Abstract
Breast augmentation remains one of the most commonly performed aesthetic procedures in the United States and worldwide. Throughout the last few decades, the implants used for this procedure have undergone significant advancements, which has allowed surgeons to provide safer and more aesthetically pleasing outcomes. This article discusses the history of breast implants since their invention in 1962. Particular emphasis is given to the evolution of silicone implants with its many challenges, which has resulted in the development of the currently used fourth- and fifth-generation devices. Knowledge of these advances will allow physicians to more critically evaluate their results, and also will encourage them to provide more up-to-date scientific data on these devices to further improve the clinical outcomes of their patients.
Keywords: breast implant, silicone breast implant, evolution, aesthetic surgery, cosmetic surgery
Over the last few decades, especially with the recent evolution of social media, the emphasis on body image is evident. This coupled with the increasing acceptance of aesthetic surgery has led to a tremendous growth in breast augmentation surgery. In fact, since 1997, the annual number of breast augmentations has increased by 206.8%. 1 Augmentation mammoplasty is currently the second most common cosmetic surgical procedure in the United States, with 310,444 procedures performed in 2016 alone. 1 The wide acceptance of the procedure is further justified by the previously described positive effects it has on women, including enhanced self-image, increased self-assurance, improved sexual functioning, and better interpersonal relationships. 2
In 1895, Czerny reported the first successful breast augmentation, in which a lipoma from the trunk was transplanted to the breast of a patient after a partial mastectomy. 3 During the next 50 years, surgeons have attempted to implant various materials into the breast, such as ivory, glass, balls, rubber, cartilage, wool, gutta-percha, polyethylene chips, and sponges. 4 The results were disappointing, as these materials created hard, distorted, and unnatural-appearing breasts. In 1954, Longacre described a local dermal fat flap for augmentation of the breast. 5 Around the same time, solid alloplastic materials were used for breast augmentations including polyurethane, polytetrafluoroethylene (Teflon), and expanded polyvinyl alcohol formaldehyde (Ivalon sponge). 6 These materials were abandoned because patients developed local tissue reactions, firmness, distortion of the breast, and significant discomfort. 7 Several other solid and semisolid materials have been injected directly into the breast parenchyma for augmentation, including epoxy resin, shellac, beeswax, paraffin, and petroleum jelly. 8 Uchida reported the injection of liquid silicone (polydimethylsiloxane) into the breast for augmentation in 1961. 7 Frequent complications were observed with this technique including recurrent infections, drainage, chronic inflammation, granuloma formation, and even necrosis. 9 As a result, use of such injectable materials was ultimately discontinued.
Despite the complications and unsatisfactory results with the aforementioned techniques, the patients' desires for breast enlargement motivated surgeons to continue searching for the ideal approach for breast augmentation. In 1962, Cronin and Gerow reported the use of the first silicone gel breast implant, which marked the beginning of the modern era of breast augmentation. 10 This was composed of a viscous silicone gel that was contained within a thick silicone shell. These two-component prosthetic devices designed with a silicone elastomer shell filled with a stable filling material, consisting of either saline solution or silicone gel, showed promising results; however, they underwent several technical modifications and improvements over the next few decades.
Evolution of Saline Implants
The first inflatable saline-filled breast implant was patented in 1964 in France by Arion and used clinically the following year. 11 A few years later, saline implants were also developed in the United States, and clinical trials were performed to thoroughly evaluate these devices. 9 12 The main incentive for the development of these implants was a smaller incision. A noninflated implant could be inserted into the pocket through a relatively small incision, and then inflated in situ with its liquid filler material.
The original saline-filled implants had very high deflation rates due to the shell of the device and valve failures. The deflation rates significantly improved after addressing these issues by using room temperature vulcanized cured shells and diaphragm valves. 4 13 Filling the implant with saline has also been advocated to have its own inherent problems. The consistency of palpation is similar to that of water as opposed to the more viscous feel of natural breast tissue. In addition, volumetrically underfilled devices may transmit visible surface wrinkles and a knuckle-like feel. On the other hand, aggressive overfilling of these devices may lead to a more spherical shape and scalloping along the implant, as well as unnatural firmness. For these reasons, saline implants historically perform better under thicker tissue, with filling of the implant to the recommended volume or just beyond.
Evolution of Silicone Implants
In 1962, Cronin and Gerow introduced the first-generation silicone gel–filled implant, which was manufactured by Dow Corning Corporation. 10 This was a two-component prosthetic device, and anatomically shaped (teardrop). The shell was built with thick, smooth silicone elastomer as a two-piece envelope with seams along the periphery. The shell was filled with moderately viscous silicone gel. Dacron fixation patches were added to the posterior surface of the implant to assist with proper positioning of the device and avoid rotation ( Fig. 1 ). The incidence of capsular contracture with these implants was relatively high. This was attributed to the quality of the shells and the lack of cohesivity of the gel. As a result, a new generation of implants was developed by various manufacturers in the 1970s. 14
Fig. 1.
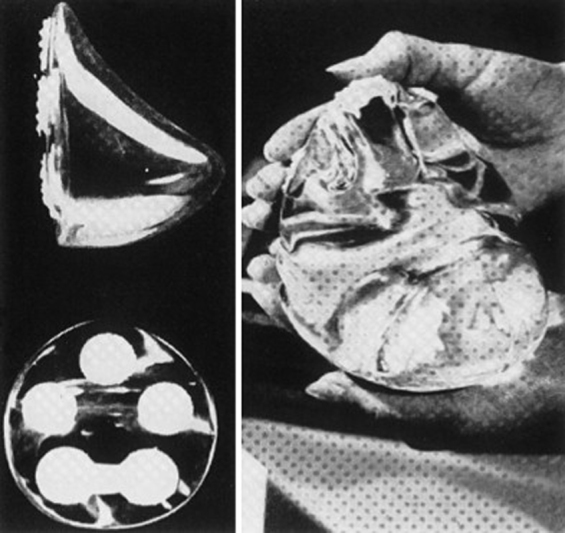
The original Cronin–Gerow silicone implant had an anatomic (teardrop) shape, smooth surface, and posteriorly placed Dacron patches to help maintain the implant's position.
The second-generation silicone gel–filled implants had a thinner shell without seams or Dacron fixation patches in an attempt to decrease the capsular contracture rate. The implants were round and filled with a less viscous silicone gel to provide a more natural feel. These changes did not only fail to significantly improve the incidence of capsular contracture, but they also led to an increased incidence of silicone gel “bleed.” This was a phenomenon whereby microscopic silicone molecules diffuse or leak through the silicone elastomer shell into the periprosthetic intracapsular space, and it was noted with both intact an ruptured implants, as well as after closed capsulotomies. 15 16 17 18 19 20 21 Leakage of silicone was reported to create an oily, sticky residue around the implant but within the periprosthetic capsule, which was evident during explantation of these devices. 22
The third generation of silicone gel–filled implants was introduced in the early 1980s. These prostheses had a new improved multilayered silicone elastomer shell characterized by two layers of high-performance elastomer with a thin fluorosilicone barrier coat in between. The main goal was to increase the strength and integrity of the shell in an attempt to eliminate the silicone gel bleed and minimize implant rupture with associated gel spillage. Indeed, these improvements enhanced the shell life of these devices, and resulted in lower rates of capsular contracture. 23 24
Despite the great evolution of these silicone gel–filled implants from the manufacturing standpoint, there were concerns related to the safety of these devices given their high complication rates. In the 1980s, several reports suggested a link between silicone prostheses and connective tissue disorders. 25 26 27 These public fears were amplified by the media coverage around 1990. The controversy over the implants reached its crescendo in 1992, when the Food and Drug Administration (FDA) announced the temporary restriction of third-generation silicone gel implants from the American market. 28 29 30 31 32 33 It was stated that these devices would be made available to women only through controlled clinical trials until more data regarding their safety become available. This moratorium urged the industry to develop the fourth- and fifth-generation silicone gel–filled implants. These new devices were designed under better quality control and more strict criteria for shell thickness and gel cohesiveness guided by both the FDA and the American Society for Testing Methodology (ASTM). 34 35 After several years of uncertainty that included enormous amounts of research and clinical testing, silicone gel–filled implants made by U.S. manufacturers were approved in November 2006.
The concept of anatomically shaped implants was also introduced with the fifth-generation silicone gel implants. 36 They were not only filled with a more cohesive gel, but they also had a textured surface. All U.S. manufacturers made these fifth-generation devices in a variety of shapes and sizes, and they were approved by the FDA between 2012 and 2013 (Sientra in 2012; Allergan in 2013; and Mentor in 2013).
Some implant characteristics, such as surface, filler, shell, and shape, are as important to the final breast form as the soft-tissue envelope. Therefore, they deserve further discussion here to allow for a more comprehensive review of the evolution of silicone gel–filled implants.
Surface
Since the early days of implant development, it has been advocated that the surface characteristics of these devices may play a role in the quality of the periprosthetic capsule that is formed after implantation. The concept of surface texturing started in the 1970s with the use of a thin layer of polyurethane foam to cover a silicone gel implant. Although initially the foam was placed on the implant mainly to maintain its position, clinical use revealed lower rates of capsular contracture. 36 37 These foam-coated implants became popular in the 1980s with reproducible results. 38 However, safety concerns were expressed a few years later, which resulted in voluntary removal of these implants from the U.S. market in 1991. It was thought that the polyurethane foam layer could not only separate from the implant but also undergo physical and chemical degradation upon implantation. 39 40 This decomposition was reported to produce a range of compounds including 2-toluenediamine, a compound that was found to be carcinogenic in rats, but not a confirmed carcinogen in humans. 41
By that point it was clear that a textured surface could potentially minimize or even disrupt capsule formation. There was strong enthusiasm by U.S. manufacturers to develop a similar textured silicone surface that would achieve the same favorable response. 42 43 Each company had a proprietary process in place; thus, the surfaces were not the same between different manufacturers. While the manufacturing process of smooth surface implants was fairly straightforward with dipping a mandrel into liquid silicone creating multilayers and then allowing the surface to cure in a laminar flow oven, the production of textured implants was more complex requiring several additional steps. 44 Sientra's Silimed implant named as TRUE Texture has small hollow pores that are made with minimal cell webbing, which reduces particle formation. It avoids the use of sodium chloride, sugar, soak/scrub, or pressure stamping. 45 46 47 Biocell (Allergan), on the other hand, is an aggressive open-pore textured silicone surface. It is created by using a “loss-salt” technique, which involves formation of a layer of salt crystals with a thin overcoat of silicone that is then cured in a laminar flow oven. 44 The third main textured surface is Siltex (Mentor), which is generated by a different method called “imprint stamping.” This technique includes dipping the chuck into uncured silicone, pushing it into polyurethane foam, and finalizing the imprint with pressure 44 ( Fig. 2 ).
Fig. 2.
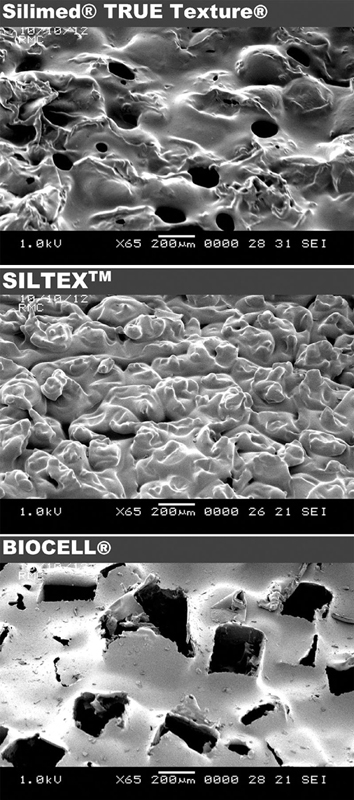
Scanning electron microscopic images of Silimed TRUE Texture, Allergan Biocell, and Mentor Siltex textured surfaces at 65-times magnification. (Reproduced with permission from Stevens W, Nahabedian M, Calobrace M, et al. Risk factor analysis for capsular contracture: A 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg 2013;132(5):1115–1123.)
In addition to the effect on capsular contracture, one of the original goals of surface texturing was to better secure the implant in the breast pocket. The shells of the aforementioned textured devices had different pore sizes, which was thought to have an effect on the integration of the prostheses. In 2001, Danino et al compared the Biocell texture with pore diameter of 600 to 800 μm and a depth of 150 to 200 μm with Siltex with pore diameter of 70 to 150 μm. 48 Tissue adherence of the device (i.e., the “adhesive effect”) was suboptimal with Siltex, which had small pore diameter. The authors concluded that pore size correlated with implant stabilization, but it was not associated with a reduction in capsular contracture.
Filler
Silicone has been used as filler since the first implants were invented in the 1960s. It did, however, undergo modifications throughout the years to provide a more reliable and durable filler with a more natural feel. In the early stages of implant development it was thought that the filler material has an effect on capsular contracture rates, but these early studies compared the third-generation silicone implants with saline implants. 49 50 51 This does not appear to be the case with the newer generations of silicone implants since their safety and long-term outcomes have been described extensively over the last decade. 45 52 53 54 55 56
Silicone is made up of silicon, oxygen, carbon, and hydrogen. It is a mixture of semi-inorganic polymeric molecules composed of varying length of chains of polydimethylsiloxane [(CH 3 ) 2 -SiO] monomers. The average length of polymer chains and the degree of cross-linking between polymer chains determine the physical properties of silicone. 57 For example, liquid silicones are composed of polymers with relatively short average length and minimal cross-linking. As the length of the polymer chains and the degree of cross-linking increases, the consistency of the silicone changes resulting in silicone gel implants with higher viscosities. Once a certain degree of cross-linking is reached, the silicone gel implant will maintain its dimensions and form producing a cohesive gel implant. These cohesive implants were also called “form-stable,” suggesting that the gel distribution within the shell remains stable. Since none of the available implants are completely form stable, this term has been challenged, and more appropriately it now indicates the ability of the implant to maintain shape. 58 Nowadays, the cohesivity of the silicone gel can be better measured, which helps compare the stiffness of both round and shaped implants among the three main U.S. manufacturers. One study by Maxwell et al revealed that the 410 implant (Allergan) had the stiffest gel representing the highest cohesivity when compared with the Contour Profile Gel (CPG) implant or now called “MemoryShape” (Mentor). 56 In another study, it was found that Sientra's form-stable implant was the least cohesive compared with both CPG and 410 implants. 54 It was also shown that Allergan's round implants were the least cohesive compared with Mentor's round implants, and that Sientra's implants were the most cohesive compared with both Allergan's and Mentor's round implants. Caution should be exercised when interpreting the results of these studies because cohesivity represents only one of the implant characteristics that can affect stiffness, and all the other implant features have to be taken into consideration when evaluating these implants. In addition, it is important to note that to date, there is no accepted standardized grading system for implant cohesivity given the proprietary manufacturing process. This makes it difficult to very accurately compare the cohesive nature of all the available devices.
Shell
The outer shell of all breast implants is composed of silicone elastomers. The structure of these elastomers is similar to silicone gels, but with much greater cross-linking and very little fluid to produce a solid shell with a flexible, rubberlike quality. Some of the favorable properties of silicone that make it an ideal material for the shell include stability across varying temperatures, low reactivity to other chemicals, and low surface tension. 59 The creation of a thicker shell with triple-shell elastomer and the addition of a barrier layer are some of the shell modifications of the modern devices that helped protect against gel bleeds, thus leading to better outcomes. 45 56 60 61 62 Another important variable that determines the shape stability of these prostheses include the relationship of the shell and the gel, with special attention paid to the bonding between the shell and the internal gel.
Implant Shape
Several factors can affect the shape of the implant including the cohesivity of the gel and its distribution within the shell, the adherence of the gel to the shell, as well as the gel–shell fill ratio. Implants maintain a better shape when the gel distribution within the shell is preserved, a more cohesive gel is used that is more adherent to the shell, and a higher gel–shell fill ratio is achieved. The gel–shell ratio differs among manufacturers, but there is no formal standardized scale to grade these devices given the proprietary process of manufacturing. The gel–shell ratio also varies within the different profiles of round implants (e.g., low, moderate, high). This variation in the gel–shell fill ratio can result in noticeable differences clinically, such as rippling and upper pole collapse, if inappropriately utilized in patients with suboptimal profiles. Interestingly, a previous study utilizing magnetic resonance imaging has demonstrated rippling of the shell of one of the most cohesive form-stable implants when the patients were in prone position. 16 Although that can raise concerns for some patients, in the majority of cases, the change of shape or form of these devices with positioning does not produce a substantial clinical or visual change.
Discussion
The evolution of breast implants has placed an increasing emphasis on improving breast aesthetics in those seeking some form of breast augmentation. Interestingly, the ideal size and shape of the female breast is inherently subjective, and is mostly influenced by personal preference and cultural norms. Despite this, an elegant and aesthetically pleasing female breast is defined by certain characteristics that are widely accepted among surgeons. For example, the profile of the breast is characterized by a sloping or full upper pole that transitions smoothly to the lower pole that is gently curved creating a well-defined inframammary fold. In addition, the nipple–areolar complex is located at the point of maximal projection of the breast. Other important characteristics include the position of the breast on the anterior chest wall, which creates the footprint that the breast parenchyma is resting on. The breast parenchyma is surrounded by the skin and subcutaneous tissue, which are also referred to as the soft-tissue envelope. Undoubtedly, the aesthetic outcome of a breast augmentation is dependent on the dynamic interaction between the breast implant and some of the native breast characteristics described earlier such as the breast footprint, parenchyma, and soft-tissue envelope.
Nowadays, with the evolution of the fourth- and fifth-generation silicone gel implants, saline implants are not commonly used for breast augmentations. In 2016, saline implants were used only in 13% of cases in the United States and 4% of cases worldwide. 1 63 Despite the extensive use of silicone gel implants and several studies confirming their safety, some individuals still questioned the association between silicone implants and autoimmune reactions. 52 53 60 Various studies comparing silicone implant breast augmentations with controls, or silicone implant—based breast reconstruction and autologous tissue breast reconstruction, revealed no difference in the incidence of autoimmune conditions. 64 65 66 67 68 Additionally, a large cohort study examining more than 87,000 women who underwent breast augmentation showed no association between silicone gel implants and connective tissue disorders. 69
In 2011, the FDA identified a possible association between breast implants and the development of a rare T cell lymphoma known as breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). 70 During the last decade, more than 500 cases of BIA-ALCL have been reported, including a few deaths. Despite that, the exact incidence is unknown due to the lack of standardized criteria for diagnosis. Interestingly, in 2016, the World Health Organization provisionally classified BIA-ALCL as a newly recognized entity for the first time, and emphasized the importance of surgical management of this disease. 71 Even though a wide variety of treatment regimens have been proposed, several points in the diagnosis and management have been agreed on by experts as noted in the 2017 National Comprehensive Cancer Network guidelines. 72 73 First, any symptomatic periprosthetic effusions greater than 1 year after implantation should be aspirated and screened for CD30 immunohistochemistry and flow cytometry. If BIA-ALCL is localized to the capsule, it may be treated with surgery alone in the majority of cases. Extended BIA-ALCL with lymph node involvement warrants adjuvant chemotherapy. Local residual or unresectable disease may require radiation therapy treatment to the chest wall in the salvage setting. Finally, distant organ metastasis follows established National Comprehensive Cancer Network guideline regimens for systemic ALCL treatment. Another important point of discussion related to BIA-ALCL is the association with different implant types. Despite the recognized connection of certain implant types to BIA-ALCL, no known restrictive action has been taken by any regulatory agency internationally, leaving the healthcare professionals in the forefront of this decision-making process.
Future Directions
A promising new device, Motiva implant, which has not been approved in the United States, is manufactured by Establishment Labs. 74 This device has a surface that is achieved in a single step with less manipulation of the shell. Controlled surface treatment is accomplished through the company's 3D inversion manufacturing process, with no foreign materials added, resulting in microsurface or nanosurface. In addition, these devices have a TrueMonobloc configuration that links all components of the implant to the same tensile strength, which allows the shell to act as a whole structure, making insertion easier and improving the mechanical qualities of the device under stress. It is advocated that this new technology combines a specific elastic elastomer shell and the special rheological properties of the gel, which allows the implant to adjust with gravity to the patient's position providing a more natural result. Another advanced component of these devices is the Q Inside Safety Technology, a passive radio frequency identification microtransponder that is biocompatible and programmed with a unique numeric sequence (15 digits) to allow access by a proprietary handheld reader when waved over the patient's breast. As a result, the healthcare provider can securely and accurately identify implant information from outside the body since the 15-digit number is linked to a secure online database that can be accessed via the Internet by authorized providers. This will potentially give increased peace of mind to the patients in the event of a safety issue or device recall, and will eliminate the need for warranty cards that can be lost or misplaced by the patient. Further research is needed to determine if the new features of these devices will actually translate to a better implant performance within the body and improve patient outcomes in the long run.
Conclusion
As the annual number of breast augmentations increases, the pursuit for the ideal device continues. Manufacturers and clinicians are working together to design the perfect implant that will not only provide a more natural result, but it will also incorporate features to further promote safety. This remains challenging because the implant characteristics on the bench are not always translatable to implant performance within the patient's body. Most of the current generation devices in the United States have been extensively studied, and are deemed safe and efficacious with reasonable aesthetic outcomes and acceptable morbidity. Nevertheless, the shape, feel, safety, and longevity of these devices remain important areas of continuing research. Healthcare providers should be encouraged to provide ongoing robust scientific data along with new ideas for improvement of the existing devices to enhance our results and provide the best possible outcome for our patients.
Footnotes
Conflicts of Interest Dr. Gabriel is a consultant for Allergan and KCI. Dr. Unger is a consultant for Allergan. None of the other authors have any conflict of interest.
References
- 1.The American Society for Aesthetic Plastic Surgery (ASAPS).Cosmetic Surgery National Data Bank StatisticsAvailable at:https://www.surgery.org/media/statistics. Published 2016. Accessed April 22, 2018 [DOI] [PubMed]
- 2.Druss R. Changes in body image following augmentation breast surgery. Int J Psychoanal Psychother. 1973;2:248. [Google Scholar]
- 3.Czerny V. Plastic replacement of the breast with a lipoma. Chir Kong Verhandl. 1895;2:216. [Google Scholar]
- 4.Young V L, Watson M E.Breast implant research: where we have been, where we are, where we need to go Clin Plast Surg 20012803451–483., vi [PubMed] [Google Scholar]
- 5.Longacre J J. Correction of the hypoplastic breast with special reference to reconstruction of the “nipple type breast” with local dermo-fat pedicle flaps. Plast Reconstr Surg (1946) 1954;14(06):431–441. doi: 10.1097/00006534-195412000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Edgerton M T, McClary A R. Augmentation mammaplasty; psychiatric implications and surgical indications; (with special reference to use of the polyvinyl alcohol sponge Ivalon) Plast Reconstr Surg Transplant Bull. 1958;21(04):279–305. [PubMed] [Google Scholar]
- 7.Maxwell G P, Gabriel A.Possible future development of implants and breast augmentation Clin Plast Surg 20093601167–172., viii [DOI] [PubMed] [Google Scholar]
- 8.Clarkson P. Local mastectomy and augmentation mammaplasty for bilateral paraffinoma of breasts. Nurs Mirror Midwives J. 1965;121(152):13–16. [PubMed] [Google Scholar]
- 9.Regnault P, Baker T J, Gleason M C et al. Clinical trial and evaluation of a proposed new inflatable mammary prosthesis. Plast Reconstr Surg. 1972;50(03):220–226. doi: 10.1097/00006534-197209000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cronin T D, Brauer R O. Augmentation mammaplasty. Surg Clin North Am. 1971;51(02):441–452. doi: 10.1016/s0039-6109(16)39388-4. [DOI] [PubMed] [Google Scholar]
- 11.Arion H.Retromammary prosthesisC R Soc Fr Gynecol1965. 5
- 12.Rees T D, Guy C L, Coburn R J. The use of inflatable breast implants. Plast Reconstr Surg. 1973;52(06):609–615. doi: 10.1097/00006534-197312000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lavine D M. Saline inflatable prostheses: 14 years' experience. Aesthetic Plast Surg. 1993;17(04):325–330. doi: 10.1007/BF00437106. [DOI] [PubMed] [Google Scholar]
- 14.Cronin T D, Greenberg R L. Our experiences with the Silastic gel breast prosthesis. Plast Reconstr Surg. 1970;46(01):1–7. [PubMed] [Google Scholar]
- 15.Feng L J, Amini S B. Analysis of risk factors associated with rupture of silicone gel breast implants. Plast Reconstr Surg. 1999;104(04):955–963. doi: 10.1097/00006534-199909040-00009. [DOI] [PubMed] [Google Scholar]
- 16.Weum S, de Weerd L, Kristiansen B. Form stability of the Style 410 anatomically shaped cohesive silicone gel-filled breast implant in subglandular breast augmentation evaluated with magnetic resonance imaging. Plast Reconstr Surg. 2011;127(01):409–413. doi: 10.1097/PRS.0b013e3181f95aba. [DOI] [PubMed] [Google Scholar]
- 17.Winding O, Christensen L, Thomsen J L, Nielsen M, Breiting V, Brandt B. Silicon in human breast tissue surrounding silicone gel prostheses. A scanning electron microscopy and energy dispersive X-ray investigation of normal, fibrocystic and peri-prosthetic breast tissue. Scand J Plast Reconstr Surg Hand Surg. 1988;22(02):127–130. doi: 10.3109/02844318809072383. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph R, Abraham J, Vecchione T, Guber S, Woodward M. Myofibroblasts and free silicon around breast implants. Plast Reconstr Surg. 1978;62(02):185–196. doi: 10.1097/00006534-197808000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Argenta L C. Migration of silicone gel into breast parenchyma following mammary prosthesis rupture. Aesthetic Plast Surg. 1983;7(04):253–254. doi: 10.1007/BF01570670. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg H V, Bartels R J. Rupture of a silicone bag-gel breast implant by closed compression capsulotomy: case report. Plast Reconstr Surg. 1977;59(06):849–850. doi: 10.1097/00006534-197706000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Huang T T, Blackwell S J, Lewis S R. Migration of silicone gel after the “squeeze technique” to rupture a contracted breast capsule. Case report. Plast Reconstr Surg. 1978;61(02):277–280. doi: 10.1097/00006534-197802000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen J L, Christensen L, Nielsen M et al. Histologic changes and silicone concentrations in human breast tissue surrounding silicone breast prostheses. Plast Reconstr Surg. 1990;85(01):38–41. doi: 10.1097/00006534-199001000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Price J E, Jr, Barker D E. Initial clinical experience with “low bleed” breast implants. Aesthetic Plast Surg. 1983;7(04):255–256. doi: 10.1007/BF01570671. [DOI] [PubMed] [Google Scholar]
- 24.Barker D E, Retsky M, Searles S L. New low-bleed implant--Silastic II. Aesthetic Plast Surg. 1985;9(01):39–41. doi: 10.1007/BF01570682. [DOI] [PubMed] [Google Scholar]
- 25.van Nunen S A, Gatenby P A, Basten A. Post-mammoplasty connective tissue disease. Arthritis Rheum. 1982;25(06):694–697. doi: 10.1002/art.1780250613. [DOI] [PubMed] [Google Scholar]
- 26.Spiera H. Scleroderma after silicone augmentation mammoplasty. JAMA. 1988;260(02):236–238. [PubMed] [Google Scholar]
- 27.Endo L P, Edwards N L, Longley S, Corman L C, Panush R S. Silicone and rheumatic diseases. Semin Arthritis Rheum. 1987;17(02):112–118. doi: 10.1016/0049-0172(87)90033-3. [DOI] [PubMed] [Google Scholar]
- 28.Kessler D A. The basis of the FDA's decision on breast implants. N Engl J Med. 1992;326(25):1713–1715. doi: 10.1056/NEJM199206183262525. [DOI] [PubMed] [Google Scholar]
- 29.Cohen I K. Impact of the FDA ban on silicone breast implants. J Surg Oncol. 1994;56(01):1. doi: 10.1002/jso.2930560102. [DOI] [PubMed] [Google Scholar]
- 30.Lundberg G D. The breast implant controversy. A clash of ethics and law. JAMA. 1993;270(21):2608. [PubMed] [Google Scholar]
- 31.Handel N, Wellisch D, Silverstein M J, Jensen J A, Waisman E.Knowledge, concern, and satisfaction among augmentation mammaplasty patients Ann Plast Surg 1993300113–20., discussion 20–22 [DOI] [PubMed] [Google Scholar]
- 32.Stombler R E.Breast implants and the FDA: past, present, and future Plast Surg Nurs 19931304185–187., 200 [DOI] [PubMed] [Google Scholar]
- 33.Fisher J C. The silicone controversy--when will science prevail? N Engl J Med. 1992;326(25):1696–1698. doi: 10.1056/NEJM199206183262511. [DOI] [PubMed] [Google Scholar]
- 34.Guidoin R, Rolland C, Fleury D et al. Physical characterization of unimplanted gel filled breast implants. Should old standards be revisited? ASAIO J. 1994;40(04):943–958. [PubMed] [Google Scholar]
- 35.Sitbon E. [Manufacturing of mammary implants: a manufacturing of high technology] Ann Chir Plast Esthet. 2005;50(05):394–407. doi: 10.1016/j.anplas.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Bengtson B P, Van Natta B W, Murphy D K, Slicton A, Maxwell G P; Style 410 U.S. Core Clinical Study Group.Style 410 highly cohesive silicone breast implant core study results at 3 years Plast Reconstr Surg 2007120070140S–48S. [DOI] [PubMed] [Google Scholar]
- 37.Capozzi A, Pennisi V R. Clinical experience with polyurethane-covered gel-filled mammary prostheses. Plast Reconstr Surg. 1981;68(04):512–520. doi: 10.1097/00006534-198110000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Handel N, Jensen J A, Black Q, Waisman J R, Silverstein M J. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96(07):1521–1533. doi: 10.1097/00006534-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Smahel J. Tissue reactions to breast implants coated with polyurethane. Plast Reconstr Surg. 1978;61(01):80–85. [PubMed] [Google Scholar]
- 40.Sinclair T M, Kerrigan C L, Buntic R.Biodegradation of the polyurethane foam covering of breast implants Plast Reconstr Surg 199392061003–1013., discussion 1014 [PubMed] [Google Scholar]
- 41.Hester T R, Jr, Nahai F, Bostwick J, Cukic J. A 5-year experience with polyurethane-covered mammary prostheses for treatment of capsular contracture, primary augmentation mammoplasty, and breast reconstruction. Clin Plast Surg. 1988;15(04):569–585. [PubMed] [Google Scholar]
- 42.Brohim R M, Foresman P A, Hildebrandt P K, Rodeheaver G T. Early tissue reaction to textured breast implant surfaces. Ann Plast Surg. 1992;28(04):354–362. doi: 10.1097/00000637-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Abramo A C, De Oliveira V R, Ledo-Silva M C, De Oliveira E L. How texture-inducing contraction vectors affect the fibrous capsule shrinkage around breasts implants? Aesthetic Plast Surg. 2010;34(05):555–560. doi: 10.1007/s00266-010-9495-9. [DOI] [PubMed] [Google Scholar]
- 44.Barr S, Hill E, Bayat A. Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty. 2009;9:e22. [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens W G, Harrington J, Alizadeh K et al. Five-year follow-up data from the U.S. clinical trial for Sientra's U.S. Food and Drug Administration-approved Silimed® brand round and shaped implants with high-strength silicone gel. Plast Reconstr Surg. 2012;130(05):973–981. doi: 10.1097/PRS.0b013e31826b7d2f. [DOI] [PubMed] [Google Scholar]
- 46.Stevens W G, Nahabedian M Y, Calobrace M B et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132(05):1115–1123. doi: 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 47.Hammond D C, Perry L C, Maxwell G P, Fisher J. Morphologic analysis of tissue-expander shape using a biomechanical model. Plast Reconstr Surg. 1993;92(02):255–259. doi: 10.1097/00006534-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Danino A M, Basmacioglu P, Saito S et al. Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg. 2001;108(07):2047–2052. doi: 10.1097/00006534-200112000-00032. [DOI] [PubMed] [Google Scholar]
- 49.Asplund O. Capsular contracture in silicone gel and saline-filled breast implants after reconstruction. Plast Reconstr Surg. 1984;73(02):270–275. doi: 10.1097/00006534-198402000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Burkhardt B R, Dempsey P D, Schnur P L, Tofield J J. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77(06):919–932. [PubMed] [Google Scholar]
- 51.Gylbert L, Asplund O, Jurell G. Capsular contracture after breast reconstruction with silicone-gel and saline-filled implants: a 6-year follow-up. Plast Reconstr Surg. 1990;85(03):373–377. doi: 10.1097/00006534-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Maxwell G P, Van Natta B W, Bengtson B P, Murphy D K. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35(02):145–155. doi: 10.1093/asj/sju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens W G, Calobrace M B, Alizadeh K, Zeidler K R, Harrington J L, d'Incelli R C.Ten-year core study data for Sientra's Food and Drug Administration-approved round and shaped breast implants with cohesive silicone gel Plast Reconstr Surg 2018141(4S Sientra Shaped and Round Cohesive Gel Implants):7S–19S. [DOI] [PubMed] [Google Scholar]
- 54.Stevens W G, Pacella S J, Gear A JL et al. Clinical experience with a fourth-generation textured silicone gel breast implant: a review of 1012 Mentor MemoryGel breast implants. Aesthet Surg J. 2008;28(06):642–647. doi: 10.1016/j.asj.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Caplin D A.Indications for the use of MemoryShape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants Plast Reconstr Surg 2014134(3, Suppl):27S–37S. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell G P, Van Natta B W, Murphy D K, Slicton A, Bengtson B P. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32(06):709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 57.Brody G S. Silicone technology for the plastic surgeon. Clin Plast Surg. 1988;15(04):517–520. [PubMed] [Google Scholar]
- 58.Tebbetts J B.Form stability of the style 410 implant: definitions, conjectures, and the rest of the story Plast Reconstr Surg 201112803825–826., author reply 826–827 [DOI] [PubMed] [Google Scholar]
- 59.LeVier R R, Harrison M C, Cook R R, Lane T H. What is silicone? Plast Reconstr Surg. 1993;92(01):163–167. [PubMed] [Google Scholar]
- 60.Cunningham B. The Mentor study on contour profile gel silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(07) 01:33S–39S. doi: 10.1097/01.prs.0000286665.91043.bc. [DOI] [PubMed] [Google Scholar]
- 61.Cunningham B.The Mentor core study on silicone MemoryGel breast implants Plast Reconstr Surg 2007120070119S–29S., discussion 30S–32S [DOI] [PubMed] [Google Scholar]
- 62.Brown M H, Shenker R, Silver S A.Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery Plast Reconstr Surg 200511603768–779., discussion 780–781 [DOI] [PubMed] [Google Scholar]
- 63.International Society of Aesthetic Plastic Surgery (ISAPS).Global Statistics Worldwide Summary for 2016 2016. Available at:https://www.isaps.org/medical-professionals/isaps-global-statistics/. Accessed April 22, 2018
- 64.Schusterman M A, Kroll S S, Reece G P et al. Incidence of autoimmune disease in patients after breast reconstruction with silicone gel implants versus autogenous tissue: a preliminary report. Ann Plast Surg. 1993;31(01):1–6. [PubMed] [Google Scholar]
- 65.Park A J, Black R J, Sarhadi N S, Chetty U, Watson A C. Silicone gel-filled breast implants and connective tissue diseases. Plast Reconstr Surg. 1998;101(02):261–268. doi: 10.1097/00006534-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Edworthy S M, Martin L, Barr S G, Birdsell D C, Brant R F, Fritzler M J. A clinical study of the relationship between silicone breast implants and connective tissue disease. J Rheumatol. 1998;25(02):254–260. [PubMed] [Google Scholar]
- 67.Gabriel S E, O'Fallon W M, Kurland L T, Beard C M, Woods J E, Melton L J., III Risk of connective-tissue diseases and other disorders after breast implantation. N Engl J Med. 1994;330(24):1697–1702. doi: 10.1056/NEJM199406163302401. [DOI] [PubMed] [Google Scholar]
- 68.Nyrén O, Yin L, Josefsson Set al. Risk of connective tissue disease and related disorders among women with breast implants: a nation-wide retrospective cohort study in Sweden BMJ 1998316(7129):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Guerrero J, Colditz G A, Karlson E W, Hunter D J, Speizer F E, Liang M H. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med. 1995;332(25):1666–1670. doi: 10.1056/NEJM199506223322502. [DOI] [PubMed] [Google Scholar]
- 70.US Food and Drug Administration. Questions and answers about breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Available at:https://www.fda.gov/medical-devices/breast-implants/questions-and-answers-about-breast-implant-associated-anaplastic-large-cell-lymphoma-bia-alcl?r=def-entry-content. Published 2019. Accessed August 28, 2019
- 71.Swerdlow S H, Campo E, Pileri S A et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clemens M W, Horwitz S M. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017;37(03):285–289. doi: 10.1093/asj/sjw259. [DOI] [PubMed] [Google Scholar]
- 73.National Comprehensive Cancer Network (NCCN). Available at:https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf. Published 2018. Accessed June 8, 2018
- 74.Establishment Labs.Why Motiva? Innovation for Enhanced Safety 2018. Available at:https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed June 8, 2018


