Summary
Regulatory T (Treg) cells are a subset of CD4+ T cells that are critical for the maintenance of self‐tolerance. The forkhead box transcription factor Foxp3 is a master regulator for the Treg phenotype and function and its expression is essential in Treg cells, as the loss of Foxp3 results in lethal autoimmunity. Two major subsets of Treg cells have been described in vivo; thymus‐derived Treg (tTreg) cells that develop in the thymus and peripherally induced Treg (pTreg) cells that are derived from conventional CD4+ Foxp3− T cells and are converted in peripheral tissues to cells that express Foxp3 and acquire suppressive ability. The transcription factor Helios, a member of the Ikaros transcription factor family, is expressed in 60–70% of Treg cells in both mouse and man, and is believed to be a marker of tTreg cells. In this review, we discuss the role and function of Helios in Treg cells, the controversy surrounding the use of Helios as a marker of tTreg cells, and how Helios controls specific aspects of the Treg cell program.
Keywords: autoimmunity, regulation/suppression, regulatory T cells, transcription factors
The Ikaros transcription factor family
Helios (Ikzf2) is a member of the Ikaros transcription factor family that is characterized by four highly conserved C2H2 zinc fingers involved in DNA binding and two C2H2 zinc fingers required for homodimeric and heterodimeric protein interactions with other family members (Fig. 1).1 There are five members of the Ikaros transcription factor family. The founding member, Ikaros (Ikzf1), was first described in 1992 and the remaining four members of the family, Aiolos (Ikzf3), Helios, Eos (Ikzf4) and Pegasus (Ikzf5) were cloned by the end of the decade.1 All members of the Ikaros family are expressed in the hematopoietic system, some widely and some more restricted. Ikaros is expressed in all hematopoietic cells, including very early hematopoietic stem cells, whereas Aiolos is expressed in most hematopoietic cells, although not in the earliest uncommitted cells.2, 3 Aiolos is observed in committed cells of the lymphoid lineage and is highly expressed in mature B cells. Helios initially appeared to be restricted to the T lymphocyte lineage.4, 5 The sequence of Eos is most homologous to Helios and was found to be expressed in a variety of hematopoietic lineages as well as other tissues.6
Figure 1.
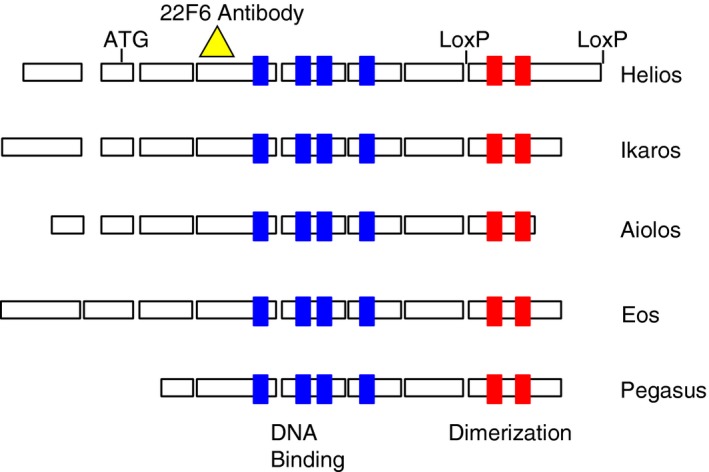
Ikaros transcription factor family. The exons for each protein are outlined with the conserved zinc fingers of the DNA‐binding domains in blue and the conserved zinc fingers of the dimerization domain in red. The epitope recognized by the monoclonal antibody 22F6 is indicated by the yellow triangle and the loxP sites used for targeted deletion are shown flanking exon 8 of Helios.
Targeted mutations of the Ikaros and Aiolos genes have demonstrated the importance of this family in lymphocyte development and homeostasis. Mice with a null mutation of Ikaros lack B cells and natural killer (NK) cells and have reduced numbers of thymic dendritic cells.7 Fetal T cells are also absent, but a small number of T cells eventually develop that are skewed towards the CD4 lineage and are hyperproliferative. Mice that are homozygous for a dominant negative mutation of Ikaros lack B cells and NK cells, but are also devoid of all T cells and early lymphoid progenitors.2 The pups fail to thrive and die by 3 weeks of age. T cells from mice with a selective deletion of Ikaros only in mature T cells were able to differentiate into T helper type 1 (Th1), Th2 and Th17 cells, but could not differentiate into regulatory T (Treg) cells in culture (iTreg) when stimulated with transforming growth factor‐β (TGF‐β).8 These mice also had enhanced expression of multiple inflammatory genes, particularly those induced by type I interferon. Mice with a targeted mutation of Aiolos have elevated serum IgG and IgE and possess autoantibodies.9 These mice have an increased number of germinal centers, their B cells display an activated phenotype and are hyperproliferative in vitro, and by 8–10 months the mice develop B‐cell lymphomas. Interestingly, old mice (> 12 months) with a germline deletion of Eos exhibit autoimmunity, whereas mice with a Treg cell‐specific deletion display immune activation and overt autoimmunity at an early (3 months) age (Gokhale and Shevach, manuscript in press). To date, no known deletions of Pegasus have been described.
Helios expression
Helios was cloned from a mouse thymoma cell line while searching for Ikaros binding partners.5 Northern blot analysis for Helios expression demonstrated very high expression in the thymus, specifically in double‐negative cells, double‐positive cells, and within a subpopulation of CD4 single‐positive cells. Further studies showed that during embryogenesis, Helios is not only expressed in sites of hematopoiesis (blood islands of the yolk sac, fetal liver and fetal thymus), but in epithelial tissues of the gut, the respiratory tract, the olfactory and salivary glands, as well as in the kidney and brain.4 However, the expression of Helios was not found in adult tissues other than the spleen and thymus. The functional analysis of Helios in these two studies was conflicting because one study concluded that Helios acted as a transcriptional activator,4 whereas neither transcriptional activation nor repression was observed in the other.5 However, analysis of human tumor lines suggested that Helios was a tumor suppressor,10, 11, 12 as patients with adult T‐cell malignancies expressed a dominant negative isoform of Helios that lacked three of the four zinc fingers in the DNA‐binding domain.13 Forced expression of this isoform of Helios in mice led to T‐cell lymphomas.14 However, ectopic expression of wild‐type Helios in B‐cell progenitors, where Helios is not normally expressed, led to increased survival and proliferation of B cells and ultimately, the development of lymphoma.15 Finally, it has been demonstrated that Helios is expressed in myeloid leukemia cells and its depletion in acute myeloid leukemia cells led to reduced colony formation and delayed oncogenesis.16 Hence, Helios appears to act as a tumor suppressor but can be tumorigenic when aberrantly expressed in inappropriate cell types. Interestingly, ectopic expression of wild‐type Helios in T cells results in cell death17, 18 (Thornton and Shevach, unpublished).
Helios as a marker of thymus‐derived Treg cells
The first suggestion that Helios was a Treg cell‐specific gene came from microarray studies that characterized the unique gene signatures of Foxp3+ Treg cells.18, 19 Using a polymerase chain reaction‐based differential screening method, we also identified Helios as a Treg cell‐specific gene.20 We also developed a hamster monoclonal antibody (22F6) to mouse and human Helios, which allowed us to characterize the expression of Helios at the single‐cell level (Fig. 1). Flow cytometry analysis (Fig. 2) showed that Helios was expressed in double‐negative thymocytes, specifically DN2 and DN3, as well as a subset of CD4+ single‐positive cells. Helios was expressed in a subset of peripheral CD4+ Foxp3+ Treg cells. The most striking observation using the 22F6 monoclonal antibody was that Helios was only expressed in approximately 70–80% of Treg cells in both human and mouse.
Figure 2.
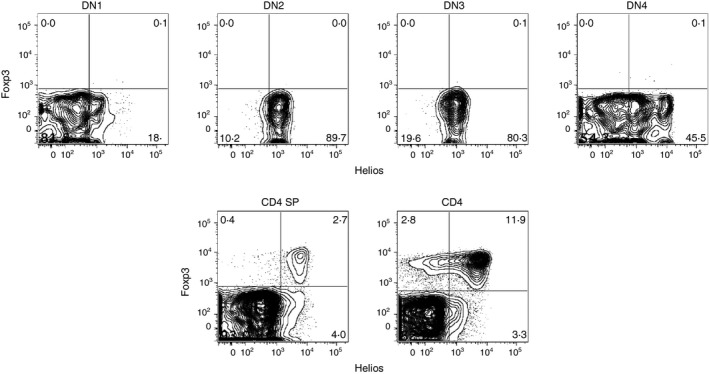
Helios expression. Thymocytes and splenocytes from C57BL/6 mice were analyzed by flow cytometry for the expression of Foxp3 and Helios. Top: CD4− CD8− double‐negative (DN) thymocytes were subdivided based on CD44 and CD25 expression (DN1: CD44+ CD25−, DN2: CD44+ CD25+, DN3: CD44− CD25+, DN4: CD44− CD25−). Bottom: CD4+ CD8− single‐positive thymocytes (left) and CD4+ CD8− splenocytes (right).
Two major subsets of Treg cells have been defined in vivo. Thymus‐derived Treg (tTreg) cells develop in the thymus and peripherally induced Treg (pTreg) cells are derived from conventional CD4+ Foxp3− T cells that are converted in peripheral tissues to cells that express Foxp3 and acquire suppressive ability.21, 22 Our initial studies strongly suggested that Helios was a marker of tTreg.23 Three main findings supported this conclusion. First, the earliest Foxp3+ cells that arise in the thymus during the first week of life are exclusively Helios+. Second, Foxp3+ Helios− cells do not begin to appear in the periphery until after the first week and do not reach their maximum percentage until after weaning. Finally, Helios expression was not observed in iTreg cells generated in vitro in the presence of exogenous TGF‐β, nor in pTreg cells generated in an oral tolerance model. A number of other studies have supported this conclusion. Expression of Bach2 is essential for TGF‐β‐induced Foxp3 expression and pTreg induction and ultimately, control of Th2‐mediated autoimmunity.24, 25 Importantly, Bach2 deficiency was associated with a specific loss of Helios− Treg cells. Similarly, a deficiency of the Kruppel‐like transcription factor 2 (KLF2) was associated with an absence of Foxp3+ Helios− Treg cells, an inability to generate iTreg cells, and a failure to develop antigen‐specific pTreg cells following oral tolerance.26 Treg cells from the colonic lamina propria of germ‐free mice are almost exclusively Helios+ 27 and Clostridium‐induced pTreg cells that accumulated in the colonic mucosa of germ‐free mice reconstituted with Clostridium, were exclusively Helios−.28 Furthermore, mice deficient in the transcription factor c‐rel lack Foxp3+ Helios+ Treg cells, but develop Foxp3+ Helios− pTreg cells in the periphery and are capable of generating iTreg cells29 and mice with a T‐cell‐specific deficiency of Satb1 also lack tTreg cells but develop Foxp3+ Helios− Treg cells in the periphery.30 Finally, Schallenberg et al.31 have described a pTreg precursor population, characterized as CD4+ CD25+ Foxp3− CD62Lint CD69+ that exists in wild‐type, unmanipulated mice. Using a unique fluorochrome‐based system to distinguish pTreg and tTreg cells, they demonstrate that Helios− cells are present only in the pTreg fraction, and that the precursors from the periphery, but not from the thymus, differentiate into Helios− Foxp3+ Treg cells.32
Although our studies, using plate‐bound anti‐CD3 as a stimulus, demonstrated that iTreg cells never expressed Helios, another study concluded that iTreg cells were frequently Helios+.33 The difference between these two studies initially appeared to be related to the use of antigen‐presenting cells for antigen presentation. However, further analysis of a variety of transgenic T‐cell receptor (TCR) cells, specific for foreign antigens, demonstrated that, for some transgenic TCR lines, but not others, Helios expression was up‐regulated on a proportion of the induced Treg cells when stimulated with antigen‐presenting cells and soluble anti‐CD3, but not with antigen‐presenting cells and the cognate antigen (Thornton and Shevach, unpublished observations). Using a transgenic TCR line specific for an autoantigen (TxA23) revealed that Helios was expressed in iTreg cells regardless of the use of anti‐CD3 or specific peptide. Hence, the requirements for Helios expression in iTreg cells, and its physiological relevance at this time, are unclear. Although most in vivo studies, described earlier, support the idea that Helios is not expressed on pTreg cells, Gottschalk et al.34 demonstrated that Helios was expressed in pTreg cells generated by low‐dose antigen administration in the absence of adjuvant. It is difficult to reconcile our findings with this study, as we were unable to observe Helios expression in iTreg cells generated from 5C.C7 TCR transgenic mice using anti‐CD3 or antigen, nor could we observe Helios expression in pTreg cells generated by low‐dose antigen administration (Thornton and Shevach, unpublished observations). Lastly, Szurek et al.,35 in three mouse models expected to lack tTreg cells or pTreg cells, demonstrated that the ratio of Foxp3+ Helios−/Foxp3+ Helios+ was unaltered, indicating that Helios was not a marker of tTreg cells. In contrast to the controversial use of Helios as a marker of mouse tTreg cells, Helios does not appear to be induced on the subset of human Foxp3+ Helios− T cells following activation in vitro 23, 36 or in vivo (Buszko and Shevach, unpublished). Although Himmel et al.37 have argued that the presence of Foxp3+ Helios− T cells within the pool of ‘naive (CD45RA+ CD31+ CCR7+ CD62L+)’ Treg cells is inconsistent with the concept that the lack of Helios expression is a marker of antigen‐experienced pTreg cells, this conclusion rests on the validity of the markers used to define ‘naive’ Treg cells. Furthermore, other studies have shown that CD45RA+ Treg cells in cord blood are almost exclusively Helios+, which is again consistent with the usefulness of Helios as a marker of human tTreg cells.38 One further confounding issue with the analysis of human Treg cell populations is that Foxp3 can be expressed in activated conventional T cells and that some of the Foxp3+ Helios− population represent activated conventional T cells.36
Helios+/Helios− Treg cell genetic differences
To directly compare the differences between Helios− and Helios+ Treg cells, we generated a Helios/Foxp3 double reporter mouse.39 A similar reporter mouse has been described, but that study only examined the suppressive and cytokine‐producing capacities of the cells.40 In our mice, comparison of the transcriptomes of Helios+ and Helios− Treg cells revealed that the two subpopulations differed by ~1000 genes and that the latter retain several features of non‐Treg cells. Notably, Foxp3+ Helios− Treg cells expressed Satb1, which is normally expressed at high levels in conventional T cells but is repressed in Foxp3+ Helios+ Treg cells. Repression of Satb1, a chromatin remodeler and super‐enhancer, has been shown to be critical for Treg cell function and ectopic expression of SATB1 in human Treg cells reprogrammed the cells into T effector cells that produced a variety of effector cytokines, even in the presence of Foxp3.30, 41, 42 Helios− Foxp3+ T cells also expressed Pde3b, which is normally expressed in naive CD4+ T cells and its expression is stable upon T‐cell activation. The physiological function of Pde3b is to break down adenosine and cyclic adenosine 3′,5′‐monophosphate (cAMP), both of which have been proposed to mediate Treg cell suppression43 and hence, Pde3b must be strongly repressed in Treg cells.44 Finally, one of the Treg cell signature genes whose expression is reduced in Helios− Treg cells is Nt5e (CD73), a 5′ ectonucleotidase that metabolizes AMP to the immunosuppressive molecule adenosine. Taken together, the higher levels of Satb1 and Pde3b and the lower levels of NT5e present in the Helios− Treg cells indicate that the Helios− Treg cell subpopulation is composed of pTreg cells that contain residual expression of certain genes normally expressed in CD4+ Foxp3− T cells. This is supported by a recent single‐cell expression analysis of gene expression in Treg cells versus conventional T cells, which identified a subpopulation (26%) of Treg cells whose expression profile overlapped with conventional T cells and was termed ‘furtive Treg cells’. This population expressed the same levels of Foxp3 transcripts as did other Treg cell subpopulations, but the ‘furtive Treg’ subpopulation expressed low levels of Helios.45 Although the large number of differentially expressed genes in the Foxp3+ Helios− subpopulation compared with Helios+ Foxp3+ indicates that the two populations are distinct, it still does not definitively rule out the possibility that the two populations of Treg cells represent different stages of differentiation.
Comparison of the pTreg and tTreg TCR repertoires is a more direct approach to determining the relationship between these Treg cell populations. Previous studies have concluded that the TCR repertoires of Treg and CD4+ Foxp3− conventional T cells are distinct and exhibit little overlap.46, 47, 48, 49 However, all of these early studies were performed in mice with restricted TCR repertoires and the results may not reflect the broader repertoires in normal mice. Wide variations in overlap (10‐60%) were also noted in studies that attempted to address the issue of peripheral conversion and pTreg cell generation by comparing tTreg cells with pTreg cells generated in a variety of ways.46, 50 Two previous studies have specifically examined the TCR repertoires of Helios− and Helios+ Treg cells. Szurek et al.35 concluded that most TCRs were expressed by both populations at similar frequencies. However, Lord et al.51 have also deep‐sequenced Helios− and Helios+ Treg cells, along with effector CD4+ T cells, from inflamed versus non‐inflamed colon of patients with ulcerative colitis. Little overlap (5–10%) of Helios− and Helios+ Treg cell sequences was observed.
Using the double reporter mice, we examined the repertoires of normal mice without having to restrict the repertoire and were able to completely sample all CD4+ T cells from the mesenteric lymph nodes.39 We concluded that the repertoires of Helios− and Helios+ Treg cells are not similar and that it is likely that Helios− and Helios+ Treg cells represent different lineages. However, this analysis was limited to cells from one location and future studies to examine the similarity of Treg cells to other populations requires an extensive analysis of these populations from multiple locations, particularly from the thymus.
Helios+/Helios− Treg functional differences
Several studies have indirectly examined the differences between Helios− and Helios+ Treg cells. Using surrogate markers for Helios expression (CD103 and GITR or TIGIT and CD226), it was demonstrated that Helios+ Treg cells had a more highly activated phenotype, were more suppressive52, 53 and had a more stable Foxp3 expression.53 Both of these studies were limited in that the surrogate markers used were not absolute. Using a single cell cloning approach of human cells, Himmel et al.37 found that Helios− Treg cells had the capacity to produce cytokines, but that the two populations had similar suppressive capacities. However, in this study, the cells were cultured for 3 weeks before cells were typed for Helios expression. We were able to use our reporter mice to clearly separate Helios+ and Helios− subsets in sufficient numbers to directly compare the two subsets in vivo and in vitro.39 Both Helios− Treg cells and Helios+ Treg cells suppress the proliferation of naive T cells in our standard co‐culture assay in vitro. However, in vivo, Helios− Treg cells and Helios+ Treg cells have differing suppressive functions. Although both subsets inhibit the ability of naive T cells to induce inflammatory bowel disease, Helios− Treg cells fail to suppress activated T effector cells in a model in which T cells from moribund scurfy mice are transferred to immunodeficient recipients. Although lower levels of cAMP or adenosine could explain the reduced function that we observe in Helios− Treg cells, the Helios− Treg cells also appear to have a defect in the regulation of the stability of Foxp3 expression in this model. Major differences in the stability of Foxp3 expression were also observed when the subpopulations were transferred to immunodeficient recipients. Helios+ Treg cells never lost Helios expression to become Helios− Treg cells, suggesting that high Helios expression is a fixed phenotype in Helios+ Treg cells. Although both subpopulations lost significant expression of Foxp3, the loss was 50% greater in the Helios− subpopulation. Consistent with this finding, both the Helios+ and the Helios− populations had substantially demethylated the Treg‐specific demethylated regions, an indication of Foxp3 stability,54 but the extent of demethylation was greater in the Helios+ subset.
In contrast to our previous conclusion that Helios was a specific marker of tTreg cells,23 the cell transfer studies also revealed that a subpopulation of Helios− Treg cells could acquire expression of Helios when transferred to both immunocompetent mice and to RAG−/− mice.39 However, the level of Helios expressed was always less than that expressed by the Foxp3+ Helios+ subset. Helios expression appears to be a stable marker that distinguishes the two subsets under physiological conditions, but it is clear that under lymphopenic conditions Helios− Treg cells can be induced to express Helios at low levels. In addition, pTreg cells generated under lymphopenic conditions from naive cells also acquire a low level of Helios. Moreover, in the studies by Schallenberg et al. and Petzold et al. described above, the induction of Helios− pTreg cells from a defined precursor was not absolute in that a population of pTreg cells that expressed Helios was also observed.31, 32 However, when compared with the Helios+ thymically derived Treg cells, Helios expression was much lower in those pTreg cells. It remains possible that some of the previous reports that claimed that pTreg expressed Helios, reflected this low level of expression as is evident in the study by Gottschalk et al.34 Whereas iTreg cells generated in vitro in the presence of accessory cells can express levels of Helios that approximate the levels seen on the Foxp3+ Helios+ subset in normal mice, the induction of Helios was TGF‐β‐dependent and may not be indicative of the normal regulation of Helios expression. Furthermore, iTreg cells never demethylate their Treg‐specific demethylated region, in contrast to pTreg cells in vivo 55 and are ultimately highly unstable in vivo when stimulated via their TCR,56 so the significance of Helios expression by iTreg cells remains unresolved.
Lessons from Helios‐deficient mice
Genetic approaches to understanding the function of Helios in lymphocytes have involved the generation of global Helios‐deficient mice as well as the use of lkzf2 fl/fl mice with deletion in selective lymphocyte subpopulations. Germline deletion of Helios resulted in almost complete neonatal lethality when the mice were generated on a mixed background,57 but complete lethality when generated on a B6 background (Thornton and Shevach, unpublished observations). Using the few surviving mice on the mixed background, Cai et al. demonstrated that the lack of Helios was not required for the development and function of T or Treg cells. We selectively deleted Helios in Treg cells (cKO) by crossing Ikzf2fl/fl mice with Foxp3YFP‐Cre mice.58 As mice with defects in Treg cell function typically exhibit autoimmunity at an early age, we were surprised to observe the lack of a prominent phenotype at 2 months of age. However, by 5–6 months of age, the mice had activated CD4+ and CD8+ T cells, hypergammaglobulinemia and markedly expanded T follicular helper (Tfh) cells. Yet, Treg cell suppressive activity was normal both in vitro and in the in vivo inflammatory bowel disease model. Similar to our studies with the double reporter mice, though, Helios‐deficient Treg cells (similar to the Helios− Treg cells from wild‐type mice) were unable to control the proliferative response of scurfy CD4+ T cells. In a manner similar to T effector cells, Treg cells can be divided into naive or activated/effector subpopulations, based on the differential expression of cell surface markers CD44 and CD62L.59 The percentage of naive Helios cKO Treg cells was unchanged, but the effector Treg subpopulation was not maintained. Moreover, the anti‐apoptotic factor Bcl‐2 was lower in Helios cKO Treg cells and was even lower upon stimulation. Hence, Helios maintains Bcl‐2 expression upon activation in Treg cells, allowing the differentiation of effector Treg cells that control Th1 and Tfh responses. Examining X‐linked genes, such as Foxp3, has a unique benefit in that X‐linked genes are subject to allelic exclusion. Only one allele of each gene on the X chromosome is expressed and this is done in a random manner. Hence, targeting one allele with a Foxp3‐driven Cre recombinase results in half of the Treg cells expressing Cre and able to delete a floxed gene of interest while the other half remain as wild‐type Treg cells. This results in a heterozygous female in which the wild‐type Treg cells are dominant and the mice are phenotypically normal. Hence, the role and function of the cKO Treg cells can be examined in a non‐lymphopenic and non‐inflammatory environment. Using the lkzf2 fl/fl mice crossed to Foxp3wt/Cre‐YFP mice to establish a competitive setting, we confirmed that Helios was required for stability of the CD44hi CD62Llo effector Treg cells (Fig. 3).
Figure 3.
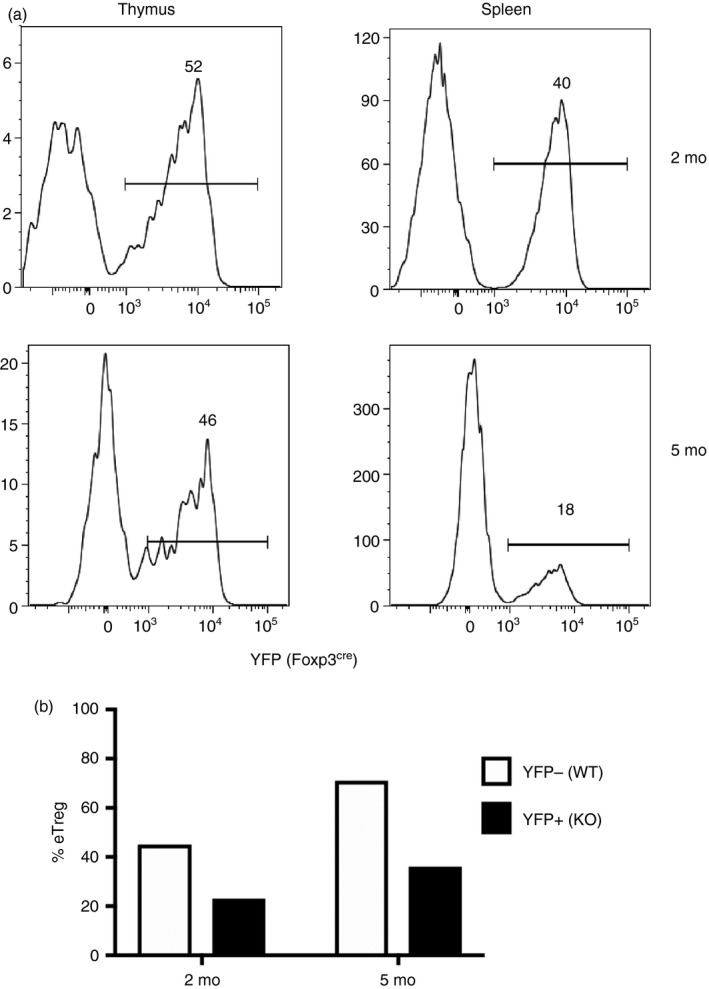
Analysis of heterozygous Foxp3 females. (a) CD4+ Foxp3+ thymocytes or splenocytes from 2‐ or 5‐month‐old Ikzf2fl/fl × Foxp3wt/ YFP ‐Cre mice were analyzed by flow cytometry for YFP expression, a reporter for the Helios‐deficient cells. (b) YFP − (wild‐type; WT) or YFP + (knockout; KO) CD4+ Foxp3+ Treg cells were analyzed for CD44 and CD62L to calculate the percent of eTreg cells defined as CD44+ CD62L−.
Kim et al.60 analyzed the Helios Treg conditional knockout mice that we had generated and concluded that Helios was required for Treg cell stability. They proposed that under inflammatory conditions Helios deficiency results in diminished signal transducer and activator of transcription 5 (STAT5) phosphorylation with resultant reduced Foxp3 expression and defective suppressor function. In contrast, we were unable to demonstrate defects in STAT5 activation. Subsequent studies by this group61 have claimed that the instability of Helios‐deficient Treg cells could be beneficial in the tumor environment and would result in the conversion of Treg cells to effector cells that produce inflammatory cytokines. However, there were fewer intratumoral Treg cells in cKO mice and the level of cytokine production from the converted Treg cells was low in comparison to that of CD4+ and CD8+ effector T cells. It is difficult to imagine that the small percentage of converted Treg cells played a significant role in antitumor activity. This group also postulated that abnormal immune responses secondary to Helios deficiency would only be apparent under inflammatory conditions. This is clearly not the case, as the survival of Helios‐deficient Treg cells is compromised in heterozygous female mice that are phenotypically normal. Regardless of the mechanism of instability (Bcl‐2 or STAT5), the consensus from these two studies is that Helios plays a role in maintaining the activation/effector phenotype of Treg cells.
Despite the clear importance of the Ikaros gene family members, their molecular mechanisms of action are still poorly understood. Ikaros family members are thought to regulate gene transcription through chromatin remodeling and studies have shown that Ikaros and Helios interact in nucleosome remodeling and the DNA methylation (NuRD) complex, an ATP‐dependent complex that has both nucleosome disruption activity and histone deacetylase activity that condenses chromatin and generally serves to inhibit transcription.62 Indeed, Ikaros can bind to the interleukin‐2 (IL‐2) promoter and repress expression while reduced binding of Ikaros releases the requirement for CD28 co‐stimulation‐mediated IL‐2 expression.63 It has been reported that Eos interacts with Foxp3 to mediate gene silencing through chromatin modifications64 and that small interfering RNA‐mediated down‐regulation of Eos resulted in IL‐2 production by Treg cells and loss of Treg suppressive capacity in vitro and in the inflammatory bowel disease model. Although mice with a conditional deletion of Eos in Treg cells developed a moderate autoimmune phenotype by 3 months of age, we found no evidence of loss of Foxp3 expression or a lethal autoimmune phenotype (Gokhale and Shevach, in press), which would have been expected from the observations of Pan et al.64
In Treg cells, it was claimed that Helios promotes binding of Foxp3 to the IL‐2 promoter and controls IL‐2 expression.65 In addition, it was reported that Helios directly bound to the Foxp3 gene and small interfering RNA knockdown experiments resulted in decreased Foxp3 expression and impaired Treg cell function in vitro.17 However, Ikaros and Aiolos, but not Helios or Eos, were identified as members of the groups of 361 proteins associated with Foxp3 in mass spectrometric analyses of Foxp3 complexes.66 Nor was Helios also identified as one of the critical transcription factors involved in the regulation of the Treg gene signature.67 Further studies of the molecular functions of Helios and its interactions with other members of the Ikaros gene family in different cell types are clearly needed.
Helios in other immune cells
This review focuses on Helios in Treg cells, but its expression and function in other cells may shed light on its role in Treg cells. Although it initially appeared that Helios was expressed primarily in Treg cells, it is expressed in several other subsets in the immune system. As we described earlier, Helios is initially expressed in all T cells during thymic development.23 Helios also appears to be a marker of autoreactive CD4+ cells undergoing negative selection in the thymus.68, 69 Helios expression was also demonstrated in CD4+ Foxp3− from TCR transgenic mice specific for a gastric autoantigen both in cells undergoing negative selection in the thymus, but also in peripheral T cells in the stomach and draining gastric lymph node. The latter were shown to be functionally anergic.69 Because of this potential functional role of Helios during negative selection in the thymus, one might expect that mice with a global deletion of Helios or a T‐cell‐specific deletion of Helios would possess a high proportion of autoreactive cells and would develop autoimmunity. However, this is clearly not the case as Helios‐deficient mice have normal thymic development.57, 58 It is possible that other Ikaros family members can compensate for the lack of Helios during thymic development.
Although we have described Helios as a marker of tTreg cells, several groups have suggested that Helios is merely a marker of T‐cell activation.35, 69, 70, 71 In fact, a small population of CD4+ Foxp3− cells in normal unmanipulated mice express Helios.23 One explanation for the failure of Ikzf2 flfl × CD4 Cre to develop immune defects is that the Helios expression in CD4+ Foxp3− cells must also play a significant functional role in normal CD4+ T‐cell activation. Although mice with a deletion of Helios in all CD4+ T cells possess Helios‐deficient Treg cells, the additional lack of Helios in CD4+ Foxp3− T cells must somehow balance out this defect to prevent the activation of Th1 and Tfh cells observed in the Ikzf2 flfl × Foxp3 Cre mice. However, Helios expression could be demonstrated in Th2 and Tfh cells, and the absence of Helios in CD4+ Foxp3− cells did not have any functional consequences.71
We recently investigated the functional role of Helios in CD4+ Foxp3− cells and found that Helios did not play a role in the primary immune response, but was critical for a secondary memory response.72 Surprisingly, the function of Helios was not cell intrinsic, but in the absence of Helios, a small proportion of the activated cells generated during the primary response differentiated to pTreg cells that were responsible for inhibiting the secondary response in an antigen‐dependent manner. This is seemingly contradictory, as we believe Helios expression is important for Treg stability but in this study, a small proportion of antigen‐specific T cells were more prone to pTreg cell differentiation in the absence of Helios.
In addition to thymic T cells and peripheral CD4+ Foxp3− cells, Helios expression had also been noted in NK cells and mucosa‐associated invariant T cells.73, 74, 75, 76, 77 Helios expression seems to be a marker of an intermediate stage in the differentiation of NK cells, but in mucosa‐associated invariant T cells it is expressed in the effector‐memory subset. The functional role of Helios in these cells has not been addressed.
In cells outside the immune system, Helios expression has been noted in neurons and in cochlear hair cells.78, 79, 80, 81 In the brain, Helios expression is confined to the embryo (beginning E14.5) and early postnatal stages and has been described as a marker of precursors to striatal matrix neurons that express enkaphalin.78 Further experiments demonstrated that Helios retains neural progenitor cells in the G1/G0 phase. The loss of Helios (using the mice generated by Cai et al.) results in an increase of cells in S phase entry and of S phase length that ultimately leads to cell death during the postnatal stage, a decrease in, and issues with the initial acquisition of motor skills.79 In the cochlear hair cells, Helios is essential for the development of outer hair cells.80 Loss of Helios leads to the functional impairment of outer hair cells and hearing loss, whereas forced expression in inner hair cells imparts an outer hair cell phenotype and restoration of electromobility function.
Conclusion
Although Helios expression was initially thought to be restricted to Treg cells, it is quite clear that Helios is expressed in a wide variety of cells. Taken together, the studies on Helios indicate that Helios appears to function by maintaining cells, or maintaining a set of genes, in a fixed, differentiated state through the modification of chromatin structure. In Treg cells specifically, Helios maintains only a subset of the Treg cell program, as Helios‐deficient mice have a moderate phenotype in comparison to Foxp3‐deficient mice. Helios is clearly a marker of stable and highly suppressive Treg cells and we advocate the use of Helios as a marker of Treg cell stability in the mouse, but particularly in man. In the treatment of autoimmunity, cellular therapy depends upon the stability of the Treg cells during the long‐term culture required to generate sufficient cells. Helios can be used as a marker to monitor Treg cell stability and methods to target Helios and Foxp3, such as oligodeoxynucleotide treatment, can improve stability and will yield an optimal population for use in biotherapy.36, 82 The issue of whether Helios is a specific marker of tTreg cells versus pTreg cells remains open. We believe that most of the data strongly supports its utility as a tTreg marker, although caution should still be exercised in drawing a definitive conclusion. Furthermore, Helios is certainly a more specific marker of tTreg cells than neuropilin‐1 (Nrp1), which has also been proposed as a tTreg cell marker.83, 84 The major problem with Nrp1 as a tTreg cell marker is that its expression can be induced by TGF‐β both in vivo and in vitro.83 The percentage of Nrp1+ Treg cells in the steady state is also dependent on TGF‐β derived from Treg cells, as mice with deficiency of GARP, a protein on Treg cells that binds latent TGF‐β, have markedly reduced percentages of Nrp1+ Treg cells (Fig. 4; Edwards and Shevach, unpublished).
Figure 4.
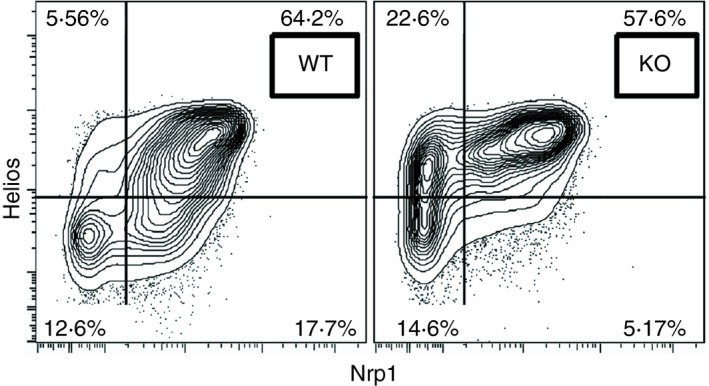
Expression of neuropilin‐1 (Nrp1) on regulatory T (Treg) cells is partially dependent on GARP‐derived transforming growth factor‐β (TGF‐β). CD4+ Foxp3+ cells from wild‐type (WT) or GARP fl/fl × CD4‐Cre (knockout; KO) mice were analyzed for expression of Helios and Nrp1.
Although progress has been made in defining the role and function of Helios in Treg cells, there are still many unanswered questions. As previously noted, the in vivo requirements for Helios induction in pTreg cells remain to be determined, as does the question of whether this lower level of Helios expression in pTreg cells is stable. In addition, the mechanism of how the up‐regulation of Helios in conventional CD4+ T cells prevents the induction of pTreg cells remains to be established. Most importantly for Treg cells, it is uncertain why the lack of Helios results in a seemingly modest phenotype, despite the overt systemic activation of T cells. The mechanisms that lead to the failure of Treg cells to control CD4 and CD8 T‐cell activation must be clarified and the interactions of Helios with chromatin and chromatin binding complexes to alter gene expression remain to be to elucidated.
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Heizmann B, Kastner P, Chan S. The Ikaros family in lymphocyte development. Curr Opin Immunol 2018; 51:14–23. [DOI] [PubMed] [Google Scholar]
- 2. Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S et al The Ikaros gene is required for the development of all lymphoid lineages. Cell 1994; 79:143–56. [DOI] [PubMed] [Google Scholar]
- 3. Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E et al Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J 1997; 16:2004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K et al Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol 1998; 8:508–15. [DOI] [PubMed] [Google Scholar]
- 5. Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R et al Helios, a T cell‐restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev 1998; 12:782–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perdomo J, Holmes M, Chong E, Crossley M. Eos and Pegasus, two members of the Ikaros family of proteins with distinct DNA binding activities. J Biol Chem 2000; 275:38347–54. [DOI] [PubMed] [Google Scholar]
- 7. Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M et al Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5:537–49. [DOI] [PubMed] [Google Scholar]
- 8. de Ana CL, Arakcheeva K, Agnihotri P, Derosia N, Winandy S. Lack of Ikaros deregulates inflammatory gene programs in T cells. J Immunol 2019; 202:1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A et al Aiolos regulates B cell activation and maturation to effector state. Immunity 1998; 9:543–53. [DOI] [PubMed] [Google Scholar]
- 10. Sun L, Kerawalla H, Wu XA, Lehnert MS, Uckun FM. Expression of a unique Helios isoform in human leukemia cells. Leuk Lymphoma 2002; 43:841–9. [DOI] [PubMed] [Google Scholar]
- 11. Nakase K, Ishimaru F, Fujii K, Tabayashi T, Kozuka T, Sezaki N et al Overexpression of novel short isoforms of Helios in a patient with T‐cell acute lymphoblastic leukemia. Exp Hematol 2002; 30:313–7. [DOI] [PubMed] [Google Scholar]
- 12. Fujii K, Ishimaru F, Nakase K, Tabayashi T, Kozuka T, Naoki K et al Over‐expression of short isoforms of Helios in patients with adult T‐cell leukaemia/lymphoma. Br J Haematol 2003; 120:986–9. [DOI] [PubMed] [Google Scholar]
- 13. Tabayashi T, Ishimaru F, Takata M, Kataoka I, Nakase K, Kozuka T et al Characterization of the short isoform of Helios overexpressed in patients with T‐cell malignancies. Cancer Sci 2007; 98:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Swindle CS, Bates JT, Ko R, Cotta CV, Klug CA. Expression of a non‐DNA‐binding isoform of Helios induces T‐cell lymphoma in mice. Blood 2007; 109:2190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dovat S, Montecino‐Rodriguez E, Schuman V, Teitell MA, Dorshkind K, Smale ST. Transgenic expression of Helios in B lineage cells alters B cell properties and promotes lymphomagenesis. J Immunol 2005; 175:3508–15. [DOI] [PubMed] [Google Scholar]
- 16. Park SM, Cho H, Thornton AM, Barlowe TS, Chou T, Chhangawala S et al IKZF2 drives leukemia stem cell self‐renewal and inhibits myeloid differentiation. Cell Stem Cell 2019; 24:153–65 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC et al A role for the transcription factor Helios in human CD4+CD25+ regulatory T cells. Mol Immunol 2010; 47:1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T et al Foxp3‐dependent and ‐independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol 2006; 18:1197–209. [DOI] [PubMed] [Google Scholar]
- 19. Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R et al Foxp3 transcription‐factor‐dependent and ‐independent regulation of the regulatory T cell transcriptional signature. Immunity 2007; 27:786–800. [DOI] [PubMed] [Google Scholar]
- 20. Rebrikov D, Desai S, Kogan YN, Thornton AM, Diatchenko L. Subtractive cloning: new genes for studying inflammatory disorders. Ann Periodontol 2002; 7:17–28. [DOI] [PubMed] [Google Scholar]
- 21. de Lafaille MAC, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009; 30:626–35. [DOI] [PubMed] [Google Scholar]
- 22. Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 2014; 259:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184:3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim EH, Gasper DJ, Lee SH, Plisch EH, Svaren J, Suresh M. Bach2 regulates homeostasis of Foxp3+ regulatory T cells and protects against fatal lung disease in mice. J Immunol 2014; 192:985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M et al BACH2 represses effector programs to stabilize T‐reg‐mediated immune homeostasis. Nature 2013; 498:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pabbisetty SK, Rabacal W, Maseda D, Cendron D, Collins PL, Hoek KL et al KLF2 is a rate‐limiting transcription factor that can be targeted to enhance regulatory T‐cell production. Proc Natl Acad Sci USA 2014; 111:9579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N et al Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y et al Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luu M, Jenike E, Vachharajani N, Visekruna A. Transcription factor c‐Rel is indispensable for generation of thymic but not of peripheral Foxp3+ regulatory T cells. Oncotarget 2017; 8:52678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R et al Guidance of regulatory T cell development by Satb1‐dependent super‐enhancer establishment. Nat Immunol 2017; 18:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3− precursor to Foxp3+ regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med 2010; 207:1393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petzold C, Steinbronn N, Gereke M, Strasser RH, Sparwasser T, Bruder D et al Fluorochrome‐based definition of naturally occurring Foxp3+ regulatory T cells of intra‐ and extrathymic origin. Eur J Immunol 2014; 44:3632–45. [DOI] [PubMed] [Google Scholar]
- 33. Verhagen J, Wraith DC. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol 2010; 185:7129; author reply 30. [DOI] [PubMed] [Google Scholar]
- 34. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 2012; 188:976–80. [DOI] [PubMed] [Google Scholar]
- 35. Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P et al Differences in expression level of Helios and Neuropilin‐1 do not distinguish Thymus‐derived from extrathymically‐induced CD4+Foxp3+ regulatory T cells. PLoS One 2015; 10:e0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ et al Oligodeoxynucleotides stabilize Helios‐expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood 2012; 119:2810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol 2013; 190:2001–8. [DOI] [PubMed] [Google Scholar]
- 38. Ayyoub M, Raffin C, Valmori D. Comment on “Helios− and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans”. J Immunol 2013; 190:4439–40. [DOI] [PubMed] [Google Scholar]
- 39. Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD et al Helios+ and Helios− Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol 2019; 49:398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sugita K, Hanakawa S, Honda T, Kondoh G, Miyachi Y, Kabashima K et al Generation of Helios reporter mice and an evaluation of the suppressive capacity of Helios+ regulatory T cells in vitro . Exp Dermatol 2015; 24:554–6. [DOI] [PubMed] [Google Scholar]
- 41. Beyer M, Thabet Y, Muller RU, Sadlon T, Classen S, Lahl K et al Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol 2011; 12:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kondo M, Tanaka Y, Kuwabara T, Naito T, Kohwi‐Shigematsu T, Watanabe A. SATB1 plays a critical role in establishment of immune tolerance. J Immunol 2016; 196:563–72. [DOI] [PubMed] [Google Scholar]
- 43. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA et al Foxp3‐dependent programme of regulatory T‐cell differentiation. Nature 2007; 445:771–5. [DOI] [PubMed] [Google Scholar]
- 45. Zemmour D, Zilionis R, Kiner E, Klein AM, Mathis D, Benoist C. Single‐cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat Immunol 2018; 19:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lathrop SK, Santacruz NA, Pham D, Luo JQ, Hsieh CS. Antigen‐specific peripheral shaping of the natural regulatory T cell population. J Exp Med 2008; 205:3105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong J, Obst R, Correia‐Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self‐peptides in regulatory and nonregulatory CD4+ T cells. J Immunol 2007; 178:7032–41. [DOI] [PubMed] [Google Scholar]
- 48. Hsieh CS, Liang YQ, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 2004; 21:267–77. [DOI] [PubMed] [Google Scholar]
- 49. Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxo3+CD4+CD25+ T cells. Immunity 2006; 25:249–59. [DOI] [PubMed] [Google Scholar]
- 50. Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B et al A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 2011; 35:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lord J, Chen J, Thirlby RC, Sherwood AM, Carlson CS. T‐cell receptor sequencing reveals the clonal diversity and overlap of colonic effector and FOXP3+ T cells in ulcerative colitis. Inflamm Bowel Dis 2015; 21:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC et al Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One 2012; 7:e34547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L et al Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol 2015; 195:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J et al Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U et al DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008; 38:1654–63. [DOI] [PubMed] [Google Scholar]
- 56. Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL‐2 controls the stability of Foxp3 expression in TGF‐β‐induced Foxp3+ T cells in vivo . J Immunol 2011; 186:6329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cai Q, Dierich A, Oulad‐Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. J Immunol 2009; 183:2303–11. [DOI] [PubMed] [Google Scholar]
- 58. Sebastian M, Lopez‐Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios controls a limited subset of regulatory T cell functions. J Immunol 2016; 196:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smigiel K, Richards E, Thomas K, Campbell D. CCR59 coordinates paracrine interleukin‐2 cross‐talk between central memory and regulatory T cells and controls the homeostatic balance between regulatory T cell subsets. J Immunol 2013; 190. [Google Scholar]
- 60. Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME et al Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015; 350:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ. Instability of Helios‐deficient Tregs is associated with conversion to a T‐effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci USA 2016; 113:6248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem 2007; 282:30227–38. [DOI] [PubMed] [Google Scholar]
- 63. Thomas RM, Chunder N, Chen CX, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol 2007; 179:7305–15. [DOI] [PubMed] [Google Scholar]
- 64. Pan F, Yu H, Dang EV, Barbi J, Pan XY, Grosso JF et al Eos mediates Foxp3‐dependent gene silencing in CD4+ regulatory T cells. Science 2009; 325:1142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baine I, Basu S, Ames R, Sellers RS, Macian F. Helios induces epigenetic silencing of IL2 gene expression in regulatory T cells. J Immunol 2013; 190:1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM et al Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 2012; 13:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fu WX, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS et al A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol 2012; 13:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD‐1 or NF‐κB. J Exp Med 2013; 210:269–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ross EM, Bourges D, Hogan TV, Gleeson PA, van Driel IR. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur J Immunol 2014; 44:2048–58. [DOI] [PubMed] [Google Scholar]
- 70. Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One 2011; 6:e24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R et al Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One 2011; 6:e20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Skadow M, Penna VR, Galant-Swafford J, Shevach EM, Thornton AM. Helios deficiency predisposes the differentiation of CD4+ Foxp3- T cells into peripherally derived regulatory T cells. J Immunol 2019; 203:370–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Narni‐Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A et al Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012; 335:344–8. [DOI] [PubMed] [Google Scholar]
- 74. Glasner A, Simic H, Miklic K, Roth Z, Berhani O, Khalaila I et al Expression, function, and molecular properties of the killer receptor Ncr1‐Noe. J Immunol 2015; 195:3959–69. [DOI] [PubMed] [Google Scholar]
- 75. Leeansyah E, Svard J, Dias J, Buggert M, Nystrom J, Quigley MF et al Arming of MAIT cell cytolytic antimicrobial activity is induced by IL‐7 and defective in HIV‐1 infection. PLoS Pathog 2015; 11:e1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci USA 2017; 114:E5434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dias J, Boulouis C, Gorin JB, van den Biggelaar R, Lal KG, Gibbs A et al The CD4−CD8− MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc Natl Acad Sci USA 2018; 115:E11513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Martin‐Ibanez R, Crespo E, Esgleas M, Urban N, Wang B, Waclaw R et al Helios transcription factor expression depends on Gsx2 and Dlx1&2 function in developing striatal matrix neurons. Stem Cells Dev 2012; 21:2239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Martin‐Ibanez R, Pardo M, Giralt A, Miguez A, Guardia I, Marion‐Poll L et al Helios expression coordinates the development of a subset of striatopallidal medium spiny neurons. Development 2017; 144:1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chessum L, Matern MS, Kelly MC, Johnson SL, Ogawa Y, Milon B et al Helios is a key transcriptional regulator of outer hair cell maturation. Nature 2018; 563:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yamashita T, Zheng F, Finkelstein D, Kellard Z, Robert C, Rosencrance CD et al High‐resolution transcriptional dissection of in vivo Atoh1‐mediated hair cell conversion in mature cochleae identifies Isl1 as a coreprogramming factor. PLoS Genet 2018; 14:e1007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim YC, Kim KK, Yoon J, Scott DW, Shevach EM. SAMHD1 posttranscriptionally controls the expression of Foxp3 and Helios in human T regulatory cells. J Immunol 2018; 201:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weiss JM, Bilate AM, Gobert M, Ding Y, de Lafaille MAC, Parkhurst CN et al Neuropilin 1 is expressed on thymus‐derived natural regulatory T cells, but not mucosa‐ generated induced Foxp3+ T reg cells. J Exp Med 2012; 209:1723–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yadav M, Louvet C, Davini D, Gardner JM, Martinez‐Llordella M, Bailey‐Bucktrout S et al Neuropilin‐1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo . J Exp Med 2012; 209:1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


