To the Editor:
Cell‐based hair follicle regeneration provides an alternative treatment for alopecia.1 Dermal papilla (DP) is a cluster of specialized fibroblasts located at the base of the hair follicle (HF) which serves as an instructive niche for hair development, cycle and morphogenesis, and plays an indispensable part in HF reconstitution.2 However, the limited number of DP cells and the gradual loss of hair‐inducing capacity in isolated DP cells during long‐term subculture in vitro3 make it a big challenge to acquire abundant hair‐inducing DP cells for successful hair reconstruction. In a recent pioneering work, Fan et al4 revealed that treatment of adult fibroblasts with cell‐free extract from embryonic skin conferred upon them the competency to regenerate hair follicles. Here, we report a chemical cocktail combined suspension culture strategy to induce the generation of hair‐inducing DP‐like cells from foetal‐derived or adult foreskin‐derived fibroblasts. And these transformed cells prompt and integrate in reconstructed HF in vivo. This simple and practical methodology makes it possible to obtain abundant hair‐inducing cells for hair loss treatment.
Microarray data showed that DP cells and dermal fibroblasts originate from common fibroblast progenitors in the developing embryonic mouse skin and have highly correlated gene expression profiles (96%).5, 6 Besides, adult dermal fibroblasts can be reprogrammed into a neonatal state with the capacity of inducing ectopic follicle formation.7 Based on these, we speculated that transformation of human fibroblasts to HF‐inducing cells might also be realized by applying appropriate signals in vitro. In vivo studies of DP development give us some guidance: epidermal FGF20 and PDGF‐A participate in the regulation of DP formation, as well as Shh8, 9, 10, 11; TGF‐β2 and BMP7 secreted by the β‐catenin‐over‐activating epidemic cells direct ectopic HF formation.7 Besides, administration of FGF2, BMP2, BIO, and suspension culture in vitro showed maintains hair‐inductive activity of DP cells.3, 12 Therefore, candidate factors mentioned above (Shh, PDGF, FGF20, TGF‐β2, BMP7, FGF2, BMP2, BIO) and suspension culture were applied in the transformation of human fibroblasts.
Human DP signature genes, including signal transduction components (Bmp2, BMP4), transcription factors (Foxo1, Lef1, Rgs2, Sox2, Trps1) and extracellular adhesion gene (Vcan),3, 6 are enriched in DP cells by several folds to a thousand folds compared with human foetal fibroblasts (Figure S1). These signature genes were picked as markers to evaluate the transformation. The efficiencies of different combinations of factors (Figure S1), treatment times and suspension culture (Figure S2) were evaluated, respectively, by DP signature genes. Results showed that treatment with the combination of FGF2, PDGF and BIO for 6 days in adherent culture followed by suspension culture with these three factors for 24 hours (Figure 1A) could be the best transformation inducing strategy which was conducted in both human foetal fibroblasts and adult fibroblasts. After treatment, foetal fibroblasts gradually changed into less flattened morphology (Figure 1B). The results of RT‐PCR, immunohistochemistry and Western blotting showed outstanding up‐regulation of DP signature genes (Figure 1C, Figure S3). Similar results were seen with adult fibroblasts (Figure 1D,E). Despite some minor differences in the gene expression between foetal and adult fibroblasts, it was easy to tell that transformed human fibroblasts acquire some DP characteristics which we called DP‐like cells.
Figure 1.
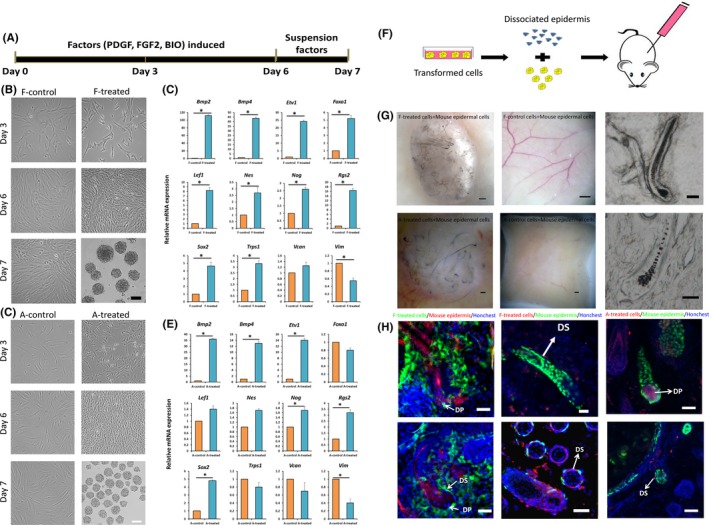
Chemically induced transformation of human fibroblasts to hair‐inducing DP‐like cells. A, Schematic representation of the chemically induced transformation of human fibroblasts to hair‐inducing DP‐like cells. B, Morphology changes of foetal fibroblasts and transformed cells induced by the transformation strategy represented by (A) from foetal fibroblasts. C, qRT‐PCR analysis (mean ± SD; n = 3) of DP signature genes in foetal fibroblasts and transformed cells induced by factors from foetal fibroblasts. D, Morphology changes of adult fibroblasts and transformed cells induced by the transformation strategy represented by (A) from adult fibroblasts. E, qRT‐PCR analysis (mean ± SD; n = 3) of DP signature genes in adult fibroblasts and transformed cells induced by factors from adult fibroblasts. F, Schematic diagram of hair follicle reconstruction. G, Stereo images of skin whole mounts from nude mice transplanted of newborn mouse epidermal cells combined with DP‐like cells (left) or untreated fibroblasts (middle) and the images of reconstructed hair follicle (right). H, Section staining of the reconstructed hair follicles induced with transformed cells from foetal fibroblasts (left and middle) or adult fibroblasts (right). Hoechst labels nuclei in blue. *P < 0.05, scale bar = 100 μm for (B) and (D), 300 μm for (G) and 50 μm for (H)
To access the hair‐inducing capacity of DP‐like cells, “patch assay” was performed as described13 (Figure 1F). To track the implanted cells, human fibroblasts were labelled with EGFP before transformation and then implanted into the back of nude mice together with RFP‐expressing neonatal mouse epidermal cells. Three weeks after implantation, we found HF formation in all positive control implanted with newborn mice dermis (Figure S4A), and no hair follicles were observed in control groups with foetal or adult fibroblasts (Figure 1G, Table 1). HF structures were reconstructed in 13 of 20 (65%) nude mice implanted with foetal DP‐like cells and in seven of 10 (70%) nude mice with adult DP‐like cells (Figure 1G, Table 1). RFP+ epidermal cells and EGFP+ dermal cells were observed in the back skin of the mice implanted with DP‐like cells, while only EGFP+ dermal cells were observed in mice implanted with fibroblasts (Figure S4B). Section assay further showed that foetal/adult DP‐like cells integrated in the DP and dermal sheath (DS) which can be recruited to replenish DP of de novo generated hair follicles (Figure 1H).14 In addition, human fibroblasts labelled with mCherry were also treated and implanted with K14‐H2B‐GFP neonatal mouse epidermal cells in patch assay. The results further confirmed transformed cells integrated into regenerated hair follicles (Figure 1H) which suggested that DP‐like cells obtained certain hair follicle‐inducing capacity.
Table 1.
Results of hair follicle formation of different groups in the patch assay
| Experimental groups | Epidermal cellsa | Dermal cellsb | Number of grafted constructs with hair follicles/total number of grafts (%) |
|---|---|---|---|
| Positive control group | Mouse epidermal cells | Mouse dermal cells | 15/15 (100) |
| Foetal treatment group | Mouse epidermal cells | Foetal DP‐like cells | 13/20 (65) |
| Foetal control group | Mouse epidermal cells | Untreated foetal fibroblasts | 0/20 (0) |
| Adult treatment group | Mouse epidermal cells | Adult DP‐like cells | 7/10 (70) |
| Adult control group | Mouse epidermal cells | Untreated adult fibroblasts | 0/10 (0) |
Epidermal cells included mouse epidermal cells.
Dermal cells included mouse dermal cells, transformed foetal/adult DP‐like cells and foetal/adult fibroblasts.
The differences of DP signature gene expression levels between foetal and adult DP‐like cells were obvious, which were consistent with previous studies that foetal fibroblasts are more sensitive than adult fibroblasts to the change in the culture condition.15 Nevertheless, it is remarkable that human fibroblasts isolated from hairless foreskin without hair‐inducing capacity could be transformed into hair follicle‐inducing cells in vitro and induce new HF formation in vivo. Intriguingly, we found that in the control group implanted with fibroblasts, implanted epidermal cells were all gone after 3 weeks. We speculated that the transformed cells might provide a more suitable niche which could offer essential epithelial‐mesenchyme interaction for the epidermal cells than fibroblasts and finally contribute to the HF formation in vivo. However, HF regeneration was not always observed in the treatment group, and the number of de novo hair follicles was much smaller compared with that in positive control group. Given the fact that the up‐regulation of DP signature genes was moderate in the transformed cells compared with DP cells, it is likely that the portion of transformed cells is relatively small in the entire culture or/and the degree of transformation is relative low. Besides, it might also attribute to the fact that human cells have poorer interaction with mouse epidermal cells than mouse dermal cells.16 The previous research reported a cocktail of organ‐specific extracellular proteins (apolipoprotein‐A1, galectin‐1 and lumican) are able to induce HF neogenesis from adult fibroblasts, which were selected by proteomics analysis of embryonic whole‐skin extracts.4 However, from the perspective of epithelial‐mesenchymal interactions in DP development in vivo and intrinsic properties maintain in vitro, we revealed another different induction system that three factors of PDGF, FGF2 and BIO composed chemical cocktail along with suspension culture to induce human fibroblasts especially adult foreskin fibroblasts into hair‐inducing DP‐like cells and contribute to HF regeneration. Given the trans‐differentiation efficiency, the induction strategy of factors in two studies or additional other factors might further enhance the efficiency. In conclusion, we demonstrated a simple and feasible way to acquire abundant hair follicle‐inducing donor cells by our chemical cocktail in hair loss treatment with the propagation ability of fibroblasts.
CONFLICT OF INTEREST
The authors have no financial conflicts of interest.
AUTHORS' CONTRIBUTIONS
QZ and NL performed the experiments; QZ collected data and prepared the manuscript. HZ, XL, YC and GX assisted in the experiments and analysed the data. QZ, SL and ED designed the study, analysed data and edited the manuscript.
Supporting information
ACKNOWLEDGEMENTS
We thank Dr. Chunsheng Han (Institute of Zoology, Chinese Academy of Sciences) for help with EGFP plasmid. We also thank Dr. Masahito Ikawa (Osaka University, Japan) for providing RFP mitochondria mice. This work was supported by the National Natural Science Foundation of China (31471287 to E.D., 31600683 to X.L, 31201099 to S.L. and U1738103 to E.D.)
Zhao and Li contributed equally to this work.
Contributor Information
Enkui Duan, Email: duane@ioz.ac.cn.
Shuang Liu, Email: liushuang@ioz.ac.cn.
REFERENCES
- 1. Zhang P, Kling RE, Ravuri SK, et al. A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J Tissue Eng. 2014;5:2041731414556850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuchs E. Scratching the surface of skin development. Nature. 2007;445(7130):834‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three‐dimensional culture enables dermal papilla cells to induce de novo human hair‐follicle growth. Proc Natl Acad Sci U S A. 2013;110(49):19679‐19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan S‐Y, Tsai C‐F, Yen C‐M, et al. Inducing hair follicle neogenesis with secreted proteins enriched in embryonic skin. Biomaterials. 2018;167:121‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4(7):a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal‐epithelial interactions in the hair follicle. PLoS Biol. 2005;3(11):e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of β‐catenin. Development. 2011;138(23):5189‐5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huh S‐H, Narhi K, Lindfors PH, et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Gene Dev. 2013;27(4):450‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF‐A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126(12):2611‐2621. [DOI] [PubMed] [Google Scholar]
- 10. Pummila M, Fliniaux I, Jaatinen R, et al. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134(1):117‐125. [DOI] [PubMed] [Google Scholar]
- 11. Biggs LC, Makela OJ, Myllymaki SM, et al. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. eLife. 2018;7:e36468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132(1):237‐239. [DOI] [PubMed] [Google Scholar]
- 13. Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin‐derived cells. J Invest Dermatol. 2005;124(5):867‐876. [DOI] [PubMed] [Google Scholar]
- 14. Tao Y, Yang Q, Wang L, et al. β‐catenin activation in hair follicle dermal stem cells induces ectopic hair outgrowth and skin fibrosis. J Mol Cell Biol. 2019;11(1):26‐38. [DOI] [PubMed] [Google Scholar]
- 15. Corkins MR, Fillenwarth MJ. Fetal intestinal fibroblasts respond to insulin‐like growth factor (IGF)‐II better than adult intestinal fibroblasts. BMC Dev Biol. 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehama R, Ishimatsu‐Tsuji Y, Iriyama S, et al. Hair follicle regeneration and human cells using grafted rodent and human cells. J Invest Dermatol. 2007;127(9):2106‐2115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


