Abstract
Objective:
Chronic rhinosinusitis (CRS) involves inflammation of the nasal and para-nasal mucosa. Due to its heterogeneous nature, unknown pathogenesis, and high recurrence rate, effective treatment is difficult. Nasal cytology is presently not a part of the routine diagnosis or treatment decision for CRS.
Data sources:
A literature search was performed for published papers in English between January 1990 and June 2019 using MEDLINE.
Study selection:
Terms used were chronic rhinosinusitis, eosinophils, etiology, immunopathology, inflammation, mast cells, nasal cytology, polyps, and treatment. Both reviews and original articles were collected and studied.
Results:
There is no standard nasal fluid, mucus sampling, or staining techniques for identifying inflammatory cell types. Results were divergent from different countries. Moreover, the main focus of these papers on the cells in nasal washings was eosinophils, with infrequent mentioning of other cell types that may imply different etiology and pathology. The heterogeneous cell profile of CRS and the role of mast cells have been unappreciated due to the lack of specific immunohistochemical technique or study of its unique mediators.
Conclusions:
Nasal cytology could help distinguish the type and the activation state of inflammatory cells. Thus it can help in providing a clearer picture of CRS pathogenesis, identifying different patient groups, and developing effective treatments.
Keywords: Rhinitis, Sinusitis, Nasal polyps, Nasal mucosa, Cytology, Inflammation, Eosinophils, Mast cells
Introduction
Chronic rhinosinusitis (CRS) is defined as inflammation of the nose and the para-nasal sinuses, which lasts more than 3 months and is featured by nasal obstruction and nasal discharge with or without facial pain or loss of smell. CRS is typically classified into two types: (a) CRS with nasal polyps (CRSwNP) and (b) CRS without nasal polyps (CRSsNP).[1] About one-third of patients with CRS have NP and tend to have concomitant asthma and aspirin sensitivity. Also, they tend to have more severe symptoms, worse quality of life (QoL), higher recurrence rates and are more resistant to treatment.[2]
With its etiology still uncertain, the current treatment of CRS largely targets mucosal inflammation. As different cell types imply different etiology and pathology, it would be rational to identify the inflammatory cell types for the proper treatment.
Nasal cytology is a convenient way to assess the number and types of inflammatory cells and could thus aid the prognosis and treatment of CRS. In this article, the role of different inflammatory white blood cell types in the nasal mucosa of CRS was reviewed, and the necessity to facilitate nasal cytology for individualized treatment of CRS was discussed including a specific focus on the role of mast cells in CRS. Studies about their density and distribution in CRS are intriguing. The contribution of mast cells in the pathology of CRS has been a mystery for a long time. Mast cell stabilizer might be a therapeutic choice for at least some specific types of CRS if mast cells are proved to dominate or orchestrate the inflammation of CRS.
Role of Inflammatory White Blood Cell Types in CRS Necessitates the Application of Nasal Cytology
The nasal mucosa is composed of pseudostratified ciliated epithelia and serves as the doorway of the airways. Normally, four types of cells can be identified using nasal cytology in healthy individuals: (a) ciliated cells, (b) mucinous cells, (c) basal cells, and (d) striated cells. Sparse neutrophils could also be seen across the epithelium. The detection of eosinophils (Eos) as well as bacteria, or fungal hyphae, indicates a pathological condition. Neutrophils are indicative of an infection that may require antibiotic treatment, while the presence of Eos, basophils, and mast cells is regarded as a sign of allergic inflammation which may respond to corticosteroid and anti-histamine treatment.[3]
Zhang et al[4] studied the normal value of four types of inflammatory cells, that is, Eos, neutrophils, macrophages, and lymphocytes in nasal lavages of 500 healthy Chinese adults. They established a normative value as 2 for Eos, and 17 for neutrophils while basophils and mast cells were only detected in a few cases (15/1620).
Nasal cytology has been a useful tool for understanding the pathophysiology and phenotypes of allergic rhinitis (AR) and non-AR and thus was recommended to be used in CRS to indicate the possible pathology.[5,6] It has been demonstrated that patients with Eos and mast cells infiltration in nasal cytology had not only the worst QoL in most periods but were also related to asthma and nasal polyposis, which underlined the importance of this method for diagnosis.[7,8] Nasal cytology can reveal the endotype rather than the clinically-observable phenotype. This could partially explain the mechanism behind why sometimes the same treatment results in different responses and help clinicians initiate therapeutic approaches [Table 1]. Recent studies showed that treating the underlying inflammation improved clinical outcomes in AR.[9,10]
Table 1.
Classification of the inflammatory cell types according to nasal cytology and treatment of choices.

CRS is characterized histologically by the infiltration of inflammatory cells. While Eos are the major cell type found in most CRSwNP specimens in Western countries,[11–13] specimens from Asian nations show a greater diversity of inflammatory cells.[14–16] Mast cells, which produce a variety of inflammatory mediators, cytokines and chemokines, are suggested to contribute to the immune and inflammatory events in CRSwNP.[17] The fact that mast cell-deficit mice do not develop NP supported the hypothesis that mast cells may play a critical role in CRSwNP pathogenesis.
Gelardi suggested that inflammatory cell types in NP are classified as eosinophilic type (Eos >20% of total cells recovered from nasal scraping, including both inflammatory and epithelial cells), mast cell type (>10% of total nasal cells), neutrophilic type (neutrophils >50% of total nasal cells), and eosinophilic and mast cell type (Eos >20% and mast cells >10% of total nasal cells).[8]
Eosinophils
According to studies from Western countries, it was concluded that most CRSwNP patients were of eosinophilic type.[11–13] As a result, Eos are considered the main players in the development of NP. However, tailored treatment had only moderate effects.[18] Meanwhile, CRSwNP in Asian countries is reported to involve neutrophils infiltration. The eosinophilic type was found to be less than 30% to 50% in Japan and Korea,[14,15] and less than 30% in the mainland of China.[16] CRS is further divided into “eosinophilic” and “non-eosinophilic.” CRSwNP with or without eosinophil infiltration within NP is known as ECRSwNP and non-ECRSwNP. CRSsNP with or without eosinophil infiltration within mucosa is known as ECRSsNP and non-ECRSsNP [Figure 1]. There is still inconsistency in the definition of the eosinophilic type. Kountakis suggested that mucosal eosinophilia is defined as more than 5 Eos/high power fields (HPF).[19] Soler defined it as more than 10 Eos/HPF,[20] while some Japanese studies defined mucosal eosinophilia as ≥70, >100, or >120 Eos/HPF.[14,21,22] Thus, the definition of eosinophilic infiltration is still uncertain, which may bring in confusion in diagnosis and treatment.
Figure 1.
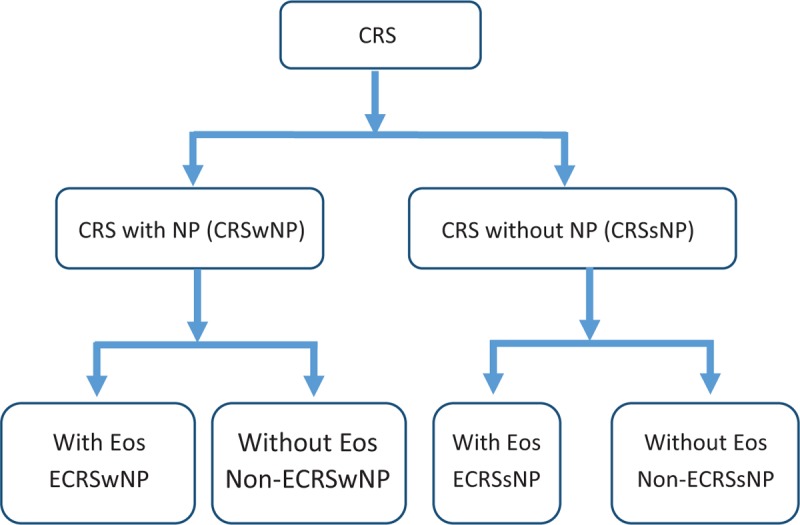
Sub-division of chronic rhinosinusitis (CRS) with/without nasal polyps (NP) and/or eosinophils (Eos). CRSwNP: Chronic rhinosinusitis with nasal polyps; CRSsNP: Chronic rhinosinusitis without nasal polyps; ECRSwNP: Chronic rhinosinusitis with nasal polyps with eosinophil dominated; Non-ECRSwNP: Chronic rhinosinusitis with nasal polyps without eosinophil dominated; ECRSsNP: Chronic rhinosinusitis with nasal polyps without eosinophil dominated; Non-ECRSsNP: Chronic rhinosinusitis without nasal polyps without eosinophil dominated.
ECRSwNP is considered to be associated with asthma, aspirin intolerance, fungal rhinosinusitis, severe symptoms, resistance to treatment, and a higher recurrence rate after surgery.[15] Lou et al[23] suggested that >27% of tissue Eos percentage or an absolute count of >55 Eos/HPF could predict recurrence with a sensitivity of 96.4% and a specificity of 92.7%. Another study showed CRSwNP patients with ≥70 Eos/HPF had the highest recurrence rate.[24] McHugh et al recommended a cut-off value of >55 Eos/HPF for predicting the likelihood of recurrence of ECRS based on the results of one meta-analysis.[25]
Neutrophils and lymphocytes
Neutrophils are known to increase in CRSsNP, CRSwNP, and severe asthma.[26] CRSwNP is more common with neutrophils in Asian patients and is resistant to corticosteroid.[18] The mechanisms of neutrophils accumulation are not yet confirmed. Pothoven et al reported that neutrophils were a major producer of the epithelial barrier-disrupting cytokine oncostatin M in CRS.[27]
T cells have been implicated in the pathogenesis of CRS. It has been shown that the sinus mucosa in CRSwNP was characterized by a significant infiltration of CD8+ T cells and a relative decrease of CD4+ T cells, even though there was no significant difference in peripheral blood T cells between CRSwNP, CRSsNP, and healthy controls.[28,29] The T cell profile in CRSsNP also seemed similar to that of control samples.[30] Kim et al showed that regulatory T cells were decreased in NP of CRSwNP compared with CRSsNP.[31] B cells were increased in the sinus mucosa of CRSwNP compared to patients with CRSsNP, and there was no correlation with the increased number of circulatory B cells.[32]
Mast cells and basophils
Mast cells and basophils are both metachromatic cells that share a lot in common while having differences.[33] Both mast cells and basophils can be detected from nasal secretion and the nasal mucosa, and mast cells account for more than 80% of the metachromatic cells in nasal scrapings, with basophils more predominant in nasal secretion.[34–36]
Basophils may play a key role in the pathogenesis of ECRS. Basophil count increased significantly in NP from patients with ECRS and is positively correlated with the severity of Lund-Mackay scores.[37]
Mast cells are multifunctional cells mostly related to allergies.[38] Mast cells have been recognized as important mediators in allergic inflammation, as well as other inflammatory conditions[39,40] such as autism and psoriasis.[41,42]
The role of the mast cell in the pathogenesis of CRS and NP has been a mystery for a long time. Studies about its density, distribution in CRS are intriguing partially due to the insufficient efforts in the detection of mast cells.
Recent studies supported that mast cells may play a role in the development of NP in CRS. Mice with mast cells deficiencies did not develop polypoid changes in the nose and sinuses.[43] The number of mast cells increased in NP of both ECRS and non-ECRS. The number of mast cells and the expression of mast cell tryptase mRNA were increased in ECRS and non-ECRS polyps in Japanese patients with CRSwNP.[44] Percentages of mast cells in the sinonasal mucosa of CRSwNP patients were elevated as compared to CRSsNP patients, regardless of atopic status.[45]
Mast cell secretion was also increased in NP. Histamine level was found to have increased in polyp fluid than in serum.[46] Tryptase protein was elevated in NP, nasal lavage fluids, and nasal secretion from patients with CRSwNP.[47] The number of mast cells positive for both tryptase and chymase and activated mast cells was increased in eosinophilic CRSwNP.[48] The expression of prostaglandin D2 ( PGD2) was up-regulated in NP compared to the normal nasal mucosa.[49]
Mast cells can produce an abundance of cytokines that activate Eos such as interleukin 5 (IL-5), granulocyte-macrophage colony-stimulating factor, eotaxin, and regulated on activation, normal T cell expressed and secreted (RANTES).[50] By releasing different kinds of cytokines, mast cells may be the initial effector cells which recruit and activate other inflammatory cells, such as Eos and play a key role in orchestrating the development of NP.[51]
The roles of mast cells in CRS and NP have been controversial, partially because of the adoption of detection methods. Mast cells were described by Ehrlich in 1879 as cells containing granules which stain metachromatically with a basic dye. Basic dyes like toluidine blue or Alcian blue have been the classical methods for identifying mast cells ever since.
Problems with basic dye included mainly two parts, one is formaldehyde-fixing would lead to some loss of the stain, and the other problem is mast cells loss of the stain after degranulation which leads to underestimating of the numbers of mast cells.
Immunohistochemistry with antibodies advances the technology of mast cell detection. Mediators stored in the granules of mast cells (tryptase, chymase, carboxypeptidase) and immunoglobulin E (IgE) and the membrane component c-kit have been used as markers for mast cell detection, with c-kit being the only non-granule-associated.
Nasal Cytology Could Help Identify More Precise Treatments
Currently, the treatments of CRS and NP primarily focus on symptoms because of the unknown etiologies. Nasal cytology may provide some insight into the immune-inflammatory profile of CRS, thus providing more specific guidance on treatment. One study showed NP had similar cellular composition to that of the mucosa.[52]
Nasal cytology is easy to perform, minimally-invasive, relatively low-cost, and can be repeated in the same subject at short intervals, therefore it qualifies as a convenient way to assess the number and type of inflammatory cells and help with diagnosis and treatment of CRS. Mast cell stabilizers can be considered as treatment solutions for CRS subsequent to the approval of the hypothesis that mast cells are causal to the inflammation underlying CRS.
Nasal Cytology Needs to be Standardized
Samples should be collected from the middle portion of the inferior turbinate where the ratio of ciliate/mucinous cells is expected to be well balanced. The sample is usually immediately smeared on a glass slide and air-dried. Then, the slide should be stained with May-Grunwald-Giemsa. This staining method allows us to easily identify all the cellular components along with bacterial and fungal spore/hyphae.[3]
Although the diagnostic value of nasal cytology is well known, different evaluation systems and unstandardized procedures make it difficult to be adopted as a reliable guideline.[1] Several new studies aimed to standardize the nasal secretion collection methods and explore the adoption of nasal cytology for the guidance of treatment of options.[10,53,54]
Conclusions
CRS is a heterogeneous chronic inflammatory disease of the sinonasal cavities. The precise etiology of CRS is still unknown and there is currently no way to prevent its progression or relapses. Nasal cytology is a simple tool to reflect the inflammation profile of CRS and, if standardized, could lead to a better understanding of the pathophysiology and better treatment. Immunohistochemical methods need to be used to accurately detect all inflammatory cells. A closer look at the activation of mast cells and secretion of mediators could help understand CRS pathogenesis and contribute to the development of more effective therapies.
Conflicts of interest
None.
Footnotes
How to cite this article: Ren HL, Li JD, Yue FS, Sun JL, Rebeiz EE, Theoharides TC. Nasal cytology with emphasis on mast cells can improve the diagnosis and treatment of chronic rhinosinusitis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000387
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012; 50:1–12. doi: 10.4193/Rhino50E2. [DOI] [PubMed] [Google Scholar]
- 2.Wu D, Bleier BS, Li L, Zhan X, Zhang L, Lv Q, et al. Clinical phenotypes of nasal polyps and comorbid asthma based on cluster analysis of disease history. J Allergy Clin Immunol Pract 2018; 6:1297–1305.e1. doi: 10.1016/j.jaip.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. NASAL cytology: practical aspects and clinical relevance. Clin Exp Allergy 2016; 46:785–792. doi: 10.1111/cea.12730. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang Q, Xie Y, Wang Z, Li D, Ma L, et al. The normative value of inflammatory cells in the nasal perfusate of Chinese adults: a pilot study. J Thorac Dis 2014; 6:905–912. doi: 10.3978/j.issn.2072-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffler E, Landi M, Caruso C, Fichera S, Gani F, Guida G, et al. Nasal cytology: methodology with application to clinical practice and research. Clin Exp Allergy 2018; 48:1092–1106. doi: 10.1111/cea.13207. [DOI] [PubMed] [Google Scholar]
- 6.Gelardi M, Fiorella ML, Leo G, Incorvaia C. Cytology in the diagnosis of rhinosinusitis. Pediatr Allergy Immunol 2007; 18 (Suppl 18):50–52. doi: 10.1111/j.1399-3038.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 7.Gelardi M, Maselli Del Giudice A, Fiorella ML, Soleti P, Di Gioacchino M, Conti CM, et al. Quality of life in non-allergic rhinitis depends on the predominant inflammatory cell type. J Biol Regul Homeost Agents 2008; 22:73–81. [PubMed] [Google Scholar]
- 8.Gelardi M, Russo C, Fiorella ML, Fiorella R, Ciprandi G. Inflammatory cell types in nasal polyps. Cytopathology 2010; 21:201–203. doi: 10.1111/j.1365-2303.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 9.Canonica GW, Compalati E. Minimal persistent inflammation in allergic rhinitis: implications for current treatment strategies. Clin Exp Immunol 2009; 158:260–271. doi: 10.1111/j.1365-2249.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zhou Y, Zhang L, Wang Y, Pepper AN, Cho SH, et al. Individualized treatment of allergic rhinitis according to nasal cytology. Allergy Asthma Immunol Res 2017; 9:403–409. doi: 10.4168/aair.2017.9.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Håkansson K, Bachert C, Konge L, Thomsen SF, Pedersen AE, Poulsen SS, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: the United Airways concept further supported. PLoS One 2015; 10:e0127228.doi: 10.1371/journal.pone.0127228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy 2006; 61:1275–1279. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 13.Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am 2004; 24:19–43. doi: 10.1016/S0889-8561(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Lee HM, Lee SH, Choe H, Kim HK, Lee JH, et al. Expression and distribution patterns of the stem cell marker, nestin, and the stem cell renewal factor, BMI-1, in normal human nasal mucosa and nasal polyps. Acta Otolaryngol 2009; 129:996–1001. doi: 10.1080/00016480802527560. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, Kawata R, Haruna S, Moriyama H, Hirakawa K, Fujieda S, et al. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol Int 2011; 60:491–496. doi: 10.2332/allergolint.10-OA-0234. [DOI] [PubMed] [Google Scholar]
- 16.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 2009; 124:478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy 2005; 87:111–129. doi: 10.1159/000087639. [DOI] [PubMed] [Google Scholar]
- 18.Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 2012; 129:1522–1528.e5. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 19.Wang CS, Lou HF, Meng YF, Piao YS, Zhang L. Predictive significance of tissue eosinophilia for nasal polyp recurrence. Chin J Otorhinolaryngol Head Neck Surg 2016; 51:268–272. doi: 10.3760/cma.j.issn.1673-0860.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg 2010; 142:64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013; 131:110–116.e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016; 315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 23.Lou H, Meng Y, Piao Y, Wang C, Zhang L, Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy 2015; 29:350–356. doi: 10.2500/ajra.2015.29.4231. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology 2011; 49:392–396. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
- 25.McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2018; 8:1421–1429. doi: 10.1002/alr.22194. [DOI] [PubMed] [Google Scholar]
- 26.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol 2017; 139:1966–1978.e9. doi: 10.1016/j.jaci.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pant H, Hughes A, Miljkovic D, Schembri M, Wormald P, Macardle P, et al. Accumulation of effector memory CD8+ T cells in nasal polyps. Am J Rhinol Allergy 2013; 27:e117–e126. doi: 10.2500/ajra.2013.27.3958. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Nayak JV, Sun Y, Huang Q, Zhou B. Peripheral blood T-helper cells and eosinophil populations in patients with atopic and nonatopic chronic rhinosinusitis. Am J Rhinol Allergy 2017; 31:8–12. doi: 10.2500/ajra.2017.31.4405. [DOI] [PubMed] [Google Scholar]
- 30.Derycke L, Eyerich S, Van Crombruggen K, Pérez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One 2014; 9:e97581.doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YM, Munoz A, Hwang PH, Nadeau KC. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol 2010; 137:111–121. doi: 10.1016/j.clim.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaltis AJ, Schlosser RJ, Yawn JR, Henriquez O, Mulligan JK. Characterization of B-cell subpopulations in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2013; 3:621–629. doi: 10.1002/alr.21173. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann HJ. News in cellular allergology: a review of the human mast cell and basophil granulocyte literature from January 2013 to May 2015. Int Arch Allergy Immunol 2015; 168:253–262. doi: 10.1159/000443960. [DOI] [PubMed] [Google Scholar]
- 34.Bryan WT, Bryan MP. Significance of mast cells in nasal secretions. Trans Am Acad Ophthalmol Otolaryngol 1959; 63:613–627. [PubMed] [Google Scholar]
- 35.Otsuka H, Dolovich J, Befus D, Bienenstock J, Denburg J. Peripheral blood basophils, basophil progenitors, and nasal metachromatic cells in allergic rhinitis. Am Rev Respir Dis 1986; 133:757–762. [PubMed] [Google Scholar]
- 36.Sakaguchi K, Okuda M, Ushijima K, Sakaguchi Y, Tanigaito Y. Study of nasal surface basophilic cells in patients with nasal polyp. Acta Otolaryngol Suppl 1986; 430:28–33. [PubMed] [Google Scholar]
- 37.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC study. Allergy 2015; 70:995–1003. doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med 2015; 373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 39.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, et al. Mast cell activation and autism. Biochim Biophys Acta 2012; 1822:34–41. doi: 10.1016/j.bbadis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Theoharides TC, Stewart JM, Panagiotidou S, Melamed I. Mast cells, brain inflammation and autism. Eur J Pharmacol 2016; 778:96–102. doi: 10.1016/j.ejphar.2015.03.086. [DOI] [PubMed] [Google Scholar]
- 42.Suttle MM, Harvima IT. Mast cell chymase in experimentally induced psoriasis. J Dermatol 2016; 43:693–696. doi: 10.1111/1346-8138.13234. [DOI] [PubMed] [Google Scholar]
- 43.Hua X, Naselsky WC, Jania CM, Chason KD, Huang JJ, Doerschuk CM, et al. Mast cell deficiency limits the development of chronic rhinosinusitis in mice. Ann Otol Rhinol Laryngol 2016; 125:290–296. doi: 10.1177/0003489415610775. [DOI] [PubMed] [Google Scholar]
- 44.Baba S, Kondo K, Suzukawa M, Ohta K, Yamasoba T. Distribution, subtype population, and IgE positivity of mast cells in chronic rhinosinusitis with nasal polyps. Ann Allergy Asthma Immunol 2017; 119:120–128. doi: 10.1016/j.anai.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Shaw JL, Ashoori F, Fakhri S, Citardi MJ, Luong A. Increased percentage of mast cells within sinonasal mucosa of chronic rhinosinusitis with nasal polyp patients independent of atopy. Int Forum Allergy Rhinol 2012; 2:233–240. doi: 10.1002/alr.21021. [DOI] [PubMed] [Google Scholar]
- 46.Drake-Lee AB, Bickerton R, McLaughlan P. Free histamine in nasal polyp fluid. Rhinology 1984; 22:133–138. [PubMed] [Google Scholar]
- 47.König K, Klemens C, Haack M, Nicoló MS, Becker S, Kramer MF, et al. Cytokine patterns in nasal secretion of non-atopic patients distinguish between chronicrhinosinusitis with or without nasal polys. Allergy Asthma Clin Immunol 2016; 12:19.doi: 10.1186/s13223-016-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 2014; 44:690–700. doi: 10.1111/cea.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okano M, Fujiwara T, Yamamoto M, Sugata Y, Matsumoto R, Fukushima K, et al. Role of prostaglandin D2 and E2 terminal synthases in chronic rhinosinusitis. Clin Exp Allergy 2006; 36:1028–1038. [DOI] [PubMed] [Google Scholar]
- 50.Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol 2003; 3:1–6. doi: 10.1111/j.1365-2222.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim DW, Kulka M, Jo A, Eun KM, Arizmendi N, Tancowny BP, et al. Cross-talk between human mast cells and epithelial cells by IgE-mediated periostin production in eosinophilic nasal polyps. J Allergy Clin Immunol 2017; 139:1692–1695.e6. doi: 10.1016/j.jaci.2016.09.026; doi: 10.1097/01.all.0000053260.61007.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Cellular comparison of sinus mucosa vs polyp tissue from a single sinus cavity in chronic rhinosinusitis. Int Forum Allergy Rhinol 2015; 5:14–27. doi: 10.1002/alr.21417. [DOI] [PubMed] [Google Scholar]
- 53.She W, Yang J, Wang C, Zhang L. Diagnostic value of nasal cytology in chronic rhinosinusitis assessed by a liquid-based cytological technique. Am J Rhinol Allergy 2018; 32:181–187. doi: 10.1177/1945892418768581. [DOI] [PubMed] [Google Scholar]
- 54.Ciofalo A, Pasquariello B, Iannella G, Manno A, Angeletti D, Gulotta G, et al. The role of nasal cytology in the diagnosis of allergic and non-allergic rhinitis in adult and children. Eur Rev Med Pharmacol Sci 2019; 23:5065–5073. doi: 10.26355/eurrev_201906_18170. [DOI] [PubMed] [Google Scholar]


