Abstract
Background:
The association between peripheral leukocyte count and bleeding events in nonvalvular atrial fibrillation (NVAF) patients treated with dabigatran remains unclear. This study aimed to explore the association between leukocyte count and bleeding events after excluding other confounders in NVAF patients taking dabigatran.
Methods:
A total of 851 NVAF patients treated with dabigatran (110 mg bid) were recruited from 12 centers in China from February 2015 to December 2017. Follow-up was completed by May 2018. The exposure and outcome variables were leukocyte count measured at baseline and the number of bleeding events within the subsequent 6 months. Multivariate Cox proportional hazards models were constructed to analyze independent associations, and a Cox proportional hazards regression with cubic spline functions and smooth curve fitting (penalized spline method) was used to address nonlinearity between leukocyte count and bleeding. The inflection point was calculated using a recursive algorithm, and then a two-piecewise Cox proportional hazards model for both sides of the inflection point was constructed.
Results:
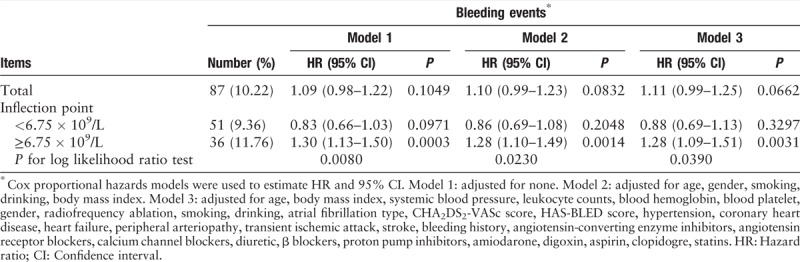
During 6-month follow-up, 87 participants occurred bleeding events. For every 1 × 109/L increase in leukocyte count, the risk of bleeding increased by 11% (hazard ratio [HR]: 1.11, 95% confidence interval [CI]: 0.99–1.25). The smooth curve showed nonlinear relationship between leukocyte count and bleeding events. The inflection point of the leukocyte count was 6.75 × 109/L. For leukocyte counts < 6.75 × 109/L, the HR (95% CI) was 0.88 (0.69–1.13), and for leukocyte counts ≥ 6.75 × 109/L, the HR (95% CI) was 1.28 (1.09–1.51).
Conclusion:
This study found a J-shaped association between baseline leukocyte count and risk of bleeding in NVAF patients treated with dabigatran.
Clinical trial registration:
Keywords: Peripheral leukocyte count, Bleeding, Nonvalvular atrial fibrillation, Threshold effect
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia that has been associated with increased mortality and morbidity from stroke and thromboembolism.[1] The introduction of oral anti-coagulants dramatically reduces the risk of stroke and thromboembolism.[2] Warfarin is the most well-documented and widely administered oral anti-coagulant in patients with both AF and non-valvular atrial fibrillation (NVAF); nevertheless, coagulation indicators need to be frequently monitored due to great differences among individual patients, and a narrow safety range exists.[3,4] Dabigatran is a competitive direct thrombin inhibitor approved worldwide for the prevention of stroke and systemic embolism in patients with NVAF based on findings of the randomized evaluation of long-term anti-coagulant therapy (RE-LY) trial.[5] Compared with warfarin, dabigatran, as a new oral anti-coagulant, had a better risk/benefit ratio in patients with NVAF, further reducing the incidence of stroke and thromboembolic events and being more convenient for use.[6–9] However, anti-coagulation therapy for thromboprophylaxis is associated with a risk of bleeding. With the increase in the use of dabigatran, there have been documented episodes of minor or even major bleeding events.[10–12]
The purpose of this study was to evaluate the efficacy and safety on anti-coagulant therapy for dabigatran in patients with NVAF, and to search for potential indicators which influenced bleeding events. It is known that leukocytosis could indicate infection, bleeding, stressful conditions, inflammation, trauma, allergy, or other diseases.[13] Previous studies have shown that bleeding events were correlated to leukocytosis in patients with sub-arachnoid hemorrhage.[14–16] So, we speculated that high leukocyte count would be associated with bleeding events in patients with NVAF treated with dabigatran, and we explored the association through the data from a prospective study.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. Informed written consent was obtained from all patients before their enrollment in this study.
Study design and participants
The present study was an observational study carried out in 12 hospitals throughout China (ClinicalTrials.gov Identifier: NCT02414035). The exposure and outcome variables were leukocyte count measured at baseline and the number of bleeding events within the subsequent 6 months. Data collection utilized an electronic data capture system. Participants with NVAF who initiated dabigatran (110 mg bid) orally after diagnosis were non-selectively and consecutively enrolled from February 2015 to December 2017, and were followed up at 1 month, 3 months, and 6 months. NVAF was diagnosed based on the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for the management of patients with AF.[17] The inclusion criteria were as follows: (1) patients diagnosed with NVAF; (2) patients aged >18 years at the start of the study; (3) patients had a CHA2DS2-VASc score ≥1; (4) dabigatran was initiated after diagnosis of NVAF; and (5) patient signed voluntarily an informed consent form. The exclusion criteria included the following: (1) patients with history of heart valve disorders, or stroke within the previous 14 days; acute coronary syndrome (ACS) within 1 year; hematuria; severe liver dysfunction (alanine aminotransferase ≥120 U/L); severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL·min−1·1.73 m−2); or major surgery in the previous month; (2) patients with history of intra-cranial, intra-ocular, spinal, retroperitoneal, or atraumatic intra-articular bleeding; gastrointestinal hemorrhage or hematuria; (3) alcohol abuse or drug addiction; (4) poor compliance; and (5) participation in any other clinical trial for investigational drugs and medical devices.
Study variables and definition of terms
The primary outcome was the occurrence of all bleeding events. The leukocyte count obtained at baseline was recorded as a continuous variable and determined with automatic blood analysis equipment in accordance with consistent standard methods at the laboratories of the different centers. Laboratory staffs were not aware of the research protocol. According to published guidelines and studies, we obtained the final outcome variable (bleeding). Major bleeding was defined as a reduction in hemoglobin concentration by at least 20 g/L, transfusion of at least two units of blood, and symptomatic bleeding in a crucial area or organ that required hospitalization. All other bleeding events were regarded as minor.[18,19] Bleeding was defined independently of leukocyte count.
Covariates in the present study included demographic data, general information, variables that affect leukocyte counts or bleeding events reported by previous studies and our clinical experiences. Therefore, the following variables were used to construct the fully adjusted model: (1) continuous variables included age; body mass index (BMI, kg/m2); CHA2DS2-VASc score (congestive heart failure, hypertension, 75 years of age and older, diabetes mellitus, previous stroke or transient ischemic attack [TIA], vascular disease, 65–74 years of age, female); HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly), obtained at baseline; systemic blood pressure (SBP, mmHg); diastolic blood pressure (DBP, mmHg); blood hemoglobin (HGB, g/L); blood platelet (PLT, ×109/L); and eGFR (mL·min−1·1.73 m−2) obtained at baseline and follow-up. (2) Categorical variables included gender; smoking; drinking; AF type; radiofrequency ablation; self-reported medical history, including hypertension, coronary heart disease (CHD), heart failure (HF), peripheral arteriopathy (PAD), TIA, stroke, bleeding history; and concomitant drugs, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), diuretic, β blockers, proton pump inhibitors (PPIs), digoxin, aspirin, clopidogrel, and statins, obtained at baseline. The CHADS2-VASc score was calculated as the sum of all points for a given patient and need ≥1. A history of previous stroke or TIA was assigned two points; congestive HF, hypertension, an age of 75 years or older, and diabetes were each assigned one point. The eGFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation.[20] BP was measured twice in the right arm after 10 min of rest. The average of the two measurements was used. Hypertension was defined as a SBP ≥140 mmHg or a DBP ≥90 mmHg or a self-reported physician diagnosis of hypertension. Information about current smoking and drinking habits, previous medical history, and use of concomitant drugs was based on a questionnaire and medical records. Participants were categorized in terms of smoking/drinking as never smokers/drinkers, former smokers/drinkers (ie, non-smokers/drinkers who previously smoked/drank daily for at least 1 year) and current smokers/drinkers (ie, daily smoking/drinking).
Follow-up
Follow-up visits were scheduled at 1, 3, and 6 months at outpatient review after the first dabigatran dose. Monitoring indicators included routine blood, routine urine, routine stool, liver, and kidney function tests, and information on self-reported bleeding events. The cut-off date for participant follow-up was May 2018. Follow-up data management was performed by specialized staff who was unaware of the outcome event. The data were stored in an electronic data acquisition system. All participants were followed for 6 months unless dabigatran was discontinued or bleeding events occurred.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequency or percentage. We used Chi-square test, one-way analysis of variance, or Kruskal-Wallis H tests to compare differences among the different leukocyte count groups (tertiles). To investigate whether leukocyte count was correlated with bleeding events in selected participants, the statistical analyses consisted of three main steps. Step 1 included univariate and multivariate Cox proportional hazards models. We constructed three models: model 1, with no covariates adjusted; model 2, with only adjustment for sociodemographic data; and model 3, which included model 2 plus the other covariates presented in Table 1. Step 2 involved addressing the non-linearity of leukocyte count and bleeding events in patients with NVAF, and Cox proportional hazards regression models with cubic spline functions and smooth curve fitting (penalized spline method) were conducted. If non-linearity was detected, we first calculated the inflection point using a recursive algorithm and then constructed a two-piecewise Cox proportional hazards model for both sides of the inflection point. We determined the best fit model based on the P values for the log-likelihood ratio test. Step 3 involved ensuring the robustness of the data analysis; therefore, we performed a sensitivity analysis in which we converted leukocyte count into a categorical variable and calculated the P for the trend. The purpose was to verify the results of leukocyte count as the continuous variable and to observe the possibility of non-linearity. All analyses were performed with statistical software packages in R (http://www.R-project.org; The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA). A P < 0.05 (two-sided) was considered statistically significant.
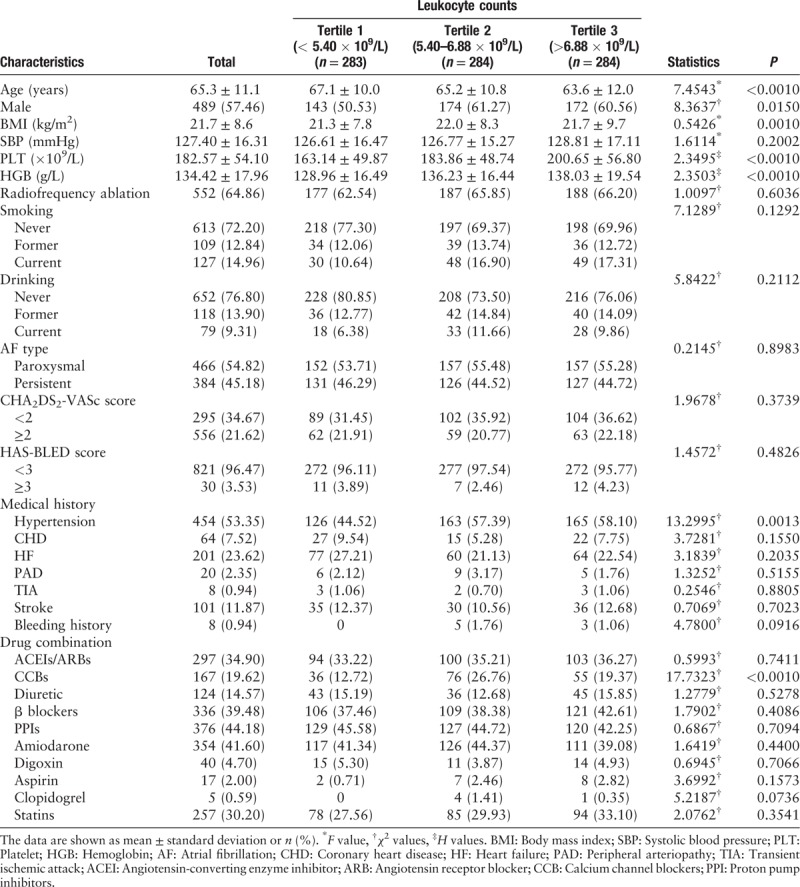
Table 1.
Baseline characteristics of all participants in this study (N = 851).
Results
Baseline characteristics of selected participants
Finally, the analysis dataset consisted of data from 851 participants with mean age of 65.3 ± 11.1 years and 489 (57.46%) males. The 87 participants occurred bleeding events (83 minor bleeding events and four major bleeding events). Bleeding events included 51 hematuria cases, 11 gingival bleeding cases, ten gastrointestinal bleeding cases, ten skin ecchymosis cases, two hemoptysis cases, one epistaxis, and two other bleeding cases. The baseline characteristics of participants are presented in Table 1 according to leukocyte count tertiles (tertile 1: leukocyte count <5.40 × 109/L; tertile 2: leukocyte count of 5.40–6.88 × 109/L; tertile 3: leukocyte count >6.88 × 109/L). No statistically significant differences were detected for the following factors among different leukocyte count tertiles: SBP, smoking, drinking, radiofrequency ablation, AF type, CHA2DS2-VASc score, HAS-BLED score, CHD, HF, PAD, TIA, stroke, bleeding history, ACEI/ARB, diuretic, β blockers, PPIs, amiodarone, digoxin, aspirin, clopidogrel, and statins (all P > 0.05). Among three leukocyte count tertiles, participants with leukocyte count >6.88 × 109/L (tertile 3) was the youngest (P < 0.0010) and had highest PLT value (P < 0.0010), HGB value (P < 0.0010), hypertension rate (P = 0.0010), and CCB rate (P < 0.0010); participants with leukocyte count of 5.40 to 6.88 × 109/L (tertile 2) had highest BMI values (P = 0.0010) and proportion of male (P = 0.0150).
Association between leukocyte count and risk of bleeding
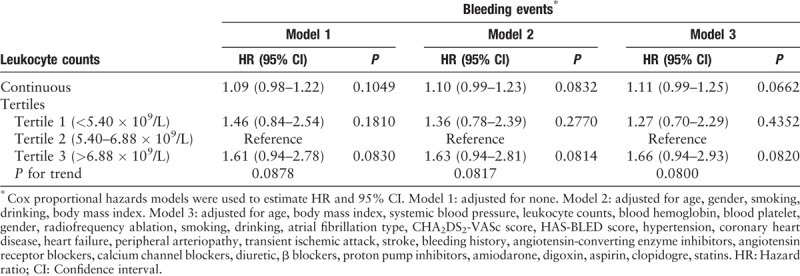
In this study, we constructed three models for analyzing the independent effects of leukocyte count on bleeding events after adjusting for other potential confounders. The hazard ratios (HRs) and 95% confidence intervals (CIs) for these three equations are listed in Table 2. In the unadjusted model (model 1), for every 1 × 109/L increase in leukocyte count, the risk of bleeding increased by 9.00% (HR: 1.09, 95% CI: 0.98–1.22). In the minimally adjusted model (model 2, adjusted for age, gender, smoking, drinking, BMI), for every 1 × 109/L increase in leukocyte count, the risk of bleeding increased by 10.00% (HR: 1.10, 95% CI: 0.99–1.23). In the fully adjusted model (model 3, adjusted for all the covariates presented in Table 1), for every 1 × 109/L increase in the leukocyte count, the risk of bleeding increased by 11.00% (HR: 1.11, 95% CI: 0.99–1.25). We also converted leukocyte count from a continuous variable to a categorical variable (tertiles). When leukocyte count was used as a categorical variable, the results of P for the trend in the fully adjusted model were consistent with the results of P when leukocyte count was a continuous variable. In addition, when leukocyte count was applied to the fully adjusted model as a categorical variable, the trend of the effect in different leukocyte count groups was non-equidistant. These results suggested that the association between leukocyte count and bleeding events was likely to be non-linear.
Table 2.
Unadjusted and adjusted association between leukocyte count and bleeding events.
Non-linearity and threshold effect between leukocyte count and risk of bleeding
In the present study, we analyzed the non-linear relationship between leukocyte count and bleeding events [Figure 1]. The result of the smooth curve showed that the relationship between leukocyte count and bleeding was non-linear (after adjusting for other covariates presented in Table 1). We fitted the association between leukocyte count and bleeding using the Cox proportional hazards regression model and the two-piecewise Cox proportional hazards regression model. The P for the log-likelihood ratio test was 0.0390. This result indicated that the two-piecewise Cox proportional hazards regression model was more suitable for analyzing the association between leukocyte count and bleeding events because it could accurately represent the relationship between leukocyte count and bleeding. Using a two-piecewise Cox proportional hazards regression and recursive algorithm, we calculated the inflection point to be 6.75 × 109/L. For leukocyte counts <6.75 × 109/L, the HR (95% CI) was 0.88 (0.69–1.13), and for leukocyte counts ≥6.75 × 109/L, the HR (95% CI) was 1.28 (1.09–1.51) [Table 3].
Figure 1.
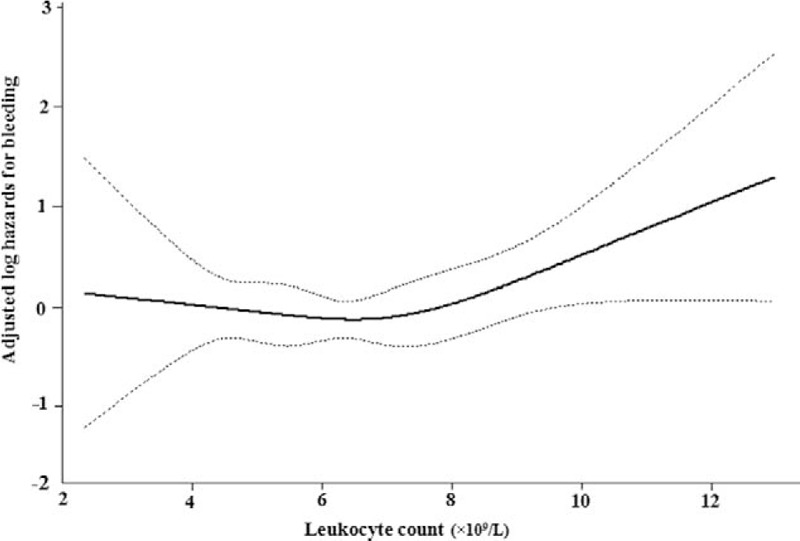
Association between Leukocyte count and bleeding events. A threshold, non-linear association between leukocyte count and bleeding events was found (P = 0.0379). The solid line and dashed line represent the estimated values and their corresponding 95% confidence interval. All adjusted for age, body mass index, systemic blood pressure, leukocyte counts, blood hemoglobin, blood platelet, gender, radiofrequency ablation, smoking, drinking, atrial fibrillation type, CHA2DS2-VASc score, HAS-BLED score, hypertension, coronary heart disease, heart failure, peripheral arteriopathy, transient ischemic attack, stroke, bleeding history, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretic, β blockers, proton pump inhibitors, amiodarone, digoxin, aspirin, clopidogre, statins.
Table 3.
Threshold effect analysis of leukocyte count on bleeding events.
Discussion
In our study, we reported the association between leukocyte count and risk of bleeding in patients with NVAF taking oral dabigatran. We found a non-linear relationship using a smoothing curve and demonstrated a threshold effect between leukocyte count and the risk of bleeding. On two sides of the inflection point of 6.75 × 109/L, the leukocyte count and risk of bleeding were not consistent, with the left side at 0.88 (95% CI: 0.69–1.13) and the right side at 1.28 (95% CI: 1.09–1.51).
Previous evidences revealed that elevated leukocyte count was a risk factor for future cardiovascular events in individuals apparently free of cardiovascular disease[21] and a prognostic marker for patients already with cardiovascular diseases.[22,23] Several studies have shown that leucocytosis was associated with increased bleeding in multiple cardiac conditions and procedures. Palmerini et al[24] reported that elevated leukocyte count was strongly related to major bleeding in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (PCI) and patients with non-ST-segment elevation ACS. Ndrepepa et al[25] also described that elevated leukocyte count was correlated with a higher incidence of major bleeding within the first 30 days following PCI, in patients presenting with ACS. Moreover, among patients undergoing cardiac surgery with cardiopulmonary bypass, high leukocyte count has been reported to be an independent predictor of increased peri-operative bleeding.[26] Therefore, the correlation between elevated leukocyte count and bleeding in considerable clinical settings has been established. There was a potential mechanism, through which leukocytosis might indirectly contribute to bleeding events, was that increases in leukocyte count concentrations might influence whole blood procoagulant activity by reducing platelet mediated procoagulation via an interaction with leukocytes.[27] George et al[28] indicated that the magnitude of the inflammatory response might be important when attempting to identify whether patients were at risk for bleeding. As we all known, leukocytosis was an important manifestation of inflammatory response. However, the mechanism linking high leukocyte count and bleeding treated with dabigatran remains to be further studied.
In our study, most of the bleeding events were minor bleeding, and the number of the patients experienced major bleeding was low. Nevertheless, there were some clinical implications of this study. First, a minor bleeding event could predict a major bleeding event.[29] Second, a minor bleeding event may lead to discontinuation of anti-coagulant treatment.[30] We prescribed a 110-mg dose instead of a 150-mg dose of dabigatran for patients with NVAF. The reasons are as follows: first, there was no obvious advantage of 150 mg over 110 mg in further reducing the risk of ischemic stroke and all-cause mortality in Asian patients, as demonstrated in the RE-LY trial.[31,32] Second, Asians have a substantially smaller average body size, lower average body weight, and lower average BMI than those in non-Asians[33]; therefore, a lower dose of dabigatran should be more suitable in China. Third, physicians may conservatively prescribe a lower dose of dabigatran because of the concern regarding bleeding problems.
Some limitations should be noted. First, the study participants were from a Chinese population; thus, the generalizability of the results to other populations remained to be verified. Second, the findings in this study were based on 110 mg instead of 150 mg dabigatran doses, so conclusions could not be applied to patients taking 150 mg of dabigatran. Third, single measurement of leukocyte count might have resulted in the misclassification of some comorbidities. However, measurement error was not equal to measurement bias, and it was reasonable to assume that the potential measurement errors would not bias our findings. Fourth, as in all observational studies, even though known potential confounding factors were controlled for, there might have been still uncontrolled confounding due to unmeasured differences in behaviors or other factors. Despite these limitations, our research also had advantages. We reported the association between leukocyte count and risk of bleeding in patients with NVAF taking oral dabigatran. In addition, we addressed the non-linearity between the leukocyte count and risk of bleeding and further explained the non-linearity. We provided adequate statistical rationale for the evaluation of independent risk due to leukocyte count, a feature that was lacking in previous studies.
In conclusion, this study found a J-shaped association between baseline leukocyte count and risk of bleeding in patients with NVAF treated with dabigatran. Patients with NVAF taking dabigatran with leukocyte counts greater than 6.75 × 109/L may be more prone to bleeding. The present findings may contribute to the construction of a prediction model for bleeding among patients with NVAF treated with dabigatran in the future.
Funding
This study was supported by grants from the Major New Drug Creation Program from National Science and Technology Major Project (No. 2014ZX09303305), the Science and Technology Planning Project of Jiangxi Province (No. 20161ACG70012), and the Key Project of Jiangxi Provincial Education Department (No. GJJ170013).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhou W, Wang T, Zhu LJ, Wen MH, Hu LH, Huang X, You CJ, Li JX, Wu YQ, Wu QH, Bao HH, Cheng XS. Peripheral leukocyte count and risk of bleeding in patients with non-valvular atrial fibrillation taking dabigatran: a real-world study. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000423
References
- 1.Lip GY, Boos CJ. Antithrombotic treatment in atrial fibrillation. Heart 2006; 92:155–161. doi: 10.1136/hrt.2005. 066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahid F, Rahmat NA, Lip GYH, Shantsila E. Prognostic implication of monocytes in atrial fibrillation: the West Birmingham Atrial Fibrillation Project. PLoS One 2018; 13:e0200373.doi: 10.1371/journal.pone. 0200373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrail fibrillation in China. J Am Coll Cardio 2008; 52:865–868. doi: 10.1016/j.jacc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Society of Cardiology, Chinese Medical, Association. Retrospective investigation of hospitalized patients with atrial fibrillation in mainland China. Chin Med J 2004; 117:1763–1767. doi: 10.1016/j.ijcard.2004.12. 042. [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrail fibrillation. N Engl J Med 2013; 369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 9.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trails. Lancet 2014; 383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 11.Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012; 366:864–866. doi: 10.1056/NEJMc1112874. [DOI] [PubMed] [Google Scholar]
- 12.Legrand M, Mateo J, Aribaud A, Ginisty S, Eftekhari P, Ba PT, et al. The use of dabigatran in elderly patients. Arch Intern Med 2011; 171:1285–1286. doi: 10.1001/archinternmed.2011.314. [DOI] [PubMed] [Google Scholar]
- 13.Neil-Dwyer G, Cruickshank J. The blood leucocyte count and its prognosis significance in subarachnoid hemorrhage. Brain 1974; 97:79–86. doi: 10.1093/brain/97.1.79. [DOI] [PubMed] [Google Scholar]
- 14.Kayhanian S, Weerasuriya CK, Rai U, Young AMH. Prognostic value of peripheral leukocyte counts and plasma glucose in intracerebral haemorrhage. J Clin Neurosci 2017; 41:50–53. doi: 10.1016/j.jocn.2017.03. 032. [DOI] [PubMed] [Google Scholar]
- 15.Söderholm M, Zia E, Hedblad B, Engström G. Leukocyte count and incidence of subarachnoid haemorrhage: a prospective cohort study. BMC Neurol 2014; 14:71.doi: 10.1186/1471-2377-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajapathy SK, Idris Z, Kandasamy R, Hieng AWS, Abdullah JM. Inflammatory biomarkers and their value in predicting survival and outcome among patients with spontaneous intracerebral haemorrhage. Malays J Med Sci 2017; 24:51–65. doi: 10.21315/mjms2017.24.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 2014; 64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376:975–983. doi: 10.1016 /S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata M, Yokoyama Y, Sasano T, Hachiya H, Tanaka Y, Yagishita A, et al. Bleeding events and activated partial thromboplastin time with dabigatran in clinical practice. J Cardiol 2013; 62:121–126. doi: 10.1016 /j.jjcc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med 1974; 290:1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 22.Schlant RC, Forman S, Stamler J, Canner PL. The natural history of coronary heart disease: prognostic factors after recovery from myocardial infarction in 2789 men. The 5-year findings of the coronary drug project. Circulation 1982; 66:401–414. doi: 10.1161/01.cir.66.2.401. [DOI] [PubMed] [Google Scholar]
- 23.Lowe GD, Machado SG, Krol WF, Barton BA, Forbes CD. White blood cell count and haematocrit as predictors of coronary recurrence after myocardial infarction. Thromb Haemost 1985; 54:700–703. doi: 10.1055/s-0038-1660101. [PubMed] [Google Scholar]
- 24.Palmerini T, Mehran R, Dangas G, Nikolsky E, Witzenbichler B, Guagliumi G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation 2011; 123:2829–2837. doi: 10.1161/CIRCULATIONAHA.110.985564. [DOI] [PubMed] [Google Scholar]
- 25.Ndrepepa G, Braun S, Iijima R, Keta D, Byrne RA, Schulz S, et al. Total leucocyte count, but not C-reactive protein, predicts 1-year mortality in patients with acute coronary syndromes treated with percutaneous coronary intervention. Clin Sci (Lond) 2009; 116:651–658. doi: 10.1042/CS20080298. [DOI] [PubMed] [Google Scholar]
- 26.Koskenkari J, Rimpiläinen J, Biancari F, Surcel HM, Kaukoranta P, Kiviluoma K, et al. Leukocyte depleting filter attenuates myocardial injury during elective coronary artery bypass surgery. Scand Cardiovasc J 2005; 39:358–368. doi: 10.1080/14017430510035943. [DOI] [PubMed] [Google Scholar]
- 27.Perussia B, Jankiewicz J, Trinchieri G. Binding of platelets to human monocytes: a source of artifacts in the study of the specificity of antileukocyte antibodies. J Immunol Methods 1982; 50:269–276. doi: 10.1016/0022-1759(82)90164-8. [DOI] [PubMed] [Google Scholar]
- 28.George JD, Vladimir L, Lawrence TG. Relationship between leukocyte count and patient risk for excessive blood loss after cardiac surgery. Crit Care Med 1997; 25:1338–1346. doi: 10.1097/00003246-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Flaker GC, Eikelboom JW, Shestakovska O, Connolly SJ, Kaatz S, Budaj A, et al. Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke 2012; 43:3291–3297. doi: 10.1161/STROKEAHA.112.664144. [DOI] [PubMed] [Google Scholar]
- 30.Ho MH, Ho CW, Cheung E, Chan PH, Hai JJ, Chan KH, et al. Continuation of dabigatran therapy in “real-world” practice in Hong Kong. PLoS One 2014; 9:e101245.doi: 10.1371/journal.pone.0101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013; 44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 32.Chan YH, Yen KC, See LC, Chang SH, Wu LS, Lee HF, et al. Cardiovascular, bleeding, and mortality risks of dabigatran in asians with nonvalvular atrial fibrillation. Stroke 2016; 47:441–449. doi: 10.1161/STROKEAHA.115.011476. [DOI] [PubMed] [Google Scholar]
- 33.Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: implications for definitions of overweight and obesity for Asians. Am J Clin Nutr 2004; 79:31–39. doi: 10.1093/ajcn/79.1.31. [DOI] [PubMed] [Google Scholar]


