Summary
Hepatitis B virus (HBV) is a main cause of chronic and acute hepatitis. Healthcare workers (HCWs), including medical students and resident doctors, have an occupational risk of HBV infection.
The study aimed to evaluate the long-term persistence of protective anti-HBs antibody levels in healthcare students and resident doctors at risk for occupational exposure to HBV at 15 years after primary vaccination course. Further objective was to evaluate the anamnestic response observed in non-seroprotected subjects receiving a booster dose.
Data were collected from the clinical documentation filled in during the occupational medical check of medical students and resident doctors undergoing Occupational Health Surveillance by the University of Ferrara.
Of the 621 included individuals, 27.7% had an anti-HBs concentration < 10 mIU/mL. Subjects vaccinated during infancy had more frequently a concentration < 10 mIU/mL than those vaccinated during adolescence (42.7% vs 6.9%; p-value < 0.001). Multivariate analysis confirmed the statistical significance of the vaccination age. 94 subjects who had an anti-HBs concentration < 10 mIU/mL received a booster dose. The proportion of subjects who had an anamnestic response was higher in those vaccinated in infancy rather than during adolescence (94.1% vs 77.8% respectively).
These findings suggest that the anti-HBs concentration decreases below 10 mIU/mL more frequently in subjects vaccinated during infancy. Immunological memory seems to persist after the decline of the anti-HB titer, as observed in response to a booster dose. In conclusion, vaccinated subjects at increased risk of HBV infection should be monitored and a booster dose administered if anti-HBs titer is below 10 mIU/mL.
Keywords: Hepatitis B virus, Occupational health, Vaccination, Immunological memory
Introduction
Hepatitis B virus (HBV) is a serious global health concern and an important occupational risk for healthcare workers. HBV is one of the main causes of chronic and acute hepatitis, leading to a high risk of death for cirrhosis and liver cancer [1, 2].
The World Health Organization (WHO) estimates that, globally, 257 million people are infected by HBV or positive for hepatitis-B-surface-antigen (HBsAg). In 2015 HBV caused 887.000 deaths, mostly by complications [2, 3].
In Europe, a downward trend in the rate of acute cases was observed, due to the implementation of immunization programmes across several countries in this region. However, through the changes in local testing and reporting practices, the rate of newly diagnosed chronic cases is rising over time [4].
In Italy, according to the National Surveillance System (SEIEVA) data, the incidence rate of hepatitis B acute cases decreased from 5.4 per 100,000 inhabitants in 1990 to 2 per 100,000 inhabitants in 2000. This decline was even more noticeable among 15-24-year-old people, whose incidence rate decreased from 17.4 per 100,000 to 2 per 100,000 in the same lapse [5-7].
Since 1982, safe and efficacious vaccines are available [8]. According to the 2017-2019 National Plan of Vaccination Prevention (PNPV), healthcare workers (HCWs) are at maximum risk to get HBV infection and an adequate immunization intervention is essential to prevent and control the transmission of HBV in order to guarantee the protection in this group of workers. Furthermore, HBV vaccination is strongly advised for students involved in healthcare setting. The recommended schedule of HBV vaccine consists of three doses administered at the third, fifth and eleventh months of life. The target for HBV vaccine is a coverage rate of at least 95% in new-borns [9].
Anti-HBs antibody levels (anti-HBs) ≥ 10 mIU/mL are considered protective [10, 11].
Available studies in the literature suggested that immunological memory persists even if anti-HBs titer decreases under 10 mIU/mL, as confirmed by an amnnestic response after the administration of a booster dose of HBV vaccine [3, 12-16].
Monitoring anti-HBsAg antibodies titer in serum and the eventual administration of a booster dose could be necessary in individuals at risk of exposure, like HCWs [10, 17].
According to the Italian law on Occupational Health and Safety (Law Decree 81/2008) [18] the students, the interns and the resident doctors are also included in HCWs. When exposed to biological risk, blood tests, including HBsAg assessment and anti-HBs titer [19], are evaluated. Moreover, the Italian Law Decree 81/2008 requires the employers to adopt protective measures for at risk workers, including providing safe and effective vaccines [9, 18, 20].
The recent guidelines developed by Italian region Emilia-Romagna [20] emphasise the importance of hepatitis B vaccine for all HCWs considered at risk even affecting, in some specific cases, fitness to work provided by the occupational physicians (e.g., refusing vaccination, high risk tasks, exposure prone procedures).
The aim of the study was to assess the prevalence and the persistence of a positive anti-HBs titer (≥ 10 mIU/mL) in medical students and in resident doctors with an occupational risk of HBV infection, approximately 20 years after the completion of a full regular vaccination series. The immunological memory through the anamnestic response to a booster dose in subjects with an anti-HBs concentration < 10mIU/mL was also evaluated.
Methods
STUDY DESIGN
A cross-sectional, observational study was conducted. Demographic, clinical and laboratory data were collected through the review of clinical records filled in during the Occupational Health Surveillance Program.
SETTING AND STUDY POPULATION
The study was conducted on medical students and resident doctors undergoing periodical medical examination provided for Occupational Health Surveillance Program by the University of Ferrara from January 2011 to February 2018. Program is offered free of charge to all workers, including students, who have an occupational risk for exposure to biological, chemical or physical agents [18].
Data were collected from the clinical documentation drafted during the Occupational Health Surveillance examination and included: age, gender, occupational category, vaccination status concerning HBV (date of vaccination, age at vaccination, number of doses, type of vaccine if available), blood tests (date of blood test, anti-HBs concentration, markers of HBV infection). The vaccination status was verified through vaccination certificates (a document certifying the administration of vaccines by the Public Health Department) provided by the subjects at the time of the first visit.
Only students and residents who received three doses of recombinant HBV vaccine, at 0 and subsequent 1 and 6 months from the first dose were included.
All subjects who received incomplete cycles, doses at inappropriate intervals, additional doses prior to the assessment of anti-HBs titre or who provided insufficient documentation were excluded. All subjects with a positive HBsAg surface antigen were also excluded.
The enrolled students and residents were classified according to the age at vaccination in “vaccinated during infancy”, if the vaccination course was administered between 0 and 3 years and “vaccinated during adolescence”, if the vaccination course was administered between 10 and 16 years. Subjects vaccinated at other ages were classified as “other age” and were excluded from the study.
The received dose was classified as “pediatric” or “adult” according to the indications of the Ministry of Health: the compulsory vaccination for HBV was introduced in Italy in 1991 [21]; from 1991 to 2000, 3 pediatric doses were administered to all new-borns starting from the third month of life and 3 adult doses to all 12-year-old individuals.
Since 2000, however, the pediatric dose is administered up to 16 years of age to subjects who have not been vaccinated at birth [19].
According to the WHO recommendations, an anti-HBs titer equal to or greater than 10 mIU/mL was considered as protective [22]. To all subjects with an anti-HBs concentration < 10 mIU/mL a booster dose of monovalent HBV vaccine was offered, as established by Italian law [23]. The anti-HBs titer was re-evaluated at least four weeks after the administration of the booster dose, considering it protective when ≥ 10 mIU/mL.
STATISTICAL ANALYSIS
Categorical variables were analysed as frequencies and percentages and compared by the Pearson’s Chi-square test. The outcome of interest was anti-HBs concentration, considered as not protective when < 10 mIU/mL. Levels of anti-HBs concentrations have been dichotomized in “0” when < 10 mIU/mL and “1” when ≥ 10 mIU/mL.
The anamnestic response to a booster dose among subjects vaccinated during infancy and among those vaccinated in adolescence has been evaluated with the Fisher’s exact test.
To allow the calculation of the geometric mean concentrations (GMC), in the case of undetectable concentrations, an arbitrary value of 0.5 mIU/mL was assigned, while for concentrations > 1,000 mIU/mL, an arbitrary value of 1000 was assigned.
A multivariate stepwise logistic regression was performed considering as outcome an anti-HBs concentration < 10 mIU/mL. The logistic regression model was build testing the following variables: gender (0 = male, 1 = female), age at vaccination (0 = infancy, 1=adolescence), vaccine dose (0 = pediatric, 1 = adult), time since vaccination (0 = < 15 years, 1 = 15-19 years, 2 = ≥ 20 years). Odds Ratios (OR) and 95% confidence intervals (95% CI) have been calculated and a p-value < 0.05 has been considered as statistically significant. Statistical analysis was performed with IBM® SPSS® Statistics, version 20.
ETHICAL APPROVAL ASPECTS
The study protocol was approved by the Local Ethics Committee. The collection of data was conducted following the principles of the Declaration of Helsinki, according to current national legislation and in compliance with the protection of personal data. As no information which may identify the subjects was collected, no informed consent was obtained.
Results
The overall population was composed of 678 students and resident doctors. 57 subjects were excluded: 23 because they received a fourth dose before the study, 27 had incomplete documentations, 6 were not vaccinated either in infancy or adolescence, 1 resulted HbsAg positive. 621 individuals met the inclusion criteria. 172 (27.7%) had an anti-HBs concentration < 10 mIU/mL and 449 (72.3%) had an anti-HBs titer ≥ 10 mIU/mL.
None of the included subjects was vaccinated with the hexavalent vaccines Hexavac or Infarix.
The univariate analysis (Tab. I) showed that the percentage of subjects vaccinated during infancy with a titer < 10 mIU/mL was significantly higher than in subjects immunized during adolescence (154/361 or 42.7% vs 18/260 or 6.9%; OR = 0.1; 95% CI = 0.1-0.2; p-value < 0.001) (Fig. 1). The percentage of subjects who received a complete vaccination course with pediatric doses and had an anti-HBs concentration < 10 mIU/mL was significantly higher than that of the subjects vaccinated with adult doses (162/450 or 36.0% vs 10/171 or 5.8%; OR 0.1; 95% CI = 0.1-0.2; p-value < 0.001).
Tab. I.
Demographic and epidemiological characteristics.
| Anti-HBs < 10 mIU/mL (N = 172) |
Anti-HBs ≥ 10 mIU/mL (N = 449) |
P-value | |
|---|---|---|---|
Gender
|
74, 43.0% 98, 57.0% |
170, 37.9% 279, 62.1% |
n.s. n.s. |
Age at HBV vaccination
|
89.5 10.5 |
46.1 53.9 |
< 0.001 |
Vaccine dose
|
94.2 5.8 |
64.1 35.9 |
< 0.001 |
| Time since vaccination mean ± SD (years) | 20.4 ± 2.2 | 18.3 ± 3.2 | < 0.001 |
| Age at testing mean ± SD (years) | 22.6 ± 2.4 | 25.3 ± 3.4 | < 0.001 |
Fig. 1.
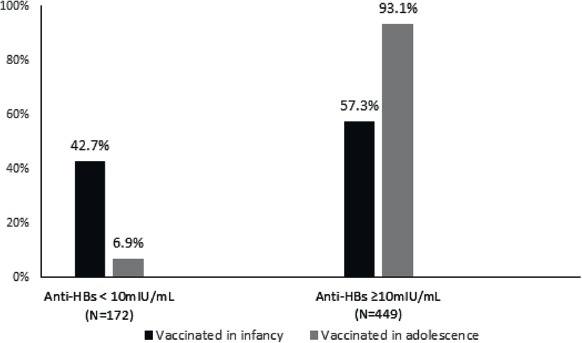
Seroprevalence against HBV according to the age at primary vaccination course.
The average period between the completion of the first immunization cycle and the evaluation of anti-HBs was 20.4 years (SD = 2.2) for the subjects with titer < 10 mIU/mL and 18.3 years (SD = 3.3) for the subjects with concentration ≥ 10 mIU/mL. The mean age of immune subjects was 25.3 years (SD = 3.4) while in seronegative individuals was 22.6 years (SD = 2.4).
The logistic regression model (Tab. II) confirmed the statistical significance of age at vaccination (infancy versus adolescence, OR = 0.2; 95% CI = 0.1-0.8; p-value = 0.02) as independent predictive variable associated with an outcome of anti-HBs concentration < 10 mIU/mL.
Tab. II.
Results of a logistic regression model estimating predictors of an anti-HBs titer < 10 mIU/mL in HBV vaccinated subjects.
| Outcome: anti-HBs titer < 10 mIU/mL N = 621 |
OR | 95% CI | P-value |
|---|---|---|---|
Gender
|
1.0 1a |
0.6-1.4 | 0.81 |
Age at HBV vaccination
|
0.2 1a |
0.1-0.8 | 0.02 |
Vaccine dose
|
0.7 1a |
0.2-1.9 | 0.48 |
Time since vaccination
|
1a 1.9 0.9 |
0.7-5.5 0.2-3.4 |
0.12 0.84 |
CI: Confidence Interval
a: reference category.
As regards to age at first immunization, the 27.4% of subjects vaccinated during infancy and 2.3% of those immunized in adolescence showed undetectable anti-HBs titers (Tab. III).
Tab. III.
Anti-HBs concentrations (mIU/mL) and Geometric Mean Concentration (GMC) before booster dose.
| Anti-HBs concentrations | Infancy (0-3 years) N = 361 (%) |
Adolescence (10-16 years) N = 260 (%) |
|---|---|---|
| Undetectable | 99 (27.4) | 6 (2.3) |
|
55 (15.2) 150 (41.6) 55 (15.2) 7 (1.9) |
12 (4.6) 82 (31.5) 92 (35.4) 68 (26.2) |
| GMC before booster dose | ||
| mIU/mL (95% CI) | 11.1 (8.7-14.0) | 158.2 (128.2-194.8) |
The GMC of anti-HBs before booster dose was higher among subjects vaccinated during adolescence (158.2 mIU/mL with 95% CI = 128.2-194.8) than in those immunized during infancy (11.1 mIU/mL with 95% CI = 8.7-14.0).
Although a booster dose was offered to all seronegative subjects, only 94 subjects had an available blood test at the moment of the study as showed in Table IV: 85 vaccinated during infancy (55.2%) and 9 vaccinated during adolescence.
Tab. IV.
Anti-HBs concentrations and Geometric Mean Concentration (GMC) after booster dose.
| Infancy (0-3 years) after booster dose N = 85 (%) |
Adolescence (10-16 years) after booster dose N = 9 (%) |
P-value | |
|---|---|---|---|
Anti-HBs concentration before booster dose:
|
5 (5.9) 80 (94.1) |
2 (22.2) 7 (77.8) |
0.13 |
|
GMC after booster dose mIU/mL (95% IC) |
241.2 (167.7-348.7) | 72.7 (21.9-278.6) |
The rate of subjects showing an anamnestic response after the booster dose was higher in individuals vaccinated during infancy (80/85 or 94.1% vs 7/9 or 77.8%). This difference was however not statistically significant (p-value = 0.13).
After receiving the booster dose, 91.9% (57 out of 62) of subjects with a previously undetectable anti-HBs concentration and 93.8% (30 out of 32) of those with a previously detectable but < 10 mIU/mL titer, achieved an anamnestic response (p-value = n.s.). None of the subjects resulted non-responder since they all reached an anti-HBs titer ≥ 10 mIU/mL within the secondary vaccination course (at 4th, 5th or 6th dose).
The GMC of anti-HBs after booster dose resulted 241.2 mIU/mL (95% CI = 167.7-348.7) in subjects immunized during the infancy and 72.7 mIU/mL (95% CI = 21.9-278.6) in subjects vaccinated during adolescence.
Discussion
Most medical students and resident doctors included in the study (72.3%) showed, at the occupational health surveillance check, a protective anti-HBs titer even 15-20 years after the first cycle of vaccination. This finding confirms the current knowledge concerning the long-term persistence of seroprotection levels in appropriately vaccinated subjects, as described in the literature [24, 25].
The study showed that, 15-20 years after the primary HBV vaccination course, there is a significant difference between the rate of subjects vaccinated during infancy who did not maintain an anti-HBs concentration ≥ 10 mIU/mL and that of those immunized during adolescence (42.7% and 93.1% respectively). Similarly to other studies [3, 26, 27], the 27.4% of subjects vaccinated during infancy also had an undetectable anti-HBs concentration and a GMC before booster dose of 11.1 mIU/mL.
Factors such as age at vaccination, vaccine dosage [1, 3] and host genetic factors [28] play a fundamental role in maintaining the anti-HBs concentration. Furthermore, the higher the antibody response after the primary vaccination cycle, the longer the persistence of an anti-HBs concentration above the 10 mIU/mL threshold [1]. The results arisen in this study reinforce the importance of age at vaccination, as highlighted by the statistical significance in the multivariate analysis (OR 0.2; 95% CI = 0.1-0.8).
The immune system in childhood is characterized by an impaired T-cell function, by lower interactions between B and T cells, by a reduced assortment of immunoglobulins and by a low affinity antibody response [29].
The anamnestic response to a booster dose in individuals with an anti-HBs concentration < 10 mIU/mL was detected, particularly in subjects vaccinated during infancy: 80 subjects out of 85 (94.1%) recovered a protective titer with a GMC of 241.2 mIU/mL. The response was less pronounced in subjects with anti-HBs concentrations < 10 mIU/mL and vaccinated during adolescence, although no statistically conclusive hypotheses can be conceived due to the small number of available blood test. Among the medical students and resident doctors who received a booster dose, no significant difference was found in the anamnestic response between those who had an undetectable anti-HBs titer and those who had a detectable but not protective anti-HBs titer. These results are consistent with previous studies and seem to confirm the hypothesis that the immunological memory is preserved even after the loss of anti-HBs antibody titer [10, 30]. Such evidence acquires significance in an occupationally exposed population at greater risk for HBV. As recently pointed out by the review of Zhao and Zhou [31], whether booster or revaccination after a period of time following the primary vaccination is required remains a debated issue. Nonetheless a booster response could be observed in most of subjects vaccinated 30 years ago, as confirmed by breakthrough HBV infection with severe consequences in successfully vaccinated individuals is extremely rare.
The waning immunity against HBV should be particularly monitored in high-risk groups. Occupational Physicians should consider with particular attention the evaluation of the persistence of anti-HBV specific antibodies in medical students (in most cases vaccinated at birth age) and HCWs specifically at the time of the first employment in order to identify subjects with non-protective anti-HBs titer [32, 33]. Occupational Physicians’ contribution to reduce vaccine delays or refusals and vaccine hesitancy that are also affecting HCWs is crucial [34]. A recent study of Riccò and colleagues [35] draw attention to the knowledge, attitudes, beliefs and practices of Occupational Physicians towards vaccination in HCWs showing that severity perception of the disease and misconceptions about vaccines still influence the immunization coverage. As only evidence-based recommendations should guide occupational physicians’ behaviour towards vaccination, specific training programs and formations courses are strongly needed.
The main limitations of this study are represented by the intrinsic characteristics of the study design and the incomplete data of a part, albeit limited, of the clinical documentation.
In particular, at the moment of the study, only a part of blood tests was available.
Some anamnestic data could play an important role on the long-term immunogenicity of the vaccine [36]. The reduced number of subjects with anti-HBs concentration < 10mIU/mL vaccinated during adolescence did not allow to detect significant differences in the booster dose response in this subgroup. Furthermore, not all subjects eligible for the administration of a booster dose accepted the recommendation.
Conclusions
The results of the study, accordingly to literature, suggest that anti-HBs titers have a tendency to decrease below the threshold of 10 mIU/mL in vaccinated subjects. Immunological memory, however, seems to persist in almost all individuals even after the reduction of the anti-HBs concentrations to undetectable levels, as observed in response to a booster dose. In conclusion, assessing seroprotection within public health programmes is not considered a cost-effective practice. In order to fill this gap, the Advisory Committee on Immunization Practices (ACIP) recommends testing health care personnel for anti-HBs antibodies levels before their occupational exposure. A positive result of the test (≥ 10mUI/mL) provides no further intervention. On the other hand, one or more additional doses of HBV vaccine and retesting is recommended in non-seroprotected subjects [37]. Occupational Health Surveillance Programs represent the best opportunity to perform these activities and recommendations, allowing also both to verify the anamnestic response to booster vaccination and the persistence of immunological memory. All these actions are supported from our results and contribute to the control of HBV transmission among HCWs and undergraduate healthcare students.
Figures and tables
Acknowledgements
Funding sources: this research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
GG received grants from Sanofi Pasteur MSD, GSK Biologicals SA, Novartis, Pfizer, Sanofi Pasteur, MSD Italy, PaxVax and Seqirus for taking part to advisory boards, expert meetings, for acting as speaker and/or organizer of meetings/congresses and as principal investigator and chief of O.U. in RCTs. Others authors declare no conflict of interest.
Authors’ contributions
AS and GG contributed to the overall design of the study, analysed the data and drafted the manuscript. NB, FS, GD, AM, EM and SL contributed to the study design, collected the data, contributed to data analysis and reviewed the manuscript. All authors critically read and revised the drafts of the manuscript. All authors read and approved the final manuscript.
References
- [1].Romanò L, Galli C, Tagliacarne C, Tosti ME, Velati C, Fomiatti L, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, Parlato A, Zotti CM, Mele A, Zanetti AR, Study Group Persistence of immunity 18-19 years after vaccination against hepatitis B in 2 cohorts of vaccinees primed as infants or as adolescents in Italy. Hum Vaccin Immunother 2017;13:981-5. doi: 10.1080/21645515.2017.1264795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization (WHO). Hepatitis B, 2017. Available at: www.who.int/mediacentre/factsheets/fs204/en (accessed 10/06/2019).
- [3].Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, Parlato A, Zamparo E, Zotti C, Stroffolini T, Mele AStudy Group. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet 2005;366:1379-84. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- [4].European Centre for Disease Prevention and Control (ECDC) Surveillance Report. Annual Epidemiological Report for 2015 Hepatitis B. 2017. Available at: https://ecdc.europa.eu/en/publications-data/hepatitis-b-annual-epidemiological-report-2015-0 (accessed 10/06/2019).
- [5].Mele A, Stroffolini T, Zanetti AR. Hepatitis B in Italy: where we are ten years after the introduction of mass vaccination. J Med Virol 2002;67:440-3. doi: 10.1002/jmv.10092. [DOI] [PubMed] [Google Scholar]
- [6].Stroffolini T, Mele A, Tosti ME, Gallo G, Balocchini E, Ragni P, Santonastasi F, Marzolini A, Ciccozzi M, Moiraghi A. The impact of the hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J Hepatol 2000;33:980-5. [DOI] [PubMed] [Google Scholar]
- [7].Zanetti AR. Update on hepatitis B vaccination in Italy 10 years after its implementation. Vaccine 2001;19:2380-3. [DOI] [PubMed] [Google Scholar]
- [8].Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol 2009;50:805-16. doi: 10.1016/j.jhep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [9].Italian Minister of Health. Italian National Vaccine Prevention Plan 2017-2019. Available at: www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf. Italian (accessed 10/06/2019).
- [10].Romanò L, Galli C, Tagliacarne C, Zanetti AR. Lessons learnt over two decades of vaccination against hepatitis B in Italy. J Prev Med Hyg 2015;56:E12-4. [PMC free article] [PubMed] [Google Scholar]
- [11].West DJ. Clinical experience with hepatitis B vaccines. Am J Infect Control 1989;17:172-80. [DOI] [PubMed] [Google Scholar]
- [12].Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011;53:68-75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- [13].van der Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, Kahn A, Jeffries D, Akum AA, Hall AJ, Bah E, McConkey SJ, Hainaut P, Whittle HC. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. PLoS One 20075;2:e753 doi: 10.1371/journal.pone.0000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 2000;19:877-85. doi:10.1016/S0264-410X(00)00224-3. [DOI] [PubMed] [Google Scholar]
- [15].Banatvala JE, Van Damme P. Hepatitis B vaccine - do we need boosters? J Viral Hepat 2003;10:1-6. doi: 10.1046/j.1365-2893.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- [16].European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 2000;355:561-5. doi: 10.1016/S0140-6736(99)07239-6. [PubMed] [Google Scholar]
- [17].West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine 1996;14:1019-27. doi: 10.1016/0264-410X(96)00062-X. [DOI] [PubMed] [Google Scholar]
- [18].Legislative Decree no. 81, April 9th 2008. Comprehensive law on health and safety at work (and subsequent amendments and additions). Gazzetta Ufficiale della Repubblica Italiana 2008;101. [Google Scholar]
- [19].Ministerial Decree, November 20th 2000. Update of the protocol for vaccination against viral hepatitis B. Gazzetta Ufficiale della Repubblica Italiana 2000. [Google Scholar]
- [20].Regulation no. 351 of Emilia-Romagna Region of 12 March 2018 on biological risk in health environment. Available at: http://servizissiir.regione.emilia-romagna.it/deliberegiunta/servlet/AdapterHTTP?action_name=ACTIONRICERCADELIBERE&operation=leggi&cod_protocollo=GPG/2018/376&ENTE=1. Italian (accessed 10/06/2019).
- [21].Legislative Decree no. 165 May 27th 1991 on mandatory vaccination against viral hepatitis B. Gazzetta Ufficiale della Repubblica Italiana 1991. [Google Scholar]
- [22].World Health Organization. Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec 2009;84:405-20.19817017 [Google Scholar]
- [23].Regulation of the Italian Minister of Health of 20 November 2000 on scheme of beahavior for vaccination against viral hepatitis B. Circolare no. 19, 2000. [Google Scholar]
- [24].Dini G, Toletone A, Barberis I, Debarbieri N, Massa E, Paganino C, Bersi F, Montecucco A, Alicino C, Durando P. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: a cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum Vaccin Immunother 2017;13:440-4. doi: 10.1080/21645515.2017.1264788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bini C, Grazzini M, Chellini M, Mucci N, Arcangeli G, Tiscione E, Bonanni P. Is hepatitis B vaccination performed at infant and adolescent age able to provide long-term immunological memory? An observational study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother 2018;14:450-5. doi: 10.1080/21645515.2017.1398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010;51:1547-54. doi: 10.1002/hep.23543. [DOI] [PubMed] [Google Scholar]
- [27].Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 2017;35:6302-7. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- [28].Ryckman KK, Fielding K, Hill AV, Mendy M, Rayco-Solon P, Sirugo G, van der Sande MA, Waight P, Whittle HC, Hall AJ, Williams SM, Hennig BJ. Host genetic factors and vaccine-induced immunity to HBV infection: haplotype analysis. PLoS One 2010;5:e12273 doi: 10.1371/journal.pone.0012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Siegrist CA. Vaccination in the neonatal period and early infancy. Int Rev Immunol 2000;19:195-219. [DOI] [PubMed] [Google Scholar]
- [30].Dentinger CM, McMahon BJ, Butler JC, Dunaway CE, Zanis CL, Bulkow LR, Bruden DL, Nainan OV, Khristova ML, Hennessy TW, Parkinson AJ. Persistence of antibody to hepatitis B and protection from disease among Alaska natives immunized at birth. Pediatr Infect Dis J 2005;24:786-92. doi: 10.1097/01.inf.0000176617.63457.9f. [DOI] [PubMed] [Google Scholar]
- [31].Zhao H, Zhou YH. Revaccination against hepatitis B in late teenagers who received vaccination during infancy: Yes or no? Hum Vaccin Immunother 2018;14:456-63. doi: 10.1080/21645515.2017.1397243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lamberti M, De Rosa A, Garzillo EM, Corvino AR, Sannolo N, De Pascalis S, Di Fiore E, Westermann C, Arnese A, Gabriella DG, Nienhaus A, Sobrinho APR, Coppola N. Vaccination against hepatitis b virus: are Italian medical students sufficiently protected after the public vaccination programme? J Occup Med Toxicol 2015;10:41 doi: 10.1186/s12995-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Coppeta L, Pompei A, Balbi O, et al. Persistence of immunity for hepatitis B virus among heathcare workers and italian medical students 20 years after vaccination. Int J Environ Res Public Health 2019;16:1515 doi: 10.3390/ijerph16091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Betsch C, Wicker S. Personal attitudes and misconceptions, not official recommendations guide occupational physicians’ vaccination decisions. Vaccine 2014;32:4478-84. doi: 10.1016/j.vaccine.2014.06.046. [DOI] [PubMed] [Google Scholar]
- [35].Riccò M, Cattani S, Casagranda F, Gualerzi G, Signorelli C. Knowledge, attitudes, beliefs and practices of occupational physicians towards vaccinations of health care workers: a cross sectional pilot study in North-Eastern Italy. Int J Occup Med Environ Health 2017;30:775-90. doi: 10.13075/ijomeh.1896.00895. [DOI] [PubMed] [Google Scholar]
- [36].Rosenberg C, Bovin NV, Bram LV, Flyvbjerg E, Erlandsen M, Vorup-Jensen T, Petersen E. Age is an important determinant in humoral and T cell responses to immunization with hepatitis B surface antigen. Hum Vaccin Immunother 2013;9:1466-76. doi: 10.4161/hv.24480. [DOI] [PubMed] [Google Scholar]
- [37].Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67:1-31. doi: 10.15585/mmwr.rr6701a1. [DOI] [PMC free article] [PubMed] [Google Scholar]


