations. Genome. 2006;49: 1606–1615. 10.1139/g06-126 [DOI] [PubMed] [Google Scholar]
- 52.Baldoni L, Cultrera NG, Mariotti R, Ricciolini C, Arcioni S, et al. A consensus list of microsatellite markers for olive genotyping. Mol Breed. 2009;24: 213–231. [Google Scholar]
- 53.Marra FP, Caruso T, Costa F, Di Vaio C, Mafrica R, Marchese A. Genetic relationships, structure and parentage simulation among the olive tree (Olea europaea L. subsp. europaea) cultivated in Southern Italy revealed by SSR markers. Tree Genet. Genomes. 2013; 9: 961–973. 10.1007/s11295-013-0609-9 [DOI] [Google Scholar]
- 54.Di Rienzo V, Sion S, Taranto F, D’Agostino M, Montemurro C, Fanelli V, et al. Genetic flow among olive populations within the Mediterranean basin. PeerJ. 2018; 6:e5260 10.7717/peerj.5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boucheffa S, Miazzi MM, di Rienzo V, Mangini G, Fanelli V, Tamendjari A, et al. The coexistence of oleaster and traditional varieties affects genetic diversity and population structure in Algerian olive (Olea europaea) germplasm. Genet Resourc Crop Evol. 2017;64(2): 379–390. [Google Scholar]
- 56.Reale S, Doveri S, Díaz A, Angiolillo A, Lucentini L, Pilla F, et al. SNP-based markers for discriminating olive (Olea europaea L.) cultivars. Genome. 2006;49: 1193–205. 10.1139/g06-068 [DOI] [PubMed] [Google Scholar]
- 57.D’Agostino N, Taranto F, Camposeo S, Mangini G, Fanelli V, Gadaleta S, et al. GBS-derived SNP catalogue unveiled wide genetic variability and geographical relationships of Italian olive cultivars. Scientific Rep. 2018;8,15877: 1–13. 10.1038/s41598-018-34207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taranto F, D’Agostino N, Pavan S, Fanelli V, di Rienzo V, Sabetta W, et al. SNP diversity in an olive germplasm collection. Acta Hortic. 2018;1199: 27–32. [Google Scholar]
- 59.Cruz F, Julca I, Gómez-Garrido J, Loska D, Marcet-Houben M, Cano E, et al. Genome sequence of the olive tree, Olea europaea. GigaScience 2016;5: 29 10.1186/s13742-016-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unver T, Wu Z, Sterck L, Turktas M, Lohaus R, Li Z, et al. Genome of wild olive and the evolution of oil biosynthesis. PNAS. 2017;31, 114(44): E9413–E9422. 10.1073/pnas.1708621114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Essalouh L, Zine El Aabidine A, Contreras S, Ben Sadok I, Santoni S, Khadari B, et al. Genomic and EST microsatellite loci development and use in olive: Molecular tools for genetic mapping and association studies. Acta Hortic. 2014;1057: 543–549. 10.17660/ActaHortic.2014.1057.69 [DOI] [Google Scholar]
- 62.Mariotti R, Cultrera NGM, Mousavi S, Baglivo F, Rossi M, Albertini E, et al. Development, evaluation, and validation of new EST-SSR markers in olive (Olea europaea L.). Tree Genet Genomes 2016;12: 120 10.1007/s11295-016-1077-9 [DOI] [Google Scholar]
- 63.Mousavi S, Mariotti R, Regni L, Nasini L, Bufacchi M, Pandolfi S, et al. The First Molecular Identification of an Olive Collection Applying Standard Simple Sequence Repeats and Novel Expressed Sequence Tag Markers. Front Plant Sci. 2017;8: 1283 10.3389/fpls.2017.01283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muzzalupo I, Salimonti A, Caravita MA, Pellegrino M, Perri E. SSR markers for characterization and identification of cultivars of Olea europaea L. in the Abruzzo and Molise regions in south-central Italy. Adv Hortic Sci. 2008;22(2): 129–135. [Google Scholar]
- 65.Gao H, Cai S, Yan B, Chen B, Yu F. Discrepancy variation of dinucleotide microsatellite repeats in eukaryotic genomes. Biol Res. 2009;42: 365–375. [PubMed] [Google Scholar]
- 66.Weeks DE, Conley YP, Ferrell RE, Mah TS, Gorin MB. A tale of two genotypes: consistency between two high throughput genotyping centres. Genome Res. 2002;12: 430–435. 10.1101/gr.211502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Las Casas G, Scollo F, Distefano G, Continella A, Gentile A, La Malfa S. Molecular characterization of olive (Olea europaea L.) Sicilian cultivars using SSR markers. Biochem Syst Ecol. 2014;57: 15–19. 10.1016/j.bse.2014.07.010 [DOI] [Google Scholar]
- 68.Sefc KM, Lopes MS, Mendonça D, Rodrigues Dos Santos M, Da Cámara Machado L. Identification of microsatellites loci in Olive (Olea europaea L.) and their characterization in Italian and Iberian trees. Mol Ecol. 2000;9: 1171–1193. 10.1046/j.1365-294x.2000.00954.x [DOI] [PubMed] [Google Scholar]
- 69.Carriero F, Fontanazza G, Cellini F, Giorio G. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theor Appl Genet. 2002;104: 301–307. 10.1007/s001220100691 [DOI] [PubMed] [Google Scholar]
- 70.La Rosa R, James CM, Tobutt KR. Isolation and characterization of polymorphic microsatellites in olive (Olea europaea L.) and their transferability to other genera in the Oleaceae. Mol Ecol Notes. 2002;2(3): 265–267. [Google Scholar]
- 71.Park SDE. Microsatellite Toolkit 3.1.1; 2001. http://animalgenomics.ucd.ie/sdepark/ms-toolkit/.
- 72.Nei M. Molecular Evolutionary Genetics. Columbia University Press: New York; 1987. [Google Scholar]
- 73.Botstein D, White R, Skolnick M, Davis R. Construction of a genetic-linkage map in man using restriction fragment length polymorphisms. Amer J Hum Genet. 1980;32: 314–331. [PMC free article] [PubMed] [Google Scholar]
- 74.Pritchard JK, Stephens M, Donnelly P. Inference of population structure from multilocus genotype data. Genetics. 2000;155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software Structure, a simulation study. Mol Ecol. 2005;14: 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- 76.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna; 2016. [Google Scholar]
- 77.Jakobsson M, Rosenberg NA. Clumpp: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinform. 2007;23: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 78.Perrier X, Flori A, Bonnot F. Data analysis methods In: Hamon P, Seguin M, Perrier X,Glaszmann J C. Ed., Genetic diversity of cultivated tropical plants. Enfield, Science Publishers; Montpellier; 2003. 43–76. [Google Scholar]
- 79.Sokal R, Michener C. A statistical method for evaluating systematic relationships. The University of Kansas. In: Sci bull. 1958; 38(22). [Google Scholar]
- 80.Dice LR. Measures of the amount of ecologic association between species. Ecol. 1945;26: 297–302. [Google Scholar]
- 81.Rohlf FJ. NTSYS-pc. Numerical taxonomy and multivariate analysis system. Version 2.00. Exeter Software. Setauket, New York; 1998.
- 82.Smouse PE, Peakall R. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity. 1999; 82: 561–73. 10.1038/sj.hdy.6885180 [DOI] [PubMed] [Google Scholar]
- 83.Peakall R, Smouse PE (2006) genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes. 2006;6(1): 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petit RJ, El Mousadik A, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol. 1998;12: 844–855. 10.1111/j.1523-1739.1998.96489.x. [DOI] [Google Scholar]
- 85.Szpiech ZA, Jakobsson M, Rosenberg NA. ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinform. 2008;24: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammer Ø, Harper DAT, Ryan PD. Past: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4: 9pp. [Google Scholar]
- 87.Gouesnard B, Bataillon TM, Decoux G, Rozale C, Schoen DJ, David JL. Mstrat: An algorithm for building germplasm core collections by maximizing allelic or phenotypic richness. J Hered. 2001;92: 93–94. 10.1093/jhered/92.1.93 [DOI] [PubMed] [Google Scholar]
- 88.Thachuk C, Crossa J, Franco J, Dreisigacker S, Warburton M, Davenport GF. CoreHunter: an algorithm for sampling genetic resources based on multiple genetics measures. Bioinform. 2009;10: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schoen DJ, Brown AHD. Conservation of allelic richness in wild crop relatives is aided by assessment of genetic markers. PNAS. 1993;90: 10623–10627. 10.1073/pnas.90.22.10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Imperio M, Viscosi V, Scarano MT, D’Andrea M, Zulo BA, Pilla F. Integration between molecular and morphological markers for the exploitation of olive germoplasm (Olea europaea). SciHortic. 2001;130: 229–240. [Google Scholar]
- 91.Fendri M, Trujillo I, Trigui A, Rodriguez-Garcia MI, Ramirez JDA. Simple sequence repeat identification and endocarp characterization of olive tree accessions in a Tunisian germplasm collection. Hortscience. 2010;45: 1429–1436. [Google Scholar]
- 92.Terral JF, Alonso N, Capdevila RB, Chatti N, Fabre L, Fiorentino G, et al. Historical biogeography of olive domestication (Olea europaea L.) as revealed by geometrical morphometry applied to biological and archaeological material, J Biogeogr. 2004;31: 63–77. [Google Scholar]
- 93.Hannachi H, Martín Gómez JJ, Saadaoui E, Cervantes E. Stone diversity in wild and cultivated olive trees (Olea europaea L.). Dendrobiol. 2017;77: 19–32. [Google Scholar]
- 94.Baali-Cherif D and Besnard G. High genetic diversity and clonal growth in relict populations of Olea europaea subsp. laperrinei (Oleaceae) from Hoggar Algeria. Ann Bot. 2005;96: 823–830. 10.1093/aob/mci232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ibanez J, Velez MD, De Andres MT and Borrego J. Molecular markers for establishing distinctness in vegetatively propagated crops: a case study in grapevine. Theor Appl Genet. 2009;119: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 96.Charafi J, El Meziane A, Moukhli A, Boulouha B, El Modafar C, Khadari B. Menara gardens: a Moroccan olive germplasm collection identified by SSR locus genetic study. Genet Resour Crop Evol. 2007;55: 893–900. [Google Scholar]
- 97.Belaj A, Rallo L, Trujillo I. Using RAPD and AFLP Markers to Distinguish Individuals Obtained by Clonal Selection of ‘Arbequina’ and ‘Manzanilla de Sevilla’ Olive. HortScience. 2004;39(7): 1566–1570. [Google Scholar]
- 98.Ipek A, Barut E, Gulen H, Ipek M. Assessment of inter- and intra-cultivar variations in olive using SSR markers. Sci agric. 2012;69(5): 327–335. [Google Scholar]
- 99.Muzzalupo I, Chiappetta A, Benincasa C, Perri E. Intra-cultivar variability of three major olive cultivars grown in different areas of central-southern Italy and studied using microsatellite markers. Sci Hortic. 2010;126: 324–329. [Google Scholar]
- 100.Kruglyak S, Durret RT, Schug M, Aquadro CF. Equilibrium distributions of microsatellite repeat length resulting from a balance between slippage events and point mutations. PNAS. 1998;95: 10774–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bachtrog D, Agis M, Imhof M, Schlotterer C. Microsatellite variability differs between dinucleotide repeat Motifs–evidence from Drosophila melanogaster. Mol Biol Evol. 2000;17: 1277–1285. 10.1093/oxfordjournals.molbev.a026411 [DOI] [PubMed] [Google Scholar]
- 102.Soleri D, Koehmstedt A, Aradhya MK, Polito V, Pinney K. Comparing the historic olive trees (Olea europaea L.) of Santa Cruz Island with contemporaneous trees in the Santa Barbara, CA area: a case study of diversity and structure in an introduced agricultural species conserved in situ. Genet Resour Crop Evol. 2010;57: 973–984. [Google Scholar]
- 103.Boucheffa S, Tamendjari A, Sanchez-Gimeno AC, Rovellini P, Venturini S, di Rienzo V, et al. Diversity Assessment of Algerian Wild and Cultivated Olives (Olea europeae L.) by Molecular, Morphological, and Chemical Traits. Eur J Lipid Sci Technol. 2019;121(1), 1800302 10.1002/ejlt.201800302 [DOI] [Google Scholar]
- 104.Cimato A, Cantini C, Sani G. L’olivo in Toscana: il germoplasme autoctono. Istituto sulla propagazione delle specie legnose, CNR: ARSIA; 2001: 217pp.
- 105.Rafalski JA. Association genetics in crop improvement. Curr Opinion Plant Biol. 2010;13: 174–180. [DOI] [PubMed] [Google Scholar]
- 106.Kaya HB, Cetin O, Kaya HS, Sahin M, Sefer F, Tanyolac B. Association Mapping in Turkish Olive Cultivars Revealed Significant Markers Related to Some Important Agronomic Traits. Biochem Genet. 2016;54(4): 506–533. 10.1007/s10528-016-9738-9 [DOI] [PubMed] [Google Scholar]
- 107.İpek A, Yılmaz K, Sıkıcı P, Tangu NA, Öz AT, Bayraktar M, et al. SNP Discovery by GBS in Olive and the Construction of a High-Density Genetic Linkage Map. Biochem Genet. 2016;54(3): 313–25. 10.1007/s10528-016-9721-5 [DOI] [PubMed] [Google Scholar]
- 108.İpek A, İpek M, Ercişli S, Tangu NA. Transcriptome-based SNP discovery by GBS and the construction of a genetic map for olive. Funct Integr Genomics. 2017;17(5): 493–501. 10.1007/s10142-017-0552-1 [DOI] [PubMed] [Google Scholar]