Abstract
Intracranial aneurysm (IA) is a cerebrovascular disease with a high mortality rate. The pathogenesis of IA remains unclear and the treatment limited. The purpose of the present study was to identify the key genes expressed in IAs and provide the basis for further research and treatment. The raw dataset GSE75436 was downloaded from Gene Expression Omnibus, including 15 IA samples and 15 matched superficial temporal artery (STA) samples. Then, differentially expressed genes (DEGs) were identified using the limma package in R software. Hierarchical clustering analysis was performed on the DEGs using the gplot2 package in R. Database for Annotation, Visualization, and Integrated Discovery (DAVID) online tools were used to perform gene ontology (GO) functional enrichment analysis. DAVID and gene set enrichment analysis were separately used to perform the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. The intersections of the two results were selected as common KEGG pathways. Protein-protein interaction (PPI) analysis among the DEGs involved in the common KEGG pathways was performed using Search Tool for the Retrieval of Interacting Genes online tools, and visualized with Cytoscape software. A total of 782 DEGs were identified, comprising 392 upregulated and 390 downregulated DEGs. Hierarchical clustering demonstrated that the DEGs could precisely distinguish the IAs from the STAs. The GO enrichment analysis demonstrated that the upregulated DEGs were mainly involved in the inflammatory response and the management of extracellular matrix, and the downregulated DEGs were mainly involved in the process of vascular smooth muscle contraction. The KEGG pathway enrichment analysis demonstrated that the common pathways were ‘leishmaniasis’, ‘Toll-like receptor signaling pathway’ and ‘vascular smooth muscle contraction’. In the PPI network, tumor necrosis factor (TNF), interleukin 8 and Toll-like receptor 4 had the highest degrees; they were associated with the inflammatory response. The Toll-like receptor signaling pathway and TNF gene may serve as targets for future research and treatment.
Keywords: IA, TNF, Toll-like receptor 4, Toll-like receptor signaling pathway, Leishmania infection
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is a severe cerebrovascular disease with high morbidity and mortality (1). Although improvements in treatment strategies have reduced SAH mortality >50% over the past 20 years, the incidence of SAH has not notably decreased (2). It has been demonstrated that 85% of spontaneous SAH is caused by intracranial aneurysm (IA) (3). For IAs, particularly unruptured IAs, the current treatment methods are few, and since the balance of risks and benefits varies greatly for each patient, choices of treatment methods are also difficult to determine (4).
The incidence of IAs worldwide is 3–5% (1), and its exact pathogenesis remains unclear. There are numerous risk factors for IA, including genetic factors, cardiovascular risk factors (including hypertension, smoking and alcohol), hemodynamics and inflammation of local vascular smooth muscle cells (VSMCs) and endothelial cells (5). Genome wide association studies have demonstrated that single nucleotide polymorphisms of SRY-box 17 and cyclin dependent kinase inhibitor 2A can increase the risk of IAs (6). Hypertension and smoking also have a synergistic effect on the occurrence of IAs (7). High and low blood vessel shear force can promote the production of IAs, but the mechanisms between these differ (8). Almost all the influencing factors cause inflammation, thus inflammation performs a decisive role in the occurrence of IAs. Inflammatory factors eventually cause an imbalance in the repair and destruction of the extracellular matrix, resulting in the formation of IAs (9).
Gene expression microarrays can analyze tissue samples to obtain high-throughput gene expression data, and find significant differences in gene expression within the aneurysm tissue as a whole. The gene expression microarray is an important approach for the study of the pathogenesis of IA. The National Center of Biotechnology Information Gene Expression Omnibus (GEO) database provides a large number of gene expression data, which facilitates the reanalysis of gene expression data by researchers (10).
Wei et al (11) analyzed Japanese IA data and identified that CD40, CD40 ligand, and microRNA-125b may be potential therapeutic targets for IA. Bo et al (12) also analyzed sample data from three IAs and three STAs, and identified that one cut homeobox 1, hepatocyte nuclear factor 4α and E2F transcription factor 4 are important genes in the formation of IAs. The present study analyzed the dataset GSE75436, which has a large number of samples from Chinese patients (13).
GSE75436, submitted on November 27th 2015 by Wang et al (13), comprises gene expression chip data that have been analyzed with microarray technology using the Affymetrix Human Genome U133 plus 2.0 Array GPL570 platforms (Thermo Fisher Scientific, Inc.), including 30 samples. The aneurysm samples were taken from 15 patients diagnosed with saccular cerebral aneurysms who underwent microsurgical clipping, and the control group samples (STAs) were from pterional craniotomies and lateral frontal craniotomies. From a general perspective, there was no significant difference in gender, age, hypertension, smoking and alcohol consumption between the aneurysm group and the control group. However, as Wang et al (13) was a clinical trial, a perfect match between the two groups was not easily achieved. In addition, and despite the rigorousness of the study, gene GO enrichment analysis, KEGG enrichment analysis and analysis of the interactions among genes were not completed.
By analyzing the gene expression data of GSE75436, differentially expressed genes were identified, GO enrichment analysis conducted, and the Database for Annotation, Visualization, and Integrated Discovery (DAVID) and gene set enrichment analysis (GSEA) used for enrichment analysis of the KEGG pathways, prior to the construction of protein-protein interaction (PPI) networks for the genes in the common KEGG pathways enriched according to the two approaches. Key genes in the pathogenesis of IA were identified by the present study, which will provide a new insight into the treatment of IA.
Materials and methods
Microarray data and data preprocessing
The gene expression microarray raw data of GSE75436 was downloaded from the GEO (http://www.ncbi.nlm.nih.gov/geo/) database. Since the present study used gene expression data downloaded from a public database, no patient consent or ethics committee approval were necessary. The raw data were preprocessed by utilizing R software (version 3.4.4; http://www.R-project.org/). First, the relative logarithm expression (RLE) box plot and the normalized unscaled standard errors (NUSE) box plot methods were used to perform quality control on the raw chip data, and chips with abnormal expression values were rejected. The robust multichip average (RMA) normalization method justrma was then used to transform the gene expression values in all selected chips to a comparable level through background correction, normalization and aggregation. The justrma function in the affy package (http://bioconductor.org/packages/affy/) only standardizes the data of the perfectly matched type probes. The gene annotation file was downloaded from the official website (https://www.thermofisher.com/cn/en/home/life-science/microarray-analysis/microarray-data-analysis/genechip-array-annotation-files.html). Probes without gene annotation or with multiple gene symbols were rejected. Next, the average expression values of multiple probe IDs which matched one official gene symbol were calculated, and these values taken to represent the expression intensity of the corresponding gene symbol. The effect of the RMA standardization method was compared by using RLE boxplots and signal intensity profiles.
Differentially expressed gene (DEG) screening
By using the ggplot2 package (http://ggplot2.org) in R, a volcano map was plotted to assess overall gene differential expression. Then, the limma package was used to select the DEGs. The empirical Bayes moderated t-test method was used to calculate the P-values of genes. Then, Benjamini and Hochberg's method (14) was used to calculate the adjusted P-values [the false discovery rate (FDR)]. Only the genes with |log2 fold change (FC)|>2 and FDR<0.01 were considered to be DEGs. Finally, the DEGs were divided into upregulated and downregulated DEGs.
Heatmap and hierarchical clustering analysis
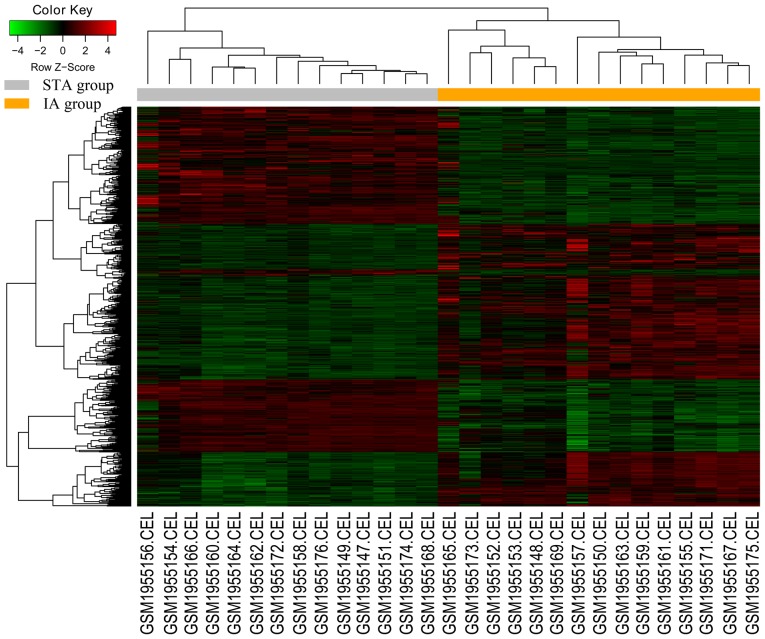
The package gplot 2 in R was used to plot the heatmap and perform the hierarchical clustering analysis. The heatmap facilitated the comparison of the overall expression of DEGs in the IA and STA groups. Hierarchical clustering can be used to group similar elements in a binary tree, and is extensively used for microarray data analysis.
GO enrichment analysis of DEGs
Functional enrichment analysis of DEGs was performed using DAVID online tools (version DAVID 6.8; http://david.ncifcrf.gov/). The GO terms were classified into three categories: Biological process (BP); cellular component (CC); and molecular function (MF). The upregulated DEGs and downregulated DEGs were entered separately and P<0.01 was considered to indicate a statistically significant difference.
KEGG pathway enrichment analysis
KEGG pathway enrichment analysis was separately performed using DAVID online tools and the GSEA tools (java GSEA Desktop Application version 3.0; http://software.broadinstitute.org/gsea/downloads.jsp). Then, the common pathways from the two methods were selected for the subsequent analysis.
For the DEGs, KEGG pathway enrichment was performed using DAVID online tools. The upregulated DEGs and downregulated DEGs were entered separately; P<0.01 and FDR <0.01 were selected as the cutoff criteria to identify the enriched pathways.
For the GSEA KEGG pathway enrichment analysis of DEGs, the RMA normalized gene expression data were entered and the Kolmogorov-Smirnov test mean of the DEGs was computed in each KEGG pathway using a permutation test with 1,000 replications. The significantly enriched pathways were defined by nominal P<0.01 and FDR<0.25.
As GSEA is a comprehensive analysis of all genes, the amount of information obtained is more complete; and DAVID allows for pathway analysis of DEGs with more significant differential expression levels, so the results of the analysis are more comprehensive. The intersection of the two may prove valuable for further research. A Venn diagram in InteractiVenn (http://www.interactivenn.net/) was used to show the common enriched KEGG pathways between the DAVID and GSEA analyses.
Construction of PPI network
The PPI networks of DEGs in the present study were constructed using the Search Tool for the Retrieval of Interacting Genes (STRING; version 10.5; http://string-db.org/cgi/input.pl) database. STRING is an online tool for predicting PPI information, and can provide a system-wide view of cellular processes. The DEGs involved in the common KEGG pathways of the DAVID and GSEA analysis methods were imported. A confidence score >0.7 was used as the cut-off criterion. Then, Cytoscape software (version 3.5.1; http://www.cytoscape.org/) was used to construct the PPI network.
Results
Microarray data and data preprocessing
The raw data of GSE75436 were downloaded from GEO, which had 15 IA samples and 15 STA samples. The RLE and NUSE boxplots indicated that the sample GSM1955170 in the STA group had abnormal expression values, so it was removed. Following RMA normalization, all chip data reached comparable levels.
Identification of DEGs
A total of 15 IA samples and 14 STA samples in the GSE75436 dataset were analyzed. The volcano plot in Fig. 1 demonstrates an overview of the differential expression of all genes, where the threshold in the volcano plot was -log10 adjusted P>2 (adjusted P<0.01) and |log2 FC|>2; the red plots indicate the upregulated genes and the green plots indicate the downregulated genes. Then, based on the cut-off criteria (adjusted P<0.01 and |log2 FC|>2), a total of 782 differentially expressed genes (DEGs) were identified, including 392 upregulated and 390 downregulated DEGs.
Figure 1.
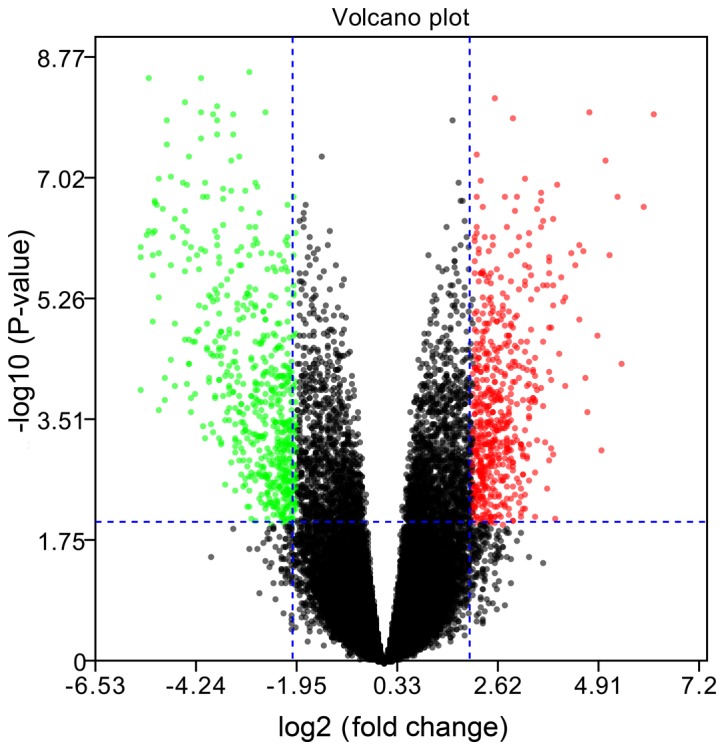
Volcano plot demonstrating an overview of the differential expression of all genes. The threshold in the volcano plot was -log10 adjusted P>2 and |log2 fold change| >2; red indicates upregulated genes, and green indicates downregulated genes.
There are currently two approaches to the analysis of DEGs. The first is a combination analysis of all the DEGs; the advantage of combining them is an analysis from a larger scale perspective. The second is to analyze the DEGs separately; the separate analysis emphasizes whether the pathway is activated or suppressed. It is suggested that DEGs divided into upregulated and downregulated groups are more suitable for the interpretation of the latter pathway analysis. Therefore, the separate analysis method was used. In addition, when PPI analysis was conducted, the upregulated genes and downregulated genes were analyzed as a whole, which can also reflect some of the overall interactions between genes. The DEG expression heatmap is presented in Fig. 2, and the hierarchical cluster analysis of the data demonstrated that the DEGs could be used to precisely distinguish IA samples from STA samples.
Figure 2.
Heatmap and the hierarchical cluster analysis of the differentially expressed genes. Red represents the upregulated genes and green represents the downregulated genes. STA, superficial temporal artery; IA, intracranial aneurysm.
GO enrichment analysis
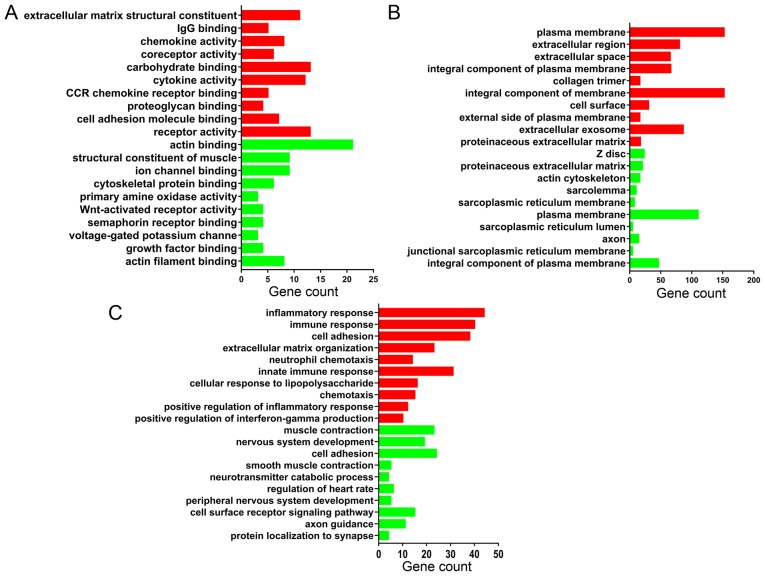
Functional enrichment analysis of DEGs was performed using DAVID. The DEGs were categorized into three functional groups: MF, CC and BP. The GO analysis results in Fig. 3 are the top 10 GO terms of upregulated and downregulated DEGs sorted by P-value.
Figure 3.
Gene ontology annotation. The top 10 upregulated (red) and 10 downregulated (green) terms in each category, (A) molecular function, (B) cellular component and (C) biological process, are shown, sorted by P-value.
According to the MF analysis, the upregulated DEGs were mainly associated with the construction of extracellular matrix components and the activation of inflammatory cytokines; downregulated DEGs were mainly associated with the synthesis of muscle contraction-related proteins and cytoskeletal proteins. The detailed results are shown in Fig. 3A.
For the CC analysis, the upregulated DEGs were mainly located in the plasma membrane and extracellular space; downregulated DEGs were mainly enriched in the muscle cells structures, as z disc, actin cytoskeleton, sarcolemma and sarcoplasmic reticulum membrane. The detailed results are shown in Fig. 3B.
In the BP, upregulated DEGs were mainly associated with infection processes, inflammatory responses and extracellular matrix organization, and downregulated DEGs were mainly associated with vascular smooth muscle contraction. The detailed results are shown in Fig. 3C.
In general, the results demonstrated that the upregulated genes were mainly involved in infection, inflammatory responses and the management of the extracellular matrix, and the downregulated genes were mainly involved in the process of muscle contraction.
Pathway enrichment analysis
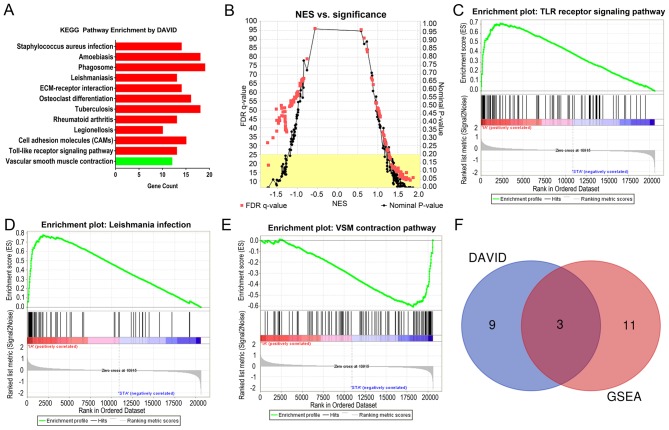
The results of the KEGG pathway analysis using the DAVID online tools are demonstrated in Fig. 4A. For the upregulated DEGs, 11 KEGG pathways were enriched according to P<0.01 and FDR<0.01. For the downregulated DEGs, only one KEGG pathways were enriched according to the criteria of P<0.01 and FDR<0.01.
Figure 4.
KEGG enrichment analysis. (A) The KEGG enrichment analysis results generated using DAVID. The upregulated pathways are in red and the downregulated pathway is in green. (B) The KEGG enrichment analysis results generated using GSEA. The red points represent the FDR q-values and the black points represent the nominal P-values. Detailed results of the three common KEGG pathways are shown: (C) TLR receptor signaling pathway; (D) Leishmania infection; and (E) VSM contraction. (F) Venn plot of the two methods. KEGG, Kyoto Encyclopedia of Genes and Genomes; DAVID, Database for Annotation Visualization and Integrated Discovery; GSEA, gene set enrichment analysis; NES, normalized enrichment score; TLR, Toll-like receptor; VSM, vascular smooth muscle.
The upregulation pathway was mainly associated with pathways related to various microbial infections, inflammatory reactions and autoimmune diseases. The downregulated pathway was ‘vascular smooth muscle contraction’.
The KEGG pathway GSEA results are demonstrated in Table I and Fig. 4B-E. According to the criteria of nominal P<0.01 and FDR<0.25, there were 13 upregulated pathways and only one downregulated pathway. Table I demonstrates the detailed results of the 14 KEGG pathways.
Table I.
KEGG enrichment analysis results by GSEA.
| Pathway name | Gene number of pathway | ES | NES | NOM P-value | FDR q-value |
|---|---|---|---|---|---|
| Vascular smooth muscle contraction | 110 | −0.608 | −1.726 | 0 | 0.188 |
| Vibrio cholerae infection | 50 | 0.566 | 1.879 | 0 | 0.119 |
| Toll like receptor signaling | 98 | 0.709 | 1.713 | 0 | 0.147 |
| Glycosphingolipid biosynthesis lacto and neolacto series | 26 | 0.603 | 1.712 | 0 | 0.119 |
| Cytosolic DNA sensing | 51 | 0.625 | 1.703 | 0 | 0.107 |
| N-glycan biosynthesis | 42 | 0.660 | 1.813 | 0.002 | 0.105 |
| Amino sugar and nucleotide sugar metabolism | 44 | 0.629 | 1.775 | 0.002 | 0.111 |
| Leishmania infection | 62 | 0.779 | 1.554 | 0.004 | 0.127 |
| Intestinal immune network for IgA production | 45 | 0.757 | 1.572 | 0.006 | 0.145 |
| Colorectal cancer | 61 | 0.438 | 1.486 | 0.006 | 0.151 |
| Rig I like receptor signaling | 65 | 0.536 | 1.675 | 0.008 | 0.119 |
| P53 signaling | 64 | 0.547 | 1.573 | 0.008 | 0.153 |
| Chronic myeloid leukemia | 72 | 0.392 | 1.504 | 0.008 | 0.167 |
| Natural killer cell mediated cytotoxicity | 129 | 0.635 | 1.621 | 0.010 | 0.127 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, gene set enrichment analysis; ES, enrichment score; NES, normalized enrichment score; NOM, nominal; FDR, false discovery rate.
Upregulated pathways were mainly associated with inflammatory infections, and the downregulated pathway was primarily responsible for the contraction of vascular smooth muscles.
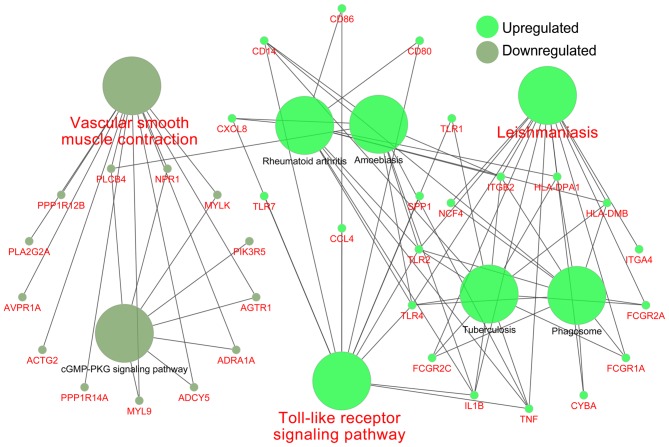
The two KEGG pathway analyses identified 11 significantly upregulated and one significantly downregulated pathway from DAVID, and 13 significantly upregulated and one significantly downregulated pathway from GSEA. All the KEGG pathways were compared using both techniques, and this identified two common upregulated pathways and one common downregulated pathway, as demonstrated in the Venn plot in Fig. 4F. The upregulated pathways were ‘Toll-like receptor signaling pathway’ and the ‘leishmaniasis’; the downregulated pathway was ‘vascular smooth muscle contraction’. Fig. 5 shows the relationship between these three important pathways and related pathways and genes, and it illustrates that the TNF, IL1B, TLR2 and TLR4 genes were involved in the Leishmania infection pathways and TLR signaling pathways.
Figure 5.
Associations between important pathways and genes (names in red). The green circles represent upregulated pathways and genes, and the gray circles represent downregulated pathways and genes.
PPI network analysis
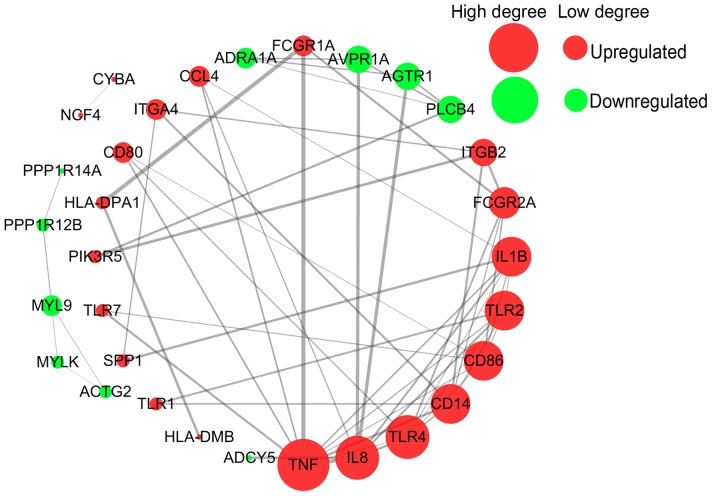
All 34 DEGs in the three common pathways were collected to perform the PPI analysis using STRING. The 34 DEGs were submitted to the STRING database to predict the protein interactions. When the interaction score was >0.7, the PPI network can be formed of 33 nodes and 57 edges. The gene Fc fragment of IgG receptor IIc could not be identified in the STRING database. The PPI network is presented in Fig. 6 As can be observed, the majority of DEGs were upregulated genes. The key points of the network include the upregulated genes, such as tumor necrosis factor (TNF; degree=10), interleukin (IL) 8 (degree=8), Toll-like receptor (TLR) 4 (degree=8), and the downregulated genes, such as PLCB4 (degree=4), AGTR1 (degree=4) and AVPR1A (degree=4).
Figure 6.
Protein-protein interaction network analysis of 34 differentially expressed genes in the three common pathways using the Search Tool for the Retrieval of Interacting Genes online tool. The upregulated genes are represented by red circles, downregulated genes are represented by green circles, and the size of the circles represents the degree value.
Discussion
In the present study, a total of 782 DEGs were identified, including 392 upregulated and 390 downregulated DEGs. GO enrichment analysis demonstrated that the upregulated genes were mainly located in the plasma membrane and extracellular space, being involved in receptor activation and the composition of the extracellular matrix, as well as infection, inflammatory responses and extracellular matrix organization bioprocesses. Inflammatory cell infiltration and inflammatory mediators in the wall of IAs critically weaken the wall and eventually cause its rupture (15). Extracellular matrix organization in the vessel walls can degrade elastic and collagen fibers, causing the occurrence and progression of aneurysms, and finally leading to vessel rupture (16). The downregulated genes were mainly located in the vascular muscle cells, and have a pivotal role in muscle contraction, including as structural constituents of muscle and in actin binding; the downregulated genes perform an important role in muscle contraction bioprocesses. Alterations in VSMCs contribute to the pathogenesis of IAs and activated VSMCs undergo dedifferentiation characterized by a reduction in contractile and cytoskeletal gene expression and increased expression of genes involved in proliferation, migration and matrix remodeling (17). VSMC phenotypic switching and inflammation also promotes atherosclerosis, which explains why numerous atherosclerosis risk factors are also risk factors for IA (18,19).
The KEGG pathway enrichment analysis in the present study demonstrated that infection, inflammation and vascular smooth muscle contraction were the major pathways in IA.
TLRs are a major family of pattern recognition receptors that perform a key role in innate host defenses, and in the initiation of adaptive immune responses. TLRs can recognize conserved molecular structures and products of various microorganisms. Following ligand recognition, TLRs recruit signal transducers to initiate pro-inflammatory signaling cascades and eventually activate several transcription factor families (20). This explains why there were a number of infection-related pathways in the results of the KEGG pathway enrichment analysis, since TLR signaling is involved in these pathways. The role of the TLR pathway is also important in autoimmune diseases, neuropsychiatric disorders, tumors, myocardial ischemia-reperfusion injury, atherosclerosis and hypertension (21–26). In the central nervous system, TLR signaling in immune cells, glia cells, and neurons may perform a function in the pathogenesis of stroke, Alzheimer's disease and multiple sclerosis. TLR also affects the development of the central nervous system in adults, including neurogenesis, axonal growth and structural plasticity. TLRs are involved in the regulation of behaviors including learning and memory as well as anxiety (27). TLR also performs an important role in vascular-related diseases. The activation of TLR signaling pathways can affect vascular function and vascular remodeling, and the proteins in these TLR signaling pathways can also activate antigen-specific adaptive immune responses (28). In the present study, TLR4 was significantly upregulated. It is known that the activation of TLR4-mediated signaling pathways can determine the activation and dysregulation of the angiotensin converting enzyme, nitric oxide, matrix metalloproteinase (MMP) and transforming growth factor pathways in endothelial cells and VSMCs (29–31). These pathways are closely associated with endothelial dysfunction, extracellular matrix remodeling and chronic inflammation, and these pathological changes can lead to the occurrence of sporadic thoracic aortic aneurysms (32). TLR4 may have the same effect in the pathogenesis of IAs. Kurki et al (33) compared the whole-genome expression profile of 11 ruptured saccular IA wall samples with eight unruptured saccular IA wall samples and the results demonstrated that the TLR pathway was significantly upregulated, indicating that TLRs play an important role in the rupture of IAs. Combined with the above findings, the present study suggested that TLRs may also play an important role in the formation of IAs. This conclusion requires confirmation by further experiments.
The association between Leishmania infection and IAs had not yet been reported, to the best of our knowledge, but it was identified in the present study that Leishmania was closely associated with the TLR pathway. The expression of TLR2 and TLR4 in the spleen tissue of Leishmania-infected patients is increased significantly (34). Leishmania is a genus of protozoan parasites that live in macrophages. Leishmania express lipopolysaccharide (LPS), proteoglycans, flagellin and profilin, so they may be recognized by TLRs expressed in host cells (35). TLRs recognized pathogen-associated molecular patterns (PAMPs) and activated innate immune cells to induce cytokines and co-stimulatory molecules, such as CD40, and enhance antigen presentation to T cells, which may eliminate or support pathogens upon activation (36). The recognition of PAMPs by TLRs leads to different immune responses, attenuating or exacerbating the Leishmania infection. TLR4 expressed by T cells promotes the suppressive function of T-reg cells, while TLR6 eliminates its inhibitory function (37). Therefore, TLR4 and TLR6 antagonize each other in the regulation of T-reg cell function. However, LPS, another TLR2 ligand, induces an anti-inflammatory response. TLR1/TLR2 heterodimers induce a pro-inflammatory response, whereas TLR2/TLR6 heterodimers induce an anti-inflammatory response (38,39). The ‘leaky gut’ hypothesis has been put forward in the study of neuropsychiatric diseases: Stress leads to increase intestinal permeability and intestinal bacterial translocation, and gram-negative bacterial LPS can therefore cause TLR4 activation, which in turn causes stress and stress-related neuropsychiatric disorders (40). In the central nervous system infectious diseases, there is the ‘pathogen-necrosis-autoantigen triplet’ hypothesis: TLRs cause damage to normal tissues by promoting an immune response to pathogens, and recognize newly released self-antigens, which become a direct trigger for TLR-mediated cellular responses to a prolonged inflammatory response (41). The above two hypotheses may be summarized as follows: The invasion of exogenous microorganisms leads to the activation of TLR4, which further promotes the inflammatory response. Due to the destructive effect of inflammatory reactions, exposure to autoantigens occurs, leading to the long-term existence of autoimmune chronic inflammation. These conclusions are only based on previous research data. Whether Leishmania infection is associated with IA requires further research.
Phenotypic changes in VSMCs are an important part of the pathological process of IA. VSMCs can alter their phenotype from one primarily associated with contraction to a pro-inflammatory and matrix remodeling phenotype. This phenotypic alteration in VSMCs also causes peripheral vascular disease and atherosclerosis (17). In the present study, the downregulation of ‘vascular smooth muscle contraction’ indicated that VSMCs undergo phenotypic changes. The upregulated gene TNF may induce the phenotypic modulation of cerebral vascular VSMCs, resulting in the downregulation of contractile genes, namely myocardin, smooth muscle-α-actin, smooth muscle myosin heavy chain and smooth muscle 22α, and the upregulation of pro-inflammatory genes, namely Kruppel like factor (KLF)4, MMP3, MMP-9, monocyte chemotactic protein 1, vascular cell adhesion molecule-1 and IL-1β (42). IL-1β was significantly upregulated in the present study and it can be induced in the early stages of aneurysm formation in mice, leading to inhibition of collagen production and promotion of VSMC apoptosis and aneurysm progression (43). In the early stages following vascular VSMC phenotypic changes, the synthesis of collagen and the degradation of the extracellular matrix caused by MMPs coexist. However, sustained stimulation of inflammatory factors such as TNF finally results in the loss of all phenotypes of VSMCs. Eventually, VSMCs progress to apoptotic and necrotic states (44,45). The loss of VSMCs ultimately leads to aneurysm rupture (46).
From the PPI network diagram, it can be observed that TNF was the key node of the PPI network, with the highest degree value. TNF is an important cytokine, involved in a variety of diseases. In IA, TNF performs a critical role in the formation and rupture of aneurysms (47). The single nucleotide polymorphism of TNF at gene locus −308 G<A has a significant association with IA and aneurysmal SAH (48). TNF can induce cerebral VSMC phenotypic modulations through myocardin and KLF4-regulated pathways, promoting a pro-inflammatory and matrix-remodeling phenotype of VSMCs, and then promoting the IA formation process (42). Therapeutic administration of a TNF-α inhibitor significantly reduces the formation of aneurysms in rats (49). The anti-TNF antibody infliximab significantly suppresses TNF-α activity and reverses phenotypic modulation of VSMCs following aneurysm induction (50). TNF could be used as a target for the future treatment of IAs.
In conclusion, the present study suggested that the TNF gene, TLR signaling pathway and vascular smooth muscle contraction pathway may be associated with IA, although their specific role in IA remains to be confirmed by further experiments. The limitation of the present study was that all the conclusions were drawn from bioinformatics and analysis of previous experimental results. These conclusions have yet to be verified by further studies. In addition, analytical methods such as ingenuity pathway analysis and WebGestalt were not been applied in the present study, and the comprehensive use of these analytical methods may lead to more reliable conclusions. These analytical methods will be used in future research. GSEA has numerous gene sets available for analysis, and the main purpose of the present study was to use GSEA for KEGG pathway analysis. The analysis of numerous related disease gene sets is extensive and cannot be completed in a short time. It is hoped this can be performed in future studies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- BP
biological process
- CC
cellular component
- DAVID
Database for Annotation Visualization and Integrated Discovery
- DEGs
differentially expressed genes
- FDR
false discovery rate
- GEO
Gene Expression Omnibus
- GO
gene ontology
- GSEA
gene set enrichment analysis
- IA
intracranial aneurysm
- IL
interleukin
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MF
molecular function
- MMP
matrix metalloproteinase
- NUSE
normalized unscaled standard errors
- PPI
protein-protein interaction
- RLE
relative logarithm expression
- RMA
robust multichip average
- SAH
sub arachnoid hemorrhage
- STA
superficial temporal artery
- STRING
Search Tool for the Retrieval of Interacting Genes
- TNF
tumor necrosis factor
- VSMC
vascular smooth muscle cell
Funding
The present study was supported by the Key Scientific and Technological Project of Hainan Province (grant no. ZDXM20130066).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DY conceived and supervised the study. TG performed the bioinformatics analysis and was a major contributor in writing the manuscript. DH prepared the figures and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. doi: 10.1212/WNL.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 2.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology. 2010;74:1494–1501. doi: 10.1212/WNL.0b013e3181dd42b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 4.Etminan N, Rinkel GJ. Cerebral aneurysms: Cerebral aneurysm guidelines-more guidance needed. Nat Rev Neurol. 2015;11:490–491. doi: 10.1038/nrneurol.2015.146. [DOI] [PubMed] [Google Scholar]
- 5.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713. doi: 10.1038/nrneurol.2016.150. [DOI] [PubMed] [Google Scholar]
- 6.Bilguvar K, Yasuno K, Niemela M, Ruigrok YM, von Und Zu Fraunberg M, van Duijn CM, van den Berg LH, Mane S, Mason CE, Choi M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40:1472–1477. doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect: A case-control study. Stroke. 2013;44:984–987. doi: 10.1161/STROKEAHA.111.000329. [DOI] [PubMed] [Google Scholar]
- 8.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–1262. doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 10.Edgar R, Barrett T. NCBI GEO standards and services for microarray data. Nat Biotechnol. 2006;24:1471–1472. doi: 10.1038/nbt1206-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L, Wang Q, Zhang Y, Yang C, Guan H, Chen Y, Sun Z. Identification of key genes, transcription factors and microRNAs involved in intracranial aneurysm. Mol Med Rep. 2018;17:891–897. doi: 10.3892/mmr.2017.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bo L, Wei B, Wang Z, Li C, Gao Z, Miao Z. Bioinformatic analysis of gene expression profiling of intracranial aneurysm. Mol Med Rep. 2018;17:3473–3480. doi: 10.3892/mmr.2017.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Li H, Yu L, Zhao Z, Wang H, Zhang D, Zhang Y, Lan Q, Wang J, Zhao J. Aberrant expression of lncRNAs and mRNAs in patients with intracranial aneurysm. Oncotarget. 2017;8:2477–2484. doi: 10.18632/oncotarget.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statistics. 2001;29:1165–1188. [Google Scholar]
- 15.Tulamo R, Frosen J, Hernesniemi J, Niemela M. Inflammatory changes in the aneurysm wall: A review. J Neurointerv Surg. 2010;2:120–130. doi: 10.1136/jnis.2009.002055. [DOI] [PubMed] [Google Scholar]
- 16.van Kuijk JP, Flu WJ, Chonchol M, Bax JJ, Verhagen HJ, Poldermans D. Metabolic syndrome is an independent predictor of cardiovascular events in high-risk patients with occlusive and aneurysmatic peripheral arterial disease. Atherosclerosis. 2010;210:596–601. doi: 10.1016/j.atherosclerosis.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK, Hasan DM, Medel R, Dumont AS. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014;5:338–346. doi: 10.1007/s12975-013-0290-1. [DOI] [PubMed] [Google Scholar]
- 18.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim S, Park S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014;47:1–7. doi: 10.5483/BMBRep.2014.47.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11:3559–3567. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole JE, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis: A ‘Pandora's box’ of advances and controversies. Trends Pharmacol Sci. 2013;34:629–636. doi: 10.1016/j.tips.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Garcia Bueno B, Caso JR, Madrigal JL, Leza JC. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci Biobehav Rev. 2016;64:134–147. doi: 10.1016/j.neubiorev.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ha T, Liu L, Kelley J, Kao R, Williams D, Li C. Toll-like receptors: New players in myocardial ischemia/reperfusion injury. Antioxid Redox Signal. 2011;15:1875–1893. doi: 10.1089/ars.2010.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: Novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H184–H196. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulopoulou S, McCarthy CG, Webb RC. Toll-like receptors in the vascular system: Sensing the dangers within. Pharmacol Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Ramos M, Calleros L, Lopez-Ongil S, Raoch V, Griera M, Rodríguez-Puyol M, de Frutos S, Rodríguez-Puyol D. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int J Biochem Cell Biol. 2013;45:232–242. doi: 10.1016/j.biocel.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Xu H, Sun B. Lipopolysaccharide regulates MMP-9 expression through TLR4/NF-κB signaling in human arterial smooth muscle cells. Mol Med Rep. 2012;6:774–778. doi: 10.3892/mmr.2012.1010. [DOI] [PubMed] [Google Scholar]
- 31.Ruvolo G, Pisano C, Candore G, Lio D, Palmeri C, Maresi E, Balistreri CR. Can the TLR-4-mediated signaling pathway be ‘a key inflammatory promoter for sporadic TAA’? Mediators Inflamm. 2014;2014:349476. doi: 10.1155/2014/349476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balistreri CR, Ruvolo G, Lio D, Madonna R. Toll-like receptor-4 signaling pathway in aorta aging and diseases: ‘Its double nature’. J Mol Cell Cardiol. 2017;110:38–53. doi: 10.1016/j.yjmcc.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Kurki MI, Hakkinen SK, Frosen J, Tulamo R, von und zu Fraunberg M, Wong G, Tromp G, Niemelä M, Hernesniemi J, Jääskeläinen JE, Ylä-Herttuala S. Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: An emerging regulative role of Toll-like receptor signaling and nuclear factor-κB, hypoxia-inducible factor-1A, and ETS transcription factors. Neurosurgery. 2011;68:1666–1675. doi: 10.1227/NEU.0b013e318210f001. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Singh OP, Gautam S, Nylen S, Sundar S. Enhanced expression of Toll-like receptors 2 and 4, but not 9, in spleen tissue from patients with visceral leishmaniasis. Parasite Immunol. 2014;36:721–725. doi: 10.1111/pim.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandel HS, Pandey SP, Roy S, Doyen N, Saha B. TLR-CD40 cross-talk in anti-leishmanial immune response. Front Immunol. 2014;5:220. doi: 10.3389/fimmu.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masopust D, Picker LJ. Hidden memories: Frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Lee DS, Madrenas J. Evolving bacterial envelopes and plasticity of TLR2-dependent responses: Basic research and translational opportunities. Front Immunol. 2013;4:347. doi: 10.3389/fimmu.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 40.Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC. Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Esen N, Kielian T. Toll-like receptors in brain abscess. Curr Top Microbiol Immunol. 2009;336:41–61. doi: 10.1007/978-3-642-00549-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013;33:1564–1573. doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke. 2006;37:900–905. doi: 10.1161/01.STR.0000204028.39783.d9. [DOI] [PubMed] [Google Scholar]
- 44.Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, Niemelä M, Hernesniemi J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012;123:773–786. doi: 10.1007/s00401-011-0939-3. [DOI] [PubMed] [Google Scholar]
- 45.Sakaki T, Kohmura E, Kishiguchi T, Yuguchi T, Yamashita T, Hayakawa T. Loss and apoptosis of smooth muscle cells in intracranial aneurysms. Studies with in situ DNA end labeling and antibody against single-stranded DNA. Acta Neurochir (Wien) 1997;139:469–475. doi: 10.1007/BF01808885. [DOI] [PubMed] [Google Scholar]
- 46.Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. Biology of intracranial aneurysms: Role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, et al. Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. doi: 10.1186/1742-2094-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontanella M, Rainero I, Gallone S, Rubino E, Fenoglio P, Valfrè W, Garbossa D, Carlino C, Ducati A, Pinessi L. Tumor necrosis factor-alpha gene and cerebral aneurysms. Neurosurgery. 2007;60:663–672. doi: 10.1227/01.NEU.0000255417.93678.49. [DOI] [PubMed] [Google Scholar]
- 49.Yokoi T, Isono T, Saitoh M, Yoshimura Y, Nozaki K. Suppression of cerebral aneurysm formation in rats by a tumor necrosis factor-α inhibitor. J Neurosurg. 2014;120:1193–1200. doi: 10.3171/2014.1.JNS13818. [DOI] [PubMed] [Google Scholar]
- 50.Ali MS, Starke RM, Jabbour P, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Dumont AS. MD184 Infliximab suppresses TNF-α induced inflammatory phenotype in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm formation. Neurosurgery. 2013;60:181. doi: 10.1227/01.neu.0000432774.35977.11. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.