Abstract
Introduction
To understand the puberty-related sex shift in the prevalence of asthma and rhinitis as single entities and as respiratory multimorbidities, we investigated if there is also a sex-specific and puberty-related pattern of their incidences.
Methods
We used harmonised questionnaire data from 18 451 participants in five prospective observational European birth cohorts within the collaborative MeDALL (Mechanisms of the Development of Allergy) project. Outcome definitions for IgE-associated and non-IgE-associated asthma, rhinitis and respiratory multimorbidity (first occurrence of coexisting asthma and rhinitis) were based on questionnaires and the presence of specific antibodies (IgE) against common allergens in serum. For each outcome, we used proportional hazard models with sex–puberty interaction terms and conducted a one-stage individual participant data meta-analysis.
Results
Girls had a lower risk of incident asthma (adjusted HR 0.67, 95% CI 0.61 to 0.74), rhinitis (0.73, 0.69 to 0.78) and respiratory multimorbidity (0.58, 0.51 to 0.66) before puberty compared with boys. After puberty onset, these incidences became more balanced across the sexes (asthma 0.84, 0.64 to 1.10; rhinitis 0.90, 0.80 to 1.02; respiratory multimorbidity 0.84, 0.63 to 1.13). The incidence sex shift was slightly more distinct for non-IgE-associated respiratory diseases (asthma 0.74, 0.63 to 0.87 before vs 1.23, 0.75 to 2.00 after puberty onset; rhinitis 0.88, 0.79 to 0.98 vs 1.20, 0.98 to 1.47; respiratory multimorbidity 0.66, 0.49 to 0.88 vs 0.96, 0.54 to 1.71) than for IgE-associated respiratory diseases.
Discussion
We found an incidence ‘sex shift’ in chronic respiratory diseases from a male predominance before puberty to a more sex-balanced incidence after puberty onset, which may partly explain the previously reported sex shift in prevalence. These differences need to be considered in public health to enable effective diagnoses and timely treatment in adolescent girls.
Keywords: sex shift, allergy, asthma, rhinitis, respiratory multimorbidity, incidence, MeDALL, puberty
Key messages.
We examined whether the sex-specific incidence of asthma, rhinitis and respiratory multimorbidity differed before and after puberty onset.
A meta-analysis of longitudinal birth cohorts showed a sex shift from higher incidence in boys before puberty towards a rather sex-balanced incidence after puberty onset.
The elevated risk of asthma and rhinitis incidences in teenage girls should lead to more consideration of a sex-specific and age-specific focus on diagnosis and treatment of these respiratory diseases in public health.
Introduction
Meta-analyses of published results from cross-sectional studies suggested a sex shift in the prevalence of allergic rhinitis with and without concurrent asthma around puberty. The prevalence shifted from a clear male predominance in childhood towards a female predominance in adolescence.1 2 Similar associations were found by individual participant data (IPD) meta-analyses combining harmonised data from large European birth cohorts collaborating in MeDALL (Mechanisms of the Development of Allergy): boys were more likely than girls to have higher prevalence of asthma, rhinitis and respiratory multimorbidity (defined as concurrent asthma and rhinitis) before puberty; after the onset of puberty, a sex shift towards a sex-balanced estimated prevalence was found.3
Reasons for the considerable sex shift in the prevalence of allergic diseases remain unclear. Asthma and rhinitis are chronic diseases that can develop throughout childhood but may not persist into school age or adolescence. Prevalence may be affected by remission and by different sex-specific incidence patterns. Therefore, we aimed to investigate whether the sex-specific incidence patterns of asthma and rhinitis as single entities as well as their co-occurrence change with the onset of puberty.
Methods
Study design and setting
This study was carried out as part of the MeDALL project, a European research initiative for a better understanding of the development of asthma and allergy. Participating birth cohorts were longitudinal, observational and population-based. For the present analyses, IPD from the five oldest birth cohorts participating in the MeDALL project (BAMSE: Swedish abbreviation for Children, Allergy, Milieu, Stockholm, Epidemiology, PIAMA: Prevention and Incidence of Asthma and Mite Allergy, GINIplus: German Infant Nutritional Intervention-Program, LISA: Lebensstil, Immunsystem, Allergien and MAS: Multizentrische Allergie Studie4) from three European countries (Sweden, the Netherlands and Germany) with follow-up assessments up to 20 years of age were used. Data from the five cohorts were combined by consistent harmonisation rules and processes,5 6 while data from the most recent follow-up were derived from a common harmonised MeDALL Core Questionnaire for four of the five birth cohorts.7 A detailed description of the overall MeDALL collaboration8 9 and the inclusion and exclusion criteria of the birth cohorts for the current analysis have been reported previously.3
Definition of primary outcomes
Incident asthma, rhinitis and respiratory multimorbidity were our primary outcomes. If five or more consecutive years of follow-up of the primary outcome data were missing, the data were censored at the last available follow-up.
Definition of asthma and rhinitis
Asthma was defined as a positive answer to at least two of the three following questions:
‘Has your child (/Have you) ever been diagnosed by a doctor as having asthma?’ (yes/no).
‘Has your child (/Have you) had wheezing or whistling in your chest at any time in the last 12 months?’ (yes/no).
‘Has your child (/Have you) taken any medication for asthma (including inhalers, nebulisers, tablets or liquid medicines) or breathing difficulties (chest tightness, shortness of breath) in the last 12 months?’ (yes/no).
Rhinitis was defined according to the International Study of Asthma and Allergies in Childhood (ISAAC)10 as a positive response to the following question:
‘Has your child (/Have you) had problems with sneezing, or a runny, or blocked nose when s/he did not have a cold or flu in the past 12 months?’ (yes/no).
For each question the wording ‘Has your child’ stems from the parental questionnaire, and the wording ‘Have you’ stems from the adolescent questionnaire.
Definition of incident asthma and incident rhinitis
Incident asthma and rhinitis were rated ‘positive’ if there was:
A positive first time ever assessment of the disease at the current follow-up.
Incident asthma and rhinitis were rated ‘negative’ if:
The assessment of the disease was negative at the current follow-up and
The assessment of the disease was not positive at any earlier follow-up.
Definition of incident respiratory multimorbidity
Incident respiratory multimorbidity was rated ‘positive’ if there was:
A positive first time ever assessment of both rhinitis and asthma at the current follow-up.
Incident respiratory multimorbidity was rated ‘negative’ if:
The assessment of rhinitis and/or asthma was negative at the current follow-up and
The assessment of both asthma and rhinitis together was not positive at an earlier follow-up.
Definition of secondary outcomes
Allergic sensitisation assessed by specific IgE
All included birth cohorts had information on specific antibodies against common aeroallergens, that is, dog, cat, house dust mite and birch pollen, which were measured as specific IgE in the serum. A participant had a positive allergic sensitisation status if at least one of the four specific IgE measurements was ≥0.7 kU/L serum. Accordingly, a participant had a negative allergic sensitisation status if all of the specific IgE measurements were <0.7 kU/L.
The following were the six secondary outcomes:
IgE-related and non-IgE-related asthma (defined as a positive incident asthma/questionnaire data in combination with a positive or negative sensitisation status/IgE data, respectively).
IgE-related and non-IgE-related rhinitis (defined as a positive incident rhinitis/questionnaire data in combination with a positive or negative sensitisation status/IgE data, respectively).
IgE-related and non-IgE-related respiratory multimorbidity (defined as a positive incident respiratory multimorbidity/questionnaire data in combination with a positive or negative sensitisation status/IgE data, respectively).
If five or more consecutive years of follow-up of the secondary outcome data were missing, the data were censored at the last available follow-up. For these six secondary outcomes, questionnaire incident data were combined with either IgE data assessed at the same follow-up or with IgE data from the most recent available follow-up, and analyses were thus restricted to those with non-missing questionnaire incident and IgE data. Please see online supplementary table 5 for a general overview of available questionnaire and IgE data at different follow-ups.
bmjresp-2019-000460supp001.pdf (41.1KB, pdf)
Definition of the time-dependent covariable puberty
Participant puberty status was self-assessed by the validated Pubertal Development Scale (PDS), which is widely used in population studies, or available data covering information from the PDS using the items ‘body hair growth’, ‘voice change’ and ‘facial hair growth’ for boys, and the items ‘body hair growth’, ‘breast development’ and ‘menstruation’ for girls.11 Based on these items the dichotomous variable ‘onset of puberty’ (yes/no) was calculated. Absence of any signs of puberty was rated as a negative onset of puberty status, while mid-pubertal, late pubertal or postpubertal category scores were all summarised as a positive onset of puberty status. Further details of this definition are presented elsewhere.3
Definition of confounder variables
The model was adjusted for the following previously identified variables: age (continuous), parental allergies, that is, one or both parents with self-reported rhinitis and/or asthma (yes/no), maternal smoking during pregnancy (yes/no), and cohort.
Statistical analyses
Data were analysed with SAS V.9.4 and R V.3.1.2 (R Foundation for Statistical Computing). In all analyses the missing values were excluded list-wise. Baseline data and incidences of the primary and secondary outcomes were described as frequencies and percentages per age and sex for each cohort and in total.
We used one-stage IPD meta-analyses to combine the data from five European birth cohorts (BAMSE, PIAMA, GINIplus, LISA and MAS). Proportional hazard models including puberty as a time-dependent covariable with the average partial likelihood method for handling ties in the event times were used for analysing the data. Within each proportional hazard model for all primary and secondary outcomes, two adjusted HRs with 95% CIs were presented, one comparing boys versus girls before puberty onset, and one comparing them after puberty onset. P values were reported for the interaction term ‘sex*puberty’, which reflects the sex-specific changes in outcome incidences before versus after puberty onset in the model. The present clinical questions and data analyses are post-hoc, and we consider the results to be hypothesis-generating rather than confirmatory. Therefore, all results, including p values, are considered exploratory and were not adjusted for any multiple testing. In defining the primary outcome, incidence was rated ‘missing’ if asthma and/or rhinitis were missing at the current follow-up. If the incidence was rated positive in an earlier follow-up, the participant was no longer at risk and was censored for the incidence calculation at the current follow-up.
Patient and public involvement
We did not include patients, only samples of healthy infants from the general population. Participants and the public were not involved in the development of the research question, outcome measures or study design. The individual birth cohort study teams inform their participants regularly about relevant new results of these long-term prospective studies, mainly via their study-specific websites.
Results
Basic characteristics of the five included birth cohorts
Five birth cohorts collaborating in the MeDALL project with 18 451 recruited participants in total were included. Due to dropouts, the available IPD for analyses of primary and secondary outcomes varied at different follow-ups. At most follow-ups the rate of dropouts was equal between sexes, and only at some follow-ups we found more male than female dropouts (see online supplementary table 1). The included follow-up assessment time points were from 4 to 20 years of age. About half of the participants were male, and positive parental history of allergy ranged from 43% to 56% in the different cohorts. Across the three cohorts with data at age 10 years, 1.9% of male and 13.6% of female participants reported signs of puberty at the 10-year follow-up. These percentages increased to over 90% at the 15-year and 16-year follow-ups for both boys and girls, respectively (table 1).
Table 1.
Baseline characteristics and presence of signs of puberty by sex and age, separately for each birth cohort and pooled
| PIAMA (n=3963) | BAMSE (n=4089) | GINIplus (n=5991) | LISA (n=3094) | MAS (n=1314) | Total | |||||||
| Boys n/N (%) |
Girls n/N (%) |
Boys n/N (%) |
Girls n/N (%) |
Boys n/N (%) |
Girls n/N (%) |
Boys n/N (%) |
Girls n/N (%) |
Boys n/N (%) |
Girls n/N (%) |
Boys (%) | Girls (%) | |
| Sex | 2054/3963 (51.8) |
1909/3963 (48.2) |
2065/4089 (50.5) |
2024/4089 (49.5) |
2991/5830 (51.3) |
2839/5830 (49.7) |
1584/3094 (51.2) |
1510/3094 (48.8) |
684/1314 (52.1) |
630/1314 (48.0) |
51.3 | 48.8 |
| Parental allergy | 885/2025 (43.7) |
805/1889 (42.6) |
1001/2050 (48.8) |
976/2007 (48.6) |
1022/2340 (43.7) |
998/2214 (45.1) |
738/1470 (50.2) |
710/1384 (51.3) |
275/643 (42.8) |
300/601 (49.9) |
45.9 | 47.4 |
| Maternal smoking during pregnancy | 356/2037 (17.5) |
344/1889 (18.2) |
272/2065 (13.2) |
259/2023 (12.8) |
383/2474 (15.5) |
356/2327 (15.3) |
262/1524 (17.2) |
274/1450 (18.9) |
154/627 (24.6) |
154/584 (26.4) |
17.2 | 17.9 |
| Puberty at age 10 | – | – | – | – | 63/1593 (3.9) |
170/1372 (12.4) |
31/853 (3.6) |
85/702 (12.1) |
0/394 (0) |
58/326 (17.8) |
1.9 | 13.6 |
| Puberty at age 11 | 40/1316 (3.0) |
796/1295 (61.5) |
– | – | – | – | – | – | 1/368 (0.3) |
147/299 (49.2) |
1.4 | 55.6 |
| Puberty at age 12 | – | – | 424/1390 (30.5) |
1184/1353 (87.5) |
– | – | – | – | 21/361 (5.8) |
261/346 (75.4) |
16.3 | 82.0 |
| Puberty at age 13 | – | – | – | – | – | – | – | – | 117/373 (31.4) |
369/387 (95.4) |
31.4 | 95.4 |
| Puberty at age 14 | 1092/1264 (86.4) |
1363/1366 (99.8) |
– | – | – | – | – | – | – | – | 86.4 | 99.8 |
| Puberty at age 15 | – | – | – | – | 1125/1268 (88.7) |
1274/1330 (95.8) |
683/741 (92.2) |
678/723 (93.8) |
289/315 (91.8) |
390/393 (99.2) |
90.8 | 96.6 |
| Puberty at age 16 | – | – | 1231/1318 (93.4) |
1594/1623 (98.2) |
– | – | – | – | – | – | 93.4 | 98.2 |
BAMSE, Swedish abbreviation for Children, Allergy, Milieu, Stockholm, Epidemiology; GINIplus, German Infant Nutritional Intervention-Program; LISA, Lebensstil, Immunsystem, Allergien; MAS, Multizentrische Allergie Studie; n, number of participants per analysis; N, total number of participants at time of recruitment; PIAMA, Prevention and Incidence of Asthma and Mite Allergy.
Incidence and sex shift of asthma
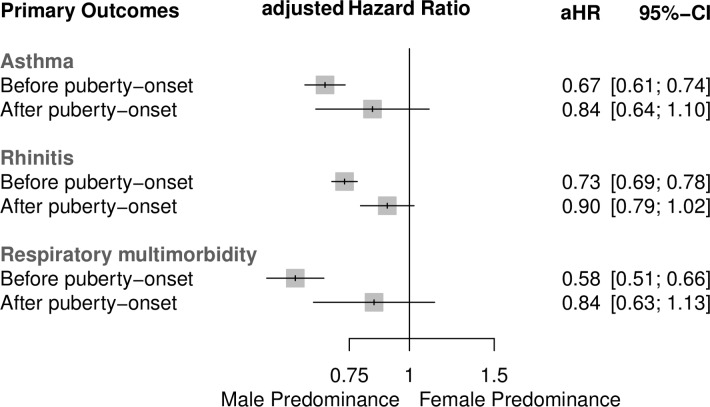
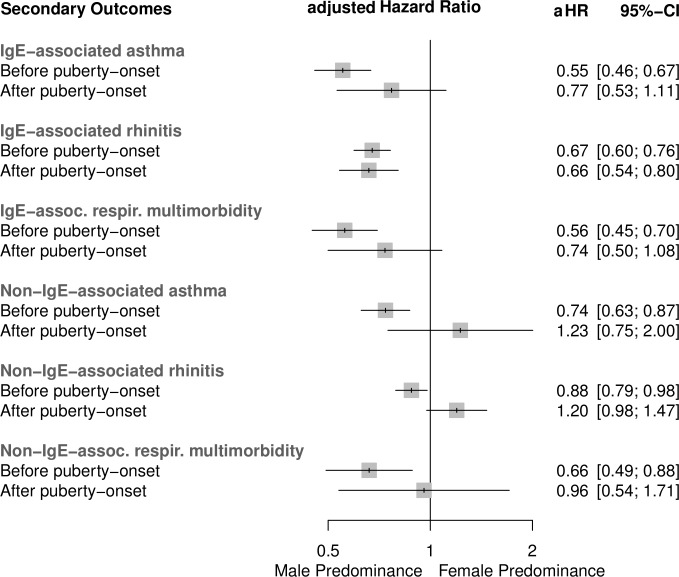
The incidence of asthma was lower for girls than for boys at preschool and early school-age follow-ups. The percentages decreased for both sexes over time (see online supplementary table 2). Before puberty onset, girls were at a considerably lower risk than boys for developing incident asthma (HR 0.67, 95% CI 0.61 to 0.74). After puberty onset, the difference was less distinct (girls vs boys; HR 0.84, 95% CI 0.64 to 1.10). The interaction term ‘sex*puberty’ described a p=0.122 (figure 1). These findings were similar for IgE-associated asthma, whereas for non-IgE-associated asthma we found a more pronounced sex shift towards female predominance after puberty onset (HR 0.74, 95% CI 0.63 to 0.87 before vs 1.23, 0.75 to 2.00 after puberty onset) (figure 2).
Figure 1.
Adjusted HRs and 95% CIs for the risk of the incidence of asthma, rhinitis and respiratory multimorbidity for boys versus girls before and after puberty onset. The presented results are based on analyses of n=14 245, n=14 266 and n=14 233 participants, respectively. aHR, adjusted HR.
Figure 2.
Adjusted HRs and 95% CIs for the risk of the incidence of IgE-associated and non-IgE-associated asthma, rhinitis and respiratory multimorbidity for boys versus girls before and after puberty onset. The presented results are based on analyses of n=10 320, n=10 331 and n=10 304 participants, respectively. aHR, adjusted HR.
Incidence and sex shift of rhinitis
In follow-ups during preschool and early school age, rhinitis incidence was more frequently reported in boys than in girls. At the latest follow-up assessments, most cohorts had either similar or slightly higher rhinitis incidences in girls compared with boys (see online supplementary table 3).
Before puberty onset, girls were at a considerably lower risk than boys for incident rhinitis (HR 0.73, 95% CI 0.69 to 0.78), whereas after puberty onset this lower risk was less distinct and almost sex-balanced (HR 0.90, 95% CI 0.79 to 1.02): interaction term ‘sex*puberty’ p=0.005 (figure 1). Incidences of IgE-associated and non-IgE-associated rhinitis differed considerably considering the effect of puberty onset: the male predominance remained almost identical before and after puberty onset for IgE-associated rhinitis, whereas for the incidence of non-IgE-associated rhinitis we found a sex switch from male to female predominance after puberty onset (HR 0.88, 95% CI 0.79 to 0.98 before vs 1.20, 0.98 to 1.47 after puberty onset) (figure 2).
Incidence and sex shift of respiratory multimorbidity
Up to the age of 6 years, the incidence of respiratory multimorbidity was higher in boys than in girls in all cohorts. At the last follow-ups, most cohorts had an either similar or higher incidence of respiratory multimorbidity for girls compared with boys (see online supplementary table 4).
Before the onset of puberty, girls were at a considerably lower risk than boys for incident respiratory multimorbidity (HR 0.58, 95% CI 0.51 to 0.66), and this effect was stronger than for asthma and rhinitis as single entities. After puberty onset, the risk for incident respiratory multimorbidity was sex-balanced (HR 0.84, 95% CI 0.63 to 1.13; interaction term ‘sex*puberty’ p=0.02) (figure 1). Considering allergic sensitisation status, these findings were similar for the incidence of IgE-associated respiratory multimorbidity and more pronounced towards a sex-balanced incidence for non-IgE-associated respiratory multimorbidity (HR 0.66, 95% CI 0.49 to 0.88 before vs 0.96, 0.54 to 1.71 after puberty onset) (figure 2).
Discussion
Main findings
Our longitudinal birth cohort meta-analyses using harmonised IPD specifically examined the sex-specific incidence in relation to puberty onset for common chronic allergic and respiratory conditions. Our results showed a sex shift from a male predominance before puberty towards a sex-balanced incidence after puberty onset for asthma and rhinitis as single entities and as a respiratory multimorbidity. These findings explain some of the previously described sex shifts in asthma and allergy prevalence from childhood to adulthood. After stratifying for allergic sensitisation, the sex-specific effect seemed stronger for non-IgE-associated conditions.
Comparison with other studies
Similar to our findings, the Isle of Wight study found a male predominance for allergic rhinitis at 4 and 18 years. For non-allergic rhinitis the study reported no sex difference at 4 years but a female predominance at 18 years.12 Observational population-based prevalence studies have been more common than incidence studies particularly for rhinitis and respiratory multimorbidity. A longitudinal British birth cohort study found the annual incidence of asthma and wheezing up to 16 years of age was higher in boys than in girls: 0.85% vs 0.58% on average per year. This effect reversed during follow-up at 17–23 years, when the incidence was considerably lower in boys than in girls: 0.56% vs 0.94%.13 This finding suggested a prevalence sex shift at a slightly older age than that of our study participants. Given the age of effect reversal, it remains unclear if it is puberty onset-related. Puberty status was not specifically evaluated in this UK birth cohort. The participants in the UK cohort were born in 1958, and the pubertal age has been decreasing over the past decades. A more recent cohort study from Canada stated a higher asthma incidence for girls than for boys aged 12–18 years: 13.2% vs 6.6% per 1000 person-years.14 A large Dutch cohort study based on medical records from a health database found an asthma incidence of 6.7 per 1000 person-years for children and adolescents aged 5–18 years. They confirmed a sex shift in asthma incidence at the age of 13, with a male predominance before and a female predominance thereafter.15 Another Dutch study found a sex shift with more girls than boys reporting asthma at the age of 16. However, an association with the assessed pubertal status could not be confirmed.16 Individuals were found to have an increased risk of asthma if they reported an early but not a normal or late puberty onset.17 A lack of information about early, normal or late puberty onset, as well as not taking into account the differentiation between IgE-associated and non-IgE-associated asthma, could account for some of the differences in the reported study results. Two recent systematic reviews of cross-sectional studies reported a sex-related shift for rhinitis prevalence with and without respiratory comorbidity from a male predominance in childhood towards a female predominance among adolescents.1 2 Our study adds to previous knowledge by examining incidence data in rhinitis and respiratory multimorbidity, the effect of puberty status, and a differentiation of IgE versus non-IgE-association for all three health outcomes. We were able to show that in the examined cohorts, puberty onset played a considerable role in a shift from male predominance towards a more sex-balanced incidence of asthma, rhinitis and respiratory multimorbidity while controlling for age.
The incidence of asthma, rhinitis and respiratory multimorbidity varied between the cohorts in our combined analyses. Potential explanations include differences in the recruitment procedures, climatic regions (from Scandinavia to Southern Germany) and/or degree of urbanisation of the study areas (from urban to mixed urban-rural). Differences in assessment methods among the included cohorts have been minimised as the specific questions have been previously harmonised (based on the ISAAC questions) and underwent a further harmonisation process for the latest assessment in adolescence.7 10 Therefore, we consider the outcome and puberty data across the included cohorts to be comparable.
In line with our results, an elevated risk of adult-onset non-IgE-associated asthma in women has been previously reported.18 Various reasons for the observed increased risk of asthma and rhinitis in women after puberty have been discussed. Contributing factors may include sex-specific and age-related anatomical differences of the lung.19 Considering puberty and the postpubertal stage, there is strong evidence that the sex hormones oestrogen and progesterone influence the development and outcome of asthma. However, the question of which hormones have protective effects or increase the risk of developing allergy and asthma is not fully understood.19 Women generally show stronger immune responses than men, which makes them potentially more vulnerable to autoimmunity and allergy. A suppressive effect of androgens on group 2 innate lymphoid cells is supposed have a protective effect for allergic asthma in men after puberty onset.20
Strengths and limitations
For this analysis, we aimed to focus on asthma, rhinitis and respiratory multimorbidity incidence data as these have relevant public health implications. An increased incidence in adolescence emphasises the need for a raised awareness for early diagnosis and treatment of formerly healthy female subjects. To date, incident asthma cases are less likely to be diagnosed in adolescence than in childhood. At 16 years, girls reported uncontrolled asthma more often but fewer doctors’ diagnoses and they were prescribed fewer medications than boys.21 As the prevalence of a disease is determined by its incidence and remission, prospectively collected longitudinal data to better understand the interplay of incidence and remission would be of interest in the future.
Among the strengths of the current study is the prospectively collected longitudinal data from five different European population-based cohorts. Furthermore, we were able to include IPD from over 18 000 European birth cohort participants for the pooled analyses rather than performing ‘traditional’ meta-analyses combining already published effect estimates. Our approach aimed to minimise heterogeneity between the cohorts by harmonising the individual data a priori, where necessary. The construction of a common database, thanks to a close trustful collaboration between the research teams, has led to a unique data source of European birth cohorts. The pooled data set has more statistical power than the individual cohort data sets and allows examination of less common (sub)phenotypes or exposures. This is the first longitudinal birth cohort evaluation of the incidence of asthma and rhinitis as single entities and as respiratory multimorbidities in relation to the puberty status of birth cohort participants.
Several limitations should be considered while interpreting the current study results. The five included European cohort studies are not representative of the general population of their countries. However, to a certain degree, they represent regional, mainly urban populations from where they were recruited.
Four out of five cohorts provided the latest follow-up for age 14–16 years and only one cohort for 20 years of age. Many girls at age 14–16 years were already in puberty at earlier follow-ups. Almost all participants were categorised as being at least in puberty, and many participants, especially the girls, had completed puberty by the last available follow-up. However, this cannot be interpreted as a long-term postpubertal observation period. Therefore, possible long-term effects of puberty-related hormone exposure cannot be evaluated with our sample and have to be subject to future investigations of these cohorts.
Another possible limitation of longitudinal studies such as birth cohorts is the systematic and unsystematic dropouts, resulting in lower participation rates and a possible bias over the time course. However, as this may influence the magnitude of the incidence estimates, we have no clear indication of a systematic sex-related loss of participants (see online supplementary table 1).
Our definition of incidence could not always be restrained to 1 year, which would have been desirable. Among the cohorts, time intervals between the follow-up assessments differed. The longest interval was 5 years. Even if the participants were asked for the occurrence of symptoms within the past 12 months, it could not be excluded that the condition might have already developed at an earlier time point within the time frame without a follow-up. This has to be considered for the definition of the onset of puberty. However, the cohorts all started with 100% prepubertal children, and we were eventually able to detect the onset of puberty in 96% of all participants. Lacking the exact timing of the onset of puberty might be negligible as it may not influence our results considerably. The variables of age and puberty onset are highly related. We aimed to disentangle these two variables by controlling for age while puberty was the time-dependent covariable.
Conclusions
Our IPD meta-analyses showed an incidence ‘sex shift’ in chronic respiratory diseases from males to females after puberty onset, which may partly explain the previously and more commonly reported prevalence ‘sex shift’. The observed sex shift, especially for non-IgE-related incidences of the diseases, suggests sex-specific and puberty-specific underlying mechanisms. Our results stress the importance of raising alertness among clinicians for incident cases of respiratory diseases in adolescent girls for effective detection and timely treatment.
Footnotes
Contributors: CH wrote the initial draft under the supervision of SR and TKei. TKel developed the statistical analysis plan and conducted the initial analyses. TKel, CH, UG, AW and HS augmented the statistical analyses plan, and TKel conducted and interpreted the statistical analyses with supervision of SR. CH, TKei, JA and JB coordinated the development of common standardised questionnaires and standard operational procedures, coordinated the follow-up assessment of the MeDALL birth cohorts, and participated in the development of the initial statistical analysis plan. MS, AvB (GINIplus), JH, IL (LISA), UG, AW, HS (PIAMA), AB, IK (BAMSE), SL, TKei and UW (MAS) coordinated the local follow-up assessments and provided the data on the harmonised follow-up and the birth cohort data. They, along with DM, participated in the planning of the common database and in the preparation of harmonised data sets for central storage and analyses. DM built the common database. DM was responsible for the correct and safe storage of the data in the common database and the data distribution to different research teams. All authors read the different versions of the manuscript, revised them and provided comments, participated in the revision of the final manuscript, and approved the final version.
Funding: This study was funded by MeDALL, a joint project conducted within the European Union under the Health Cooperation Work Programme of the 7th Framework Programme (grant agreement no 261357). The BAMSE study was supported by the Swedish Research Council, Swedish Heart and Lung Foundation, Swedish Research Council for Working Life and Social Welfare, Swedish Asthma and Allergy Association Research Foundation, Swedish Research Council Formas, Stockholm County Council (ALF), and the European Commission’s Seventh Framework 29 Programme MeDALL under grant agreement no 261357. The GINIplus study was supported by the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm), by respective budgets of the five study centres (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich, IUF - Leibniz Research-Institute for Environmental Medicine at the University of Düsseldorf) and by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the GINIplus study was supported by the Commission of the European Communities, the 7th Framework Programme: MeDALL project, as well as by the companies Mead Johnson and Nestlé. The LISA study was supported by grants from the Federal Ministry for Education, Science, Research and Technology, and in addition by Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research - UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, by the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research - UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF – Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf) and by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). Further, the 15-year follow-up examination of the LISA study was supported by the Commission of the European Communities, the 7th Framework Programme: MeDALL project. The PIAMA study was supported by the Netherlands Organization for Health Research and Development; the Netherlands Organization for Scientific Research; the Netherlands Asthma Fund (grant 4.1.14.001); the Netherlands Ministry of Spatial Planning, Housing, and the Environment; and the Netherlands Ministry of Health, Welfare and Sport. The MAS study was funded by the German Federal Ministry of Education and Research (BMBF; reference numbers 07015633, 07 ALE 27, 01EE9405/5, 01EE9406) and the German Research Foundation (DFG; reference number KE 1462/2-1).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was obtained from the local ethics committees for each study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Fröhlich M, Pinart M, Keller T, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin Transl Allergy 2017;7:44 10.1186/s13601-017-0176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinart M, Keller T, Reich A, et al. Sex-Related allergic rhinitis prevalence switch from childhood to adulthood: a systematic review and meta-analysis. Int Arch Allergy Immunol 2017;172:224–35. 10.1159/000464324 [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Hohmann C, Standl M, et al. The sex-shift in single disease and multimorbid asthma and rhinitis during puberty - a study by MeDALL. Allergy 2018;73:602–14. 10.1111/all.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau S, Matricardi PM, Wahn U, et al. Allergy and atopy from infancy to adulthood: messages from the German birth cohort MAS. Ann Allergy Asthma Immunol 2019;122 10.1016/j.anai.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 5.Fortier I, Burton PR, Robson PJ, et al. Quality, quantity and harmony: the DataSHaPER approach to integrating data across bioclinical studies. Int J Epidemiol 2010;39:1383–93. 10.1093/ije/dyq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benet M, Albang R, Pinart M, et al. Integrating clinical and epidemiologic data on allergic diseases across birth cohorts: a harmonization study in the mechanisms of the development of allergy project. Am J Epidemiol 2019;188:408–17. 10.1093/aje/kwy242 [DOI] [PubMed] [Google Scholar]
- 7.Hohmann C, Pinart M, Tischer C, et al. The development of the MeDALL core questionnaires for a harmonized follow-up assessment of eleven European birth cohorts on asthma and allergies. Int Arch Allergy Immunol 2014;163:215–24. 10.1159/000357732 [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Anto J, Auffray C, et al. MeDALL (mechanisms of the development of allergy): an integrated approach from phenotypes to systems medicine. Allergy 2011;66:596–604. 10.1111/j.1398-9995.2010.02534.x [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Anto JM, Akdis M, et al. Paving the way of systems biology and precision medicine in allergic diseases: the MeDALL success story: mechanisms of the development of allergy; EU FP7-CP-IP; project NO: 261357; 2010-2015. Allergy 2016;71:1513–25. 10.1111/all.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (Isaac): rationale and methods. Eur Respir J 1995;8:483–91. 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 11.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health 1993;14:190–5. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- 12.Kurukulaaratchy RJ, Karmaus W, Raza A, et al. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy 2011;41:851–9. 10.1111/j.1365-2222.2011.03765.x [DOI] [PubMed] [Google Scholar]
- 13.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax 1992;47:537–42. 10.1136/thx.47.7.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson JA, Janssen I, Bruner MW, et al. Asthma incidence and risk factors in a national longitudinal sample of adolescent Canadians: a prospective cohort study. BMC Pulm Med 2014;14:51 10.1186/1471-2466-14-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelkes M, Janssens HM, de Ridder MAJ, et al. Time trends in the incidence, prevalence and age at diagnosis of asthma in children. Pediatr Allergy Immunol 2015;26:367–74. 10.1111/pai.12376 [DOI] [PubMed] [Google Scholar]
- 16.Vink NM, Postma DS, Schouten JP, et al. Gender differences in asthma development and remission during transition through puberty: the tracking adolescents' individual lives survey (trails) study. J Allergy Clin Immunol 2010;126:498–504. 10.1016/j.jaci.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 17.Minelli C, van der Plaat DA, Leynaert B, et al. Age at puberty and risk of asthma: a Mendelian randomisation study. PLoS Med 2018;15:e1002634 10.1371/journal.pmed.1002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen S, Probst-Hensch N, Keidel D, et al. Gender differences in adult-onset asthma: results from the Swiss SAPALDIA cohort study. Eur Respir J 2015;46:1011–20. 10.1183/13993003.02278-2014 [DOI] [PubMed] [Google Scholar]
- 19.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 2012;33:1–47. 10.1210/er.2010-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffont S, Blanquart E, Guéry J-C. Sex differences in asthma: a key role of androgen-signaling in group 2 innate lymphoid cells. Front Immunol 2017;8:1069 10.3389/fimmu.2017.01069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ödling M, Andersson N, Ekström S, et al. Characterization of asthma in the adolescent population. Allergy 2018;73:1744–6. 10.1111/all.13469 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000460supp001.pdf (41.1KB, pdf)