Abstract
Background
Phosphatidylinositol 3-kinase isoform δ (PI3Kδ) mediates multiple events in lymphocytes, including cell proliferation, survival, and motility. Inhibition of PI3Kδ, with downstream inhibitory effects on cell growth and survival, has been utilized to treat lymphoid malignancies. ME-401 is an oral, once-daily, selective PI3Kδ inhibitor with an optimized pharmacologic profile that is in clinical development for the treatment of B-cell malignancies.
Objectives
This work examined aspects of the pharmacologic profile of ME-401 in preclinical models to investigate the basis of the clinical activity of ME-401 that may differentiate it from other currently approved PI3Kδ inhibitors.
Methods
We determined the ME-401 blood to plasma ratios, permeability, and purified enzyme-binding kinetics. The oral bioavailability and volume of distribution of ME-401 were evaluated in various species. ME-401 concentrations in plasma and tumor and brain tissues were evaluated following oral administration in an A20 syngeneic mouse model of B-cell lymphoma. Idelalisib was used as a reference compound for the measurement of purified enzyme-binding kinetics and concentrations in plasma and tumor in the A20 syngeneic mouse model.
Results
Oral administration of ME-401 to dogs resulted in 79% bioavailability compared with intravenous administration. Allometric scaling from rodents, dogs, and nonhuman primates resulted in a predicted human volume of distribution at steady state of 10.75 L/kg. ME-401 was shown to distribute into the lymph in dogs and permeate into cells readily, with a human blood to plasma ratio of 1.4 and ~ 50% retention in the Caco-2 cell monolayer at 1 μM. The high binding affinity and low dissociation rate of ME-401 resulted in an equilibrium dissociation constant (KD) of 3.03 × 10−11 M. Oral administration of ME-401 in an A20 syngeneic mouse model resulted in tumor concentrations 20–30 times higher than plasma concentrations at 4 h after the last dose. By 24 h, the tumor levels had decreased approximately 30–50% compared with levels at 4 h while remaining significantly increased relative to plasma concentrations. ME-401 was also present in brain tissue at 4 and 24 h after the last dose. In comparison, the idelalisib dissociation rate was ~ 100 times higher, resulting in a KD of 1.11 × 10−9 M. Idelalisib tumor concentrations were only approximately three times higher than plasma concentrations at 4 h, while dropping below the limit of quantitation in both tumor and plasma by 24 h.
Conclusions
These data support the capacity of ME-401 to be orally absorbed, distribute to target tissues, enter and accumulate in target cells, and bind to the target with high affinity to exert its mechanism of action. These characteristics underlie the high clinical potency seen in B-cell malignancies that may differentiate ME-401 from other PI3Kδ inhibitors currently approved or in development.
Key Points
| ME-401 is an oral, once-daily, selective PI3Kδ inhibitor, part of a signaling pathway in cells that can become hyperactive leading to tumor growth and resistance to chemotherapy. |
| In preclinical studies, ME-401 reached its target site (PI3Kδ) and bound to it more tightly and longer than idelalisib, another PI3Kδ inhibitor. ME-401 bound to its target tightly for ~ 5 h compared with ~ 2 min for idelalisib. |
| Oral ME-401 is well absorbed and distributed throughout the body in animal studies with concentrations in key sites for lymphoma including lymph, plasma, lymphoma tumor, and brain tissue. |
Introduction
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase with a catalytic subunit that exists in four different isoforms (α, β, γ, and δ). The δ isoform is highly restricted to hematopoietic cells and plays a key role in normal B-cell development and function. Once recruited to phosphorylated binding sites on the B-cell adapter of PI3K or on the cytoplasmic tail of the transmembrane protein CD19, activated PI3Kδ signaling occurs via phosphorylation of the key lipid second messenger phosphatidylinositol, with resultant phosphorylation of protein kinase B (Akt), leading to the downstream phosphorylation of a variety of effector molecules (e.g., TSC1/2, mTORC1, BAD, FOXO, GSK-3β, p27), which control cell proliferation, survival, and motility [1, 2]. These signaling pathways are commonly hyperactive in B-cell malignancies [3–5]. Disrupting these pathways with PI3Kδ inhibitors such as Zydelig® (idelalisib), Aliqopa™ (copanlisib), and Copiktra™ (duvelisib) has been shown to effectively treat such malignancies by inhibiting their growth and survival [5–7]. Although effective, these approved agents cause toxicities such as diarrhea/colitis, transaminitis, cutaneous reactions, hyperglycemia, hypertension, and pneumonitis [7–9]. These toxicities may preclude the use of these agents at their maximum clinical efficacious doses, suggesting a need for novel PI3K inhibitors that provide meaningful clinical benefit with decreased toxicity.
The primary pharmacology of the oral PI3Kδ inhibitor ME-401 has previously been characterized in several in vitro studies that demonstrated its selectivity for the δ isoform, inhibition of PI3Kδ-mediated cellular activity in activated immune cells (Raji cell line, primary basophils) with low nM 50% inhibitory concentrations even in the presence of whole blood, and activity against multiple primary B-cell malignancies [10]. Results from a first-in-human study in healthy volunteers demonstrated oral bioavailability, linear pharmacokinetics (PK), and a half-life of 28 h to support once-daily dosing. Additionally, the PK/pharmacodynamic (PD) relationship was examined with inhibition of basophil activation assessed via CD63 expression following ex vivo stimulation. Oral administration of ME-401 resulted in 90% of maximal effective concentration (EC90) at a plasma concentration of 5 ng/mL (9 nM), with trough plasma values predicted to be above the EC90, using daily dosing of 60 mg [11, 12]. Consequently, 60 mg/day was used as the starting dose for a Phase 1b dose escalation study in relapsed B-cell malignancies, even though the maximum recommended starting dose (MRSD) in patients with cancer was determined to be 140 mg/day based on preclinical toxicology data. Initial results from the Phase 1b study have shown high initial overall response rates (ORRs) in relapsed follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) [13, 14].
To be efficacious, an oral agent must overcome the hurdles of entering the bloodstream from the gastrointestinal tract, reaching the target tissues, and binding with sufficient affinity to exert its mechanism of action. Intracellular targets such as PI3Kδ have additional hurdles of entering the target cells and establishing an effective concentration at the intracellular site of action. To that end, the preclinical studies presented here examined the permeability and binding affinity of ME-401; oral bioavailability and volume of distribution in various species; and concentrations in plasma and tumor and brain tissues in an A20 syngeneic mouse model of B-cell lymphoma. Idelalisib was used as a reference compound for the measurement of purified enzyme-binding kinetics and plasma and tumor concentrations in the A20 syngeneic mouse model.
Materials and Methods
Animals
Animal studies are reported in compliance with the ARRIVE guidelines [15, 16]. CD-1 mice were from BK Laboratory Animal Co. Ltd. Sprague Dawley (SD) rats were from BK Laboratory Animal Co. Ltd and Shanghai Laboratory Animal Center, CAS (Shanghai, China). Female BALB/c mice were from Charles River Laboratories (Raleigh, NC, USA). Beagle dogs were from Marshall BioResources (Beijing, China, and North Rose, NY, USA). Cynomolgus monkeys were from Marshall BioResources (Beijing, China).
In Vitro Blood to Plasma Ratio, Permeability, and Binding Kinetics
Blood to Plasma Ratios
Blood to plasma ratios (B:Ps) were measured in fresh SD rat, beagle dog, and human (single-donor) whole blood at a concentration of 1 µM. ME-401 was incubated for 30 min at 37 °C, pH 7.4. Three replicates were carried out, and the positive markers methazolamide (B:P >>1), verapamil (B:P ~ 1), and diclofenac (B:P < 1) were included in the assay.
Caco-2 Assay
An assessment of the potential of ME-401 to bind to the tissue culture plasticware was performed in ReadyCell 24-well Transwells® without cells. ME-401 (1 μM in 0.1% DMSO, N = 3) in an experimental buffer of Hank’s Balanced Salt Solution (HBSS) containing 25 mM HEPES + 5 mM glucose + 0.1% w/v BSA at pH 7.4 was incubated at 37 °C for 2 h to mimic the Caco-2 experimental time course. Plastic binding potential was assessed by loss of ME-401 in the experimental solutions at 2 h. Propranolol (10 uM) and atenolol (10 uM) were used as positive control markers for high and low passive permeability, respectively.
Caco-2 cells in passage 63 at 24 days post-seeding were used. Only monolayers with transepithelial electrical resistance (TEER) values of > 330 Ω/cm2 (taken at the start of the investigation and at the end of the incubations before cell lysis to indicate the presence of an intact monolayer) were used in the investigation. The receiver buffer was the experimental buffer noted above. The donor buffer was the receiver buffer without BSA, containing the test compound and appropriate levels of DMSO. The ReadyCell plates were washed for 1 min with 0.25-mL receiver buffer on the apical side and 0.75 mL on the basolateral side, and washing was repeated for 30 min at 37 °C with receiver buffer. The cell wells were then transferred to a fresh receiver plate.
For the apical to basolateral (AB) permeability, 0.75 mL of the receiver buffer was placed in the basolateral plate of three transwells for each concentration. Donor buffer solution (0.25 mL) with the test compound at pH 7.4 was added to the apical compartment. For the basolateral to apical (BA) permeability, 0.75 mL of donor buffer solution with the test compound at pH 7.4 was added to the basolateral compartment of three transwells for each concentration. Receiver buffer solution (0.25 mL) was added to the apical compartment. The transwell plates were incubated at 37 °C for 2 h (without shaking). Samples (100 µL) were taken at 0 and 2 h.
The remaining donor and receiver volumes were removed, and the monolayers were washed three times for 2 min each with 0.3 mL of ice-cold HBSS solution to wash excess ME-401 from the monolayers/wells and also to stop any active processes. Cells were lysed by rinsing three times with 100 µL methanol containing 0.66 µM tolbutamide as internal standard. Extracts were pooled, and 100 µL transport buffer was added to match the composition of the other samples. All buffer samples were quenched into 100 µL methanol and 200 µL methanol containing 1 µM tolbutamide as internal standard. Allowing for dilution factors for the cell-associated samples and the buffer samples, the final internal standard concentration was 0.5 µM. All samples were kept at − 20 °C for ≥ 2 h before centrifugation at 2500×g, and for 20 min at 4 °C. Supernatants were transferred to a microtiter plate, and substrate concentrations were assessed by liquid chromatography–tandem mass spectroscopy (LC–MS/MS) against a matrix-matched standard curve.
Percent recovery was calculated from the following equation:
where S0 is the amount of substrate added to the donor side, and SA, SB, and SC are the amount of substrate in the apical buffer, basolateral buffer, and cell extract, respectively, all at the end of the experiment. Amounts were calculated as volume (mL) × concentration (nmol/mL).
Purified Enzyme-Binding Kinetics
Kinase protein immobilization: An aliquot of biotinylated PIK3CD/PIK3R1 (Carna Biosciences, Inc., Kobe, Japan) was thawed, diluted to 40 µg/mL in precooled Biacore buffer (50 mM Tris pH 7.5, 0.05% Tween, 150 mM NaCl, and 5 mM MgCl2), and immobilized with the biotin tag on a Series S Sensor Chip SA. First, the streptavidin on the sensor chip was washed three times with a washing solution (1 M NaCl, 50 mM NaOH) for a period of 60 s with a flow rate of 10 µL/min. Biotinylated PIK3CD/PIK3R1 was then captured on the chip, with an aimed immobilization level of 8000 resonance units. Remaining streptavidin was blocked with 10 µg/mL biocytin injections to circumvent nonspecific binding of compounds to the streptavidin-bound surface. To obtain a stable surface with a functional protein, the immobilization procedure was performed at 4 °C. Channel 1 was used as a reference channel to correct for buffer effects; the other channels were used for protein immobilization. After immobilization, the system was buffered with Biacore buffer containing 1% DMSO. After twice priming, a prerun was performed for a period of 60 min at a flow rate of 30 µL/min to obtain a stable surface.
Single-cycle kinetics assay: To obtain a stable signal after compound and buffer injections, the experiment was started with five start-up cycles comprising ten consecutive injections with buffer performed at 22 °C. ME-401 and idelalisib were dissolved in 100% DMSO to create 10-mM solutions, which were diluted in Biacore buffer without DMSO to obtain solutions with 1% DMSO. Further dilutions were made in Biacore buffer containing 1% DMSO. This buffer was also used as running buffer during the experiments to avoid bulk response shifts due to differences in DMSO concentration. The single-cycle kinetic experiment was performed at 22 °C with a compound concentration gradient of 1–100 nM in half-log increments, a contact time of 100 s, and a flow rate of 30 µL/min. The dissociation period was 1200 s. After every single-cycle kinetics run, an extra wash step with 50% DMSO was performed. The kinase surface was not washed during this step.
A blank run was performed before the compound range was injected with five buffer injections at the same flow rate, contact time, and dissociation time as the compound runs. This measurement was subtracted from the compound curve as a double reference to correct for surface instability.
Curve analysis: The compound curves were analyzed with the Biacore Evaluation Software using the method of double referencing. The reference channel was subtracted from the channel containing immobilized protein, and the reference curve obtained with buffer injections was subtracted. The resulting curve was fitted with the 1:1 binding model.
ME-401 Pharmacokinetics in Non-Tumor-Bearing Species
PK Studies Following a Single Oral or Intravenous (IV) Dose
The single-dose PKs of oral ME-401 were investigated in beagle dogs. ME-401 2 mg/kg was administered by oral gavage as a solution in 10% NMP/5% Solutol HS 15 + 20% PEG400 + 65% sterile water. The single-dose PK of IV ME-401 were investigated in CD-1 mice, SD rats, beagle dogs, and cynomolgus monkeys. ME-401 was administered IV as a solution in 10% NMP/5% Solutol HS 15 + 20% PEG400 + 65% sterile water (10 mg/kg for mice, 5 mg/kg for rats, 2 mg/kg for dogs and monkeys). In both studies, animals were fasted overnight before dosing and fed 4 h post-dose. Blood samples were collected in K2EDTA tubes at pre-dose and 0.083, 0.25, 0.5, 1, 2, 4, 8, and 24 h post-dose. The plasma was separated by centrifugation and analyzed for ME-401 concentration by a suitably qualified LC–MS/MS bioanalytical method, with a lower limit of quantification (LLOQ) of 1.0 ng/mL. PK parameters were estimated by noncompartmental analysis with Pharsight Phoenix WinNonlin® software. These experiments were conducted at ChemPartner, Zhangjiang Hi-Tech Park, Pudong New Area, Shanghai, China.
With data from the IV PK study, the log of body weight was plotted against the log of the apparent volume of distribution at steady state (VSS), and the best-fit line by linear regression was used to obtain the formulas for allometric scaling of VSS.
Dog Lymph/Plasma PK
In three male beagle dogs, a thoracic duct-to-external jugular (or left subclavian, depending on individual anatomy) shunt was created in which a catheter was placed via a neck incision into the thoracic duct at the junction of the left external jugular (or left subclavian) and axillary vein. This catheter was advanced to the level of the terminal bulb of the thoracic duct and was assessed for patency by allowing the lymph to freely flow. A tip of a second catheter was placed within the left subclavian vein via the axillary vein. The catheters were secured in the vessels with 2-0 silk or similar nonabsorbable suture. The catheters were connected to a three-way stopcock to create a “T-tube” port to allow for collection, and when not in use, continuous flow of lymph into the left external jugular or subclavian vein. This system allowed for lymph to be collected by occluding the catheter leading to the vein and drawing from the catheter nearest the lymph source. Similarly, venous blood was collected from the catheter within the left subclavian vein. If lymph could not be obtained because of either loss of patency or disruption of the catheter by the animal, blood was drawn from the saphenous or cephalic vein.
ME-401 10 mg/kg was administered by oral gavage as a solution in 20% vitamin E-tocopherol polyethylene glycol 1000 succinate (VE-TPGS) + 80% 100 mM citrate buffer (pH 3.5). Lymph and blood samples (~ 1 and 1.5 mL, respectively) were obtained pre-dose and 0.5, 1, 4, 6, 8, 24, and 48 h post-dose. Blood was collected in K2EDTA tubes, with the plasma separated by refrigerated centrifugation. Lymph samples also underwent refrigerated centrifugation; plasma and lymph samples were stored at − 80 °C until analysis. Plasma and lymph samples were analyzed for ME-401 concentrations by a suitably qualified LC–MS/MS bioanalytical method, with an LLOQ of 0.5 ng/mL. These experiments were conducted at Wake Forest Innovations, Winston-Salem, NC, USA (in-life) and Seventh Wave Laboratories LLC, Chesterfield, MO, USA (Bioanalysis).
ME-401 and Idelalisib Tissue Distribution in A20 Syngeneic Model
A20 tumor cells were obtained from ATCC and were injected subcutaneously on the flank of 8- to 12-week-old BALB/c mice. Body weights and tumor measurements were recorded daily. Once tumors were 100–150 mm3, pairs of matched mice received orally administered ME-401 75 mg/kg (n = 12) twice daily for 4 days. In a follow-up study, pairs of matched mice received orally administered ME-401 (n = 12) or idelalisib (n = 12) 50 mg/kg twice daily for 4 days. In both studies, ME-401 was prepared in 20% VE-TPGS + 80% 100 mM citrate buffer (pH 3.5). In the second study, idelalisib, obtained from LC Laboratories (Cat # I-7447, Lot # DLL-101), was prepared in 10% NMP/10% Solutol HS 15 + 20% PEG400 in saline. Mice were given 10 mL/kg (0.2 mL/20 g mouse) of ME-401 or idelalisib twice daily, 4 h apart, for 4 days. In both studies, six of the mice in each group were sacrificed at 4 and 24 h after the last dose. Blood was collected in K2EDTA tubes and stored on dry ice. Tissues were snap frozen and stored on dry ice. All samples were shipped to Charles River Laboratories-Massachusetts for PK analysis. The plasma was separated by centrifugation. Tissue samples were analyzed against respective homogenate curves. All samples were analyzed for ME-401 or idelalisib concentrations by a suitably qualified LC–MS/MS bioanalytical method, with an LLOQ of 1.0 ng/mL for both drugs. These experiments were conducted at Charles River Laboratories, Morrisville, NC, USA.
Results
In Vitro Blood to Plasma Ratio, Permeability, and Binding Kinetics
ME-401 B:P
The B:P for all three of the markers was within the expected range for those compounds (Table 1). The B:P for ME-401 was similar across the species and was measured as > 1. In human whole blood, the B:P of 1.4 indicated that ME-401 distributes into blood cells.
Table 1.
Blood to plasma ratios (mean ± SD)
| Compound | Sprague Dawley rat | Beagle dog | Human |
|---|---|---|---|
| ME-401 | 1.8 ± 0.35 | 1.8 ± 0.35 | 1.4 ± 0.28 |
| Methazolamide | 150 ± 70 | 185 ± 140 | 50 ± 50 |
| Verapamil | 1.1 ± 0.08 | 1.3 ± 0.13 | 1.1 ± 0.16 |
| Diclofenac | 0.8 ± 0.16 | 0.7 ± 0.18 | 0.9 ± 0.28 |
SD standard deviation
Caco-2 Permeability and Percent Recovery
The ME-401 AB and BA Papp were 3.67 ± 1.37 × 10−6 cm/s and 7.69 ± 1.39 × 10−6 cm/s, respectively. The permeability results for the marker compounds were as expected; the high permeability marker, propranolol, showed high passive permeability with Papp values > 30 × 10−6 cm/s, and the low permeability marker, atenolol, had low passive permeability (< 1 × 10−6 cm/s). The recovery data for all positive control marker conditions were acceptable as > 70%; however, the recovery for ME-401 at 1 µM was 30.3 ± 6.7% and 69 ± 5.6% for AB and BA, respectively, when only the experimental solutions were measured. When the cell lysate was included (thereby accounting for ME-401 associated with the cell monolayer), the recovery significantly improved, with 79.3 ± 5.5% for AB and 85 ± 8.5% for BA. This indicated that ~ 50% of the ME-401 was retained in the monolayer.
Equilibrium Dissociation Constant (KD) and On/Off Rates
Binding kinetics for ME-401 and idelalisib are presented in Table 2. The lower dissociation rate resulted in a KD of 3.03 × 10−11 M (i.e. 30 pM) for ME-401. In comparison, the idelalisib dissociation rate was ~ 100 times higher, resulting in a KD of 1.11 × 10−9 M (i.e. 1110 pM). ME-401 was tightly bound with a residence time of 5.3 h as compared to 2.3 min for idelalisib.
Table 2.
Binding kinetics of ME-401 and idelalisib on PIK3CD/PIK3R1
| Compound | ka (1/M·s) | kd (1/s) | KD (M) | t ½ | Τ |
|---|---|---|---|---|---|
| ME-401 | 1.72 × 106 | 5.20 × 10−5 | 3.03 × 10−11 | 3.7 h | 5.3 h |
| Idelalisib | 6.53 × 106 | 7.25 × 10−3 | 1.11 × 10−9 | 1.6 min | 2.3 min |
ka association rate constant, kd dissociation rate constant, KD equilibrium dissociation constant, t1/2 half-life, T residence time
ME-401 Pharmacokinetics in Non-Tumor-Bearing Species
Oral Bioavailability in Dogs
Following administration of ME-401 2 mg/kg IV in dogs, the area under the curve from time 0 to infinity (AUCinf) was 1247 ng∙h/mL. Following administration of ME-401 2 mg/kg orally in fasted dogs, the AUCinf was 980 ng∙h/mL. The bioavailability of ME-401 in dogs was estimated to be 79%.
VSS from IV Dosing and Extrapolation to Human VSS
VSS from the IV single-dose experiments in CD-1 mice, SD rats, cynomolgus monkeys, and beagle dogs are presented in Table 3, and linear regression fit for allometric scaling of VSS is presented in Fig. 1. Extrapolation to a hypothetical 60-kg human resulted in a VSS value of 10.75 L/kg.
Table 3.
Preclinical intravenous single-dose pharmacokinetics
| Species | Body weight (kg) | VSS (L/kg) |
|---|---|---|
| CD-1 mouse | 0.02 | 5.605 |
| Sprague Dawley rat | 0.15 | 23.733 |
| Cynomolgus monkey | 3 | 8 |
| Beagle dog | 10 | 9.7 |
VSS apparent volume of distribution at steady state
Fig. 1.
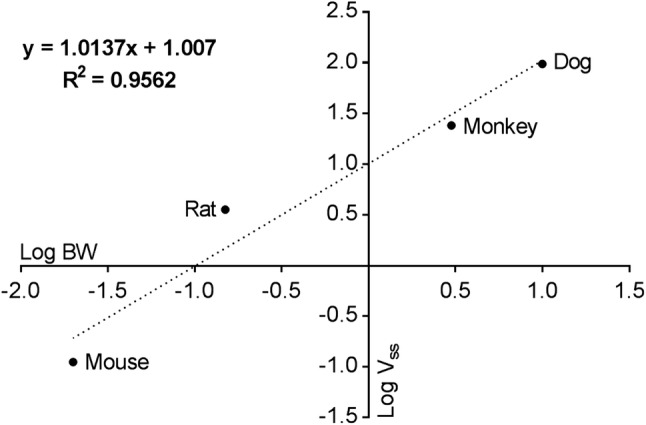
Allometric relationship between volume of distribution at steady state (VSS) and body weight
Time/Plasma Concentrations of Plasma and Lymph in Dogs
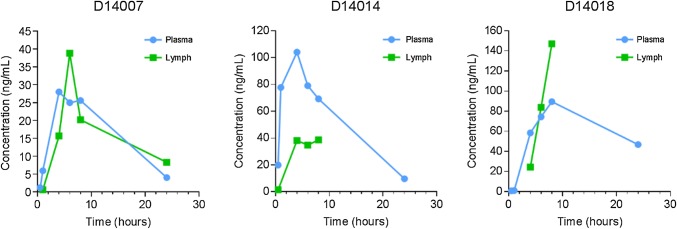
All plasma samples were collected and analyzed as planned. However, many of the corresponding lymph samples could not be collected due to catheter patency. ME-401 lymph and plasma concentrations are plotted for each dog (Fig. 2) and were above the limit of quantitation in all samples.
Fig. 2.
ME-401 concentrations in blood and lymphatic fluid following a single oral dose to male beagle dogs
ME-401 and Idelalisib Tissue Distribution in A20 Syngeneic Model
The administration of ME-401 50 and 75 mg/kg twice daily for 4 days resulted in tumor concentrations approximately 20 and 30 times higher than plasma concentrations, respectively, at 4 h after the last dose (Table 4; Fig. 3). Concentrations within the tumor were lower at 24 h after the last dose, but higher relative to plasma, when compared to the 4-h concentrations. In the ME-401 75-mg/kg group, concentrations in brain tissue were present at 4 and 24 h after the last dose at approximately 30% of plasma values, suggesting penetration into and retention of ME-401 in the central nervous system. Administration of idelalisib 50 mg/kg twice daily for 4 days resulted in tumor concentrations approximately three times higher than plasma concentrations at 4 h after the last dose, and concentrations were below the limit of quantitation at 24 h.
Table 4.
Plasma and tissue concentrations (mean ± SD) of ME-401 and idelalisib in A20 syngeneic model in BALB/c mice 4 and 24 h after the last dose
| Compound | Plasma (ng/mL), mean ± SD (CV%) | Tumor (ng/g), mean ± SD (CV%) | Brain (ng/g), mean ± SD (CV%) | |||
|---|---|---|---|---|---|---|
| 4 h (n = 6) | 24 h (n = 6) | 4 h (n = 6) | 24 h (n = 6) | 4 h (n = 6) | 24 h (n = 6) | |
| ME-401 75 mg/kg | 7225 ± 531 (7.4) | 2328 ± 416 (17.9) | 225,333 ± 40,582 (18) | 151,500 ± 35,799 (23.6) | 2603 ± 466 (17.9) | 676 ± 48 (7.1) |
| ME-401 50 mg/kg | 4897 ± 467 (9.5) | 420 ± 105 (25.1) | 99,850 ± 18,001 (18) | 48,100 ± 11,569 (24.1) | NE | NE |
| Idelalisib 50 mg/kg | 23.6 ± 4.81 (20.4) | BLQ | 61.8 ± 17.6 (28.5) | BLQ | NE | NE |
BLQ below limit of quantitation of idelalisib in plasma of 1 ng/mL or 2 ng/mL after 2 × assay dilution, CV coefficient of variation, NE not evaluated, SD standard deviation
Fig. 3.
Plasma and tissue concentrations (mean ± SD) at 4 (n = 6) and 24 h (n = 6) after the last dose of ME-401 or idelalisib administered orally twice daily for 4 days in an A20 syngeneic model in BALB/c mice. a ME-401 75 mg/kg plasma and tumor concentrations. b ME-401 75 mg/kg plasma and brain concentrations. c ME-401 50 mg/kg plasma and tumor concentrations. d Idelalisib 50 mg/kg plasma and tumor concentrations at 4 h; concentrations at 24 h were below the limit of quantitation
Discussion
PI3Kδ is a target for the treatment of B-cell malignancies as evidenced by the approval of the PI3K inhibitors idelalisib and duvelisib for treatment of FL and CLL/SLL and copanlisib for the treatment of FL [7–9]. However, these agents have significant toxicity; idelalisib has been shown to cause hepatotoxicity, severe diarrhea/colitis, pneumonitis, and intestinal perforation [8]. Copanlisib, an IV pan-PI3K inhibitor given weekly, is associated with severe hypertension and hyperglycemia [9]. Duvelisib has been shown to cause fatal and serious infections, diarrhea/colitis, cutaneous reactions, and pneumonitis [7]. There is a need for novel PI3Kδ inhibitors with decreased toxicity profiles that provide durable clinical activity.
PI3Kδ is located inside B-cell lymphocytes, which reside in the bone marrow and lymphatic system. Therefore, the efficacy of a drug that targets PI3Kδ is governed by its capacity to distribute into those tissues and cells, as well as the potency with which it inhibits PI3Kδ function. The results of our preclinical studies demonstrate the efficiency and potency of ME-401 in performing these tasks. Most of these studies were performed early in development and served to justify advancement of ME-401 into first-in-human clinical trials. Therefore, the concentrations of ME-401 in these studies may not reflect clinical concentrations. Consequently, these preclinical data are used to ascertain insights to the pharmacologic properties of ME-401.
We evaluated the distribution of ME-401 into cells by B:P and the Caco-2 cell assay. The B:P of 1.4 indicates that ME-401 readily distributes into blood cells, reaching equilibrium when approximately 60% of the drug in the blood distributes into blood cells. In comparison, idelalisib has a B:P of 0.5–0.7 [8, 17], suggesting it remains more in the plasma rather than permeating cells as compared with ME-401. The Caco-2 assay indicated that ME-401 not only permeates cells but is also retained by cells. The low recovery in the Caco-2 assay prevented accurate quantitation of permeability rate; the recovery was significantly improved when the cell lysate was included, indicating that the low recovery was due to retention of ME-401 inside the cells. Collectively, these results indicate that ME-401 exhibits a propensity to distribute into, and accumulate inside, cells and may account for the higher potency observed in cellular assays compared with the purified enzyme assay. It was previously reported [10, 11] that the clinical half maximal effective concentration (EC50) of 1 nM for inhibition of activation of peripheral blood basophils in healthy volunteers was in agreement with the inhibition of PI3Kδ in cellular assays (half maximal inhibitory concentration [IC50] = 0.6 nM); the IC50 was 5 nM for inhibition of purified PI3Kδ enzyme.
Kinetics of ME-401 binding to isolated, biotinylated PI3Kδ immobilized on a Biacore sensor chip were measured via surface plasmon resonance and compared with binding kinetics of idelalisib. The very low KD of 30 pM was coupled with a very slow off-rate of 5.20 × 10−5/s, equating to a half-life and residence time of several hours. In contrast, idelalisib has a higher KD of 1110 pM, due largely to a faster off-rate of 7.25 × 10−3/s, equating to half-life and residence times of a few minutes. These data show that once ME-401 reaches its target, it binds ~ 37 times more tightly, and remains on-target ~ 138 times longer, than idelalisib in vitro. With ME-401 accumulating in cells and binding tightly to its target, it is possible that prolonged inhibition of PI3Kδ could occur within the tumor or lymphoid tissue even as ME-401 levels decrease in plasma over time. This tight binding to PI3Kδ and its ability to readily distribute into tissue may partially explain the clinical results of ME-401 of high initial ORRs in early studies in FL and CLL/SLL [13, 14].
The PK profile of ME-401 was evaluated in non-tumor-bearing species following oral administration to dogs and IV administration to mice, rats, dogs, and monkeys. The high oral bioavailability of 79% indicates that the drug was well absorbed. Extrapolation of VSS from preclinical species to a hypothetical 60-kg human resulted in a very large VSS of 645 L, which is approximately 100 times higher than blood volume and approximately 15 times higher than whole body volume. This VSS is ~ 28 times larger than the 23 L reported for idelalisib, indicating greater diffusion of ME-401 away from the blood and into the tissues of the body [8, 18]. Although the mechanism behind this large VSS is unclear, and it is beyond the scope of this work to estimate the degree to which ME-401 distributes to extravascular tissue in humans, it is likely that ME-401 concentrations are significantly higher in one or more extravascular tissues compared with drug concentrations measured in human plasma.
The capacity of ME-401 to distribute to target tissues was evaluated in the dog lymph/plasma PK study and the A20 syngeneic model in mice. In the lymph/plasma study, the propensity for ME-401 to distribute into the lymphatic system was evaluated by measuring drug concentration in plasma and lymphatic fluid, following oral administration to dogs. The methodology used to collect lymphatic fluid involved surgically implanted catheters. In some cases, the catheters became occluded, preventing collection at subsequent time points. Consequently, the lymph concentration data were too sparse to calculate PK parameters with a reasonable degree of confidence. Nonetheless, the results were adequate to conclude that ME-401 readily distributes into the lymphatic system after oral administration to dogs.
In the A20 syngeneic model, concentrations of ME-401 in tumor tissue were approximately 20–30 times greater than plasma concentrations at 4 h after the last dose; these concentrations remained elevated relative to plasma concentrations 24 h after the last dose, suggesting prolonged concentrations in this targeted tissue. In comparison, plasma and tumor concentrations of idelalisib were significantly lower than those of ME-401 at 4 h following the last dose. By 24 h after the last dose, idelalisib plasma and tumor concentrations were below the limit of quantitation. Interestingly, our study demonstrated the distribution of ME-401 into brain tissue at concentrations around 30% of plasma concentrations that were still measurable at 24 h, and suggests that future investigation into the role of ME-401 in the treatment of CNS lymphomas may be warranted. Although idelalisib and ME-401 are administered to humans at different doses, potentially resulting in very different tissue concentrations, these agents were tested at identical dosing regimens in the A20 mouse model, to enable direct comparison of the molecules’ performance attributes. Notwithstanding that ME-401 plasma concentrations in our studies may not reflect clinical plasma concentrations, ME-401 does exhibit linear PKs in mice (data not shown), indicating that drug distribution does not vary with dose.
ME-401 entered clinical trials in B-cell malignancies at a starting dose of 60 mg orally, once daily, now the recommended Phase 2 dose. This dose is a fraction of the predicted 140-mg MRSD based on preclinical toxicology studies. It was previously reported [11, 12] that daily oral dosing of ME-401 60 mg was expected to result in clinical plasma concentrations that maintained maximal PI3Kδ inhibition. The high in vitro potency of ME-401, together with its capacity to distribute into target tissues and accumulate at the site of action, could underlie the high clinical potency in B-cell malignancies that may differentiate ME-401 from other PI3Kδ inhibitors [13]. These characteristics may also allow ME-401 the flexibility of intermittent dosing after a period of continuous therapy in an effort to prevent on-target immune-related adverse effects that may occur late in treatment in some patients [14]. Current studies of ME-401, as monotherapy and in combination with novel agents, are ongoing in B-cell malignancies, and future studies may provide answers to safety and efficacy concerns that have shadowed this class of agents.
Acknowledgements
The authors acknowledge Sandra Petralia, Dan Gold, and David Walsey for feedback on the manuscript and the medical writing assistance of Laura Jung, PharmD, of ETHOS Health Communications in Yardley, PA, USA, which was supported financially by MEI Pharma Inc., San Diego, CA, USA, in compliance with international Good Publication Practice guidelines.
Compliance with Ethical Standards
Funding
This study was sponsored by MEI Pharma Inc.
Conflict of interest
J. Wood and O. Moreno are employees and stockholders of MEI Pharma Inc.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Charles River Institutional Animal Care and Use Committee ASP # 990202).
References
- 1.Tzenaki N, Papakonstanti EA. p110delta PI3Kinase pathway: emerging roles in cancer. Front Oncol. 2013;3:40. doi: 10.3389/fonc.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner M, Hobeika E, Jumaa H. Role of PI3K in the generation and survival of B cells. Immunol Rev. 2010;237(1):55–71. doi: 10.1111/j.1600-065X.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 3.Herman SE, Lapalombella R, Gordon AL, Ramanunni A, Blum KA, Jones J, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117(16):4323–4327. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyling M, Santoro A, Mollica L, Leppa S, Follows GA, Lenz G, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi: 10.1200/JCO.2017.75.4648. [DOI] [PubMed] [Google Scholar]
- 7.Copiktra [product insert]. Needham, MA: Verastem, Inc; 2018.
- 8.Zydelig [product insert]. Foster City, CA: Gilead Sciences, Inc; 2018.
- 9.Aliqopa [product insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc; 2017.
- 10.O’Farrell M, Ventura R, Tai A, Tyner JW, Loriaux MM, Mahadevan D, et al. Preclinical characterization of PWT143, a novel selective and potent phosphatidylinositol 3-kinase delta (PI3K delta) inhibitor with ex-vivo activity in hematologic malignances. Blood. 2012;120:2907. doi: 10.1182/blood.V120.21.2907.2907. [DOI] [Google Scholar]
- 11.Moreno O, Butler T, Zann V, Willson A, Leung P, Connor A. Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110delta, following single ascending dose administration to healthy volunteers. Clin Ther. 2018;40(11):1855–1867. doi: 10.1016/j.clinthera.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Zann VCA, Preston M, Leung P, Duncan D, Moreno O. Formulation selection and development for ME-401, an oral, potent and selective inhibitor of phosphatidylinositol 3-kinase P110δ during a first-in-human study in healthy volunteers. Denver, CO: American Association of Pharmaceutical Scientists; 2016. [Google Scholar]
- 13.Soumerai JD, Pagel JM, Jagadeesh D, Salman HS, Kenkre VP, Asch AS, et al. Initial results of a dose escalation study of ME-401, a selective and structurally differentiated PI3Kδ inhibitor, in relapsed/refractory (R/R) follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Presented at Amercian Society of Clinical Oncology Annual Meeting, 1–5 June 2018, Chicago, IL (Abstract 7519).
- 14.Zelenetz AD, Soumerai JD, Jagadeesh D, Reddy N, Stathis A, Asch AS, et al. Preliminary safety and efficacy results with an intermittent schedule of the PI3Kδ inhibitor ME-401 along or in combination with rituximab for B-cell malignancies. Presented at the American Society of Hematology Annual Meeting, 1–4 December 2018, San Diego, CA (Abstract 2893).
- 15.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol. 2015;172(13):3189–3193. doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin FRM, Zhou H, Kwan E, Ramanathan S. Pharmacokinetics, metabolism, and excretion of idelalisib. Blood. 2013;122:5570. doi: 10.1182/blood.V122.21.5570.5570. [DOI] [Google Scholar]
- 18.European Medicines Agency. Committee for medicinal products for human use. EMEA/CHMP/324336/2014. Zydelig. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003843/WC500175379.pdf. Accessed 7 Mar 2019.