Abstract
Increasing evidence has indicated that long noncoding RNAs (lncRNAs) are involved in the tumorigenesis and progression of various types of cancer. The lncRNA deleted in lymphocytic leukemia 1 (DLEU1) has been reported to be dysregulated in cancer cells and thus associated with tumor development; however, the role of DLEU1 in renal cell carcinoma (RCC) remains unclear. In the present study, DLEU1 was knocked down using small interfering RNA in the RCC cell lines KETR3 and 786-O to determine the role of DLEU1. Cell Counting Kit-8, colony formation, Transwell and flow cytometry assays were performed to assess the effects of DLEU1 on cell proliferation, migration, invasion and apoptosis in KETR3 and 786-O cells. The protein expression levels of factors associated with apoptosis and epithelial-mesenchymal transition (EMT) were examined by western blot. The results demonstrated that silencing DLEU1 decreased the growth capacity, migration and invasion of KETR3 and 786-O cells. Additionally, loss of DLEU1 was observed to stimulate the mitochondrial pathway of cell apoptosis via regulation of the expression of Bcl-2/Bax, cleaved caspase-3 and cleaved caspase-9 in KETR3 and 786-O cells. Furthermore, DLEU1 knockdown significantly inhibited the protein kinase B (Akt) pathway by downregulating the expression of phosphorylated-Akt, cyclin D1 and P70S6 kinase. In addition, depletion of DLEU1 was observed to impair the process of EMT in RCC cells via the upregulation of E-cadherin, and downregulation of N-cadherin and vimentin. Collectively, these results indicated a pro-oncogenic role of DLEU1 in the progression and development of RCC via modulation of the Akt pathway and EMT phenotype.
Keywords: renal cell carcinoma, lncRNA DLEU1, Akt pathway, epithelial-mesenchymal transition
Introduction
Renal cell carcinoma (RCC), a urinary tract malignancy originating from the renal epithelium, is among the 10 most common types of cancer globally, accounting for ~100,000 deaths annually (1,2). Partial nephrectomy is generally used as an effective treatment for patients with early-stage RCC; however, ~1/3 of patients have already progressed to distant metastases at the time of initial diagnosis, resulting in a poor prognosis (3). The 5-year survival rate is only 10%, and the average survival time is <1 year (3–6). There are limited options for the treatment of patients with advanced or metastatic disease; therefore, it is necessary to identify novel and reliable biomarkers to target for the development of novel therapeutic strategies for RCC.
Long noncoding RNAs (lncRNAs), a group of noncoding RNAs >200 nucleotides in length, are involved in the regulation of gene expression in addition to diverse biological processes, including cell growth, migration, invasion and apoptosis (7–9). Increasing evidence has indicated that lncRNAs exert important functions in numerous diseases, including tumorigenesis and tumor progression via epigenetic, transcriptional and post-transcriptional approaches (10–12). For example, lnc-ATB has been reported to regulate the development of tumors (13–15). Lei et al (16) revealed that lnc-ATB is upregulated in gastric cancer and acts as a pro-oncogene in gastric cancer tumorigenesis via the regulation of a microRNA (miRNA/miR)-141-3p/transforming growth factor β2 feedback loop. lnc-ATB has also been reported to be involved in the growth and invasion of RCC cells (14). Additionally, there are numerous lncRNAs that are associated with the tumorigenesis and progression of RCC; for example, the lncRNA HOX transcript antisense RNA improves the migratory and invasive abilities of RCC cells in vitro by upregulating the histone H3K27 demethylase JMJD3 (5). Silencing DLX6 antisense RNA 1 inhibits the growth of RCC cells by regulating the miR-26a/PTEN axis (17).
The lncRNA deleted in lymphocytic leukemia 1 (DLEU1) is located on chromosome 13q14.3, and is frequently deleted in hematopoietic malignancies, including chronic lymphocytic leukemia and multiple myeloma (18–20). Conversely, DLEU1 has also been reported to be highly expressed in gastric cancer (21), breast cancer (22), epithelial ovarian carcinoma (23) and endometrial carcinoma (24), and is proposed to serve an oncogenic role in tumor progression. At present, to the best of our knowledge, no study has investigated the biological effects of DLEU1 on the progression and development of RCC. Therefore, in the present study, the role of DLEU1 in the tumorigenesis of RCC and the underlying mechanisms were investigated. The present findings suggested that knockdown of DLEU1 suppressed the growth, migration and invasion of RCC cells, and induced cell apoptosis. Additionally, the protein kinase B (Akt) pathway and epithelial-mesenchymal transition (EMT) were inhibited by knockdown of DLEU1. Collectively, this study indicated a pro-oncogenic role for DLEU1 in the progression of RCC via modulation of the Akt pathway and EMT.
Materials and methods
Cell culture and transfection
The human RCC cell lines KETR3 and 786-O were obtained from the Cell Bank of Chinese Academy of Sciences and cultured in DMEM (HyClone; GE Healthcare Life Sciences) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (penicillin, 100 U/ml; streptomycin, 0.1 mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2. In total, ~6×104 cells were cultured overnight until 60–70% convergence and cells were subsequently transfected with 50 nM small interfering RNA (siRNA/si)-DLEU1 (Oligobio) or scrambled siRNA [as negative control (NC); Oligobio] using Lipo6000 (Beyotime Institute of Biotechnology) and cultured for 6–8 h at 37°C. Subsequently, the medium containing the transfection reagent was removed and the cells were cultured in DMEM for 24–48 h before the next experiments. The sequence of siRNA-DLEU1 was 5′-CAACGGAAUGUAUCAAUGATT-3′, the sequence of siRNA-control was 5′-TTCTCCGAACGTGTCACGT-3′.
Reverse transcription-quantitative PCR (RT-qPCR)
A total of 24 h post-transfection, total RNA was isolated from transfected cells using an Ultrapure RNA kit (CoWin Biosciences Co., Ltd.) and reverse transcribed into cDNA using a HiFiScript cDNA Synthesis kit (CoWin Biosciences Co., Ltd.) according to the manufacturer's protocol. qPCR was performed with a SYBR Premix Ex Taq II kit (Takara Bio, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec. Data were analyzed according to the sample quantification cycle (Cq) value from three independent experiments. The relative gene expression was calculated using the 2−∆∆Cq method (25). Target gene expression was normalized to the expression of β-actin. The primer sequences were as follows: DLEU1, forward 5′-CCAGCCCACAGGCATTTAGT-3′, reverse, 5′-GTTCCGAGGCTTAAGTGCGA-3′; and β-actin, forward 5′-CCCGAGCCGTGTTTCCT-3′ and reverse, 5′-GTCCCAGTTGGTGACGATGC-3′.
Cell Counting kit-8 (CCK-8) assay
A CCK-8 (Beijing Solarbio Science & Technology, Co., Ltd.) assay was conducted to assess cell viability. Briefly, cells were seeded in each well of a 96-well plate at a density of 1×103 cells/well; after transfection for 0, 24, 48 and 72 h, cells were treated with CCK-8 reagent (10 µl) for an additional 1 h at 37°C. The optical density value of excitation was detected using a microplate reader (BioTek Instruments, Inc.) at a wavelength of 450 nm. The assay was conducted three times independently.
Colony forming assay
A total of 24 h post-transfection, cells were seeded in a 6-cm dish at a density of 200 cells/well and were cultured for ~1 week at 37°C in an atmosphere containing 5% CO2 until visible colonies were formed. The culture medium was removed, and 5 ml 4% paraformaldehyde was added to fix the colonies for 30 min at room temperature followed by staining with 0.1% crystal violet for 30 min at room temperature. The colonies were counted under a light microscope (Nikon Corporation, Inc.) and images were captured. The assay was conducted three times independently.
Transwell assay
Transwell chambers with 8-µm pore filters (EMD Millipore) coated with Matrigel (BD Biosciences) in a 24-well plate were used for the cell invasion assay; chambers were not coated with Matrigel for the migration assay. A total of 24 h post-transfection, cells were trypsinized and resuspended in serum-free culture medium at a concentration of 1×106 cells/ml. Subsequently, a 100- or 5-µl cell suspension was added to the upper compartment of Transwell chambers for the invasion and migration assays, respectively. Complete medium (600 µl) with 10% FBS was added to the bottom chamber to serve as a chemoattractant. After 48 h at 37°C, the residual cells on the upper surface were removed, and the cells that had invaded or migrated to the lower surface of the filter were fixed with 4% paraformaldehyde for 30 min at room temperature, followed by staining with 0.1% crystal violet for 20 min at room temperature. Images of the invaded and migrated cells were captured (magnification, ×40) and counted under a light microscope. The assay was conducted three times independently.
Apoptosis assay
Cells transfected with siRNA for 24 h were resuspended in 1X binding buffer [10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2] at a density of 1–5×106 cells/ml. Subsequently, the cell suspension (100 µl) was stained with 5 µl Annexin V-FITC (BD Biosciences) in the dark for 5 min at room temperature and incubated with 10 µl propidium iodide (PI) for 5 min at room temperature. The rate of apoptosis was analyzed by flow cytometry (BD FACSCanto II; BD Biosciences) and calculated using BD FACSDiva™ software (version 6.0; BD Biosciences). The assay was conducted three times independently.
Western blot analysis
After 48 h of transfection, cells were harvested and lysed using RIPA lysis buffer (CoWin Biosciences Co., Ltd.) at 4°C for protein extraction. A bicinchoninic assay kit (Beyotime Institute of Biotechnology) was used to measure the protein concentration according to the manufacturer's protocol. Proteins (20 µg/sample) were separated via 10% SDS-PAGE and were then transferred onto PVDF membranes (EMD Millipore), which were blocked with 5% non-fat milk for 1 h at room temperature, followed by incubation overnight at 4°C with primary antibodies (1:1,000). After washing with TBS-Tween-20 (20%), the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies anti-rabbit immunoglobulin G (IgG; 1:3,000; cat. no. SA00001-2; ProteinTech Group, Inc.) or anti-mouse IgG (1:3,000; cat. no. SA00001-1; ProteinTech Group, Inc.) for 1 h at room temperature. An enhanced chemiluminescence kit (CoWin Biosciences Co., Ltd.) was used for signal development, and the bands were analyzed using ImageJ software version 1.44 (National Institutes of Health). The primary antibodies used were as follows: Anti- Bcl-2 (cat. no. 60178-1-Ig; ProteinTech Group, Inc.), Bax (cat. no. 50599-2-Ig; ProteinTech Group, Inc.), total caspase-3 (cat. no. 66470-2-Ig; ProteinTech Group, Inc.), cleaved caspase-3 (cat. no. 25546-1-AP; ProteinTech Group, Inc.), total caspase-9 (cat. no.9502; Cell Signaling Technology, Inc.), cleaved caspase-9 (cat. no. 10380-1-AP; ProteinTech Group, Inc.), Akt (cat. no. 9272; Cell Signaling Technology, Inc.), phosphorylated (p)-Akt (cat. no. 66444-1-Ig; ProteinTech Group, Inc.), cyclin D1 (cat. no. 60186-1-Ig; ProteinTech Group, Inc.), P70S6K (cat. no. 14485-1-AP; ProteinTech Group, Inc.) and GAPDH (cat. no. cat. no. 60004-1-Ig; ProteinTech Group, Inc.). The assay was conducted three times independently.
Statistical analysis
SPSS 18.0 software (SPSS, Inc.) was used for statistical analysis. The data from triplicate experiments are presented as the means ± standard deviation. The difference between two groups was compared with Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
DLEU1 knockdown inhibits the viability and proliferation of RCC cells
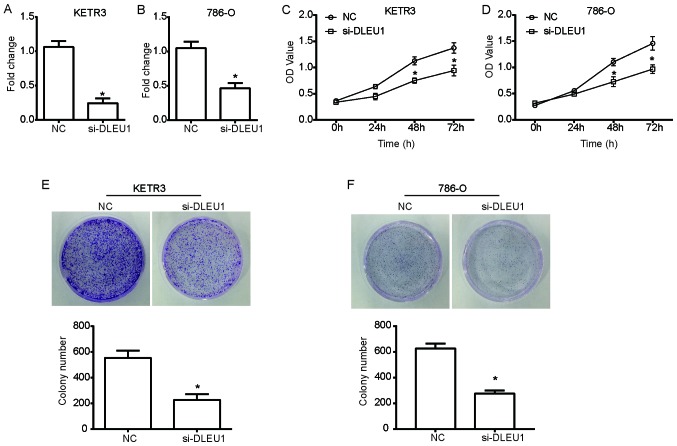
To investigate the functional role of DLEU1 in the growth of RCC cells, loss-of-function experiments were performed in the RCC cell lines KETR3 and 786-O using si-DLEU1; scrambled siRNA was used as the NC. As presented in Fig. 1A and B, the expression levels of DLEU1 were significantly downregulated in si-DLEU1-transfected cells compared with in NC cells (P<0.05). A CCK-8 assay was then performed using cells transfected with si-DLEU1. Notably, the growth curves of KETR3 cells demonstrated a significant reduction in the viability of si-DLEU1-transfected cells compared with NC cells (P<0.05; Fig. 1C). Additionally, 786-O cells transfected with si-DLEU1 exhibited a significant suppression of cell viability (P<0.05; Fig. 1D). Furthermore, a colony forming assay further revealed that DLEU1 knockdown significantly decreased the number of KETR3 and 786-O cell colonies compared with in the NC group (P<0.05; Fig. 1E and F), thus suggesting that DLEU1 knockdown inhibited the clonogenic ability of RCC cells.
Figure 1.
DLEU1 knockdown inhibits the viability and clonogenic ability of RCC cells. si-DLEU1 was transfected into the KETR3 and 786-O RCC cells; scrambled siRNA was used as the NC. A total of 24 h post-transfection, DLEU1 expression in (A) KETR3 and (B) 786-O cells was determined via reverse transcription-quantitative PCR analysis. Post-transfection, cell viability was determined in (C) KETR3 and (D) 786-O cells via a Cell Counting Kit-8 assay. The colony formation ability of (E) KETR3 and (F) 786-O cells was determined using a colony formation assay. Data are presented as the means ± standard deviation (n=3). Results were obtained from three independent experiments. *P<0.05 vs. NC. DLEU1, deleted in lymphocytic leukemia 1; NC, negative control; OD, optical density; RCC, renal cell carcinoma; si/siRNA, small interfering RNA.
DLEU1 knockdown inhibits the migratory and invasive abilities of RCC cells in vitro
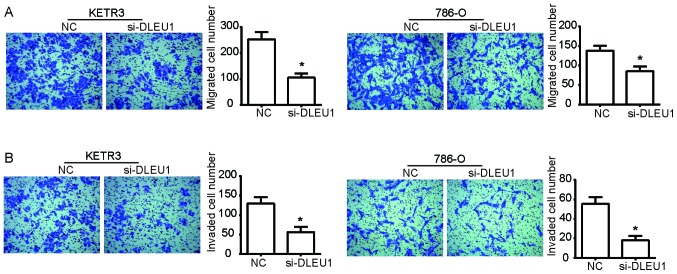
To determine the functional role of DLEU1 in the progression of RCC, Transwell assays were performed to detect the migration and invasion of KETR3 and 786-O cells. As presented in Fig. 2A, si-DLEU1 transfection significantly decreased the ability of KETR3 and 786-O cells to migrate into the lower chamber compared with NC cells (P<0.05). Additionally, compared with the NC, si-DLEU1-transfected cells exhibited a significant reduction in their ability to invade through the Matrigel into the lower chamber (P<0.05; Fig. 2B). Collectively, these data indicated that DLEU1 knockdown reduced the migratory and invasive abilities of RCC cells in vitro.
Figure 2.
DLEU1 knockdown inhibits the migratory and invasive abilities of renal cell carcinoma cells. Post-transfection for 24 h, a Transwell assay was employed to determine the effect of si-DLEU1 on (A) cell migration and (B) invasion in (left) KETR3 and (right) 786-O cells. Magnification, ×100. Data are presented as the means ± standard deviation (n=3). Results were obtained from three independent experiments. *P<0.05 vs. NC. DLEU1, deleted in lymphocytic leukemia 1; NC, negative control; si, small interfering RNA.
DLEU1 knockdown promotes apoptosis of RCC cells in vitro
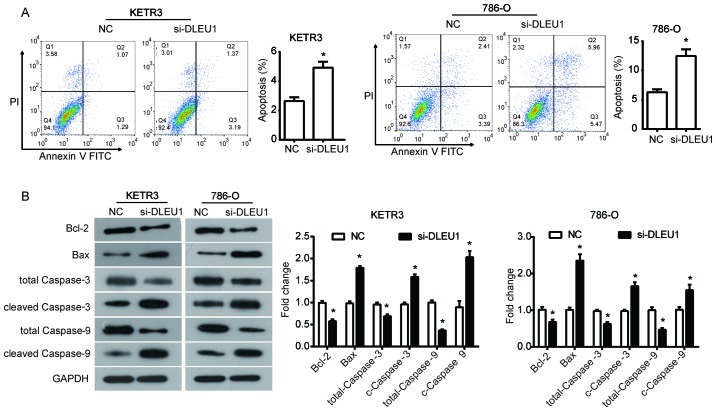
To further assess the effect of DLEU1 on the survival of RCC cells, cell apoptosis was evaluated using flow cytometry. It was demonstrated that DLEU1 knockdown significantly increased the percentage of apoptotic KETR3 cells compared with in the NC group (P<0.05; Fig. 3A). Similarly, silencing DLEU1 with siRNA significantly promoted apoptosis of 786-O cells (P<0.05; Fig. 3A). Subsequently, the expression of apoptosis-associated proteins was evaluated to further investigate the mechanisms underlying the increased apoptosis induced by the downregulation of DLEU1 in RCC cells. From the results of western blot analysis, it was revealed that DLEU1 downregulation significantly inhibited the expression of Bcl-2, total caspase-3 and total caspase-9, and increased the expression of Bax, cleaved caspase-3 and cleaved caspase-9 in KETR3 and 786-O cells compared with in the NC group (P<0.05; Fig. 3B). Collectively, these findings suggested that the increase in apoptosis induced by DLEU1 knockdown may be dependent on its regulation of the Bcl-2/Bax axis and caspase cascade in RCC cells.
Figure 3.
DLEU1 knockdown promotes the apoptosis of renal cell carcinoma cells. (A) Flow cytometric analyses were conducted to determine the effects of si-DLEU1 on cell apoptosis in (left) KETR3 and (right) 786-O cells. (B) Post-transfection for 48 h, western blot analysis of apoptosis-associated protein expression (Bcl-2, Bax, C-caspase-3 and C-caspase-9) was conducted in (left) KETR3 and (right) 786-O cells following knockdown of DLEU1. Data are presented as the means ± standard deviation (n=3). Results were obtained from three independent experiments. *P<0.05 vs. NC. C-caspase-3, cleaved caspase-3; C-caspase-9, cleaved caspase-9; DLEU1, deleted in lymphocytic leukemia 1; NC, negative control; PI, propidium iodide; si, small interfering RNA.
DLEU1 knockdown suppresses the Akt pathway and disrupts EMT in RCC cells
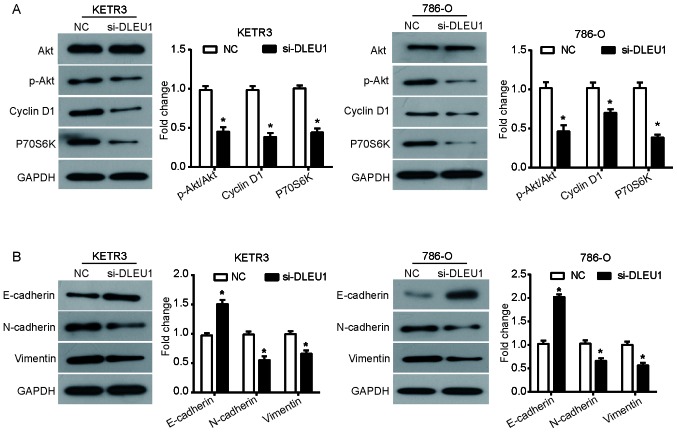
The phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway is an established pathway involved in the occurrence and development of cancer (26). To determine whether the Akt signaling pathway was involved in the function of DLEU1, the phosphorylation levels of Akt, and the expression of downstream proteins cyclin D1 and P70S6 kinase (P70S6K) were evaluated via western blot analysis. It was observed that silencing DLEU1 significantly reduced the phosphorylation levels of Akt in KETR3 and 786-O cells compared with the NC (P<0.05), but induced no notable effects on the expression of total Akt (Fig. 4A). Additionally, the expression levels of cyclin D1 and P70S6K, proteins involved in the cell cycle, were similarly downregulated in DLEU1 knockdown cells (P<0.05; Fig. 4A).
Figure 4.
DLEU1 knockdown inhibits the Akt pathway and disrupts EMT in renal cell carcinoma cells. (A) Following transfection for 48 h, western blot analysis was conducted to determine alterations in the expression of Akt pathway-associated proteins. (B) Alterations in the expression of marker proteins (E-cadherin, N-cadherin and vimentin) in the EMT process were detected by western blotting. Data are presented as the means ± standard deviation (n=3). Results were obtained from three independent experiments. *P<0.05 vs. NC. Akt, protein kinase B; DLEU1, deleted in lymphocytic leukemia 1; EMT, epithelial-mesenchymal transition; NC, negative control; p, phosphorylated; P70S6K, P70S6 kinase; si/siRNA, small interfering RNA.
Due to the critical role of EMT in promoting tumor cell migration and invasion, the expression of EMT marker proteins was further assessed to investigate whether EMT was involved in the function of DLEU1. It was revealed that si-DLEU1-transfected KETR3 cells demonstrated a significant increase in the expression of E-cadherin, an important marker of epithelial cells, with similar observations in 786-O cells (P<0.05; Fig. 4B). Furthermore, DLEU1 knockdown also induced a significant downregulation in the expression of N-cadherin and vimentin, which are important mesenchymal markers, in KETR3 and 786-O cells (P<0.05; Fig. 4B). Collectively, these results suggested that DLEU1 knockdown interfered with the mesenchymal properties of RCC cells.
Discussion
Increasing evidence has indicated that lncRNAs serve important roles in modulating a wide variety of fundamental biological processes by regulating gene expression, despite the fact that lncRNAs do not encode proteins (7–9). In view of their important regulatory functions, lncRNAs have been reported to be closely associated with tumorigenesis and metastasis (5). For example, the lncRNA metastatic renal cell carcinoma-associated transcript 1 has been reported to function as a prognostic biomarker and therapeutic target for clear cell RCC (ccRCC), as it promotes the metastasis of ccRCC (6). Therefore, further understanding of the association between lncRNAs and tumor progression may provide novel targets and therapeutic perspectives for tumor-targeted therapy (5,27). It has been demonstrated that there is recurrent deletion of DLEU1 in hematopoietic tumors, and that DLEU1 acts as a tumor suppressor gene via inhibition of the cell cycle and promotion of programmed cell death in Burkitt lymphoma (20). Recent studies have confirmed that DLEU1 is markedly upregulated in certain solid tumor tissues and cell lines, and serves as an oncogene to promote tumorigenesis in gastric cancer, ovarian carcinoma and endometrial carcinoma (21,23,24). Therefore, the present study aimed to investigate the function of DLEU1 in the progression of RCC. The present study, to the best of our knowledge, is the first to demonstrate that silencing DLEU1 in two RCC cell lines (KETR3 and 786-O cells) inhibited the viability, migration and invasion of RCC cells. This promoted the mitochondrial pathway of apoptosis by regulating the Bcl-2/Bax axis and caspase cascade, suggesting a pro-oncogenic role of DLEU1 in the progression and development of RCC in vitro. This is consistent with other findings from ovarian cancer (23).
To provide insight into the relevant mechanisms by which dysregulated DLEU1 contributes to cell proliferation, migration, invasion and apoptosis in RCC cells, potentially associated signaling pathways were further investigated. It is widely reported that the PI3K/Akt signaling pathway serves a pivotal role in the regulation of cellular processes, and tumor progression and development, including cell survival, apoptosis, cell cycle regulation and invasion (28). Additionally, the PI3K/Akt signaling pathway is frequently activated in cancer, and targeted inhibition of this pathway has become the focus for the treatment of various types of cancer, including RCC (29,30). Therefore, following silencing of DLEU1, alterations in the PI3K/Akt signaling pathway and the expression of important downstream proteins were determined, including cyclin D1 and P70S6K, which are key regulators in mediating the cell cycle and proliferation (31–33). In the present study, western blotting revealed a significant decrease in the expression of p-Akt, cyclin D1 and P70S6K following DLEU1 knockdown, which is consistent with results from endometrial cancer cells that DLEU1 knockdown reduces the expression of Akt1, mTOR and P70S6K (24). Based on these results, the Akt pathway may be involved in the mechanisms underlying the pro-oncogenic role of DLEU1 in RCC cells.
EMT is a crucial process that promotes cell invasion and enhances the metastatic potential of tumor cells (34). It has been demonstrated that decreased expression of E-cadherin, and increased expression of N-cadherin and vimentin in epithelial cells indicates that the tumors are susceptible to a metastatic phenotype (35,36). Therefore, in this study, it was further investigated as to whether DLEU1 affected the EMT process in RCC cells. It was revealed that knockdown of DLEU1 resulted in increased expression of E-cadherin, a classical epithelial cell marker, but reduced the expression of N-cadherin and vimentin, suggesting that DLEU1 may modulate EMT in RCC cells in vitro.
In conclusion, to the best of our knowledge, the present study is the first to report that DLEU1 may serve as an oncogene in the progression and development of RCC via modulation of the Akt pathway and EMT phenotype in RCC cells, thus indicating that DLEU1 may serve as a potential therapeutic target in RCC. Notably, lncRNAs usually function by binding to miRNAs or genes; therefore, the target genes of DLEU1 that are involved in the growth and survival of RCC cells will be investigated in the future, as will the role of DLEU1 in vivo.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Akt
protein kinase B
- lncRNA
long noncoding RNA
- RCC
renal cell carcinoma
Funding
The present study was supported by the Inner Mongolia Natural Science Foundation Project (grant. no. 2015MS08115), the Inner Mongolia Autonomous Region Health and Family Planning Research Project (grant. no. 201701066) and the Major Project of Affiliated Hospital of Inner Mongolia Medical University (grant. no. NYFY ZD 2014011).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GY, CC, YX and HC conceived and designed the experiments. GY, CC, LB, GW, YH, YW, HC and YX performed the experiments. GY, YX and HC contributed to the conception of the study and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JS, Kabbinavar F. Metastatic clear cell renal cell carcinoma: A review of current therapies and novel immunotherapies. Crit Rev Oncol Hematol. 2015;96:527–533. doi: 10.1016/j.critrevonc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Molina AM. Targeting renal cell carcinoma. J Clin Oncol. 2009;27:3274–3276. doi: 10.1200/JCO.2009.21.8461. [DOI] [PubMed] [Google Scholar]
- 5.Xia M, Yao L, Zhang Q, Wang F, Mei H, Guo X, Huang W. Long noncoding RNA HOTAIR promotes metastasis of renal cell carcinoma by up-regulating histone H3K27 demethylase JMJD3. Oncotarget. 2017;8:19795–19802. doi: 10.18632/oncotarget.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JK, Chen C, Liu JY, Shi JZ, Liu SP, Liu B, Wu DS, Fang ZY, Bao Y, Jiang MM, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16:111. doi: 10.1186/s12943-017-0681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 8.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Shi SJ, Wang LJ, Yu B, Li YH, Jin Y, Bai XZ. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6:11652–11663. doi: 10.18632/oncotarget.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong MS, Cai W, Yuan Y, Leong HC, Tan TZ, Mohammad A, You ML, Arfuso F, Goh BC, Warrier S, et al. ‘Lnc’-ing Wnt in female reproductive cancers: Therapeutic potential of long non-coding RNAs in Wnt signalling. Br J Pharmacol. 2017;174:4684–4700. doi: 10.1111/bph.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini ES, Meryet-Figuiere M, Sabzalipoor H, Kashani HH, Nikzad H, Asemi Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol Cancer. 2017;16:107. doi: 10.1186/s12943-017-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Li L, Xu H, Liu Y, Yang C, Cowley AW, Jr, Wang N, Liu P, Liang M. Characteristics of long non-coding RNAs in the Brown Norway rat and alterations in the Dahl salt-sensitive rat. Sci Rep. 2014;4:7146. doi: 10.1038/srep07146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iguchi T, Uchi R, Nambara S, Saito T, Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, et al. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015;35:1385–1388. [PubMed] [Google Scholar]
- 14.Xiong J, Liu Y, Jiang L, Zeng Y, Tang W. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol. 2016;46:378–384. doi: 10.1093/jjco/hyv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, Jiang X. LncRNA-ATB: An indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50 doi: 10.1111/cpr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei K, Liang X, Gao Y, Xu B, Xu Y, Li Y, Tao Y, Shi W, Liu J. Lnc-ATB contributes to gastric cancer growth through a MiR-141-3p/TGFβ2 feedback loop. Biochem Biophys Res Commun. 2017;484:514–521. doi: 10.1016/j.bbrc.2017.01.094. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Hu Z, Ke X, Tang H, Wu B, Wei X, Liu Z. Long noncoding RNA DLX6-AS1 promotes renal cell carcinoma progression via miR-26a/PTEN axis. Cell Cycle. 2017;16:2212–2219. doi: 10.1080/15384101.2017.1361072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garding A, Bhattacharya N, Claus R, Ruppel M, Tschuch C, Filarsky K, Idler I, Zucknick M, Caudron-Herger M, Oakes C, et al. Epigenetic upregulation of lncRNAs at 13q14.3 in leukemia is linked to the In Cis downregulation of a gene cluster that targets NF-κB. PLoS Genet. 2013;9:e1003373. doi: 10.1371/journal.pgen.1003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowd AA, Homeida S, Elkarem HA. Detection of chromosome 13 (13q14) deletion among Sudanese patients with multiple myeloma using a molecular genetics fluorescent in situ hybridization technique (FISH) Malays J Pathol. 2015;37:95–100. [PubMed] [Google Scholar]
- 20.Lee S, Luo W, Shah T, Yin C, O'Connell T, Chung TH, Perkins SL, Miles RR, Ayello J, Morris E, et al. The effects of DLEU1 gene expression in Burkitt lymphoma (BL): Potential mechanism of chemoimmunotherapy resistance in BL. Oncotarget. 2017;8:27839–27853. doi: 10.18632/oncotarget.15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Li Z, Liu Z, Xiao J, Yu S, Song Y. Long non-coding RNA DLEU1 predicts poor prognosis of gastric cancer and contributes to cell proliferation by epigenetically suppressing KLF2. Cancer Gene Therapy. 2018;25:58–67. doi: 10.1038/s41417-017-0007-9. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Guo L, Jiang F, Li L, Li Z, Chen F. Analysis of the miRNA-mRNA-lncRNA networks in ER+ and ER- breast cancer cell lines. J Cell Mol Med. 2015;19:2874–2887. doi: 10.1111/jcmm.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang LL, Sun KX, Wu DD, Xiu YL, Chen X, Chen S, Zong ZH, Sang XB, Liu Y, Zhao Y. DLEU1 contributes to ovarian carcinoma tumourigenesis and development by interacting with miR-490-3p and altering CDK1 expression. J Cell Mol Med. 2017;21:3055–3065. doi: 10.1111/jcmm.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Y, Wang L, Chen S, Liu Y, Zhao Y. lncRNA DLEU1 contributes to tumorigenesis and development of endometrial carcinoma by targeting mTOR. Mol Carcinog. 2018;57:1191–1200. doi: 10.1002/mc.22835. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol Res. 2009;39:177–186. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellinger J, Blondeau JJC, Deng M, Syring I, Schrödter S, Schmidt D, Perner S, Müller SC. 863 Identification and functional analysis of novel long non-coding RNAs in clear cell renal cell carcinoma. Eur Urol Suppl. 2015;14:e863. doi: 10.1016/S1569-9056(15)60851-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Re. 2013;19:5310–5319. doi: 10.1158/1078-0432.CCR-13-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, Ding Z. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42:343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335–350. doi: 10.1007/978-1-4939-7234-0_23. [DOI] [PubMed] [Google Scholar]
- 31.Diehl JA. Cycling to Cancer with Cyclin D1. Cancer Biol Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 32.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavares MR, Pavan IC, Amaral CL, Meneguello L, Luchessi AD, Simabuco FM. The S6K protein family in health and disease. Life Sci. 2015;131:1–10. doi: 10.1016/j.lfs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Bates RC, Mercurio A. The epithelial-mesenchymal tansition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 36.Mao XW, Xiao JQ, Xu G, Li ZY, Wu HF, Li Y, Zheng YC, Zhang N. CUL4B promotes bladder cancer metastasis and induces epithelial-to-mesenchymal transition by activating the Wnt/β-catenin signaling pathway. Oncotarget. 2017;8:77241–77253. doi: 10.18632/oncotarget.20455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.