Abstract
The Karnaphuli River estuary, located in southeast coast of Bangladesh, is largely exposed to heavy metal contamination as it receives a huge amount of untreated industrial effluents from the Chottagram City. This study aimed to assess the concentrations of five heavy metals (As, Pb, Cd, Cr and Cu) and their bioaccumulation status in six commercially important fishes, and also to evaluate the potential human health risk for local consumers. The hierarchy of the measured concentration level (mg/kg) of the metals was as follows: Pb (13.88) > Cu (12.10) > As (4.89) > Cr (3.36) > Cd (0.39). The Fulton’s condition factor denoted that fishes were in better ‘condition’ and most of the species were in positive allometric growth. The bioaccumulation factors (BAFs) of the contaminants observed in the species were in the following orders: Cu (1971.42) > As (1042.93) > Pb (913.66) > Cr (864.99) > Cd (252.03), and among the specimens, demersal fish, Apocryptes bato appeared to be the most bioaccumulative organism. Estimated daily intake (EDI), target hazard quotient (THQ), hazard index (HI) and carcinogenic risk (CR) assessed for potential human health risk implications suggest that the values were within the acceptable threshold for both adults and children. However, calculated CR values indicated that both age groups were not far from the risk, and HI values demonstrated that children were nearly 6 times more susceptible to non-carcinogenic and carcinogenic health effects than adults.
Introduction
Heavy metals pollution has become a major concern worldwide due to their toxicity, intrinsic persistence, non-biodegradable nature, and accumulative behaviors [1]. These metals differ from other toxic materials in a way that they are neither created nor destroyed by human. They are inert in the environment and are often considered to be conservative pollutants if left undisturbed [2]. However, the rapid industrialization, urbanization, population growth, agricultural and other human activities have resulted in severe pollution by heavy metals globally, especially in developing countries [3]. Significant quantities of heavy metals from such activities are discharged into rivers, which can be strongly accumulated and biomagnified along water, sediment, and aquatic food chain, resulting in sublethal effects or death in local fish populations [4]. As fishes occupy higher trophic level in the food chain, they are considered one of the most common bioindicators for pollutants [5, 6]. Again, fishes are consumed by human as a major source of protein for many years. Thus, the human body is largely susceptible to enriched heavy metal concentration in fishes [7]. Consequently, an analysis of the levels of heavy metals in fish could be used to investigate anthropogenic impacts on ecosystem and human health.
Generally, bioaccumulation and biomagnification occur due to longstanding anthropogenic activities within a coastal ecosystem [8]. The accumulation of heavy metals in fish organs could also be driven by physiochemical and biological variables such as pH, temperature, hardness, exposure duration, feeding habits of species and habitat complexity [9]. While terrestrial species exhibit a strong pattern of biomagnification, marine and estuarine organisms show less clear pattern [10]. Condition factor, is one of the most common tools that is widely used to assess the life, reproduction and health conditions, as well as the life cycle of a fish species [11]. Along with that, condition factor also suggests the food availability and quality, breeding duration, and process for distinct populations [12, 13]. In addition, this tool indicates the status of fish health due to stress in the population within an ecosystem [14].
Heavy metals and metalloids, when occurring at higher concentrations, become severe poisons for all living organisms including human. For example, an excessive amount of Hg, As, Pb, and Cd elements could be detrimental to the living cells, and a prolonged exposure to the body can lead to illness or death [15]. Among the metals, Hg is the most toxic metal in our environment. Methyl mercury toxicity are inhibition of protein synthesis, microtubule disruption, increase of intracellular Ca2+ with disturbance of neurotransmitter function [16]. Prostatic proliferative lesions, lung cancer, bone fractures, kidney failures are associated with chronic exposure to Cd, even at a low concentration of ~ 1 mg/kg [17, 18]. Excessive Pb can have detrimental health effect on human [19] including nervous system disorders, mental retardation, skeletal hematopoietic function disorder and even death [20]. Cr was reported to have carcinogenic effects on human health [21, 22].
The Karnaphuli River estuary is one of the potential fish habitats along the southeast coast of Bay of Bengal known to be an important breeding, feeding and nursery ground for many aquatic species. At present, the ecosystem is receiving untreated effluents from several industries including textile crafts, dyeing industry and others as it passes through the industrial zone [23]. A number of studies have attempted to assess the contamination status from river and estuarine environment from Bangladesh [3, 24–27], from China [28], from Turkey [29]. However, to date there has been no proper investigation carried out on the potential human health risk due to heavy metal contamination in the fish species harvested and consumed from the Karnaphuli estuary. Therefore, this study aimed to fill this knowledge gap by assessing the concentration of heavy metals in some selected fish species and their bioaccumulation status, and the human health risk for local adult and children consumers.
Materials and methods
Ethical statement
Specimens from wild populations were collected from local fishermen. None of the sampled species were endangered or protected. No permit was required to conduct the present study. There were no ethical considerations linked to the experiment.
Sampling
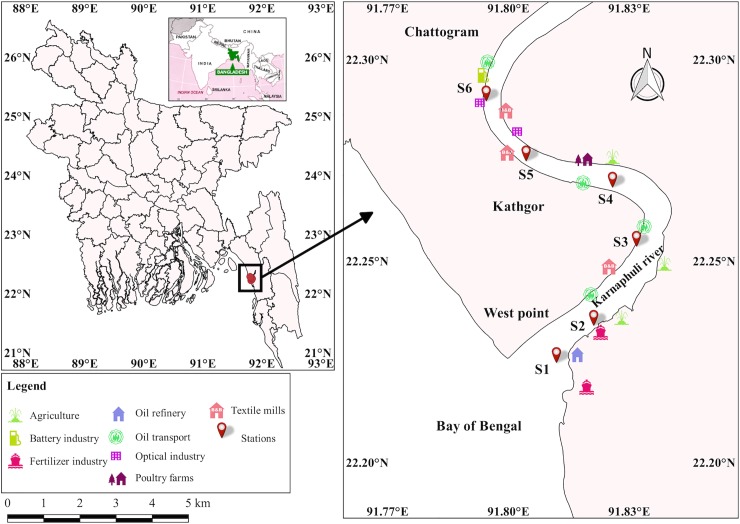
The study area (Karnaphuli River estuary) is located from 22.234008 N and 91.821105 E to 22.289695 N and 91.794403 E (Fig 1). A total of six commercial species (i.e. Apocryptes bato (Chewa), Pampus chinensis (Rup chanda), Liza parsia (Bata), Mugil cephalus (Flathead bata), Hyporhamphus limbatus (Ek Thuitta), and Tenualosa toil (Chandana ilish) were collected from fishermen for a period of seven months (February 2018 to August 2018) using seine net. The collected samples were carried in plastic ice container and immediately stored at –20°C. Afterwards in the lab, total length (cm) and weights (gm) were measured carefully to the nearest 0.1 cm using a vernier caliper; total weight was determined with an electronic balance to 0.01 g accuracy. Muscles of each specimen were dissected with stainless steel scissors for further chemical analysis using inductively coupled plasma mass spectrometry (ICP-MS, Model: ELAN9000, Perkin-Elmer, Germany) for metal detection. Data were analysed statistically by fitting a straight line adopting the least square method.
Fig 1. Six sampling locations in Karnaphuli River estuary and the source of heavy metal pollution.
[Reprinted from (www.wikipedia.org) under a CC BY license, with permission from (Wikipedia), original copyright (2007)].
Chemical analysis procedures and accuracy
A 1.5 g of dissected muscle was used for subsequent analysis. The samples were dried in a Zirbus freeze drying machine (Model: VaCo 2, Germany, CFC free, condenser dimensions-Ø200×200 mm) for 24 h [30]. After cooling, 0.5 g of muscle samples were placed in 10ml of of 100% HNO3. The solution was heated on an oil bath and added with 4 drops of H2O2 repeatedly until the mixture turned clear. The solution was mineralized using microwave digester (model: WX-6000, China). After filtering, solution was diluted for 2h and kept in a 50 ml tezaron tube [3]. A standard reagent (Merck VI; Germany) was used to analyze the metal concentration in the targeted fish species, which was prepared from the same acid matrix [1]. Moreover, certified reference material (CRM 320, Merck KGaA, Germany) was employed for validation and accuracy check [3].
Metal pollution index (MPI)
To assess the metal pollution, the metal pollution index (MPI) was adopted following [31] and [32]. The equation is as follows:
where, CM1 is the concentration value of first concerned metal, CM2 is the concentration value of second concerned metal, CM3 is the concentration of third concerned metal, CMn is the concentration of nth metal (mg/kg dry wt) in the tissue sample of a certain species.
Statistical analysis
The mean and standard deviation of metal concentrations were calculated. The Kolmogorov-Smirnov, Shapiro-Wilk and Kruskal-Wallis tests were performed using SPSS 23. The Kolmogorov-Smirnov and Shapiro-Wilk tests were performed to check the normality of the data [33]. The Kruskal-Wallis test was carried out to identify significant variance of the targeted elements in the specimens of the studied area where p ≤ 0.05 was used as the cut-off for significance (confidence level in 95%). In addition, the Levene’s test with the significant level at p ≤ 0.05 was adopted to determine the homogeneity of variances in terms of ANOVA tests. Correlation matrix (CM) and principal component analysis (PCA) were performed to determine the correlations and association between heavy metals in the studied fish species [34]. The correlation matrix used to analyze heavy metals could result in both positive and negative outcomes [35–38]. Hierarchical cluster analysis is one of the most widely used hierarchical algorithms which results in clusters in which variables or individuals are added in sequence considering hierarchy of the cluster [39]. Additionally, the similar groups of the studied elements within the sampling sites measured as a distance between the two closest members, and is conducted by Origin 9 software [3, 40]. Metals with similar properties were pooled in one or associated cluster while the dissimilar groups of elements were plotted in a separate cluster, and thus differentiated the contamination status of the samples [41–43].
Bioaccumulation factor
Bioaccumulation factors (BAFs) were calculated as a ratio between the concentration level of biota (those in water) and the living environment of the specimens, and was expressed as follows [44–46]:
where CnBiota is the concentration of metal in the tissues (mg/kg) and CnWater is the metal concentration in the aquatic environment (mg/l). BAF is categorized as follows: BAF < 1000: no probability of accumulation; 1000 < BAF < 5000: bioaccumulative; BAF > 5000: extremely bioaccumulative [47].
Length-weight relationship and condition factor
The length-weight relationship of the fish samples were calculated using Fulton condition factor following the equation [48–51]:
where W is the total body weight of fish (gm), L is the total length of fish (cm). Fulton`s Q is categorized as follows: Q = 1: Condition is poor, Q = 1.2: condition is moderate, Q ≥ 1.40: condition is proportionally good [52]. The equation can be expressed by the following formula [12, 53]:
The equation can be estimated using the least-square formula adopted with the logarithm form of the equation is shown as [54]:
where ‘a' is the calculated intercept of the regression line, and ‘b’ is the coefficient of that regression. The ‘b’ values signify the growth pattern of an organism which can be classified as follows: b < 3: negative allometric, b = 3: Isometric and b > 3: positive allometric [48, 55].
As Fulton’s Q is substantially correlated with the length-weight relationship, exponent ‘b’ acts an identical role of determining the well-being of the organisms [48]. The deviation of the condition, further, depends on the food availability and the divergence of reproductive organ development [56].
Human health risk
Estimated daily intake (EDI)
Estimated daily intake (EDI) was calculated by the following equation [22, 57, 58]:
where Cn is the concentration level of metal in the selected fish tissues (mg/kg dry-wt); IGr is the acceptable ingestion rate, which is 55.5 g/day for adults and 52.5 g/day for children [59, 60]; Bwt is the body weight: 70 kg for adults and 15 kg for children [59].
Target hazard quotient (THQ) for non-carcinogenic risk assessment
THQ was estimated by the ratio of EDI and oral reference dose (RfD). RfDs of the different metals for example As, Pb, Cd, Cr, and Cu are 0.0003, 0.002, 0.001, 0.003 and 0.3, respectively [59, 61]. The value of ratio < 1 implies a non-significant risk effects [62]. The THQ formula is expressed as follows [60, 63–65]:
Where Ed is exposure duration (65 years) (USEPA, 2008); Ep is exposure frequency (365 days/year) [21]; At is the average time for the non-carcinogenic element (Ed×Ep).
Hazard index (HI)
Hazard index (HI) was calculated for the multiple elements (Hg, As, Mn, and Cr) found in the fish samples and the equation is as follows [3, 19, 66, 67]:
where, THQs is the estimated risk value for individual metal [68]. When HI value is higher than 10, the non-carcinogenic risk effect is considered high for exposed consumers [69–71].
Carcinogenic risk (CR)
To assess the probability of developing cancer over a lifetime, the carcinogenic risk is evaluated for the consequence of exposure to the substantial carcinogens [72, 73]. The acceptable range of the risk limit is 10−6 to 10−4 [74–77]. CRs higher than 10−4 are likely to increase the probability of carcinogenic risk effect [78, 79]. The established equation to assess the CR is as follows [60, 61, 80, 81]:
Where CSf is oral slope factor of particular carcinogen (mg/kg-day) [74]. Available CSf values (mg/kg-day) are: As (1.5), Pb (0.0085) and Cd (6.3) [74].
Results
The concentration of heavy metals and source identification
The average concentration of Pb, Cu, As, Cr, and Cd from the fish tissues were 13.88 mg/kg (range: 3.19–6.19 mg/kg); 12.10 mg/kg (range: 10.27–16.41 mg/kg); 4.89 mg/kg (range: 3.19–6.19 mg/kg); 3.36 mg/kg (range: 2.46–4.17 mg/kg), and 0.39 mg/kg (range: 0.21–0.74 mg/kg) respectively. Therefore, the hierarchy of the metal concentrations was: Pb > Cu > As > Cr > Cd (please see Table 1). From the results, Pb was 1.14, 2.8, 4.13, and 35.44 folds higher than Cu, As, Cr, and Cd respectively, and contributed 40% of all the elements in the study area. Along with that, Pb attributed a higher concentration of 15.73 mg/kg at S1, whereas Cd was accounted for lower concentration of 0.29 mg/kg on average observed at S1.
Table 1. Concentration of heavy metals of different species and their feeding nature, length and weight and a comparison of other relevant studies along with various standard guideline values.
| Species | Feeding nature | Amounts | Length (cm) |
Weight (gm) |
Heavy metals (mean ± std) mg/kg | MPI | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Pb | Cd | Cr | Cu | ||||||||
| A. bato | Demersal | 18 | 13.26±1.35 | 72.64±6.39 | 4.65±1.06 | 15.22±1.32 | 0.60±0.13 | 3.54±0.54 | 15.29±3.82 | 4.70 | ||
| P. chinensis | Demersal | 18 | 6.96±0.90 | 166.58±21.68 | 5.03±0.86 | 14±1.79 | 0.44±0.14 | 3.59±0.55 | 13.10±2.49 | 4.29 | ||
| L. parsia | Demersal | 18 | 7.34±1.17 | 25.20±3.12 | 4.36±0.93 | 13.98±1.93 | 0.34±0.09 | 3.30±0.40 | 9.50±1.16 | 3.65 | ||
| M. cephalus | Pelagic | 18 | 27.17±2.30 | 711.44±111.03 | 4.89±0.48 | 12.70±1.72 | 0.31±0.09 | 3.14±0.36 | 11.48±1.27 | 3.69 | ||
| H. limbatus | Pelagic | 18 | 10.97±0.80 | 26.77±4.39 | 5.14±0.86 | 13.77±1.54 | 0.35±0.08 | 3.52±0.48 | 12.52±1.40 | 4.05 | ||
| T. toli | Pelagic | 18 | 30.68±2.54 | 646.82±41.16 | 5.26±0.49 | 13.61±0.82 | 0.31±0.07 | 3.11±0.54 | 10.72±1.47 | 3.75 | ||
| Water (mg/l) | 0.006±0.003 | 0.017±0.006 | 0.002±0.001 | 0.006±0.002 | 0.006±0.001 | |||||||
| Guidelines (mg/kg) | ||||||||||||
| FAO | 1 | 2.5 | 0.2 | 1 | 10 | [82] | ||||||
| WHO | 0.01 | 2 | – | 0.15 | 3 | [83] | ||||||
| EU | – | 0.1 | 0.05 | 1 | 3 | [84] | ||||||
| Bangladesh (fish) | 5 | 0.30 | 0.25 | – | 5.00 | [85] | ||||||
| Literature (mg/kg) | ||||||||||||
| Coastal area, Bangladesh | 0.08–13 | 0.07–0.63 | 0.03–0.09 | 0.15–2.2 | 1.3–14 | [86] | ||||||
| Arasalar River, India | – | 0.23 | 6.13 | 0.3 | – | [87] | ||||||
| North east coast, India | 0.64 | – | 0.33 | – | 3.9 | [88] | ||||||
| Ganga River, India | – | 3–6 | 0.1–2.9 | – | 10–100 | [89] | ||||||
| Pearl River, China | – | 8.64 | 8.55 | 8.73 | 2.48 | [90] | ||||||
| Meiliang Bay, China | – | 0.636 | 0.173 | 0.118 | 0.336 | [91] | ||||||
| Iskenderun Bay, Turkey | – | 0.09–6.95 | 0.01–4.16 | 0.07–6.46 | 0.04–5.43 | [92] | ||||||
The evaluated MPIs ranged from 3.65 mg/kg to 4.70 mg/kg with the mean of 4.02 mg/kg (Table 1). Due to higher concentration level, the maximum MPI value (4.70 mg/kg) was corresponded to A. bato, followed by P. chinensis (4.2 mg/kg) H. limbatus (4.05 mg/kg), T. toli (3.75 mg/kg), M. cephalus (3.69 mg/kg), and L. parsia (3.65 mg/kg).
From the statistical results, Kolmogorov-Smirnov and Shapiro-Walk test revealed that the metals in the targeted fish species were non-normally distributed along the study area. The adopted Levene’s tests showed that metals were non-homogenously distributed. Kruskal-Wallis test identified that the distribution of metal was significantly different (p ≤ 0.05) in the fish species along the sampling stations. From Table 2, among the species, L. parsia and H. limbatus exhibited significant relationship (regression line) with As only, where T. toil showed significant relationship with As and Pb (p ≤ 0.05). None of the other metals showed a significant linear relationship with the organisms. Among the species, T. toil showed the maximum response for Pb (R2 = 99.5%), followed by L. parsia for As (R2 = 83.7%).
Table 2. A regression analysis between metal distributions in the selected specimens.
| Species and metals | Regression equation | Standard error | Pearson’s r | P values | R2 (%) |
|---|---|---|---|---|---|
| A. bato | |||||
| As | y = 8.66+0.99x | 0.404 | 0.774 | 0.070 | 58.1 |
| Pb | y = 11.88+0.09x | 0.511 | 0.088 | 0.867 | 0.8 |
| Cd | y = 13.44–0.31x | 5.166 | -0.029 | 0.955 | 0.01 |
| Cr | y = 12.70+0.16x | 1.25 | 0.063 | 0.904 | 0.4 |
| Cu | y = 11.56+0.11x | 0.168 | 0.313 | 0.545 | 9.8 |
| P. chinensis | |||||
| As | y = 4.19 + 0.55x | 0.449 | 0.522 | 0.287 | 27.3 |
| Pb | y = 5.14 + 0.13x | 0.243 | 0.257 | 0.621 | 6.6 |
| Cd | y = 5.72 + 2.81x | 3 | 0.425 | 0.4 | 18.1 |
| Cr | y = 5.75 + 0.34x | 0.8 | 0.205 | 0.695 | 4.2 |
| Cu | y = 4.58 + 0.18x | 0.157 | 0.502 | 0.309 | 25.3 |
| L. parsia | |||||
| As | y = -0.96 + 0.73x | 0.16 | 0.914 | 0.01 | 83.7 |
| Pb | y = 7.79 + 0.84x | 0.711 | 0.51 | 0.301 | 26.0 |
| Cd | y = 0.12 + 0.03x | 0.036 | 0.378 | 0.459 | 14.3 |
| Cr | y = 1.86 + 0.2x | 0.139 | 0.58 | 0.227 | 33.7 |
| Cu | y = 4.24 + 0.72x | 0.346 | 0.719 | 0.107 | 51.7 |
| M. cephalus | |||||
| As | y = 2.08+0.11x | 0.09 | 0.5 | 0.311 | 25.0 |
| Pb | y = 23.98–0.42x | 0.309 | -0.557 | -0.557 | 31.0 |
| Cd | y = 0.87–0.02x | 0.016 | -0.547 | 0.26 | 30.0 |
| Cr | y = 4.04–0.03x | 0.075 | -0.217 | 0.68 | 4.7 |
| Cu | y = 16.95–0.21x | 0.257 | -0.364 | 0.477 | 13.3 |
| H. limbatus | |||||
| As | y = -4.63+0.89x | 0.309 | 0.821 | 0.045 | 67.5 |
| Pb | y = 1.09+1.156x | 0.778 | 0.596 | 0.211 | 35.6 |
| Cd | y = 0.36–0.001x | 0.048 | -0.014 | 0.978 | 0.01 |
| Cr | y = -0.6+0.38x | 0.238 | 0.620 | 0.189 | 38.5 |
| Cu | y = 2.54+0.91x | 0.749 | 0.519 | 0.291 | 26.9 |
| T. toli | |||||
| As | y = 0.48+ 0.16x | 0.059 | 0.8 | 0.05 | 63.9 |
| Pb | y = 3.74+0.32x | 0.012 | 0.997 | 1.11E-5 | 99.5 |
| Cd | y = 0.35–0.01x | 0.013 | -0.042 | 0.936 | 0.2 |
| Cr | y = -1.22+ 0.14x | 0.08 | 0.662 | 0.152 | 43.8 |
| Cu | y = 0.37 + 0.34x | 0.237 | 0.581 | 0.227 | 33.7 |
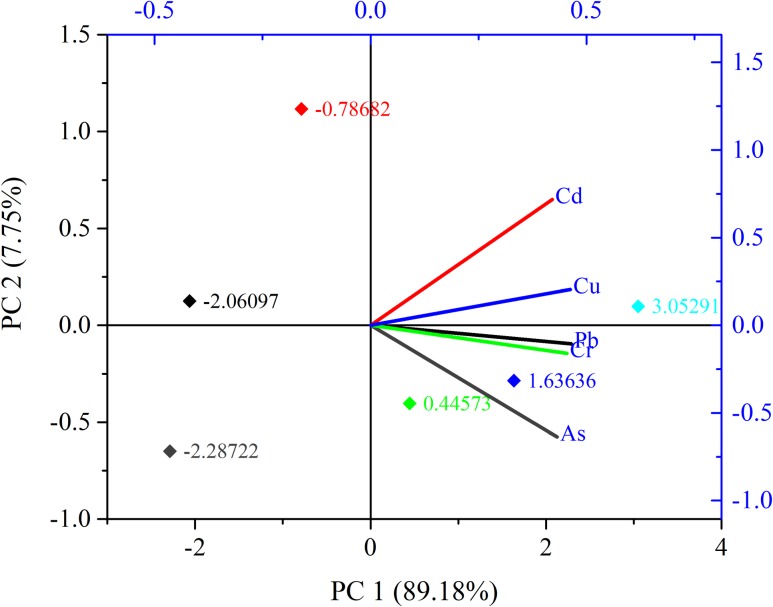
Table 3 depicts the correlation matrix among the metals in different fish species. The most significant positive correlations were observed between As—Pb (r = 0.912), As–Cr (r = 0.904), Pb–Cr (r = 0.917), Pb–Cu (r = 0.967), and Cd–Cu (r = 0.911) at p <0.05 level. Based on CM, the rotated component plot, PCA was depicted in Fig 2. The PCA exhibited two factors PC 1 and PC 2, which resulted in the corresponded variance of 89.18% and 7.75% respectively. Both factors were responsible for cumulative variance of 96.93%. Cu and Pb were the most dominant elements in PC 1, corresponded with the loadings of 20.78% and 20.70% respectively. On the contrary, in PC 2, Cd and Cu showed the domination with the loadings of 77.81% and 22.19% respectively.
Table 3. Pearson’s correlation coefficient between heavy metal elements and matrix of PCA loadings.
| Correlation analysis | Principal component analysis | ||||||
|---|---|---|---|---|---|---|---|
| As | Pb | Cd | Cr | Cu | PC 1 | PC 2 | |
| As | 1 | 0.432 | -0.637 | ||||
| Pb | 0.912* | 1 | 0.465 | -0.106 | |||
| Cd | 0.640 | 0.828 | 1 | 0.421 | 0.718 | ||
| Cr | 0.904* | 0.917* | 0.820 | 1 | 0.455 | -0.160 | |
| Cu | 0.840 | 0.967* | 0.911* | 0.890 | 1 | 0.463 | 0.205 |
| Eigenvalues | 4.459 | 0.387 | |||||
| % of Variance | 89.18 | 7.75 | |||||
| Cumulative % | 89.18 | 96.93 | |||||
* Significant at p ≤ 0.05
Fig 2. Loading plots of principal component analysis (PCA) of elements.
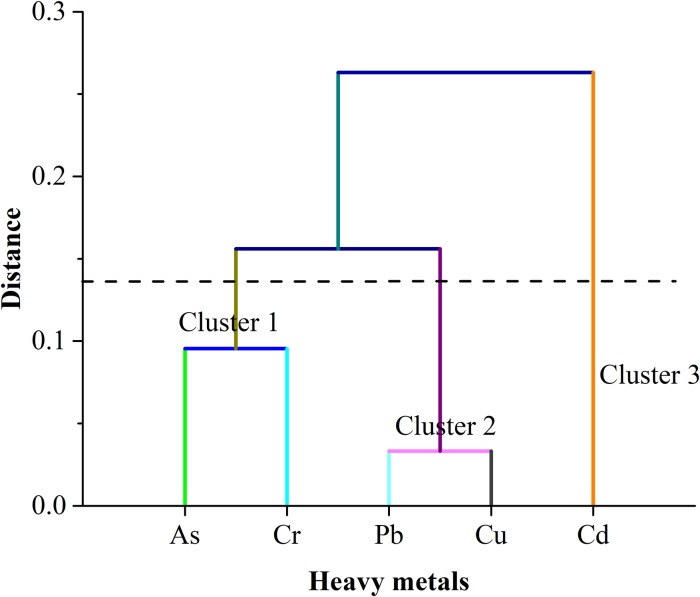
To execute hierarchical cluster dendrogram, the Ward-Linkage method was employed with Euclidean distance, which resulted in three distinct clusters, presented in Fig 3. Cluster 1 included As and Cr that could have been originated from anthropogenic activities like chemical industries. Pb, and Cu confined in cluster 2 could have been attributed from the textile organization, fertilization and oil droppings from the boats/ships in the study area. Lastly, Cd was included in cluster 3, and source of the Cd could have come from battery industry.
Fig 3. Hierarchical cluster (dendrogram) using Ward linkage method among the experimented metals in fish species.
Length-weight Relationship and condition factor evaluation
Higher ‘b’ value reflected the appetite state and reproductive organ development of the species [55]. The identified b value of L. parsia from the length weight relationship was close to 3, hence, it represented isometric growth pattern that was considered as ideal shape (Table 4). Meanwhile, among all, species, P. chinensis exhibited the highest positive allometric growth, which was 37 times higher, than average value and 14 folds, on average, higher than other species.
Table 4. Length-weight relationship, growth pattern and Fulton condition factor for the targeted fishes.
| Species | a (intercept) ± SE | b (slope regression) ± SE | Group (Growth pattern) | W = aLb | Fulton`s Q ± std | Fish Condition |
|---|---|---|---|---|---|---|
| A. bato | 19.62 ±16.63 | 3.99±1.24 | Positive allometric | W = 19.62×L3.99 | 3.22±0.78 | Good |
| P. chinensis | 7.23±25.94 | 22.88±3.7 | Positive allometric | W = 7.23×L22.88 | 51.77±15.26 | Good |
| L. parsia | 7.13±3.8 | 2.76±0.51 | Isometric | W = 7.13×L2.46 | 7.10±3.44 | Good |
| M. cephalus | 0.002±0.0008 | 10.85± 4.98 | Positive allometric | W = 0.002×L10.85 | 3.56±0.45 | Good |
| H. limbatus | 0.01±0.002 | 2.43±0.41 | Negative | W = 0.01×L2.43 | 2.03±0.23 | Good |
| T. toli | 164.30±60.97 | 15.72±1.98 | Positive allometric | W = 164×L15.72 | 2.29±0.46 | Good |
Bioaccumulation (BAF) status of targeted species
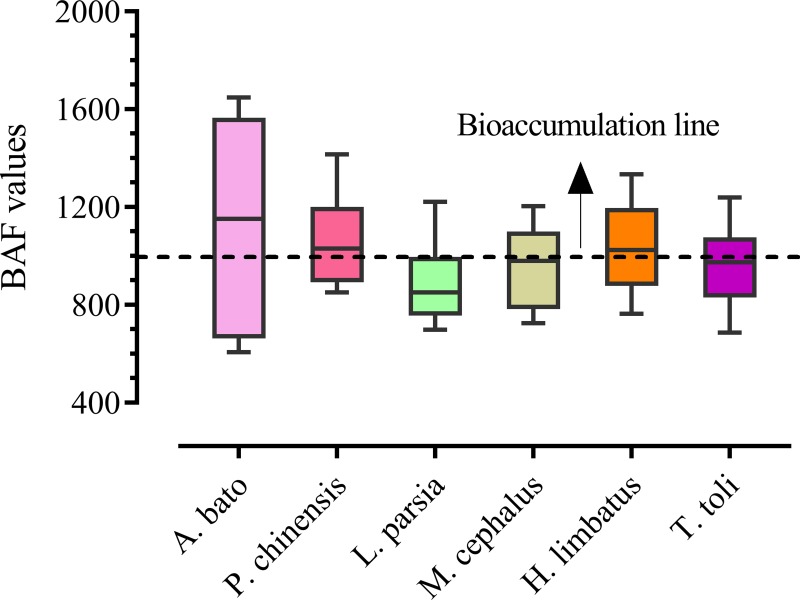
The estimated BAFs are depicted in Fig 4. The BAFs were ranged from 110.53 for Cd observed in S4 to 3353.7 for Cu as well. The minimum value was found for T. toil, on the other hand, A. bato showed maximum bioaccumulation result. Moreover, the mean BAFs of the metals were observed in the species as follows: Cu (1971.42) > As (1042.93) > Pb (913.66) > Cr (864.99) > Cd (252.03).
Fig 4. Bioaccumulation factor among the species that varied from particular metals and species.
Health risk evaluation
Estimated daily intake (EDI)
The explored EDI of two concerned age groups, adults and children, is presented and summarized in Table 5. Adults and children showed comparably higher EDIs for demersal species than pelagic ones. In the consequence, high doses of demersal species were exposed to the consumers through consuming metal affected fish species as the food items. The EDIs for both groups were organized in the following order: Pb > Cu > As > Cr > Cd.
Table 5. A comparison between recommended daily allowance (RDA) and estimated daily intake (EDI) for adults and children.
| Elements | Mean concentration (mg/kg) |
RDA (mg/kg/person) [93] | EDIs (mg/day/person) | |
|---|---|---|---|---|
| Adult | Child | |||
| As | 4.89 | 0.15 | 0.005 | 0.029 |
| Pb | 13.88 | 0.25 | 0.011 | 0.049 |
| Cd | 0.39 | 0.07 | 0.001 | 0.001 |
| Cr | 3.36 | 0.23 | 0.003 | 0.012 |
| Cu | 12.10 | 35 | 0.010 | 0.042 |
Target hazard quotient (THQ) and Hazard Index (HI) for non-carcinogenic risk
The assessed target hazard quotient (THQ) for the studied fish species are displayed in Table 6. THQs in the adult group induced to As, Pb, Cd, Cr, and Cu were 0.016, 0.003, 3.0E-04, 0.001 and 2.38E-04, respectively, whereas for children the values were 0.097, 0.014, 0.001, 0.004 and 0.001, respectively. Moreover, the rank of the THQs of the elements was as follows: As > Pb > Cr > Cd > Cu. While, for the cumulative scenario of HI, children were 5.83 times more susceptible than adults. However, the investigated HI was not surpass the recommended limit (Table 6).
Table 6. Calculated THQ, HI and CR for the selected two aged groups.
| Species | THQ (As) | THQ (Pb) | THQ (Cd) | THQ (Cr) | THQ (Cu) | HI | CR (As) | CR (Pb) | CR (Cd) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | Ad | Ch | |
| A. bato | 0.012 | 0.054 | 0.003 | 0.015 | 0.000 | 0.002 | 0.001 | 0.004 | 0.000 | 0.001 | 0.017 | 0.077 | 5.47E-06 | 2.44E-05 | 1.79E-05 | 8.00E-05 | 2.99E-06 | 1.30E-05 |
| P. chinensis | 0.013 | 0.059 | 0.003 | 0.014 | 0.000 | 0.002 | 0.001 | 0.004 | 0.000 | 0.001 | 0.018 | 0.08 | 5.93E-06 | 2.64E-05 | 9.35E-08 | 7.40E-05 | 2.18E-06 | 9.70E-06 |
| L. parsia | 0.011 | 0.051 | 0.003 | 0.014 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.016 | 0.071 | 5.14E-06 | 2.29E-05 | 9.33E-08 | 4.20E-07 | 1.68E-06 | 7.50E-06 |
| M. cephalus | 0.013 | 0.057 | 0.003 | 0.013 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.017 | 0.076 | 5.77E-06 | 2.57E-05 | 8.48E-08 | 3.80E-07 | 1.52E-06 | 6.80E-06 |
| H. limbatus | 0.013 | 0.06 | 0.003 | 0.014 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.018 | 0.08 | 6.06E-06 | 2.7E-05 | 9.2E-08 | 4.10E-07 | 1.72E-06 | 7.70E-06 |
| T. toli | 0.030 | 0.3 | 0.003 | 0.014 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.034 | 0.319 | 1.35E-05 | 1.35E-04 | 9.09E-08 | 4.00E-07 | 1.54E-06 | 6.90E-06 |
| Mean | 0.016 | 0.097 | 0.003 | 0.014 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.015 | 0.079 | 6.98E-06 | 4.36E-05 | 3.07E-06 | 2.58E-05 | 1.94E-06 | 8.64E-06 |
Carcinogenic risk (CR)
Exposure of CR was estimated for a particular element and summarized in Table 6. The measured CR values of As, Pb and Cd were ranged from 5.14E-06- 1.35E-05, 8.48E-08- 1.79E-05 and 1.52E-06- 2.99E-06 respectively in adults and 2.29E-05- 1.35E-04, 3.78E-07- 7.99E-05 and 6.76E-06- 1.33E-05 in children. The results showed that children were exposed to higher CRs than adults. But, calculated CR values for both age groups were noted far from the risk as acceptable range is 10−6 to 10−4.
Discussion
Concentration of heavy metals and source identification
In the present study, T. toil had the highest concentration of As, whereas A. bato exhibited the maximum concentration for Pb, Cd and Cu, and P. chinensis had the highest concentration of Cr. The metal concentrations in the edible tissues were ranked in the following sequence: A. bato > P. chinensis > H. limbatus > T. toli > M. cephalus > L. parsia. Data from the previous literatures showed that metal concentrations in fish muscles varied widely depending on the location and species. The As and Pb concentrations in this study were higher than all other findings and recommended guidelines. Although Cd concentration was at the lower level comparing Northeast coast, Ganga River, and Pearl River, it surpassed all the guideline values along with other coastal environments, Meiliang Bay, Iskenderun Bay, Arasalar River and coastal area of Bangladesh. The results reported by [89] from the Ganga River were generally lower than present results, except Cu. The concentrations of Cd and Cu in the fish collected from the Pearl River were higher than present study. Different fish species of Bangladesh’s coastal area have been reported to contain 0.08–13 As, 0.07–0.63 Pb, 0.03–0.09 Cd, 0.15–2.2 Cr and 1.3–14 Cu mg/kg dry weight of muscles [86], which were generally lower than the present findings. In this study, the demersal species had comparatively higher concentration level of metals than pelagic ones as they inhabit close to the bottom or sediment. The MPI values of 3.65 to 4.70 in the present study were much lower than those of Blackchin tilapia, Sarotherodon melanotheron and Silver catfish, Chrysichthys nigrodigitatus (8.1 to 17.76) from Okrika Estuary, Nigeria. This is most likely due to the oil bunkering and transportation activities along the study sites [94]. The findings of MPI in the present study were almost similar to that of Rutilus rutilus in Pluszne Lake [12]. However, the metal accumulation in fishes could be highly influenced by sampling locations and habitats [95, 96].
Previous studies have shown that heavy metals in the aquatic environment could come from different natural and anthropogenic sources [1, 3, 39, 97], where many factors influence their concentrations e.g., the original levels of rocks and parent materials, processes of soil formation, contamination by human activities, and other anthropogenic factors [98]. Generally, high correlations between specific heavy metals in the environment may reflect similar levels of contamination and/or release from the same sources of pollution [40]. In this study, strong positive correlations were found between Cd and Pb, Cr and Pb, Cr and Cd, and Cr and Cu indicating that they had the same source either natural or anthropogenic. The employed PCA revealed that the source of origin of the metal was anthropogenic. Pb, Cd, and Cu were the dominant compounds in PCA analysis due to their high loading scores. PCA also revealed the changes of geochemical composition and the justification of cluster analysis [99]. In the cluster analysis, Pb, Cd and Cu are grouped together in cluster 2, indicating that the anthropogenic sources of these heavy metals are closely related to the sediments of the study area. There are several manufacturing industries near the study area which largely use alloy, paints and poisonous chemicals containing As. The commercial uses of heavy metals in modern microelectronic and optical industries are notable sources of As intrusion in the aquatic environment [100]. Non-essential element, Pb originated from extreme agriculture, poultry farms, industries and textile mills might end up in the aquatic ecosystem [101], thus contributing to the metal in the study area. Thus the benthic feeders are to be greatly affected by the deposited Pb in the ecosystem [91]. Cd is typically found at a low concentration in the aquatic environment, however, indiscriminate use of phosphate fertilizer and industries are two primary sources of Cd introduction [102]. In the study area, nickel-cadmium battery manufacturing along with industries engaged with Cd metal incineration and production might increase the Cd concentration level in the aquatic environment [103, 104]. Besides Cd and Pb, Cr is also widely introduced in the textile industries [105]. Near the bank of the Karnaphuli estuary, such commercial textile industries produce colour pigment and thus become a common contaminant for the aquatic ecosystem [106]. Notably, a considerable level of Cu become swelled up in the study area due to oil droppings from ships and boats, recurrent usage of antifouling paints and other boating interferes [107].
Bioaccumulation (BAF) status of target species
The bioaccumulation potential of metals was assessed in the muscles of various fish species, and was found to vary from species to species. The hierarchy suggested that most of the species were tend to be bioaccumulative (BAF value close to 1000). A. bato exhibited the highest bioaccumulation in the studied area. The accumulation of the metal elements in an aquatic organism depends upon the classification of species, invasion pathways, metabolic characters of the sampled tissues and finally, the surrounding environmental condition in which the species live [108]. In our results, the BAFs of As, Cd, Cr, Pb and Cu were relatively higher than those of Pearl River estuary [90], where the BAFs in Tilapia were reported to be Cd > Cu > Pb > Cr. Such reports were mostly in line with our results. The fact is that, Cu is actively persistent in muscles due to being an essential element of living tissue [60, 109]. Notably, the bioaccumulation capability of Cd takes a long time to spare, thus making it relatively infirm [20].
Human health risk evaluation
EDI, based on the oral reference dose (RfD) for an individual element [110], reflects the daily exposure to the toxic element, and is executed to avoid any harmful effect on human health [63]. The records of EDI of the people were compared with recommended daily allowance (RDA), provided by WHO, and introduced that, mean EDI values of the metals were still lower than RDAs. The values lower than RDA guidelines suggested a lower possible health effect of the elements to the consumers. However, it would not be wise to take it as a permanent measurement to reach a final conclusion [61, 63].
The <1 THQ values for both adult and children suggested that the adverse effect on human health might not occur. Similarly, the HI results also followed the THQ trend. Hence, there is no potential non-carcinogenic effect for the consumers due to intake of the fish species. Studies carried out by several authors in similar conditions were in line with our results [32, 69, 111–113]. In general, the assessment of THQ for human health risk evaluation has no dose-response relation of the examined elements [114]. However, human can dramatically suffer in the long run due to multiple simultaneous pollutants [77].
The CR values lower than 10−6 indicates a negligible health risk whereas values in the range of 10−6–10−4 are in the acceptable belt [63]. The CR values found in this study suggested an acceptable limit, and consumers are therefore less prone to carcinogenic risk. In fact, 90% of the carcinogenic risk is observed in the As contaminated aquatic food items. The inorganic state of As is more lethal than organic one [20, 115], and only 10% of the total As can be assessed as inorganic form [63]. The findings of the present study were in the acceptable range (10−6 to 10−4) compared with Okogwu et al. [116], except for Cr which surpassed the CR limits. Again, in the Persian Gulf, consumers at the threshold limit for As were in concern of carcinogenic risk [117]. For this reason, carcinogenic risk should be given more attention due to intake of aquatic products, especially for the study area.
Conclusions
Five heavy metals in the muscles of six fish species from the Karnaphuli River estuary were measured to investigate their potential sources, bioaccumulation rate and human health risk. Relatively high concentration of Cu was observed in A. bato. In most cases, the metal concentrations exceeded the recommended limits. The maximum metal accumulation was recorded in A. bato. A. bato, P. chinensis and H. limbatus were observed to be extreme bio-accumulative species. The mean bioaccumulation factors of the metals were observed in the studied species as follows: Cu > As > Pb > C > Cd.). Among the fishes studied, demersal fishes had higher levels of heavy metals compared with those fishes in the upper layers, suggesting higher level of heavy metals in the bottom water or sediment compared with the surface water. Accumulation of heavy metals in fish could be resulted from surface contact with the water, by breathing, and via the food chain. Heavy metals in the sediment enter the food chain through the feeding of benthic animals. Pearson correlation analysis showed strong positive correlations between Cd and Pb, Cr and Pb, Cr and Cd, and Cr and Cu indicating that they had the same source, either natural or anthropogenic. As per cluster analysis, Pb, Cd, and Cu, were grouped together, indicating the local anthropogenic sources. Existing textile mills, fertilizer factory, leather industry and agricultural activities in the catchment area of the river might be the sources of high concentration of Pb and Cd. Carcinogenic risk assessment suggested local consumers were free from the risk of cancer for the time being but they might be affected in future upon consumption of fish from studied region. Finally, it is found that children were six times more vulnerable to non-carcinogenic and carcinogenic risks than adults. Nonetheless, further study required ensuring the same conclusions are reached.
Acknowledgments
The authors gratefully acknowledge the Rapid Action Battalion Headquarter, Bangladesh for providing necessary fund for heavy metal analyses and instrumental facilities. Comments from the editor and reviewers have significantly improved the manuscript.
Data Availability
All relevant data are with the manuscript.
Funding Statement
Sampling was performed by self-funded. Heavy metal analysis were conducted and funded by Rapid Action Battalion Headquarter, Bangladesh. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Islam MS, Hossain MB, Matin A, Sarker MSI. Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere. 2018; 202:25–32. 10.1016/j.chemosphere.2018.03.077 10.1016/j.chemosphere.2018.03.077 [DOI] [PubMed] [Google Scholar]
- 2.Wilcock D. River and inland water environments In: Nath B, Hens L, Compton P, Devuyst D (Eds), environmental management in practice (volume 3), Routledge, New York: 1999. p.328 [Google Scholar]
- 3.Hossain MB, Ahmed ASS, Sarker MSI. Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environmental Science and Pollution Research. 2018; 25:31727–31736. 10.1007/s11356-018-3158-9 10.1007/s11356-018-3158-9 [DOI] [PubMed] [Google Scholar]
- 4.Xu S, Tao S. Coregionalization analysis of heavy metals in the surface soil of Inner Mongolia. Science of the total environment. 2004; 320:73–87. 10.1016/S0048-9697(03)00450-9 [DOI] [PubMed] [Google Scholar]
- 5.Idriss A, Ahmad A. Heavy metal concentrations in fishes from Juru River, estimation of the health risk. Bulletin of Environmental Contamination and Toxicology. 2015; 94:204–208. 10.1007/s00128-014-1452-x 10.1007/s00128-014-1452-x [DOI] [PubMed] [Google Scholar]
- 6.Authman MM, Zaki MS, Khallaf EA, Abbas HH. Use of fish as bio-indicator of the effects of heavy metals pollution. Journal of Aquaculture Research & Development. 2015; 6:1–13. 10.4172/2155-9546.1000328 [DOI] [Google Scholar]
- 7.Ali H, Khan E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environmental Chemistry Letters. 2018; 16:903–917. 10.1007/s10311-018-0734-7 [DOI] [Google Scholar]
- 8.Bayen S, Wurl O, Karuppiah S, Sivasothi N, Lee HK, Obbard JP. Persistent organic pollutants in mangrove food webs in Singapore. Chemosphere. 2005; 61:303–313. 10.1016/j.chemosphere.2005.02.097 10.1016/j.chemosphere.2005.02.097 [DOI] [PubMed] [Google Scholar]
- 9.Zeitoun MM, Mehana E. Impact of water pollution with heavy metals on fish health: overview and updates. Global Veterinaria. 2014; 12:219–231. DOI: 10.5829/idosi.gv.2014.12.02.82219 [DOI] [Google Scholar]
- 10.Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FA. Food web–specific biomagnification of persistent organic pollutants. Science. 2007; 317:236–239. DOI: 10.1126/science.1138275 [DOI] [PubMed] [Google Scholar]
- 11.Siddique M, Arshad A, Amin S. Length-weight and length-length relationships of two tropical fish Secutor megalolepis (Mochizuki & Hayashi, 1989) and Rhabdamia gracilis (Bleeker, 1856) from Sabah, Malaysia. Journal of Applied Ichthyology. 2015; 31:574–575. 10.1111/jai.12752 [DOI] [Google Scholar]
- 12.Luczynska J, Paszczyk B, Luczynski MJ. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer's health. Ecotoxicology and Environmental Safety. 2018; 153:60–67. 10.1016/j.ecoenv.2018.01.057 10.1016/j.ecoenv.2018.01.057 [DOI] [PubMed] [Google Scholar]
- 13.Zamri Z, Arshad A, Amin SN, Rahman MA, Al Khayat JA. Sex ratio, gonad development and fecundity of Miyakella nepa (Crustacea, Stomatopoda) of Pantai Remis coastal waters of Malaysia. Journal of Environmental Biology. 2016; 37:677–683 [PubMed] [Google Scholar]
- 14.Parente T, Hauser-Davis R. The use of fish biomarkers in the evaluation of water pollution. Pollution and Fish Health in Tropical Ecosystems. 2013:164–181. 10.1201/b16298-8 [DOI] [Google Scholar]
- 15.Azaman F, Juahir H, Yunus K, Azid A, Kamarudin MKA, Toriman ME, et al. Heavy metal in fish: Analysis and human health-a review. Jurnal Teknologi. 2015; 77:61–69. 10.11113/jt.v77.4182 [DOI] [Google Scholar]
- 16.Castro-González M, Méndez-Armenta M. Heavy metals: Implications associated to fish consumption. Environmental Toxicology and Pharmacology. 2008; 26:263–271. 10.1016/j.etap.2008.06.001 10.1016/j.etap.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Rahman MS, Hossain MB, Babu SOF, Rahman M, Ahmed AS, Jolly Y, et al. Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Marine Pollution Bulletin. 2019; 141:137–146. 10.1016/j.marpolbul.2019.02.032 10.1016/j.marpolbul.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 18.Rahman MA, Mustafa MG, Barman BK. Impacts of aquaculture extension activities on female fish farmers in different areas of Bangladesh. Bangladesh Journal of Zoology. 2012; 39:213–221. 10.3329/bjz.v39i2.10591 [DOI] [Google Scholar]
- 19.Saha N, Mollah M, Alam M, Rahman MS. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016a; 70:110–118. 10.1016/j.foodcont.2016.05.040 [DOI] [Google Scholar]
- 20.Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, et al. Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicology and Environmental Safety. 2018; 157:343–349. 10.1016/j.ecoenv.2018.03.048 10.1016/j.ecoenv.2018.03.048 [DOI] [PubMed] [Google Scholar]
- 21.Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, et al. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environmental Science and Pollution Research. 2015; 22:15880–15890. 10.1007/s11356-015-4813-z 10.1007/s11356-015-4813-z [DOI] [PubMed] [Google Scholar]
- 22.Varol M, Kaya GK, Alp A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: Risk-based consumption advisories. Science of the Total Environment. 2017; 599:1288–1296. 10.1016/j.scitotenv.2017.05.052 10.1016/j.scitotenv.2017.05.052 [DOI] [PubMed] [Google Scholar]
- 23.Dey S, Das J, Manchur M. Studies on heavy metal pollution of Karnaphuli river, Chittagong, Bangladesh. IOSR Journal of Environmental Science, Toxicology and Food Technology. 2015; 9:79–83. DOI: 10.9790/2402-09817983 [DOI] [Google Scholar]
- 24.Islam MM, Rahman SL, Ahmed SU, Haque MKI. Biochemical characteristics and accumulation of heavy metals in fishes, water and sediments of the river Buriganga and Shitalakhya of Bangladesh. Journal of Asian Scientific Research. 2014; 4:270. [Google Scholar]
- 25.Ahmed MK, Baki MA, Kundu GK, Islam MS, Islam MM, Hossain MM. Human health risks from heavy metals in fish of Buriganga river, Bangladesh. SpringerPlus. 2016; 5:1697 10.1186/s40064-016-3357-0 10.1186/s40064-016-3357-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman MS, Molla AH, Saha N, Rahman A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chemistry. 2012; 134:1847–1854. 10.1016/j.foodchem.2012.03.099 10.1016/j.foodchem.2012.03.099 [DOI] [PubMed] [Google Scholar]
- 27.Amin MN, Begum A, Mondal MK. Trace element concentrations present in five species of freshwater fish of Bangladesh. Bangladesh Journal of Scientific and Industrial Research. 2011; 46:27–32. 10.3329/bjsir.v46i1.8101 [DOI] [Google Scholar]
- 28.Xie W, Chen K, Zhu X, Nie X, Zheng G, Pan D, et al. Evaluation of heavy metal contents in water and fishes collected from the waterway in Pearl River Delta in south China. Journal of Agro-Environment Science. 2010; 29:1917–1923. [Google Scholar]
- 29.Ciftci N, Ay O, Karayakar F, Cicik B, Erdem C. Effects of zinc and cadmium on condition factor, hepatosomatic and gonadosomatic index of Oreochromis niloticus. Fresenius Environmental Bulletin. 2015; 24:3871–3874. [Google Scholar]
- 30.Hossein KP, Takhsha M, Aein JK, Aghshenas A. Assessment level of heavy metals (Pb, Cd, Hg) in four fish species of Persian Gulf (Bushehr-Iran). International Journal of Advanced Technology & Engineering Research. 2014; 4:7–11. [Google Scholar]
- 31.Usero J, González-Regalado E, Gracia I. Trace metals in the bivalve mollusc Chamelea gallina from the Atlantic coast of southern Spain. Oceanographic Literature Review. 1996; 10:1058 10.1016/0025-326x(95)00209-6 [DOI] [Google Scholar]
- 32.Abdel-Khalek AA, Elhaddad E, Mamdouh S, Marie M- AS. Assessment of metal pollution around sabal drainage in River Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turkish Journal of Fisheries and Aquatic Sciences. 2016; 16:227–239. 10.4194/1303-2712-v16_2_02 [DOI] [Google Scholar]
- 33.Keshavarzi B, Hassanaghaei M, Moore F, Mehr MR, Soltanian S, Lahijanzadeh AR, et al. Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Marine Pollution Bulletin. 2018; 129:245–252. 10.1016/j.marpolbul.2018.02.032 10.1016/j.marpolbul.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Liao Y, Shou L. Concentration and potential health risk of heavy metals in seafoods collected from Sanmen Bay and its adjacent areas, China. Marine Pollution Bulletin. 2018; 131:356–364. 10.1016/j.marpolbul.2018.04.041 10.1016/j.marpolbul.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 35.Monsefrad F, Imanpour Namin J, Heidary S. Concentration of heavy and toxic metals Cu, Zn, Cd, Pb and Hg in liver and muscles of Rutilus frisii kutum during spawning season with respect to growth parameters. Iranian Journal of Fisheries Sciences. 2012; 11:825–839. [Google Scholar]
- 36.Yi YJ, Zhang SH. Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environmental Science and Pollution Research. 2012; 19:3989–3996. 10.1007/s11356-012-0840-1 10.1007/s11356-012-0840-1 [DOI] [PubMed] [Google Scholar]
- 37.Tekin-Özan S, Aktan N. Relationship of heavy metals in water, sediment and tissues with total length, weight and seasons of Cyprinus carpio L., 1758 from Işikli Lake (Turkey). Pakistan Journal of Zoology. 2012; 44:1405–1416. [Google Scholar]
- 38.Merciai R, Guasch H, Kumar A, Sabater S, García-Berthou E. Trace metal concentration and fish size: Variation among fish species in a Mediterranean river. Ecotoxicology and Environmental Safety. 2014; 107:154–161. 10.1016/j.ecoenv.2014.05.006 10.1016/j.ecoenv.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 39.Varol M, Şen B. Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena. 2012; 92:1–10. 10.1016/j.catena.2011.11.011 [DOI] [Google Scholar]
- 40.Li J, He M, Han W, Gu Y. Analysis and assessment on heavy metal sources in the coastal soils developed from alluvial deposits using multivariate statistical methods. Journal of Hazardous Materials. 2009; 164:976–981. 10.1016/j.jhazmat.2008.08.112 10.1016/j.jhazmat.2008.08.112 [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Wang Y, Shen Z, Niu J, Tang Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. Journal of Hazardous Materials. 2009; 166:1186–1194. 10.1016/j.jhazmat.2008.12.034 10.1016/j.jhazmat.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 42.Sundaray SK, Nayak BB, Lin S, Bhatta D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. Journal of Hazardous Materials. 2011; 186:1837–1846. 10.1016/j.jhazmat.2010.12.081 10.1016/j.jhazmat.2010.12.081 [DOI] [PubMed] [Google Scholar]
- 43.Chung C-Y, Chen J-J, Lee C-G, Chiu C-Y, Lai W-L, Liao S-W. Integrated estuary management for diffused sediment pollution in Dapeng Bay and neighboring rivers (Taiwan). Environmental Monitoring and Assessment. 2011; 173:499–517. 10.1007/s10661-010-1401-z 10.1007/s10661-010-1401-z [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Chen M, Shan G, Chen P, Cui S, Yi S, et al. Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Science of The Total Environment. 2017; 598:814–820. 10.1016/j.scitotenv.2017.04.167 10.1016/j.scitotenv.2017.04.167 [DOI] [PubMed] [Google Scholar]
- 45.Qin D, Jiang H, Bai S, Tang S, Mou Z. Determination of 28 trace elements in three farmed cyprinid fish species from Northeast China. Food Control. 2015; 50:1–8. 10.1016/j.foodcont.2014.08.016 [DOI] [Google Scholar]
- 46.Zhang L, Shi Z, Jiang Z, Zhang J, Wang F, Huang X. Distribution and bioaccumulation of heavy metals in marine organisms in east and west Guangdong coastal regions, South China. Marine Pollution Bulletin. 2015; 101:930–937. 10.1016/j.marpolbul.2015.10.041 10.1016/j.marpolbul.2015.10.041 [DOI] [PubMed] [Google Scholar]
- 47.Arnot JA, Gobas FA. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews. 2006; 14:257–297. 10.1139/a06-005 [DOI] [Google Scholar]
- 48.Sekitar PKA, Hamid M, Mansor M, Nor SAM. Length-weight relationship and condition factor of fish populations in Temengor reservoir: Indication of environmental health. Sains Malaysiana. 2015; 44:61–66. [Google Scholar]
- 49.Luczynska J, Luczynski MJ, Paszczyk B, Tonska E. Concentration of mercury in muscles of predatory and non-predatory fish from lake Pluszne (Poland). Journal of Veterinary Research. 2016; 60:43–47. DOI: 10.1515/jvetres-2016-0007 [DOI] [Google Scholar]
- 50.Froese R. Cube law, condition factor and weight–length relationships: history, meta‐analysis and recommendations. Journal of Applied Ichthyology. 2006; 22:241–453. 10.1111/j.1439-0426.2006.00805.x [DOI] [Google Scholar]
- 51.Jin S, Yan X, Zhang H, Fan W. Weight–length relationships and Fulton’s condition factors of skipjack tuna (Katsuwonus pelamis) in the western and central Pacific Ocean. PeerJ. 2015; 3:e758 10.7717/peerj.758 10.7717/peerj.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.C B A B. Condition Factor, K, for Salmonid Fish, Fisheries Notes. 1998. [Google Scholar]
- 53.Datta SN, Kaur VI, Dhawan A, Jassal G. Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. SpringerPlus. 2013; 2(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricker W. Linear regressions in fishery research. Journal of the fisheries board of Canada. 1973; 30(3):409–34. [Google Scholar]
- 55.Pervin MR, Mortuza MG. Notes on length-weight relationship and condition factor of fresh water fish, Labeo boga (Hamilton)(Cypriniformes: Cyprinidae). University Journal of Zoology, Rajshahi University. 2008; 27:97–98 [Google Scholar]
- 56.Gupta B, Sarkar U, Bhardwaj S, Pal A. Condition factor, length–weight and length–length relationships of an endangered fish Ompok pabda (Hamilton 1822) (Siluriformes: Siluridae) from the River Gomti, a tributary of the River Ganga, India. Journal of Applied Ichthyology. 2011; 27:962–964. doi: 10.1111/j.1439-0426.2010.01625.x [DOI] [Google Scholar]
- 57.USEPA. Guideline for Assessing Chemical Contaminant Data for Use in Fish Advisories, Vol. I: Fish Sampling and Analysis. Third Edition Office of Water. U.S. Environmental Protection Agency; Washington, DC: Document No. EPA 823-B-00-007. November 2000.; 2000a. [Google Scholar]
- 58.Wei Y, Zhang J, Zhang D, Tu T, Luo L. Metal concentrations in various fish organs of different fish species from Poyang Lake, China. Ecotoxicology and Environmental Safety. 2014; 104:182–188. 10.1016/j.ecoenv.2014.03.001 10.1016/j.ecoenv.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 59.USEPA. Integrated risk information system, United States Environmental Protection Agency, Washington, DC, USA: https://www.epa.gov/iris. 2008. Accessed on 25 September 2018. [Google Scholar]
- 60.Traina A, Bono G, Bonsignore M, Falco F, Giuga M, Quinci EM, et al. Heavy metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): Potential human health risk estimation. Ecotoxicology and Environmental Safety. 2019; 168:466–478. 10.1016/j.ecoenv.2018.10.056 10.1016/j.ecoenv.2018.10.056 [DOI] [PubMed] [Google Scholar]
- 61.Vu CT, Lin C, Yeh G, Villanueva MC. Bioaccumulation and potential sources of heavy metal contamination in fish species in Taiwan: assessment and possible human health implications. Environmental Science and Pollution Research. 2017; 24:19422–19434. 10.1007/s11356-017-9590-4 10.1007/s11356-017-9590-4 [DOI] [PubMed] [Google Scholar]
- 62.Abtahi M, Fakhri Y, Oliveri Conti G, Keramati H, Zandsalimi Y, Bahmani Z, et al. Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: a systematic review. Toxin Reviews. 2017; 36:331–341. 10.1080/15569543.2017.1354307 [DOI] [Google Scholar]
- 63.Baki MA, Hossain MM, Akter J, Quraishi SB, Shojib MFH, Ullah AA, et al. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicology and Environmental Safety. 2018; 159:153–163. 10.1016/j.ecoenv.2018.04.035 10.1016/j.ecoenv.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 64.Rahmani J, Fakhri Y, Shahsavani A, Bahmani Z, Urbina MA, Chirumbolo S, et al. A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food and Chemical Toxicology. 2018; 118:753–765. 10.1016/j.fct.2018.06.023 10.1016/j.fct.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 65.Heshmati A, Ghadimi S, Khaneghah AM, Barba FJ, Lorenzo JM, Nazemi F, et al. Risk assessment of benzene in food samples of Iran's market. Food and Chemical Toxicology. 2018; 114:278–284. 10.1016/j.fct.2018.02.043 10.1016/j.fct.2018.02.043 [DOI] [PubMed] [Google Scholar]
- 66.Yi Y, Yang Z, Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution. 2011; 159:2575–2585. 10.1016/j.envpol.2011.06.011 10.1016/j.envpol.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 67.Xiao C-C, Chen M-J, Mei F-B, Fang X, Huang T-R, Li J-L, et al. Influencing factors and health risk assessment of microcystins in the Yongjiang river (China) by Monte Carlo simulation. PeerJ. 2018; 6:e5955 10.7717/peerj.5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.USEPA. Region III Risk-Based Concentration Table: Technical background information. pp 227 2006. [Google Scholar]
- 69.Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y. Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Reviews. 2017; 36:222–227. 10.1080/15569543.2016.1274762 [DOI] [Google Scholar]
- 70.Lei M, Tie B-q, Song Z-g, Liao B-H, Lepo JE, Huang Y-z. Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Security. 2015; 7:45–54. 10.1007/s12571-014-0414-9 [DOI] [Google Scholar]
- 71.Fakhri Y, Mohseni-Bandpei A, Oliveri Conti G, Keramati H, Zandsalimi Y, Amanidaz N, et al. Health risk assessment induced by chloroform content of the drinking water in Iran: systematic review. Toxin Reviews. 2017; 36:342–351. 10.1080/15569543.2017.1370601 [DOI] [Google Scholar]
- 72.Fantke P, Friedrich R, Jolliet O. Health impact and damage cost assessment of pesticides in Europe. Environment International. 2012; 49:9–17. 10.1016/j.envint.2012.08.001 10.1016/j.envint.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 73.Pepper I, Gerba C, Brusseau M. Environmental and pollution science (pollution science series). Academic Press; 2012:212–232. [Google Scholar]
- 74.USEPA. Risk-based concentration table. United States Environmental Protection Agency, Washington, DC: 2000b. [Google Scholar]
- 75.FAO. The State of the World Fisheries and Aquaculture. Opportunities and Challenges. FAO Fisheries and Aquaculture Dept; Rome, Italy, pp 243 2014. [Google Scholar]
- 76.Yin S, Feng C, Li Y, Yin L, Shen Z. Heavy metal pollution in the surface water of the Yangtze Estuary: a 5-year follow-up study. Chemosphere. 2015; 138:718–725. 10.1016/j.chemosphere.2015.07.060 10.1016/j.chemosphere.2015.07.060 [DOI] [PubMed] [Google Scholar]
- 77.Li PH, Kong SF, Geng CM, Han B, Lu B, Sun RF, et al. Assessing the hazardous risks of vehicle inspection workers’ exposure to particulate heavy metals in their work places. Aerosol and Air Quality Research. 2013; 13:255–265. doi: 10.4209/aaqr.2012.04.0087 [DOI] [Google Scholar]
- 78.Hu B, Jia X, Hu J, Xu D, Xia F, Li Y. Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze river delta, China. International Journal of Environmental Research and Public Health. 2017; 14:1042 10.3390/ijerph14091042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.USEPA. Integrated Risk Information System (IRIS); United States Environmental Protection Agency: Washington, DC, USA: Available online: http://www.epa.gov/ncea/iris/index.html (Accessed on 15 September 2018). 2010. [Google Scholar]
- 80.Cao S, Duan X, Zhao X, Ma J, Dong T, Huang N, et al. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Science of The Total Environment. 2014; 472:1001–1009. 10.1016/j.scitotenv.2013.11.124 10.1016/j.scitotenv.2013.11.124 [DOI] [PubMed] [Google Scholar]
- 81.Vieira C, Morais S, Ramos S, Delerue-Matos C, Oliveira M. Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra-and inter-specific variability and human health risks for consumption. Food and Chemical Toxicology. 2011; 49:923–932. 10.1016/j.fct.2010.12.016 10.1016/j.fct.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 82.Nauen CE. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular (FAO) no 764; 1983. [Google Scholar]
- 83.WHO. Guideline for drinking water quality, 2nd edition Recommendation. World Health Organization General; 1:30–113. 1985. [Google Scholar]
- 84.EU. Commission Regulation as Regards Heavy metals, Directive, 2001/22/EC, No: 466. 2001. [Google Scholar]
- 85.MOFL. Bangladesh Gazette, Bangladesh Ministry of Fisheries and Livestock, SRO no. 233/Ayen. 2014. [Google Scholar]
- 86.Raknuzzaman M, Ahmed MK, Islam MS, Habibullah-Al-Mamun M, Tokumura M, Sekine M, et al. Trace metal contamination in commercial fish and crustaceans collected from coastal area of Bangladesh and health risk assessment. Environmental Science and Pollution Research. 2016; 23:17298–17310. 10.1007/s11356-016-6918-4 10.1007/s11356-016-6918-4 [DOI] [PubMed] [Google Scholar]
- 87.Lakshmanasenthil S, Vinothkumar T, AjithKumar TT, Marudhupandi T, Veettil DK, Ganeshamurthy R, et al. Harmful metals concentration in sediments and fishes of biologically important estuary, Bay of Bengal. Journal of Environmental Health Science and Engineering. 2013; 11:33 10.1186/2052-336X-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar B, Sajwan K, Mukherjee D. Distribution of heavy metals in valuable coastal fishes from North East Coast of India. Turkish Journal of Fisheries and Aquatic Sciences. 2012; 12:81–88. DOI: 10.4194/1303-2712-v12_1_10 [DOI] [Google Scholar]
- 89.Mitra A, Chowdhury R, Banerjee K. Concentrations of some heavy metals in commercially important finfish and shellfish of the River Ganga. Environmental Monitoring and Assessment. 2012; 184:2219–2230. 10.1007/s10661-011-2111-x 10.1007/s10661-011-2111-x [DOI] [PubMed] [Google Scholar]
- 90.Kwok C, Liang Y, Wang H, Dong Y, Leung S, Wong MH. Bioaccumulation of heavy metals in fish and Ardeid at Pearl River Estuary, China . Ecotoxicology and Environmental Safety. 2014; 106:62–67. 10.1016/j.ecoenv.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 91.Rajeshkumar S, Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicology Reports. 2018; 5:288–295. 10.1016/j.toxrep.2018.01.007 10.1016/j.toxrep.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Türkmen A, Türkmen M, Tepe Y, Akyurt I. Heavy metals in three commercially valuable fish species from Iskenderun Bay, Northern East Mediterranean Sea, Turkey. Food Chemistry. 2005; 91:167–172. 10.1016/j.foodchem.2004.08.008 [DOI] [Google Scholar]
- 93.WHO. World Health Organization technical report series. Evaluation of certain food additives and contaminants. Fifty-third report of the joint FAO/WHO expert committee on food additives (JECFA)', World Health Organization, Geneva, Switzerland. 2000. http://www.Who.Int/foodsafety/publications/jecfa-reports/en/. Accessed on 26 March 2019.
- 94.Ogunola OS, Onada OA, Falaye AE. Ecological Risk Evaluation of Biological and Geochemical Trace Metals in Okrika Estuary. International Journal of Environmental Research. 2017; 11:149–173. 10.1007/s41742-017-0016-4 [DOI] [Google Scholar]
- 95.Rejomon G, Nair M, Joseph T. Trace metal dynamics in fishes from the southwest coast of India. Environmental Monitoring and Assessment. 2010; 167:243–255. 10.1007/s10661-009-1046-y 10.1007/s10661-009-1046-y [DOI] [PubMed] [Google Scholar]
- 96.Weber P, Behr ER, Knorr CDL, Vendruscolo DS, Flores EM, Dressler VL, et al. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchemical Journal. 2013; 106:61–66. 10.1016/j.microc.2012.05.004 [DOI] [Google Scholar]
- 97.Bing H, Wu Y, Sun Z, Yao S. Historical trends of heavy metal contamination and their sources in lacustrine sediment from Xijiu Lake, Taihu Lake Catchment, China. Journal of Environmental Sciences. 2011; 23:1671–1678. 10.1016/S1001-0742(10)60593-1 [DOI] [PubMed] [Google Scholar]
- 98.Li S, Zhang Q. Geochemistry of the upper Han River basin, China, 1: spatial distribution of major ion compositions and their controlling factors. Applied Geochemistry. 2008; 23:3535–3544. 10.1016/j.apgeochem.2008.08.012 [DOI] [Google Scholar]
- 99.Liu H, Liu G, Yuan Z, Ge M, Wang S, Liu Y, et al. Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay, China. Marine Pollution Bulletin. 2019; 140:388–394. 10.1016/j.marpolbul.2019.01.067 10.1016/j.marpolbul.2019.01.067 [DOI] [PubMed] [Google Scholar]
- 100.WHO. Guideline for Drinking Water Quality, Recommendation vol. 1, WHO, Geneva: p.130 2002. [Google Scholar]
- 101.Rashed M. Monitoring of environmental heavy metals in fish from Nasser Lake. Environment International. 2001; 27:27–33. 10.1016/S0160-4120(01)00050-2 [DOI] [PubMed] [Google Scholar]
- 102.SS M. Forms of cadmium in soils of Western Australia. University of Western Australia; 1993. [Google Scholar]
- 103.Bustueva KA, Revich BA, Bezpalko LE. Cadmium in the environment of three Russian cities and in human hair and urine. Archives of Environmental Health: An International Journal. 1994; 49:284–288. 10.1080/00039896.1994.9937481 [DOI] [PubMed] [Google Scholar]
- 104.Bennet-Chambers M, Davies P, Knott B. Cadmium in aquatic ecosystems in Western Australia: A legacy of nutrient-deficient soils. Journal of Environmental Management. 1999; 57:283–295. 10.1006/jema.1999.0304 [DOI] [Google Scholar]
- 105.Sanyal T, Kaviraj A, Saha S. Deposition of chromium in aquatic ecosystem from effluents of handloom textile industries in Ranaghat–Fulia region of West Bengal, India. Journal of Advanced Research. 2015; 6:995–1002. 10.1016/j.jare.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manzoor S, Shah MH, Shaheen N, Khalique A, Jaffar M. Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. Journal of Hazardous Materials. 2006; 137:31–37. 10.1016/j.jhazmat.2006.01.077 10.1016/j.jhazmat.2006.01.077 [DOI] [PubMed] [Google Scholar]
- 107.Sivaperumal P, Sankar T, Nair PV. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chemistry. 2007; 102(3):612–620. 10.1016/j.foodchem.2006.05.041 [DOI] [Google Scholar]
- 108.Ozmen M, Ayas Z, Güngördü A, Ekmekci GF, Yerli S. Ecotoxicological assessment of water pollution in Sariyar Dam Lake, Turkey. Ecotoxicology and Environmental Safety. 2008; 70:163–173. 10.1016/j.ecoenv.2007.05.011 10.1016/j.ecoenv.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 109.ATSDR. Agency for Toxic Substances and Disease Registry, Toxicological Profile for Zinc. U.S. Department of Health and Human Services, Public Health Service, Atlanta; 2005. [Google Scholar]
- 110.USEPA. Region 9, Regional Screening Level (RSL) Summery Table (TR = 1E-6, HQ = 1.0). 2014. https://www.epa.gov/region9/superfund/prg.htm [Google Scholar]
- 111.Zafarzadeh A, Bay A, Fakhri Y, Keramati H, Hosseini Pouya R. Heavy metal (Pb, Cu, Zn, and Cd) concentrations in the water and muscle of common carp (Cyprinus carpio) fish and associated non-carcinogenic risk assessment: Alagol wetland in the Golestan, Iran. Toxin Reviews. 2018; 37(2):154–160. 10.1080/15569543.2017.1386684 [DOI] [Google Scholar]
- 112.Tvermoes BE, Banducci AM, Devlin KD, Kerger BD, Abramson MM, Bebenek IG, et al. Screening level health risk assessment of selected metals in apple juice sold in the United States. Food and Chemical Toxicology. 2014; 71:42–50. 10.1016/j.fct.2014.05.015 10.1016/j.fct.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 113.Saha N, Mollah M, Alam M, Rahman MS. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016b; 70:110–118. 10.1016/j.foodcont.2016.05.040 [DOI] [Google Scholar]
- 114.USEPA. Risk Assessment Guideline for Superfund Volume 1 Human Evaluation Manual (Part A) 291; 1989. [Google Scholar]
- 115.Kalantzi I, Pergantis S, Black K, Shimmield T, Papageorgiou N, Tsapakis M, et al. Metals in tissues of seabass and seabream reared in sites with oxic and anoxic substrata and risk assessment for consumers. Food Chemistry. 2016; 194:659–670. 10.1016/j.foodchem.2015.08.072 10.1016/j.foodchem.2015.08.072 [DOI] [PubMed] [Google Scholar]
- 116.Okogwu OI, Nwonumara GN, Okoh FA. Evaluating Heavy Metals Pollution and Exposure Risk Through the Consumption of Four Commercially Important Fish Species and Water from Cross River Ecosystem, Nigeria. Bulletin of Environmental Contamination and Toxicology. 2019: 102:867–872. 10.1007/s00128-019-02610-4) 10.1007/s00128-019-02610-4 [DOI] [PubMed] [Google Scholar]
- 117.Fakhri Y, Saha N, Miri A, Baghaei M, Roomiani L, Ghaderpoori M, et al. Metal concentrations in fillet and gill of parrotfish (Scarus ghobban) from the Persian Gulf and implications for human health. Food and Chemical Toxicology. 2018; 118:348–354. 10.1016/j.fct.2018.05.041 10.1016/j.fct.2018.05.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are with the manuscript.