Abstract
p53 and mouse double minute 2 homolog (MDM2) serve key regulatory roles in the apoptosis of synovial cells. The present study aimed to investigate the effects of electroacupuncture (EA) at the ‘Zusanli’ (ST36) and ‘Xuanzhong’ (GB39) acupoints on apoptosis in an adjuvant arthritis (AA) rat model. A total of 40 male Sprague-Dawley rats were randomly divided into Control, AA, AA + EA and AA + sham EA groups (n=10 rats in each group). Rats in all the groups, with the exception of the control group, were injected with Complete™ Freund's adjuvant into the bilateral hindlimb footpad to establish the AA model. Rats in the AA + EA group were treated with EA at the ST36 and GB39 acupoints. Rats in the AA + sham EA group were treated with percutaneous electrical stimulation at a position of 5 mm away from the ST36 and GB39 acupoints. The arthritis index scores and hindlimb paw volumes of the rats in each group were recorded. Subsequently, pathological changes in the synovial tissue were evaluated by hematoxylin and eosin (H&E) staining, and the apoptotic rate of the synovial cells was detected by TUNEL staining. In addition, the expression levels of the apoptosis-associated proteins, Bax, phorbol-12-myristate-13-acetate-induced protein 1 (Noxa) and p53 upregulated modulator of apoptosis (PUMA), were determined by western blot analysis. The expression of both the gene and protein of p53 and MDM2 in synovial tissue was detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis, respectively. The results indicated that the arthritis index scores and hindlimb paw volumes upon EA stimulation were significantly decreased compared with those of the AA group (P<0.05). H&E staining revealed that the synovial inflammation of EA stimulation was significantly decreased compared with the AA group (P<0.05). The TUNEL assay results indicated that the apoptotic rate of synovial cells in the AA + EA group was significantly increased compared with that in the AA group (P<0.05). Furthermore, an increased expression of proapoptotic proteins was confirmed by the increased expression levels of Bax, Noxa and PUMA in the AA + EA group. The results of RT-qPCR and western blot analysis demonstrated that, compared with the AA group, EA stimulation led to a marked increase in p53 (P<0.05) and a significant decrease in MDM2 (P<0.05) gene and protein expression. Taken together, these results demonstrated that EA performed on the ST36 and GB39 acupoints led to a significant amelioration in AA injury of model rats, by regulating the p53 signaling pathway and inducing apoptosis.
Keywords: electroacupuncture, adjuvant arthritis, synovial cells, apoptosis, p53 signaling pathway
Introduction
Rheumatoid arthritis (RA) is a clinically common, chronic, systemic autoimmune disease characterized by ‘tumor-like’ abnormal excessive proliferation of joint synovial cells, inflammatory cell infiltration, pannus formation, and cartilage and bone destruction (1–4). However, the precise mechanisms associated with the pathogenesis of RA have yet to be fully elucidated. With the deepening of research on the pathogenesis of RA, it has been revealed that an imbalance between cell proliferation of the joint synovial membrane and apoptosis is closely associated with the progression of RA (5–8). The tumor suppressor gene, p53, exerts a crucial role in the induction of cell apoptosis, and serves as the transcription factor for some anti- and pro-apoptotic proteins; namely, Bax, phorbol-12-myristate-13-acetate-induced protein 1 (Noxa), p53 upregulated modulator of apoptosis (PUMA) and mouse double minute 2 homolog (MDM2) (9–14). MDM2 is an important negative regulator of p53, which, upon combining with p53, inhibits apoptosis and stimulates cellular inflammation via forming a negative feedback regulatory loop, therefore functioning in a p53-dependent capacity (15,16). Bax, Noxa, and PUMA are pro-apoptotic proteins belonging to the B-cell lymphoma-2 (Bcl-2) protein family, and are closely associated with mitochondrial-dependent apoptosis, serving as major effectors of p53-mediated cell death (17–19). A previously published study suggested that the upregulated p53 gene in chondrocytes of patients with RA was closely associated with the apoptosis of RA cells (20). Overexpression of the p53 gene in the early stages of disease led to an increase in synovial fibroblast apoptosis, thereby alleviating progression of the disease in an adjuvant arthritis (AA) rat model (21). The expression of MDM2 in fibroblasts of patients with RA was significantly increased and positively correlated with the disease activity of RA, and inhibition of MDM2 effectively inhibited the inflammatory response of RA (16). Upregulation of Bax, Noxa and PUMA has been observed in the apoptosis model of RA fibroblast-like synoviocytes (22–26).
Clinical studies have confirmed that acupuncture has the potential to be an effective therapy for patients with RA via inhibiting the inflammatory reaction and autoimmunity (27–29). Animal experimental studies have also confirmed that electroacupuncture (EA) intervention may lead to a significant inhibition of infiltration of local inflammatory cells, a decrease in the expression of inflammatory cytokines, and the proliferation of synovial cells, thereby effectively hindering disease progression in an RA rat model (28,30–32). An additional previously published study disclosed that the regulation of apoptosis was involved in the protective effects of acupuncture (33). It has been demonstrated that acupuncture intervention is able to improve memory and cognitive function by means of inhibiting neuronal apoptosis via increases in the expression of p53 protein in the cerebral cortex of Alzheimer's disease model mice (34). However, knockout of the p53 gene in the midbrain dopamine neurons of Alzheimer's disease model mice was able to eliminate the protective effect of acupuncture (35). Furthermore, previous evidence has also suggested that EA protects rats from ischemic brain injury by inhibiting the protein level of MDM2 (36). EA stimulation was also able to inhibit cerebral ischemia (CI) injury-induced cell apoptosis of cerebral and myocardial tissues in CI rats, which was discussed as being potentially associated with the downregulation of Bax expression, and upregulation of Bcl-2 expression, in both myocardial and cerebral tissues (37). Furthermore, it has been revealed that EA improves learning and memory ability, and protects pyramidal cells from apoptosis, by blocking expression of p53 and Noxa in the hippocampal CA1 region of vascular dementia (VD) rats. Increasing expression levels of p53 and Noxa, therefore, have important roles in the pathogenesis of VD (38). Considered collectively, these results suggest that acupuncture may be able to treat certain diseases by regulating p53, Bax, Noxa, and MDM2. However, to the best of our knowledge, whether EA is able to suppress proliferation by regulating the p53 signaling pathway has not been previously investigated. In the present study, an AA rat model was established, and the molecular mechanism was investigated via the simultaneous EA of the acupoints ‘Zusanli’ (ST36) and ‘Xuanzhong’ (GB39).
Materials and methods
Animals and experimental design
The present study was approved by the Scientific Investigation Board of the Chengdu University of Traditional Chinese Medicine. All animal experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (39). A total of 40 7-week-old male Sprague-Dawley rats (weight, 170–180 g) were provided by Chengdu Dashuo Laboratory Animal Co., Ltd and housed under standard conditions of temperature and humidity (12: 12 h light: Dark cycle; indoor temperature, 23±3°C; humidity, 60±10%). In addition, rats had ad libitum access to standard rodent chow and water. After a 7 day period of acclimation, rats were randomly divided into four groups, with 10 rats/group: Control; AA; AA + EA; and AA + sham EA.
Experimental induction of AA in rats and EA treatment
With the exception of the control rats, all rats received an intradermal injection of 0.1 ml Complete™ Freund's adjuvant (CFA; cat. no. F5881; Merck KGaA) into both hind paws to induce AA. An equivalent volume of saline was administered to each rat in the control group by intradermal injection. On day 3 following AA induction, EA stimulation was performed with sterile metallic needles (Beijing Tianyuheng Technology Co., Ltd.) with a width of 0.25 mm and a length of 25 mm, which synchronously entered the ST36 (7 mm depth) and GB39 (3 mm depth) acupoints, as described previously (31). The pattern of stimulus frequency and duration was 2 Hz for 15 min using a commercial electric acupuncture apparatus (SDZ-II; Suzhou Medical Appliance Factory). EA treatment was administered every other day for 16 days. In the AA + sham EA group, similar EA procedures were performed; however, the needles were inserted into inappropriate acupoints (specifically, rats in the AA + sham EA group were treated with percutaneous electrical stimulation at a position of 5 mm away from the acupoints of ST36 and GB39).
Evaluation of development of arthritis
The arthritic index and foot swelling (i.e., measurement of the hindlimb paw volume) were measured on days 0, 3, 8, 13 and 18 following AA induction, as previously described (30,40). In brief, the polyarthritis severity was graded on a scale of 0–4, and scored as follows: 0, no swelling; 1, swelling of finger joints; 2, mild swelling of ankle or wrist joints; 3, severe inflammation of the entire paws; 4, paws with deformity or ankylosis. The foot swelling was determined by a volume drainage method using a plethysmograph apparatus (YLS-7A; Yiyan Sci, Ltd.).
Histopathological analysis
The rats were sacrificed on day 18 following AA induction by intraperitoneal injection with 200 mg/kg sodium pentobarbital. Following previous studies of histological analysis (41,42), the ankle joints were harvested and fixed in 4% paraformaldehyde for 48 h at 4°C, and then were subsequently decalcified using a commercial tissue decalcification reagent according to the manufacturer's protocol (cat. no. G1107; Wuhan Servicebio Technology Co., Ltd.) and embedded in paraffin. The tissues were then sliced into 4-µm thick sections. Following washing with xylene I and II for 20 min and dehydration with 100, 95, 80 and 70% ethanol for 5 min each, the sections were stained with hematoxylin for 30 min at 25°C, washed with water for 20 min, stained with eosin for 5 min at 25°C. Finally, images of the sections were captured under a light microscope (BX53; Olympus Corporation) at magnification, ×400. The stained sections were scored blindly by 2 investigators, as described previously (30).
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) staining
The effect of EA on apoptosis was determined using a TUNEL assay, according to the manufacturer's protocol (cat. no. ab15251; Roche Diagnostics). Briefly, following fixation in 4% paraformaldehyde for 48 h at 4°C, and after deparaffinization and dehydration, the ankle joint sections were treated with proteinase K (20 µg/ml) for 15 min at room temperature. Subsequently, equilibration buffer was applied for 10 sec, and the specimens were immersed and incubated for 1 h in working strength terminal deoxynucleotidyl transferase (TdT) enzyme solution at 37°C. Following incubation in stop/wash buffer for 10 min to terminate the reaction, sections were incubated for 30 min in working strength anti-digoxigenin conjugate at room temperature in the dark to visualize the DNA fragments. Proteinase K, equilibration buffer, and stop/wash buffer were all included in the TUNEL assay kit. Finally, the slides were counterstained with 0.5 µg/ml DAPI, and mounted in fluorescence mounting medium. Sections treated only with reaction buffer lacking the TdT enzyme were used as negative controls. To quantify the numbers of apoptotic cells, images of 6 randomly chosen high power fields/slide at magnification, ×200, were captured by a blinded examiner using a wide-field fluorescence microscope (Olympus BX53; Olympus Corporation). TUNEL+ nuclei were quantified by automatic counting using ImageJ software (v.1.47t; National Institutes of Health).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from synovial tissues using an RNA extraction kit (cat. no. G3013; Wuhan Servicebio Technology Co., Ltd). RNA was quantified using a NanoDrop™ 2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc.), and then immediately reverse-transcribed into cDNA using a cDNA synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocols. Subsequently, qPCR was performed with SYBR® Premix EX Taq™ II (Takara Biotechnology Co., Ltd.) on an ABI PRISM® 7900HT Sequence Detection system (Thermo Fisher Scientific, Inc.). The thermocycling protocol was as follows: Initial denaturation at 95°C for 10 min, followed by 45 cycles of 15 sec at 95°C (denaturation), 60 sec at 60°C (primer annealing) and 15 sec at 60°C (elongation), and a final extension step for 10 min at 72°C. The primer sequences used for qPCR are presented in Table I. GAPDH was used as the internal reference, and relative gene expression levels were calculated using the 2−ΔΔCq method (43).
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction in the present study.
| Gene | Primer sequences (5′-3′) |
|---|---|
| GAPDH | F: CTGGAGAAACCTGCCAAGTATG |
| R: GGTGGAAGAATGGGAGTTGCT | |
| p53 | F: GGAATCTTCTGGGACGGGA |
| R: CCACGGATCTTAAGGGTGAAAT | |
| MDM2 | F: AGGCAGAAGAAGGCTTAGATGTG |
| R: CGGCTGGGAATAGTCGTCAC |
MDM2, mouse double minute 2 homolog. F, forward; R, reverse.
Western blot analysis
As described previously (44), frozen rat ankle synovial tissues were homogenized in cold whole cell lysis buffer (cat. no. G2002; Wuhan Servicebio Technology Co., Ltd.) and subsequently centrifuged at 14,000 × g for 5 min at 4°C. Protein concentration in the supernatant was standardized using a protein concentration assay kit (cat. no. G2026; Wuhan Servicebio Technology Co., Ltd.). A total of 40 µg protein per lane was separated using 10% SDS-PAGE gels, and subsequently transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% bovine serum albumin (cat. no. G2013; Wuhan Servicebio Technology Co., Ltd.) for 2 h at 25°C, followed by incubation with primary antibodies against p53 (dilution, 1:1,000; cat. no. ab131442; Abcam), MDM2 (1:1,000; cat. no. ab38618; Abcam), PUMA (1:1,000; cat. no,. bs-1573R; BIOSS), Bax (1:500; cat. no. bs-0127M; BIOSS), NOXA (1:1,000; cat. no. bs-19322R; BIOSS), β-actin (1:3,000; cat. no. GB12001; Wuhan Servicebio Technology Co., Ltd.), and GAPDH [1:1,000; cat. no. 70-ab011-100; MultiSciences (Lianke) Biotech Co., Ltd.] overnight at 4°C. The following day, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibodies (1:5,000; cat. no. 70-GAR007; MultiSciences) for 2 h at 25°C. Finally, protein bands were developed using a Chemidoc™ XRS Imaging system (Bio-Rad Laboratories, Inc.). The bands of interest were quantified, and normalized against GAPDH using Image-Pro Plus v.6.0 software (Media Cybernetics, Inc.).
Statistical analysis
The data are presented as the mean ± standard deviation. Differences between groups were analyzed using one-way analysis of variance, followed by Tukey multiple comparison post-hoc tests. Statistical analyses were performed with SPSS 19.0 software (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
EA stimulation decreases the arthritis index scores of AA rats
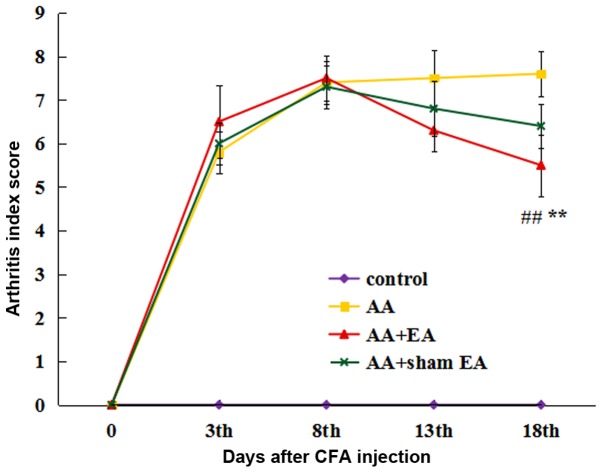
As presented in Fig. 1, the arthritis index scores in the AA, AA + EA and AA + sham EA groups were significantly increased on day 3 following AA induction (P<0.01), indicating that the AA model had been successfully established. During the course of the experiment, the arthritis index score in the AA group increased gradually. EA intervention significantly decreased the arthritis index score (P<0.01). However, no significant differences were identified between the AA + sham EA and AA groups (P>0.05).
Figure 1.
Effect of EA on the arthritic index in rats with AA. Data are expressed as the mean ± standard deviation (n=10). **P<0.01 vs. the control group; ##P<0.01 vs. the AA group. EA, electroacupuncture; AA, adjuvant arthritis.
EA stimulation inhibits foot swelling in AA rats
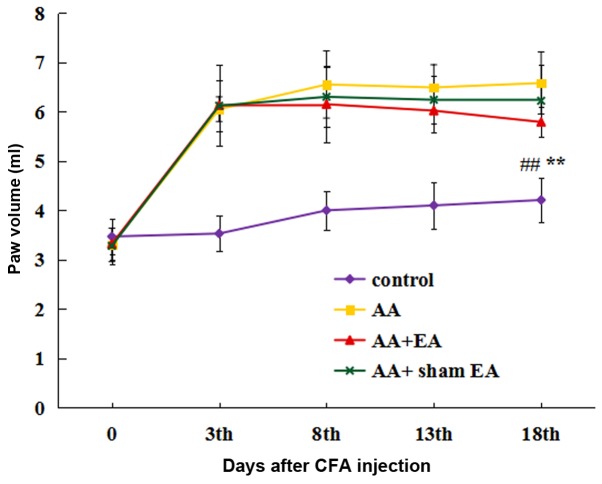
The foot swelling was measured by the volume drainage method (Fig. 2). Prior to initiation of the experiment, no significant differences in the size of foot swelling were observed between the groups. At 3 days after the injection of CFA, a significant increase in foot volume was observed in all groups, with the exception of the control group. After 8 sessions of EA stimulation, foot swelling in the AA + EA group was markedly alleviated compared with that in the AA group (P<0.01); by contrast, foot swelling in the AA + sham EA group was not decreased compared with that in the AA group (P>0.05).
Figure 2.
Effect of EA on foot swelling in rats with AA. Data are expressed as the mean ± standard deviation (n=10). **P<0.01 vs. the control group; ##P<0.01 vs. the AA group. EA, electroacupuncture; AA, adjuvant arthritis.
EA stimulation improves the pathological morphology of synovial tissue in AA rats
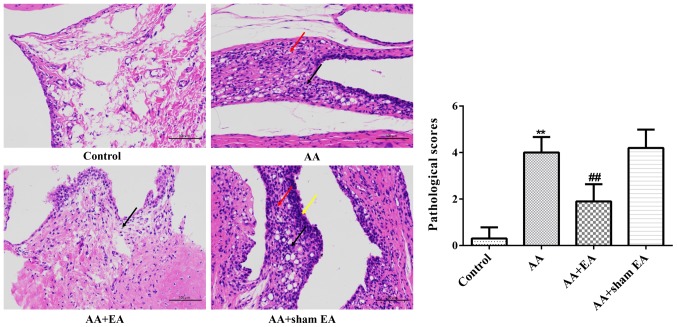
Histological analysis revealed that the inner-surface-layer synovial cells in the ankle joint of the control group were smooth and flat, and the cells were neatly arranged without any indications of inflammatory cell infiltration or synovial cell proliferation (Fig. 3). By contrast, the synovial tissue of the ankle joint was highly proliferated, with irregular cell arrangement, significant infiltration of inflammatory cells, increased new capillary development, pannus formation, and synovitis in the AA group. However, in the AA + EA group, the results of H&E staining appeared to be similar to those of the control group.
Figure 3.
Effects of EA on ankle joint damage in AA rats. Representative microphotographs of ankle joint sections from various groups (H&E stain, magnification, ×400). Data are expressed as the mean ± standard deviation (n=10). **P<0.01 vs. the control group; ##P<0.01 vs. the AA group. The red arrows indicate hyperplastic synoviocytes, the black arrows indicate infiltrating inflammatory cells, and the yellow arrows indicate hyperplastic synovial lining cells. EA, electroacupuncture; AA, adjuvant arthritis; H&E, hematoxylin and eosin.
EA stimulation increases the apoptotic rate of synovial cells in AA rats
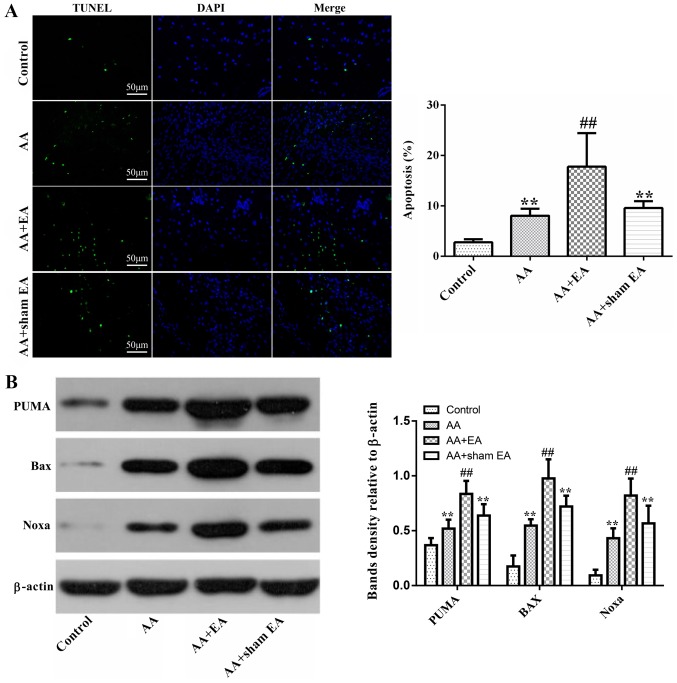
Subsequently, cell death was observed using a TUNEL assay. Compared with the control group, the percentage of TUNEL-positive cells increased markedly in the AA group (P<0.01; Fig. 4A). However, compared with the AA + sham EA and AA groups, EA stimulation led to a further, marked increase in the number of TUNEL-positive cells (P<0.01). Additionally, no significant difference in the apoptotic cell ratio was observed between the AA + sham EA and AA groups (P>0.05).
Figure 4.
Effects of EA on synovial cell apoptosis. (A) Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling staining of apoptotic cells in AA rats. (B) Expression of the proapoptotic proteins, Bax, Noxa and PUMA. Data are expressed as the mean ± standard deviation (n=10). **P<0.01 vs. the control group; ##P<0.01 vs. the AA group. EA, electroacupuncture; AA, adjuvant arthritis; Noxa, phorbol-12-myristate-13-acetate-induced protein 1; PUMA, p53 upregulated modulator of apoptosis.
Subsequently, expression levels of the pro-apoptosis--associated proteins, Bax, Noxa and PUMA, were detected by western blot analysis. As demonstrated in Fig. 4B, treatment of rats with AA resulted in increased Bax, Noxa and PUMA protein levels compared with the control group (P<0.01). EA stimulation caused an additional increase in Bax, Noxa and PUMA protein expression (P<0.01). However, compared with the AA group, sham acupuncture stimulation elicited no effect on Bax, Noxa and PUMA protein expression (P>0.05).
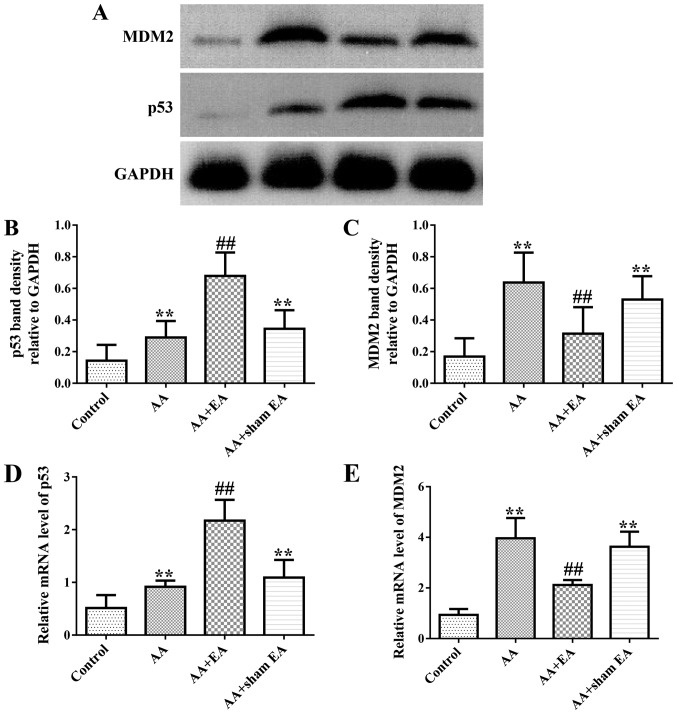
EA stimulation regulates the expression of p53 and MDM2 in synovial tissue of AA rats
As demonstrated in Fig. 5A-C, increased levels of p53 and MDM2 protein in synovial tissue were observed in the AA group compared with the control group (P<0.01). Additionally, EA stimulation resulted in an additional increase in p53 protein expression (P<0.01). However, by contrast, EA stimulation inhibited MDM2 protein expression (P<0.01). Consistently, sham acupuncture stimulation exerted no effect on p53 and MDM2 protein expression compared with the AA group (P>0.05). In addition, treatment of rats with AA led to significantly increased gene expression levels of the p53 and MDM2 genes (Fig. 5D and E) compared with the control group (P<0.01). However, increased and decreased expression mRNA levels of p53 and MDM2, respectively, were detected following EA stimulation (P<0.01). Compared with the AA group, sham acupuncture stimulation produced no effect on p53 and MDM2 gene expression (P>0.05).
Figure 5.
Effects of EA on the gene and protein expression of p53 and MDM2 in synovial tissues of the AA rats. (A) Typical examples of p53 and MDM2 protein expressions in synovial tissues from various groups. (B and C) Semi-quantitative statistical analysis of the relative p53 and MDM2 protein values. (D and E) Semi-quantitative statistical analysis of the p53 and MDM2 mRNA levels. Data are expressed as the mean ± standard deviation (n=10). **P<0.01 vs. the control group; ##P<0.01 vs. the AA group. EA, electroacupuncture; AA, adjuvant arthritis; MDM2, mouse double minute 2 homolog.
Discussion
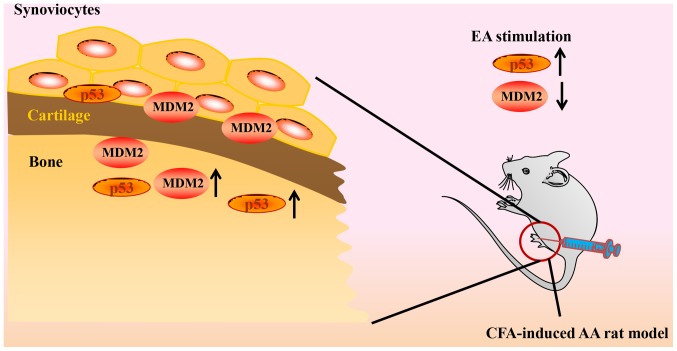
Neovascularization and synovial cell proliferation are two key factors in the progression of RA. Our recently published article focused on the inhibitory effect of electroacupuncture (EA) on synovial neovascularization in RA rats. The results of this study suggested that EA intervention may inhibit neovascularization by downregulating the expression of HIF-1α and VEGF (45). The present study aimed to investigate the effect of EA on the abnormal proliferation of synovial cells in RA rats. The present study has demonstrated that EA is able to effectively inhibit arthritis in AA rats, promote synovial cell apoptosis, increase the expression levels of p53 and its downstream factors, and decrease the expression of MDM2, a negative regulator of p53 (Fig. 6).
Figure 6.
Proposed model demonstrating how EA alleviates AA rats. EA, electroacupuncture; AA, adjuvant arthritis; MDM2, mouse double minute 2 homolog; CFA, Complete™ Freund's adjuvant.
Synovial cell proliferation is a hallmark pathology of RA, which is largely due to the abnormal proliferation and insufficient apoptosis of fibroblast-like synoviocytes of RA, which exhibit ‘tumor-cell-like’ properties, including unlimited proliferation, resistance to cell death, and aggressive invasion and migration (3,7,46,47). Previous studies have suggested that the abnormal apoptosis of synovial cells is associated with RA, and decreased levels of apoptosis may lead to excessive proliferation of synovial tissue, thus promoting the deterioration of RA (5,6,48). Previous studies have demonstrated that inducing apoptosis of synovial cells effectively leads to an inhibition of cellular proliferation (49,50). An additional study revealed that acupuncture therapy may be involved in the regulation of apoptosis, and that the underlying mechanism is predominantly governed by regulating apoptosis-associated genes, cytokines and other intracellular small molecules, including p53, MDM2, Bax, Noxa and PUMA (51).
One previous study identified that fibroblast-like synoviocytes (FLS) obtained from AA rats significantly proliferated and exhibited increased levels of pro-inflammatory cytokines TNF-α and IL-β compared with the control group. Concomitantly, the apoptotic rate of FLS was significantly decreased, the expression level of anti-apoptotic protein Bcl-2 was upregulated, and the relative expression of Bax was decreased. A potential mechanism is that the mitochondrial apoptotic pathway of AA-FLS is inhibited and exhibits anti-apoptotic properties, while arthritic symptoms are manifested (52). A second study indicated that increasing the apoptotic rate of synovial cells in AA rats may significantly improve the symptoms of arthritis. Its mechanism may be associated with decreases in Bcl-2 and increases in Bax expression levels, and increases in caspase-3 activity (53).
In the present study, AA rats were selected as a model of RA, which is the most commonly used animal model (54,55). The results indicated that, compared with the control group, after 72 h, the toes and ankles of RA rats possessed different degrees of swelling, deformity and increased arthritis index scores. The results of the H&E staining of synovial tissue revealed abnormal proliferation of synovial cells, connective tissue and blood capillaries. Furthermore, a large number of inflammatory cells had infiltrated the synovium. Concomitantly, the proportion of TUNEL-positive cells increased markedly in the AA group. These pathological changes in the AA rat model were consistent both with the symptoms of patients with clinical RA and with pathological lesions in previously described experimental animal models (32,56).
Previous studies have suggested that p53, a tumor suppressor factor, fulfills a crucial role in mitochondrial-dependent apoptosis (12,13). MDM2 is a key negative regulator of p53, affecting the transcriptional activity and stability of p53, and thereby forms a negative feedback pathway with p53 (15,16). Previous studies have indicated that in wild-type cells, p53 expression is maintained at a very low level under normal physiological conditions. However, in the state of cellular stress including hypoxia and DNA damage, p53 is stabilized and accumulated. In addition, enhanced transcriptional activity of p53 may increase the expression of MDM2, a downstream regulator of p53 (57–59). In accordance with previous studies (31,60), in the present study, EA performed on the ST36 and GB39 acupoints resulted in an inhibition of the proliferation of synovial cells and joint inflammation in rats. Notably, the results of the present study also indicated that EA stimulation led to a marked increase in synovial cell apoptosis, as evidenced by intensive TUNEL-positive staining. Furthermore, compared with the AA group, the protein and gene expression levels of p53 were demonstrated to increase in the synovial cells of AA rats, whereas the protein and gene expression levels of MDM2 were inhibited.
Previous data have suggested that increased levels of p53-induced apoptosis occur in fibroblasts of AA rat synovial tissue during increasing and chronic arthritis (16,21,31,61–65). Additionally, inhibited expression of the p53 gene in synovial fibroblasts may lead to upregulation of inflammatory factors in CD4+ T lymphocytes of patients with RA, including interleukin-17 and interferon-γ (66). Taken together, an accumulation of the p53 gene product may alleviate RA via inhibiting the inflammatory factor-induced subsequent spread of inflammatory reactions, and inducing apoptosis in synovial tissue. Zhang et al (16) demonstrated that the expression of MDM2 protein was significantly increased in fibroblast-like synovial cells of patients with RA, and that this was positively correlated with the disease activity of RA. In addition, inhibition of MDM2 protein expression may serve an anti-inflammatory role by regulating the mitogen-activated protein kinase and NF-κB signaling pathways in collagen-induced arthritic mice (67).
Previous studies have indicated that local hypoxia of synovial joints caused by RA may activate the p53-mediated mitochondrial apoptotic pathway (18,19). It has been suggested that, as a downstream effector of the Bcl-2 protein family, p53 fulfills a crucial role in mitochondrial-dependent apoptosis, a process that results in abnormal expression of Bax, Noxa and PUMA (12–14). Noxa may inhibit the expression of anti-apoptotic Bcl-2 family members, while PUMA increases the expression and conformational changes of Bax, which in turn contributes to pore formation (13,17,68–70). The results of the present study indicated that compared with the AA group, EA intervention may increase the expression of Bax, Noxa and PUMA proteins in the downstream regulatory genes of p53.
The aforementioned results of the present study suggested that EA was able to effectively treat RA disease by regulating the expression of p53 and MDM2, and promoting further expression of the proapoptotic proteins, Bax, Noxa and PUMA, data that were consistent with previous studies (16,20,21).
Prior clinical studies have suggested that the effects of acupuncture or EA intervention at acupoints are markedly improved compared with those of sham acupuncture intervention at non-acupoints near to acupoints (71–73). In an animal experiment on the anti-inflammatory effects of EA, pretreatment of the Hegu acupoint led to an improvement in the survival rate of rats with lethal endotoxemia, whereas acupuncture at non-acupoints, located on the ulna side of the metacarpus, failed to elicit a similar effect (74). These results also suggested that the anti-inflammatory effects of EA on acupoints is markedly improved compared with that of non-acupoints. The results of the present study also confirmed that sham EA stimulation exerted no effect on the arthritis scores, pathological lesions of synovial joints, apoptosis, or gene or protein expression of p53 and its downstream factors including Bax, Noxa, PUMA, or MDM2, compared with the AA group; these data were consistent with previous studies (6,21,26).
In conclusion, the results from the present study have demonstrated that EA stimulation of the ST36 and GB39 acupoints exhibited potential therapeutic effects on AA rats. Furthermore, the underlying mechanisms may be associated with increases in the expression of the p53 signaling pathway, thereby inducing apoptosis in synovial tissue. However, further investigations are required to determine the precise mechanisms underlying activation of the p53 signaling pathway by EA.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National Natural Science Foundation of China (grant nos. 81704152 and 81503599) and the project of the Science and Technology Department in Sichuan province (grant no. 2019YJ0491).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JZ designed and performed the experiments, and evaluated the data and reviewed the manuscript. CS performed the experiments and wrote the manuscript. YuzC, QL, HL and XL also performed the experiments. YZ, YunC and LL analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Scientific Investigation Board of the Chengdu University of Traditional Chinese Medicine. All animal experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Matsui T, Nakata N, Nagai S, Nakatani A, Takahashi M, Momose T, Ohtomo K, Koyasu S. Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J Nucl Med. 2009;50:920–926. doi: 10.2967/jnumed.108.060103. [DOI] [PubMed] [Google Scholar]
- 3.Bartok B, Firestein GS. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Wang X, Lv T, Liu J, Ren Y, Zhang J, Zhang Y. Effects of fhrelin on the apoptosis of rheumatoid arthritis fibroblast-like synoviocyte MH7A cells. Biol Pharm Bull. 2019;42:158–163. doi: 10.1248/bpb.b18-00285. [DOI] [PubMed] [Google Scholar]
- 5.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–588. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Chou CT, Yang JS, Lee MR. Apoptosis in rheumatoid arthritis-expression of Fas, Fas-L, p53, and Bcl-2 in rheumatoid synovial tissues. J Pathol. 2001;193:110–116. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH746>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Hou YN, Guo LH. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Chin J Cell Biol. 2009;31:157e162. [Google Scholar]
- 8.Li R, Cai L, Tang WJ, Lei C, Hu CM, Yu F. Apoptotic effect of geniposide on fibroblast-like synoviocytes in rats with adjuvant-induced arthritis via inhibiting ERK signal pathway in vitro. Inflflammation. 2016;39:30–38. doi: 10.1007/s10753-015-0219-9. [DOI] [PubMed] [Google Scholar]
- 9.Aupperle KR, Boyle DL, Hendrix M, Seftor EA, Zvaifler NJ, Barbosa M, Firestein GS. Regulation of synoviocyte proliferation, apoptosis, and invasion by the p53 tumor suppressor gene. Am J Pathol. 1998;152:1091–1098. [PMC free article] [PubMed] [Google Scholar]
- 10.Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: How oxidative stress, might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82. doi: 10.1016/S0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamanishi Y, Boyle DL, Rosengren S, Green DR, Zvaifler NJ, Firestein GS. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 2002;99:10025–10030. doi: 10.1073/pnas.152333199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doroshevskaya AY, Kondratovskii PM, Dubikov AI, Eliseikina MG. Apoptosis regulator proteins: Basis for the development of innovation strategies for the treatment of rheumatoid arthritis in patients of different age. Bull Exp Biol Med. 2014;156:377–380. doi: 10.1007/s10517-014-2353-z. [DOI] [PubMed] [Google Scholar]
- 13.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/S1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 14.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 15.Thomasova D, Mulay SR, Bruns H, Anders HJ. p53-Independent roles of MDM2 in NF-κB signaling: Implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia. 2012;14:1097–1101. doi: 10.1593/neo.121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Luo J, Wen H, Zhang T, Zuo X, Li X. MDM2 promotes rheumatoid arthritis via activation of MAPK and NF-κB. Int Immunopharmacol. 2016;30:69–73. doi: 10.1016/j.intimp.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 18.Bouvard V, Zaitchouk T, Vacher M, Duthu A, Canivet M, Choisy-Rossi C, Nieruchalski M, May E. Tissue and cell-specific expression of the p53-target genes: Bax, Fas, MDM2 and Waf1/p21, before and following ionising irradiation in mice. Oncogene. 2000;19:649–660. doi: 10.1038/sj.onc.1203366. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, Aaronson SA, Lee SW. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Hase H, Kojima H, Saotome K, Nishioka K, Kobata T. Central role of mitochondria and p53 in Fas-mediated apoptosis of rheumatoid synovial fibroblasts. Rheumatology (Oxford) 2004;43:277–285. doi: 10.1093/rheumatology/keh039. [DOI] [PubMed] [Google Scholar]
- 21.Tak PP, Klapwijk MS, Broersen SF, van de Geest DA, Overbeek M, Firestein GS. Apoptosis and 53 expression in rat adjuvant arthritis. Arthritis Res. 2000;2:229–235. doi: 10.1186/ar92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, He N, Shen L, Liu M. Apoptotic effect of Aralia echinocaulis extract on fibroblast-like synoviocytes in rats with adjuvant-induced arthritis via inhibiting the Akt/Hif-1α signaling pathway in vitro. J Pharmacol Sci. 2019;139:340–345. doi: 10.1016/j.jphs.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Cha HS, Rosengren S, Boyle DL, Firestein GS. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:587–592. doi: 10.1002/art.21631. [DOI] [PubMed] [Google Scholar]
- 24.Mazumder S, Choudhary GS, Al-Harbi S, Almasan A. Mcl-1 phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res. 2012;72:3069–3079. doi: 10.1158/0008-5472.CAN-11-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idrus E, Nakashima T, Wang L, Hayashi M, Okamoto K, Kodama T, Tanaka N, Taniguchi T, Takayanagi H. The role of the BH3-only protein Noxa in bone homeostasis. Biochem Biophys Res Commun. 2011;410:620–625. doi: 10.1016/j.bbrc.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 26.Cottier KE, Fogle EM, Fox DA, Ahmed S. Noxa in rheumatic diseases: Present understanding and future impact. Rheumatology (Oxford) 2014;53:1539–1546. doi: 10.1093/rheumatology/ket408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He G, Wu Z, Wang Q, et al. Therapeutic observation of electroacupuncture plus electromagnetic therapy for rheumatoid arthritis. Shanghai J Acupuncture Moxibustion. 2014;33:247–250. (In Chinese) [Google Scholar]
- 28.Ouyang BS, Gao J, Che JL, Zhang Y, Li J, Yang HZ, Hu TY, Yang M, Wu YJ, Ji LL. Effect of Electro-acupuncture on tumor necrosis Factor-α and vascular endothelial growth factor in peripheral blood and Joint synovia of patients with rheumatoid arthritis. Chin J Integr Med. 2011;17:505–509. doi: 10.1007/s11655-011-0783-2. [DOI] [PubMed] [Google Scholar]
- 29.Chou PC, Chu HY. Clinical efficacy of acupuncture on rheumatoid arthritis and associated mechanisms: A systemic review. Evid Based Complement Alternat Med. 2018;2018:8596918. doi: 10.1155/2018/8596918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He TF, Yang WJ, Zhang SH, Zhang CY, Li LB, Chen YF. Electroacupuncture inhibits inflammation reaction by upregulating vasoactive intestinal peptide in rats with adjuvant-induced arthritis. Evid Based Complement Alternat Med. 2011;2011:1–8. doi: 10.1093/ecam/nep095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Chen XY, Li LB, Yu XT, Zhou Y, Yang WJ, Liu Z, Zhao N, Fu C, Zhang SH, Chen YF. Electroacupuncture attenuates collagen-induced arthritis in rats through vasoactive intestinal peptide signalling-dependent re-establishment of the regulatory T cell/T-helper 17 cell balance. Acupunct Med. 2015;33:305–311. doi: 10.1136/acupmed-2014-010732. [DOI] [PubMed] [Google Scholar]
- 32.Dong ZQ, Zhu J, Lu DZ, Chen Q, Xu YL. Effect of electroacupuncture in ‘Zusanli’ and ‘Kunlun’ acupoints on TLR4 signaling pathway of adjuvant arthritis rats. Am J Ther. 2018;25:e314–e319. doi: 10.1097/MJT.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Wu Q, Yang M, Deng S, Bai L, Chen L, Liang L. Research review of the action mechanism of acupuncture based on cell apoptosis. Shanghai J Acupuncture Moxibustion. 2016;35:1143–1146. (In Chinese) [Google Scholar]
- 34.Liu J, Zhang Y, Sun J, Xu S, Zhang X. Effect of acupuncture on the p53 Protein expression of mice with Alzheimer's disease. Chin J Integrated Traditional Western Med. 2013;33:1367–1371. (In Chinese) [PubMed] [Google Scholar]
- 35.Park JY, Choi H, Baek S, Jang J, Lee A, Jeon S, Kim J, Park HJ. p53 signalling mediates acupuncture-induced neuroprotection in Parkinson's disease. Biochem Biophys Res Commun. 2015;460:772–779. doi: 10.1016/j.bbrc.2015.03.105. [DOI] [PubMed] [Google Scholar]
- 36.An N, Xu T, Sun Q, Zhang H. Research on effect of electroacupuncture on MDM2 expression in hypoxic-ischemic encephalopathy rat model. Chin Arch Traditional Chin Med. 2016;34:289–292. (In Chinese) [Google Scholar]
- 37.Shi WY, Yan J, Chang XR, Lou BD, Li JX, Huang J, Lin H, Wang C, Zhang W. Observation of electroacupuncture intervention on cell apoptosis and Bax and Bcl-2 expression in cerebral and myocardial tissues in cerebral ischemia rats based on ‘heart-brain correlation’ theory. Zhen Ci Yan Jiu. 2019;44:107–112. doi: 10.13702/j.1000-0607.170332. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Zeng Y. Electroacupuncture protected pyramidal cells in hippocampal CA1 region of vascular dementia rats by inhibiting the expression of P53 and Noxa. CNS Neurosci Ther. 2011;17:599–604. doi: 10.1111/j.1755-5949.2010.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalev M. APHIS, FDA, and NIH issue memorandum of understanding on laboratory animal welfare. Lab Anim (NY) 2006;15:13. doi: 10.1038/laban0906-13b. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Xie G, Song S, Huang P, Wu Y, Wang Q, Chang Y, Zhang Y, Zhou A, Liu L, et al. Clinical manifestations and the main evaluation method on adjuvant-induced arthritis model in rats. Chin J Immunol. 2012;28:453–457. (In Chinese) [Google Scholar]
- 41.Ai X, Hou Y, Wang X, Wang X, Liang Y, Zhu Z, Wang P, Zeng Y, Li X, Lai X, et al. Amelioration of dry eye syndrome in db/db mice with diabetes mellitus by treatment with Tibetan Medicine Formula Jikan mingmu drops. J Ethnopharmacol. 2019;241:111992. doi: 10.1016/j.jep.2019.111992. [DOI] [PubMed] [Google Scholar]
- 42.Hou Y, Wang X, Chen X, Zhang J, Ai X, Liang Y, Yu Y, Zhang Y, Meng X, Kuang T, Hu Y. Establishment and evaluation of a simulated high-altitude hypoxic brain injury model in SD rats. Mol Med Rep. 2019;19:2758–2766. doi: 10.3892/mmr.2019.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Hou Y, Li Q, Li X, Wang W, Ai X, Kuang T, Chen X, Zhang Y, Zhang J, et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J Ethnopharmacol. 2019;241:111801. doi: 10.1016/j.jep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, Su CG, Chen YZ, Hao XY, Jiang JZ. Electroacupuncture on ST36 and GB39 acupoints inhibits synovial angiogenesis via downregulating HIF-1α/VEGF expression in a rat model of adjuvant arthritis. Evid Based Complement Alternat Med. 2019;2019:5741931. doi: 10.1155/2019/5741931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller-Ladner U, Pap T. Pathogenesis of RA: More than just immune cells. Z Rheumatol. 2005;64:396–401. doi: 10.1007/s00393-005-0772-y. (In German) [DOI] [PubMed] [Google Scholar]
- 47.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baier A, Meineckel I, Gay S, Pap T. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:274–279. doi: 10.1097/00002281-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Peng C, Luo L, Hu L, Wu Z, Cai R, Hao F, Hu W, Hong J. Study on the effect of moxibustion in treating rhreumatoid arthritis rats and its mechanism. J Acupuncture Tuina Sci. 2012;10:336–341. doi: 10.1007/s11726-012-0632-7. (In Chinese) [DOI] [Google Scholar]
- 50.Zhang C, Cai R, Tang Z. Influences of moxibustion on inflammatory factors and synoviocytes in rats with rheumatoid arthritis. J Beijing Univ Traditional Chin Med. 2014;37:190–194. (In Chinese) [Google Scholar]
- 51.Chen L, Wu QF, Yang MX, et al. Research review of the action mechanism of acupuncture based on cell apoptosis. Shanghai J Acupuncture Moxibustion. 2016;35:1143–1146. (In Chinese) [Google Scholar]
- 52.Gao W, Deng Q, Bai S, Tong L. Establishment and characteristic analysis of fibroblast-like synoviocytes in rats with adjuvant arthritis. Chin Pharmacol Bulletin. 2015;12:1693–1698. [Google Scholar]
- 53.Meng Q, Du X, Wang H, Gu H, Zhan J, Zhou Z. Astragalus polysaccharides inhibits cell growth and pro-inflammatory response in IL-1β-stimulated fibroblast-like synoviocytes by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Apoptosis. 2017;22:1138–1146. doi: 10.1007/s10495-017-1387-x. [DOI] [PubMed] [Google Scholar]
- 54.Dekkers JS, Schoones JW, Huizinga TW, Toes RE, van der Helm-van Mil AH. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: A systematic literature review and meta-analysis. Ann Rheum Dis. 2017;76:458–467. doi: 10.1136/annrheumdis-2016-209830. [DOI] [PubMed] [Google Scholar]
- 55.Taşçi Bozbaş G, Yilmaz M, Paşaoğlu E, Gürer G, Ivgin R, Demirci B. Effect of ozone in Freund's complete adjuvant-induced arthritis. Arch Rheumatol. 2018;33:137–142. doi: 10.5606/ArchRheumatol.2018.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 57.Ren XY, Liu QX, Wang HY. p53 and cell death. Chin J Bioch Mol Biol. 2018;34:588–594. [Google Scholar]
- 58.Zhen D, Zhao F, Song H. Progress of polysaccharides regulating p53 signal network. Chin J Cell Biol. 2017;39:1234–1242. [Google Scholar]
- 59.Peng L, Liu J, Xie X, et al. The research progress of p53 regulating the balance of tumor and aging. Chem Life. 2017;37:515–520. (In Chinese) [Google Scholar]
- 60.Zhu J, Chen X, Li L, Zhou Y, Bing X, Chen Y. Experimental study on vasoactive intestinal peptide mediated by electroacupuncture for the treatment of collagen-induced arthritis in rats. Shanghai J Traditional Chin Med. 2015;49:72–76. (In Chinese) [Google Scholar]
- 61.Lassus P, Bertrand C, Zugasti O, Chambon JP, Soussi T, Mathieu-Mahul D, Hibner U. Anti-apoptotic activity of p53 maps to the COOH-terminal domain and is retained in a highly oncogenic natural mutant. Oncogene. 1999;18:4699–4709. doi: 10.1038/sj.onc.1202841. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima T, Aono H, Hasunuma T, Yamamoto K, Shirai T, Hirohata K, Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- 63.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi T, Okamoto K, Kobata T, Hasunuma T, Nishioka K. Apomodulation as a novel therapeutic concept for the regulation of apoptosis in rheumatoid synoviocytes. Curr Opin Rheumatol. 1999;11:188–193. doi: 10.1097/00002281-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Yao Q, Wang S, Glorioso JC, Evans CH, Robbins PD, Ghivizzani SC, Oligino TJ. Gene transfer of p53 to arthritic joints stimulates synovial apoptosis and inhibits inflammation. Mol Ther. 2001;3:901–910. doi: 10.1006/mthe.2001.0343. [DOI] [PubMed] [Google Scholar]
- 66.Tang B, You X, Zhao L, Zhang T, Zhang L, Zhang X, Tang F. The effect of p53 expression in fibroblast-like synoviocytes on CD4+ T lymphocytes in patients with rheumatoid arthritis. Chin J Rheumatol. 2009;13:587–591. (In Chinese) [Google Scholar]
- 67.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 68.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 69.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 70.Liu FT, Newland AC, Jia L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem Biophys Res Commun. 2003;310:956–962. doi: 10.1016/j.bbrc.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 71.Seca S, Patrício M, Kirch S, Franconi G, Cabrita AS, Greten HJ. Effectiveness of acupuncture on pain, functional disability, and quality of life in rheumatoid arthritis of the hand: Results of a double-blind randomized clinical trial. J Altern Complement Med. 2019;25:86–97. doi: 10.1089/acm.2018.0297. [DOI] [PubMed] [Google Scholar]
- 72.Liu Z, Liu Y, Xu H, He L, Chen Y, Fu L, Li N, Lu Y, Su T, Sun J, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: A randomized clinical trial. JAMA. 2017;317:2493–2501. doi: 10.1001/jama.2017.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z, Yan S, Wu J, He L, Li N, Dong G, Fang J, Fu W, Fu L, Sun J, et al. Acupuncture for chronic severe functional constipation: A randomized trial. Ann Intern Med. 2016;165:761–769. doi: 10.7326/M15-3118. [DOI] [PubMed] [Google Scholar]
- 74.Song JG, Li HH, Cao YF, Lv X, Zhang P, Li YS, Zheng YJ, Li Q, Yin PH, Song SL, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology. 2012;116:406–414. doi: 10.1097/ALN.0b013e3182426ebd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.