Circovirus is the smallest virus known to replicate autonomously. Knowledge of viral genome release may provide understanding of viral replication and a method to artificially inactivate viral particles. Currently, little is known about the release model of porcine circovirus type 2 (PCV2). Here, we report the release of the PCV2 genome from assembled capsid and the intracellular trafficking of infectious PCV2 by alterations in the capsid conformation. Knowledge of PCV2 capsid stability and dynamics is essential to understanding its infectious cycle and lays the foundation for discovering powerful targets for therapeutic and prophylactic intervention.
KEYWORDS: porcine circovirus, capsid, conformational change, integrity, life cycle
ABSTRACT
A relatively stable and flexible capsid is critical to the viral life cycle. However, the capsid dynamics and cytosol trafficking of porcine circovirus type 2 (PCV2) during its infectious cycle are poorly understood. Here, we report the structural stability and conformation flexibility of PCV2 virions by genome labeling and the use of three monoclonal antibodies (MAbs) against the native capsid of PCV2. Genome labeling showed that the infectivity of the PCV2 virion was not affected by conjugation with deoxy-5-ethynylcytidine (EdC). Heat stability experiments indicated that PCV2 capsids started to disassemble at 65°C, causing binding incompetence for all antibodies, and the viral genome was released without capsid disassembly upon heating at 60°C. Antibody binding experiments with PCV2 showed that residues 186 to 192 were concealed in the early endosomes of epithelial PK-15 and monocytic 3D4/31 cells with or without chloroquine treatment and then exposed in PK-15 cytosol and the 3D4/31 nucleus. Viral propagation and localization experiments showed that PCV2 replication and cytosol trafficking were not significantly affected by microtubule depolymerization in monocytic 3D4/31 cells treated with nocodazole. These findings demonstrated that nuclear targeting of viral capsids involved conformational changes, the PCV2 genome was released from the assembled capsid, and the transit of PCV2 particles was independent of microtubules in 3D4/31 cells.
IMPORTANCE Circovirus is the smallest virus known to replicate autonomously. Knowledge of viral genome release may provide understanding of viral replication and a method to artificially inactivate viral particles. Currently, little is known about the release model of porcine circovirus type 2 (PCV2). Here, we report the release of the PCV2 genome from assembled capsid and the intracellular trafficking of infectious PCV2 by alterations in the capsid conformation. Knowledge of PCV2 capsid stability and dynamics is essential to understanding its infectious cycle and lays the foundation for discovering powerful targets for therapeutic and prophylactic intervention.
INTRODUCTION
Circovirus is known to infect mammals and birds. Among the 29 species in the genus Circovirus, only porcine circovirus (PCV) can be propagated in cell cultures. Porcine circovirus type 1 (PCV1) is nonpathogenic, but PCV2 is the main pathogen of porcine circovirus-associated disease, including postweaning multisystemic wasting syndrome (PMWS) and porcine dermatitis and nephropathy syndrome (PDNS) (1). As the smallest virus known to replicate autonomously, circovirus adopts a nonenveloped icosahedral structure (triangulation number, T=1) with equal interactions between each identical subunit approximately 15 to 20 nm in diameter (2, 3). It contains a single-stranded circular DNA (ssDNA) genome 1.7 to 2.0 kb in length (4). The genomes of viruses in the genus Circovirus contain four open reading frames (ORFs) that encode viral proteins, namely, the ORF1-encoded replicase protein (Rep) and spliced Rep′ (5), the ORF2-encoded capsid protein (Cap) (6), and the apoptosis-related ORF3- and ORF4-encoded proteins (7, 8). PCV2 releases genomic DNA into the infected cells to replicate, and the circular ssDNA is converted to a double-stranded DNA (dsDNA) intermediate by host DNA polymerases in the nucleus (9). The complex of Rep and Rep′ binds to the replication origin of the dsDNA intermediate to initiate genome replication (10, 11). However, how PCV2 genomic DNA is released is unclear.
Accumulated evidence showed that PCV2 Cap was clearly detected in the nuclei of infected cells during infection (12). Significantly, Cap appeared not to be involved in the synthesis of viral DNA (vDNA) and Rep-associated proteins (13). Moreover, some small-diameter viruses are recognized to enter the nucleus in intact forms (14–16). The N terminus of Cap (N-Cap) contains a nuclear localization signal (NLS) that is necessary and sufficient for its specific nuclear accumulation (17). However, the N-Cap was completely buried in the interior of the virion (3). Therefore, whether the conformation of the PCV2 capsid is dynamic in transit into the nucleus is unknown.
Porcine epithelial PK-15 cells and monocytic 3D4/31 cells are two major target cells of PCV2. PCV2 was shown to be predominantly internalized through clathrin-mediated endocytosis in 3D4/31 cells (18), through an actin/small GTPase-dependent pathway in PK-15 cells (19), to be localized in the endosomes of both cells (18, 20). After escape from the early endosomes, Cap binds to dynein directly to transport along microtubules and moves through the cytoplasm in PK-15 cells (12, 21). However, many steps in the infectious cycle of PCV2, including uncoating, nuclear entry, and assembly, are poorly understood.
Multiple antigen epitopes of Cap, i.e., the epitopes L-102 (residues 85 to 102), L-162 (residues 156 to 162), L-192 (residues 175 to 192), L-202 (residues 195 to 202), and L-233 (residues 231 to 233), were identified (22, 23). The antibodies provide effective tools to monitor structural integrity and conformational alternations observed in the present study. Here, we found that the PCV2 genome was released from the assembled capsid in vitro, and the conformation of the PCV2 capsid was dynamic during nuclear entry. Additionally, the conformational dynamics of the PCV2 capsid were different in PK-15 and 3D4/31 cells. The Cap in the nuclei of PK-15 and 3D4/31 cells at 21 hours postinfection (hpi) were reactive with all monoclonal antibodies (MAbs) but disassembled capsids were not. The Cap in the early endosomes of both cell lines at 6 hpi and that did not arrive in the nuclei of 3D4/31 cells were not recognized by MAb 3F6. This is the first study about PCV2 capsid conformational stability and flexibility. Our findings support a viral genome release model in which the PCV2 capsid does not disassemble and the cellular trafficking of PCV2 particles is independent of microtubules in 3D4/31 cells.
RESULTS
Labeling of the PCV2 genome with ethynyl-modified nucleotide analogues.
To efficiently label genomic DNA of PCV2, PK-15 cells were infected with PCV2 at a multiplicity of infection (MOI) of 2 for 11 h, and then different concentrations of the ethynyl-modified nucleotide analogues, including deoxy-5-ethynylcytidine (EdC), 5-ethynyl-2′-deoxyuridine (EdU), (2′S)-2′-deoxy-2′-fluoro-5-ethynyluridine (F-ara-EdU), and 7-deaza-7-ethynyl-2′-deoxyadenosine (EdA), were added for 61 h. The results showed that F-ara-EdU across the entire concentration range and EdA at concentrations of 1.25, 2.5, and 5 μM gave the highest titers. EdC gave medium levels of titers at concentration of 1.25, 2.5, and 5 μM, while EdU gave the lowest titers across the entire concentration range (Fig. 1A). However, when screening nucleotide analogues for labeling PCV2 DNA efficiently by laser scanning confocal microscopy (LSCM), a stronger fluorescence signal was observed in PK-15 cells infected with EdC- and EdU-incorporated PCV2 than that infected with F-ara-EdU- and EdA-incorporated PCV2 (Fig. 1B). We selected EdC at a concentration of 2.5 μM for metabolic labeling of the circovirus DNA genome.
FIG 1.
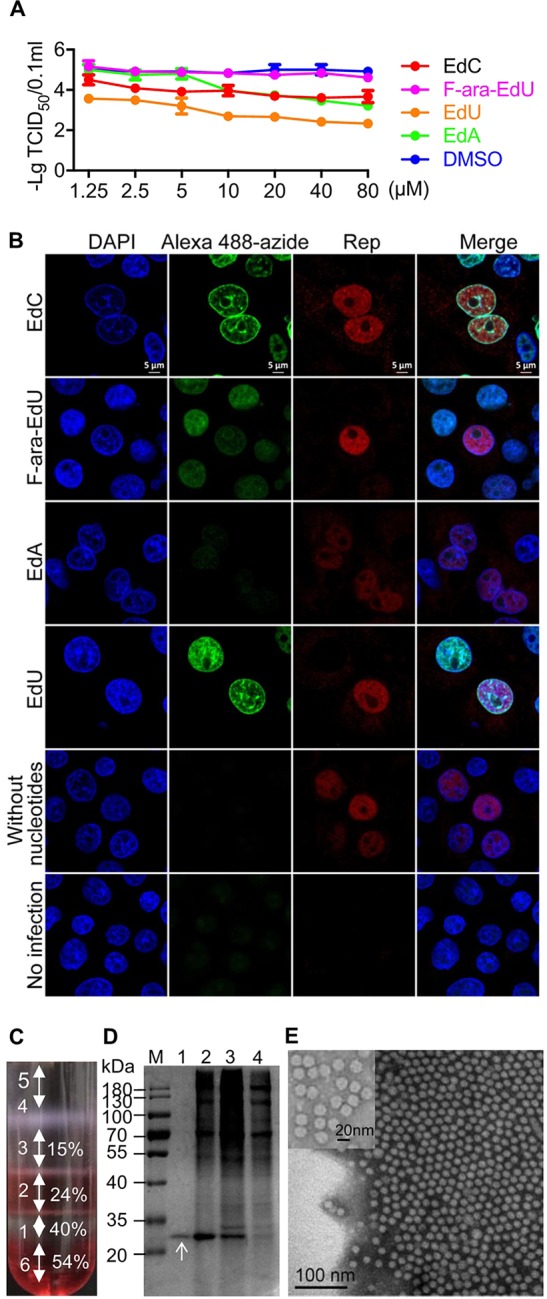
Nucleoside labeling and purification of PCV2. (A) Replication of PCV2 in the presence of EdA, EdC, EdU, and F-ara-EdU. PK-15 cells were infected with PCV2 at an MOI of 2 for 11 h and then treated with the nucleoside analogues at a concentration of 1.25, 2.5, 5, 10, 20, 40, or 80 μM for 61 h. The viral titers are indicated as the 50% tissue culture infective dose (TCID50). Dimethyl sulfoxide (DMSO) treatments were set as controls. (B) Laser scanning confocal microscopy (LSCM) images of nucleoside analogue-incorporated PK-15 cells. PK-15 cells were infected with PCV2 at an MOI of 25 for 4 h and then treated with four nucleoside analogues at a concentration of 2.5 μM for 20 h. The cells were incubated with MAb anti-Rep and then Alexa Fluor 546 IgG at 37°C for 1 h and Alexa Fluor 488 azide for 2 h. Cellular nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) and observed by LSCM (red, Rep; green, nucleotide analogues; blue, DAPI). Bar, 5 μm. (C) Iodixanol density gradient centrifugation of PCV2. PCV2 was subjected to iodixanol density gradient centrifugation at 350,000 × g for 1 h. (D) Purity analysis of ultracentrifuged viral particles in each fraction. Each fraction was subjected to SDS-PAGE and then stained with Coomassie dye. (E) Transmission electronic microscopy (TEM) observation of purified PCV2 particles in fraction 1. Bar, 100 nm.
Increasing evidence suggests that heat, one of the most common external energy sources, is widely used to trigger virus uncoating (24–26). To monitor the release of PCV2 vDNA, EdC-labeled PCV2 (EdC-PCV2) and wild-type PCV2 (wtPCV2) were purified by iodixanol density gradient centrifugation. Bands formed at each interface between different density layers after centrifugation for 1 h (Fig. 1C). Each fraction was separated by SDS-PAGE, then stained with Coomassie brilliant blue. Only one single band with a mass of approximately 28 kDa was observed in fraction 1 (the interface between 54%/40% iodixanol and 40% iodixanol layers) (Fig. 1D). A large number of pure, regular icosahedral structures with a diameter of approximately 17 nm were observed in fraction 1 by transmission electronic microscopy (TEM) (Fig. 1E). In fraction 1, the infectivity of concentrated purified PCV2 was 109.10 times the 50% tissue culture infective dose (TCID50)/0.1 ml. In summary, we obtained highly purified EdC-PCV2 particles by iodixanol density gradient centrifugation.
Integrity and conformation of the PCV2 capsid exhibited high thermal stability in vitro.
To test whether EdC was incorporated into PCV2 particles, purified EdC-PCV2 and wtPCV2 were diluted 100 times, exposed to 60 or 80°C for 3 min or not, and finally spotted on glass-bottomed dishes. The vDNA was detected with Alexa Fluor 488 azide and viral particles were indicated with anti-Cap MAb 5E11. As expected, EdC-PCV2 particles heated at 60, 80°C were positive for Alexa Fluor 488 azide, while wtPCV2 and native EdC-PCV2 were negative for Alexa Fluor 488 azide (Fig. 2A). Negative staining electron microscopy (EM) observation showed that regular icosahedral viral particles were observed in samples that were native or heated at 60°C, while almost no icosahedral particles were observed in samples heated at 80°C (Fig. 2B). This indicated that PCV2 genomic DNA efficiently labeled with EdC without colocalization with capsid was capsid-free upon heating at 60°C. As ejection of a subunit inevitably results in disassembly of the PCV2 capsid, the integrity of PCV2 could be reliably determined by EM. To further determine the temperature required for PCV2 disassembly, a series of temperature gradients was set, and the number of PCV2 icosahedral particles was counted in three random fields of view of EM in each sample. Statistical analysis of the number of particles showed that there was no significant decrease in the number of wtPCV2 and EdC-PCV2 particles heat treated at 60 and 63°C (P > 0.05), but there was a significant decrease in the number of wtPCV2 and EdC-PCV2 particles heat treated at 65°C, and almost all particles disappeared at 67°C (P < 0.01) (Fig. 2C). This showed that the PCV2 capsid started to disassemble at ≥65°C. Collectively, these data demonstrated that PCV2 particles were assembled upon heating at 60°C for 3 min, but vDNA was released from the capsid.
FIG 2.

Thermal stability of PCV2. (A and B) Purified wtPCV2 and EdC-PCV2 particles diluted 100 times were exposed to heat at 60 to 80°C for 3 min or not. (A) LSCM images of genome release from the PCV2 capsid. Samples were spotted on glass-bottomed dishes, dried, fixed with 4% paraformaldehyde (PFA) for 15 min, incubated with anti-Cap MAb 5E11, followed by Alexa Fluor 546 IgG and Alexa Fluor 488 azide, and finally observed by LSCM (red, Cap; green, nucleotide analogues). Bar, 5 μm. (B) TEM images of native and heat-treated PCV2 virions with ×57,000 magnification. Bar, 100 nm. (C) Statistical analysis of the number of assembled capsids. The number of PCV2 capsids that were native or heat-treated at 60, 63, 65, and 67°C for 3 min was counted in three random fields of view of EM. *, P < 0.05; **, P < 0.01. (D) Mapping of the epitope recognized by three MAbs on PCV2 capsid exterior surface. Red indicates residues 225 to 230 binding with MAb 5E11, green indicates residues 51 to 60 and 231 binding with MAb 7F5, and blue indicates residues 186 to 192 binding with MAb 3F6. The 59 subunits of PCV2 capsid were surfaced with grey. (E) Reactivity of purified EdC-PCV2 and PCV2 particles heat treated or not with MAbs anti-Cap. Samples exposed to 60, 63, 65, or 67°C or not for 3 min were spotted on nitrocellulose blotting membranes, probed with the anti-Cap MAbs 5E11, 7F5, and 3F6. (F) Statistical analysis of data in panel E. *, P < 0.05; **, P < 0.01.
Moreover, MAbs against PCV2 Cap were used to analyze whether uncoating or disassembly of the capsid was accompanied by conformational changes. Of the available anti-Cap MAbs in this study, only MAbs 5E11, 7F5, and 3F6 were found to recognize native PCV2 particles (data not shown), indicating that the three MAbs bound to the exterior surface of the PCV2 capsid. Fig. 2D showed that antigenic epitopes recognized by MAbs 5E11, 7F5, and 3F6 mapped on the capsid surface by using the cryo-electron microscopy (cryo-EM) structure of PCV2 virus-like particles (VLPs) (Protein Data Bank [PDB] ID 3R0R) (3, 23). Residues 225 to 230 bind with MAb 5E11 at the 5-fold axes, residues 51 to 60 and residue 231 bind with MAb 7F5 at the 5-fold axes and define the knob-like protrusions of the mountainous capsid surface, and residues 186 to 192 bind with MAb 3F6 at the 3-fold axes and generate the depression of the capsid surface. Equal volumes of purified EdC-PCV2 and wtPCV2 particles heated at 60, 63, 65, 67, 70, or 80°C for 3 min or not were dotted on nitrocellulose blotting membranes, then incubated with three MAbs (5E11, 7F5, and 3F6). The results indicated that these MAbs only exhibited a significant decrease in reactivity with wtPCV2 and EdC-PCV2 heated at 65 to 67°C for 3 min (P < 0.01) but not with those heated at 60 or 63°C for 3 min (Fig. 2E and F). Moreover, EdC-PCV2 heated at >67°C almost lost reactivity with anti-Cap MAbs (data not shown). These data demonstrated that the integrity and exterior surface conformation of the PCV2 capsid were stable at 60°C when the PCV2 genome was released.
Conformation of the PCV2 capsid is altered in transit into the early endosome of host cells.
Previous studies reported that PCV2 was localized in the early endosomes after internalization at 6 hpi without conformational changes upon attachment (12, 27). To determine and compare the conformation of Cap in the early endosomes of PK-15 and 3D4/31 cells with that of native virions, the two cell lines infected with PCV2 (MOI, 10) were incubated with MAb 5E11 against PCV2 capsid and antibody anti-early antigen 1 (EEA1), which indicated early endosomes. The results showed that PCV2 Cap protein was located to the early endosome at 6 hpi in both PK-15 and 3D4/31 cells (Fig. 3A). Antibody reaction results showed that MAbs 5E11 and 7F5 but not 3F6 bound with PCV2 Cap in both PK-15 and 3D4/31 cells in an immunofluorescence (IF) assay (Fig. 3B). Additionally, the recognition of the three MAbs with PCV2 Cap in infected cells were confirmed by Western blot assay (Fig. 3C). Moreover, Cap was scattered in dotted patterns in PCV2-infected cells without chloroquine (CQ; a lysosomotropic agent that buffers pH) treatment and appeared in mass-like clustered distributions in PCV2-infected cells with the addition of CQ immediately after infection (Fig. 3B). These data indicated that CQ did not change the reactivity of PCV2 with the MAbs against capsid and that PCV2 underwent conformational changes in transit into the early endosomes.
FIG 3.

Antibody binding with PCV2 in the early endosomes. (A) Observation of the location of PCV2 in the early endosomes in cells by immunofluorescence (IF) assay. PK-15 and 3D4/31 cells were inoculated with PCV2 (MOI, 10) for 6 h, fixed, probed with anti-Cap MAb 5E11 and antibody against EEA1, and finally observed by confocal microscopy (red, EEA1; green, Cap; blue, DAPI). Bar, 10 μm. (B) Determination of antibody binding of PCV2 in early endosomes at 6 hpi with or without CQ treatment by IF assay. PK-15 and 3D4/31 cells were inoculated with PCV2 (MOI, 10) in the absence or presence of CQ for 6 h, fixed, stained with MAbs 5E11, 7F5, and 3F6, respectively, and observed by LSCM (green, Cap; blue, DAPI). Bar, 10 μm. (C) Western blot assay. The cells infected with PCV2 (MOI, 10) for 6 or 21 h were incubated with the three MAbs as primary antibody, then with horseradish peroxidase (HRP) as secondary antibody. Actin protein was set as an internal control.
Translocation of PCV2 is independent of microtubules in 3D4/31 cells.
To track the intracellular location of PCV2, PK-15 and 3D4/31 cells were infected with PCV2 (MOI, 10), fixed at 15, 18, 21, or 24 hpi, and finally probed with the MAb 5E11. PCV2 could be detected in the nucleus at 15 hpi. As the inoculation time increased, the amount of Cap scattered in the cytoplasm decreased. The following three PCV2 particle distribution patterns appeared in infected cells: only in the cytoplasm, only in the cytosol, and both in the cytosol and nucleus, the distributions were typical at 21 hpi (Fig. 4A). Beginning at 24 hpi, diffusely distributed Cap was gradually detected in the cytoplasm (data not shown). The MAb binding assay at 21 hpi showed that PCV2 in the PK-15 cell cytosol, PK-15 cell nucleus, and 3D4/31 cell nuclei could react with all MAbs (5E11, 7F5, and 3F6), but PCV2 in the 3D4/31 cell cytosol only reacted with MAbs 5E11 and 7F5 (Fig. 4A), suggesting that PCV2 underwent different conformational changes in PK-15 and 3D4-31 cells during nuclear targeting transportation. To further determine whether cytoplasmic transport of PCV2 was different in PK-15 and 3D4/31 cells, 3D4/31 cells were pretreated with nocodazole at different concentrations for 3 h in the same way as that in PK-15 cells, then infected with PCV2 at an MOI of 2 for 48 h. The data shown in Fig. 4B indicated that cell viability was not significantly affected by nocodazole. Compared with those in dimethyl sulfoxide (DMSO)-treated controls (12), viral titers in nocodazole-treated 3D4/31 cells were not significantly different (P > 0.05). Moreover, there was no significant difference (P > 0.05) in viral titers among the nocodazole-treated groups (Fig. 4C). Confocal microscopy observations showed that nuclear targeting of PCV2 was not affected in nocodazole-treated 3D4/31 cells compared with that in nocodazole-treated PK-15 cells (Fig. 4D). These results suggested that intracellular transportation of PCV2 was independent of microtubules in 3D4/31 cells.
FIG 4.

Antibody binding during PCV2 nuclear entry and PCV2 trafficking in 3D4/31 cells. (A) Antibody binding with PCV2 during nuclear entry in 3D4/31 cells. PCV2 was inoculated into PK-15 and 3D4/31 cells at an MOI of 10, fixed at 21 hpi, stained with the anti-Cap MAbs 5E11, 7F5, and 3F6, and then observed by LSCM (green, Cap; blue, DAPI). Bar, 10 μm. (B) The 3D4/31 cells were pretreated with different concentrations of nocodazole for 3 h. Cell viability was analyzed by Cell Counting Kit-8 (CCK-8) assay. (C) The 3D4/31 cells pretreated with different concentrations of nocodazole for 3 h were infected with PCV2 at an MOI of 2 for 48 h. Viral titers were determined and are shown as TCID50 values. *, P < 0.05; **, P < 0.01. (D) Effects of microtubule depolymerization on the cytosol transport of PCV2. PK-15 or 3D4/31 cells were pretreated with nocodazole (4 μM) for 20 min, then infected with equal volume of PCV2 with nocodazole at an MOI of 10 for 6 h.
DISCUSSION
PCV2 is not only of great economic significance in the pig industry but also of important taxonomic interest as the smallest virus known to propagate in cell cultures. Its only capsid protein becomes flexible during the infectious cycle, in which it ejects and then encloses the genome and finally releases from the host cells to protect the genome from harsh conditions. Hence, this study highlighted the stability and dynamic properties of the capsid by the use of a panel of MAbs that recognize different epitopes on the exterior surface of the capsid to help unveil the life cycle of PCV2.
Nucleoside analogues added early during infection could be integrated into vDNA as raw materials during viral replication. Once the labeled genome is released, it is visible by a click reaction with fluorophore azide (28). Nucleoside analogues have been widely used for DNA labeling and for cell and organism metabolism studies (29, 30). Compatible with immunocytochemistry, DNA metabolic labeling with “clickable” nucleoside analogues and copper (I)-catalyzed azide-alkyne cycloaddition reactions allow DNA visualization without sample denaturation. Recently, the vDNA of adenovirus, vaccinia virus, and herpes simplex virus was labeled with clickable nucleoside analogues efficiently (28). In the present study, nucleoside analogues, including EdA, EdC, EdU, and F-ara-EdU, were screened for DNA labeling of PCV2. It was found that, based on toxic effects of the nucleoside analogues on cells, EdC was optimal for metabolic labeling of the circovirus vDNA (Fig. 1A and B). To the best of our knowledge, EdC labeling provided an alternative tool for visualizing PCV2 genome release in vitro for the first time.
Interestingly, many viruses, such as parvovirus (24, 26), picornavirus (31), and human immunodeficiency viruses (32), use dynamic pores to eject the viral genome or import nucleotides. In the present study, we built an in vitro model in which the vDNA was found to be released from the PCV2 assembled capsid that was exposed to heat (Fig. 2A). Upon heating at 60°C, the genome was capsid-free without disintegration of the capsid (Fig. 2A to C), which remained binding stability of MAbs 5E11, 7F5, and 3F6 (Fig. 2A, E and F). These results implied that heat induced PCV2 capsid to be dynamic to eject the genome. The residues 145 to 162 form strands F and G of the β-barrel located on the interior surface of the capsid, and the residues 205 to 230 generate strand I of the β-barrel at the C terminus (3, 23). Coincidently, disassembly induced by heat resulted in the concealment or destruction of the epitopes recognized by the MAbs 5E11 (binding to residues 225 to 230), 3F6 (binding to residues 186 to 192), and 7F5 (binding to residues 51 to 60 and 231) (Fig. 2C to F), but the denaturing conditions in the Western blot did not (Fig. 3C). It is possible that antibodies recognized both conformational epitopes and linear epitopes. We hypothesized that the 3-fold axes of the capsid allow vDNA release, which was accompanied by conformational changes of the capsid interior. These data may imply that the genome release model of some DNA viruses is similar to that of some RNA viruses.
PCV2 in PK-15 cells penetrates early from the endosome into the cytosol (12, 20), then interacts with dynein proteins directly by overlapped binding motif of L185RLQT189 with MAb 3F6 but did not block MAb 3F6 binding in the cytosol of PK-15 cells (21) (Fig. 4A). The reverse and transient binding failure of MAb 3F6 alone was thus mostly due to PCV2 capsid conformational changes. The inhibition of endosome-lysosome acidification increases PCV2 infection in porcine epithelial cells (20), while PCV2 requires an acidic environment in 3D4/31 cells (18) and colocalizes with the lysosomes in primary porcine monocytes (33). The motor-driven transport of vesicles through the cytoplasm might be actin dependent in 3D4/31 cells (34). In our experiments, antibody binding was not affected in the early endosomes of both PK-15 and 3D4/31 cells by CQ treatment (Fig. 3A and B). A previous study observed that CQ treatment caused the MAb specific for PCV2 Cap to lose reactivity (20). A possible explanation for this is that the antigen epitope read by the MAb was blocked. Interestingly, the MAb signals in PCV2-infected PK-15 cells were significantly stronger than that in 3D4/31 cells (Fig. 3B, 4A), PCV2 reacted with all three MAbs in the cytosol of PK-15 cells, but with only MAbs 5E11 and 7F5 in the cytosol of 3D4/31 cells (Fig. 4A). Moreover, we also demonstrated that PCV2 propagation and trafficking was not affected in 3D4/31 cells treated with nocodazole (Fig. 4B to D). These data demonstrated that the conformational changes of the PCV2 capsid in 3D4/31 cells were different from those in PK-15 cells and that the nuclear targeting of PCV2 was independent of microtubules in 3D4/31 cells.
In summary, we characterized the conformational stability and flexibility of the PCV2 capsid, as demonstrated by the constant reactivity of a panel of MAbs against native virions and empty capsids in vitro and the varied reactivity of MAbs against Cap proteins at different stages of nuclear entry. Our detailed findings supported a “breathing” model of PCV2, in which residues 186 to 192 were transiently concealed in the early endosomes of PK-15 and 3D4/31 cells (12, 18, 20) and then exposed after endosomal escape. PCV2 depends on the microtubule network in PK-15 cells (12) but not in 3D4/31 cells to pass through the cytosol. The PCV2 capsid carries the genome to the nucleus. After uncoating, vDNA released from the capsids is converted to a replication-competent dsDNA (Fig. 5). These findings provide new insights into the PCV2 infectious cycle.
FIG 5.

Suggested model of genome release in host cells. For PCV2, the genome of which needs to be converted to dsDNA, the particles bind to glycosaminoglycan without capsid conformational alternation, are internalized and are then received by the early endosomes. Our findings supported a “breathing” model of PCV2, in which residues 186 to 192 were transiently buried in the early endosomes of PK-15 cells or 3D4/31 cells and then exposed after endosomal escape. PCV2 depends on the microtubule network in PK-15 cells but not in 3D4/31 cells to pass through the cytosol. Finally, the capsid arrived in the nucleus and then ejected the genome without capsid disintegration.
MATERIALS AND METHODS
Cells, virus, MAbs, nucleoside analogues, and iodixanol solutions.
Permanent porcine kidney epithelial cell line PK-15 (ATCC-CCL-33, American Type Culture Collection) and continuous porcine monocytic cell line 3D4/31 (ATCC-CRL-2844) free of PCV were routinely cultured in minimum essential medium and RPMI 160 medium (Gibco; Thermo Fisher Scientific, Waltham, MA) supplemented with 5% fetal bovine serum (Gibco; Thermo Fisher Scientific) respectively, as previously described (12). PCV2 strain HZ0201 (GenBank accession no. AY188355.1) was isolated from pig herds with PMWS and propagated in PK-15 cells (35). The cells were infected with PCV2 at the MOIs and for the times indicated in the figures, figure legends, or text. Mouse MAbs anti-PCV2 Rep (36) and anti-Cap 5E11 (binding with residues 205 to 230), 7F5 (binding with residues 1 to 60 and 231 to 233), and 3F6 (binding with residues 145 to 162 and 175 to 192) were prepared and maintained in our laboratory (23, 37). Goat polyclonal antibody anti-EEA1 (catalog no. sc-6414) and rabbit MAb anti-alpha tubulin (catalog no. ab52866) were bought from Santa Cruz Biotechnology (Dallas, TX) and Abcam Biotechnology (Cambridge, UK), respectively. All fluorescent secondary antibodies were bought from Thermo Fisher Scientific. The 5-ethynyl-2′-deoxyuridine (EdU) (product no. T511285), (2′S)-2′-deoxy-2′-fluoro-5-ethynyluridine (F-ara-EdU) (product no. T511293), and deoxy-5-ethynylcytidine (EdC) (product no. T511307), were purchased from Sigma-Aldrich (St. Louis, MO), and 7-deaza-7-ethynyl-2′-deoxyadenosine (EdA) was custom-made in Carbosynth (Oxford, UK). Iodixanol (product no. D1556) was purchased from Sigma-Aldrich (St. Louis, MO).
PCV2 labeling with clickable nucleosides.
Nucleoside screening was performed as described previously (28). Briefly, PK-15 cells were infected with PCV2 at an MOI of 2. At 11 hpi, the inoculated cells were added with EdC, EdU, F-ara-EdU, and EdA at concentrations of 1.25, 2.5, 5, 10, 20, 40, or 80 μM, respectively, and continually cultured for 61 h. Cell lysates were then used to inoculate fresh PK-15 cell suspensions for 72 h to detect viral titers by IF assay. Samples were observed with an IX71 inverted fluorescence microscope (Olympus; Shinjuku, Tokyo, Japan). The TCID50 values were calculated using the Reed-Muench method. To screen the nucleoside analogues that incorporated into the PCV2 genome efficiently, PCV2 was inoculated into PK-15 cell monolayers (MOI, 25) for 24 h with 2.5 μM EdC, EdU, F-ara-EdU, or EdA added at 4 hpi, then fixed with 4% PFA for 15 min; quenched with 25 mM NH4Cl; permeabilized with 0.5% Triton X-100; stained with MAb anti-Rep, then Alexa Fluor 546-conjugated goat anti-mouse IgG (catalog no. A-11003), and then the freshly prepared click staining mix containing 10 mM Alexa Fluor 488-azide (catalog no. A10266; Thermo Fisher Scientific), 1 mM CuSO4, 10 mM sodium ascorbate, 1 mM Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA; product no. 762342-100MG; Sigma-Aldrich, St. Louis, MO), and 10 mM amino-guanidine (AG; product no. 396494-25G; Sigma-Aldrich) in phosphate-buffered saline (PBS) at room temperature (RT) for 2 h in the dark by copper(I)-catalyzed azide-alkyne cycloaddition reactions, and finally with 4′,6-diamidino-2-phenylindole (DAPI, catalog no. 10236276001; Sigma-Aldrich) for 5 min. Fluorescence images were recorded and analyzed on an LSM780 microscope (Carl Zeiss, Oberkochen, Germany).
Purification of PCV2.
Iodixanol density gradient centrifugation was performed as previously described (38). Briefly, PK-15 cells infected with EdC-PCV2 or wtPCV2 were disrupted by three consecutive freeze/thaw cycles. Lysates were centrifuged at 4,000 × g for 20 min at 4°C. Then, the supernatant was collected and filtered with 0.22-μm pore size filters (catalog no. SLGP033NB; Millipore, Burlington, MA). Viral particles were centrifuged at 160,000 × g for 2 h at 4°C using an Optima L-100XP ultracentrifuge (Beckman Coulter, Brea, CA). The pellets were soaked with PBS for at least 4 h or overnight. The viral suspension was then transferred to Ultra-Clear centrifuge tubes (catalog no. 344057; Beckman Coulter). The iodixanol solutions at various concentrations of 15% to 54% were prepared with OptiPrep density gradient medium according to the Cold Spring Harbor protocol published online (http://cshprotocols.cshlp.org/content/2016/11/pdb.rec091116.full?text_only=true). Then, 1.2 ml of 15%, 0.8 ml of 24%, 0.7 ml of 40%, and 0.7 ml of 54% iodixanol working solution were sequentially added to the bottom of the tube to form a density gradient. Samples were added on the top of the gradient, then centrifuged at 350,000 × g for 1 h at 18°C. Fractions at different iodixanol concentration interfaces were collected. Finally, purified wtPCV2 and EdC-PCV2 particles were subjected to 12% SDS-PAGE and then Coomassie brilliant blue R250 staining.
TEM observation.
TEM was conducted as previously described (39). Briefly, the native and heat-treated purified PCV2 samples were stained with 1% uranyl acetate, then observed with an H-7650 microscope (Hitachi, Tokyo, Japan) or Tecnai Spirit microscope (Thermo Fisher Scientific).
Copper (I)-catalyzed azide-alkyne cycloaddition staining.
Metabolic labeling with ethynyl-modified nucleotide analogues makes DNA visible by copper(I)-catalyzed azide-alkyne cycloaddition reactions with fluorophore azide without sample denaturation. The click reaction procedure was performed to visualize vDNA release as previously described (28). Briefly, cells were cultured in glass-bottomed dishes, while purified PCV2 and EdC-PCV2 particles were incubated in a water bath at 60 to 80°C for 3 min or not, then immediately placed on ice, spotted on glass-bottomed dishes, and air dried. The cultured cells and purified viral particles were fixed with 4% paraformaldehyde (PFA) for 15 min, incubated with MAb 5E11 anti-Cap, then Alexa Fluor 546-conjugated goat anti-mouse secondary antibody, and subsequently the freshly prepared click staining mix by copper(I)-catalyzed azide-alkyne cycloaddition reactions. Fluorescence images were recorded and analyzed on an LSM780 microscope (Zeiss, Oberkochen, Germany).
Western and dot blot assays.
Western (7) and dot blot (8) assays were performed as previously described. Briefly, the cells infected with PCV2 (MOI, 10) for 6 or 21 h were lysed with NP-40 buffer (catalog no. P0013F; Beyotime, Shanghai, China) overnight, separated by SDS-PAGE, blotted on the nitrocellulose membranes, then stained with three anti-Cap MAbs or anti-actin (catalog no. ab179467; Abcam, Cambridge, UK), respectively. Purified PCV2 particles were dotted on nitrocellulose blotting membranes. Noninfected cell lysates were prepared as negative controls. The membranes were blocked with 5% skim milk in PBS and incubated with all three anti-Cap MAbs at 37°C for 1 h, then with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (product no. 5450-0006 374-1806; KPL, Michigan) at 37°C for 1 h. Finally, the membranes were visualized using SuperSignal West Pico chemiluminescent substrate (catalog no. 34580; Thermo Fisher) under the conditions recommended by the manufacturer. Images were captured with a FluorChem M chemiluminescent imaging system (Cell Biosciences, Palo Alto, CA).
Treatment of cell cultures with chemicals.
Chemicals, including the lysosomotropic agent chloroquine (CQ) (catalog code tlrl-chq; InvivoGen, San Diego, CA) and the microtubule-destabilizing drug nocodazole (catalog no. M1404-2MG; Sigma-Aldrich), were used to pretreat PK-15 and 3D4/31 cells. Before treatment, cells were cultured to 60% to 70% confluence, and the medium was replaced with fresh medium in the absence or presence of a drug. DMSO (catalog no. 276855-100ML; Sigma-Aldrich) was used as a control. Cell viability was determined using the CCK-8 assay (catalog no. C0037; Beyotime, Shanghai, China).
Confocal microscopy.
To track the nuclear targeting and conformational changes of PCV2, PK-15 and 3D4/31 cells were infected with PCV2 at an MOI of 10 for 6, 15, 18, 21, or 24 h. The resultant cells were incubated with three anti-Cap MAbs (MAb 7F5 diluted 800 times, MAb 3F6 and 5E11 diluted 2,000 times), then with Alexa Fluor 488-conjugated goat anti-mouse MAb diluted 500 times (catalog no. A11029) at 37°C for 1 h. Early endosomes were probed with antibody anti-EEA1 and then Alexa Fluor 647 IgG (H+L) (catalog no. A-21447), and the microtubule network was stained with antibody against alpha tubulin and Alexa Fluor 546 IgG (H+L). Cellular nuclei were stained with DAPI for 5 min. Fluorescence images were recorded on an LSM780 laser confocal microscope (Zeiss, Oberkochen, Germany) and analyzed by Zen 2012 software (Zeiss).
Statistical analysis.
The reactivity of the 5E11, 7F5, and 3F6 MAbs with purified EdC-PCV2 and PCV2 was analyzed by ImageJ 1.51s and quantified with integration density. All statistical data were presented as the means ± standard deviations (SDs) and analyzed by GraphPad. Significant differences between two groups were analyzed using Student’s t tests. A P value of <0.05 or 0.01 was considered statistically significant or extremely significant, respectively.
ACKNOWLEDGMENTS
This work was supported by the National Key Technology Research and Development Program of China (grant no. 2015BAD12B01) and National Natural Science Foundation of China (grant no. 312330072).
We thank Yunqin Li and Junying Li (Bio-Ultrastructure Analysis Lab, Center of Agrobiology and Environmental Sciences, Zhejiang University), and Xing Zhang (Center of Cryo-Electron Microscopy, Zhejiang University) for technical help with confocal microscopy and electron microscopy observations.
REFERENCES
- 1.Opriessnig T, Meng X-J, Halbur PG. 2007. Porcine circovirus type 2-associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 2.Crowther RA, Berriman JA, Curran WL, Allan GM, Todd D. 2003. Comparison of the structures of three circoviruses: chicken anemia virus, porcine circovirus type 2, and beak and feather disease virus. J Virol 77:13036–13041. doi: 10.1128/jvi.77.24.13036-13041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khayat R, Brunn N, Speir JA, Hardham JM, Ankenbauer RG, Schneemann A, Johnson JE. 2011. The 2.3-angstrom structure of porcine circovirus 2. J Virol 85:7856–7862. doi: 10.1128/JVI.00737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tischer I, Gelderblom H, Vettermann W, Koch MA. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- 5.Mankertz A, Caliskan R, Hattermann K, Hillenbrand B, Kurzendoerfer P, Mueller B, Schmitt C, Steinfeldt T, Finsterbusch T. 2004. Molecular biology of porcine circovirus: analyses of gene expression and viral replication. Vet Microbiol 98:81–88. doi: 10.1016/j.vetmic.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Harms PA, Nawagitgul P, Sorden SD, Morozov I, Paul PS, Bolin SR. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- 7.Lin C, Gu J, Wang H, Zhou J, Li J, Wang S, Jin Y, Liu C, Liu J, Yang H, Jiang P, Zhou J. 2018. Caspase-dependent apoptosis induction via viral protein ORF4 of porcine circovirus 2 binding to mitochondrial adenine nucleotide translocase 3. J Virol 92. doi: 10.1128/JVI.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu J, Wang L, Jin Y, Lin C, Wang H, Zhou N, Xing G, Liao M, Zhou J. 2016. Characterization of specific antigenic epitopes and the nuclear export signal of the porcine circovirus 2 ORF3 protein. Vet Microbiol 184:40–50. doi: 10.1016/j.vetmic.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Focher F, Buhk H-J, Ferrari E, Spadari S, Hubscher U. 1988. Replication of single-stranded porcine circovirus DNA by DNA polymerases α and δ. Biochim Biophys Acta 951:280–289. [DOI] [PubMed] [Google Scholar]
- 10.Steinfeldt T, Finsterbusch T, Mankertz A. 2001. Rep and Rep′ protein of porcine circovirus type 1 bind to the origin of replication in vitro. Virology 291:152–160. doi: 10.1006/viro.2001.1203. [DOI] [PubMed] [Google Scholar]
- 11.Cheung AK. 2004. Detection of template strand switching during initiation and termination of DNA replication of porcine circovirus. J Virol 78:4268–4277. doi: 10.1128/jvi.78.8.4268-4277.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Lin C, Wang H, Wang L, Zhou N, Jin Y, Liao M, Zhou J. 2015. Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein. J Virol 89:2777–2791. doi: 10.1128/JVI.03117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung AK. 2003. The essential and nonessential transcription units for viral protein synthesis and DNA replication of porcine circovirus type 2. Virology 313:452–459. doi: 10.1016/s0042-6822(03)00373-8. [DOI] [PubMed] [Google Scholar]
- 14.Rabe B, Vlachou A, Panté N, Helenius A, Kann M. 2003. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc Natl Acad Sci U S A 100:9849–9854. doi: 10.1073/pnas.1730940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au S, Panté N. 2012. Nuclear transport of baculovirus: revealing the nuclear pore complex passage. J Struct Biol 177:90–98. doi: 10.1016/j.jsb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Marr AK, Garcin P, Panté N. 2011. Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle. J Virol 85:4863–4874. doi: 10.1128/JVI.01999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Tikoo SK, Babiuk LA. 2001. Nuclear localization of the ORF2 protein encoded by porcine circovirus type 2. Virology 285:91–99. doi: 10.1006/viro.2001.0922. [DOI] [PubMed] [Google Scholar]
- 18.Misinzo G, Meerts P, Bublot M, Mast J, Weingartl HM, Nauwynck HJ. 2005. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J Gen Virol 86:2057–2068. doi: 10.1099/vir.0.80652-0. [DOI] [PubMed] [Google Scholar]
- 19.Misinzo G, Delputte PL, Lefebvre DJ, Nauwynck HJ. 2009. Porcine circovirus 2 infection of epithelial cells is clathrin-, caveolae- and dynamin-independent, actin and rho-GTPase-mediated, and enhanced by cholesterol depletion. Virus Res 139:1–9. doi: 10.1016/j.virusres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Misinzo G, Delputte PL, Nauwynck HJ. 2008. Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells. J Virol 82:1128–1135. doi: 10.1128/JVI.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theerawatanasirikul S, Phecharat N, Prawettongsopon C, Chaicumpa W, Lekcharoensuk P. 2017. Dynein light chain DYNLL1 subunit facilitates porcine circovirus type 2 intracellular transports along microtubules. Arch Virol 162:677–686. doi: 10.1007/s00705-016-3140-0. [DOI] [PubMed] [Google Scholar]
- 22.Mahe D, Blanchard P, Truong C, Arnauld C, Le Cann P, Cariolet R, Madec F, Albina E, Jestin A. 2000. Differential recognition of ORF2 protein from type 1 and type 2 porcine circoviruses and identification of immunorelevant epitopes. J Gen Virol 81:1815–1824. doi: 10.1099/0022-1317-81-7-1815. [DOI] [PubMed] [Google Scholar]
- 23.Shang S-B, Jin Y-L, Jiang X-T, Zhou J-Y, Zhang X, Xing G, He JL, Yan Y. 2009. Fine mapping of antigenic epitopes on capsid proteins of porcine circovirus, and antigenic phenotype of porcine circovirus type 2. Mol Immunol 46:327–334. doi: 10.1016/j.molimm.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Ros C, Baltzer C, Mani B, Kempf C. 2006. Parvovirus uncoating in vitro reveals a mechanism of DNA release without capsid disassembly and striking differences in encapsidated DNA stability. Virology 345:137–147. doi: 10.1016/j.virol.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Mullapudi E, Füzik T, Přidal A, Plevka P. 2017. Cryo-electron microscopy study of the genome release of the dicistrovirus Israeli acute bee paralysis virus. J Virol 91:e02060-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng G, Zhang X, Plevka P, Yu Q, Tijssen P, Rossmann MG. 2013. The structure and host entry of an invertebrate parvovirus. J Virol 87:12523–12530. doi: 10.1128/JVI.01822-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhindwal S, Avila B, Feng S, Khayat R. 2019. Porcine circovirus 2 uses a multitude of weak binding sites to interact with heparan sulfate, and the interactions do not follow the symmetry of the capsid. J Virol 93:e02222-18. doi: 10.1128/JVI.02222-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang I-H, Suomalainen M, Andriasyan V, Kilcher S, Mercer J, Neef A, Luedtke NW, Greber UF. 2013. Tracking viral genomes in host cells at single-molecule resolution. Cell Host Microbe 14:468–480. doi: 10.1016/j.chom.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Neef AB, Samain F, Luedtke NW. 2012. Metabolic labeling of DNA by purine analogues in vivo. Chembiochem 13:1750–1753. doi: 10.1002/cbic.201200253. [DOI] [PubMed] [Google Scholar]
- 30.Kuntam S, Ayaydin F. 2015. Detection of S-phase of cell division cycle in plant cells and tissues by using 5-ethynyl-2′-deoxyuridine (EdU), p 311–322. In Yeung ECT, Stasolla C, Sumner MJ, Huang BQ. (ed), Plant microtechniques and protocols. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 31.Shingler KL, Yoder JL, Carnegie MS, Ashley RE, Makhov AM, Conway JF, Hafenstein S. 2013. The enterovirus 71 A-particle forms a gateway to allow genome release: a cryoEM study of picornavirus uncoating. PLoS Pathog 9:e1003240. doi: 10.1371/journal.ppat.1003240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacques DA, McEwan WA, Hilditch L, Price AJ, Towers GJ, James LC. 2016. HIV-1 uses dynamic capsid pores to import nucleotides and fuel encapsidated DNA synthesis. Nature 536:349–353. doi: 10.1038/nature19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei R, Trus I, Yang B, Huang L, Nauwynck HJ. 2018. Breed differences in PCV2 uptake and disintegration in porcine monocytes. Viruses 10:562. doi: 10.3390/v10100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuh M. 2011. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol 13:1431–1436. doi: 10.1038/ncb2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou JY, Chen Q-X, Ye J-X, Shen HG, Chen T-F, Shang S-B. 2006. Serological investigation and genomic characterization of PCV2 isolates from different geographic regions of Zhejiang province in China. Vet Res Commun 30:205–220. doi: 10.1007/s11259-006-3203-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Ma G, Li Y, Jiang X, He J, Zhou J. 2009. Characterization of monoclonal antibody against replication-associated protein of porcine circovirus. DNA Cell Biol 28:23–29. doi: 10.1089/dna.2008.0800. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J-Y, Shang S-B, Gong H, Chen Q-X, Wu J-X, Shen H-G, Chen T-F, Guo J-Q. 2005. In vitro expression, monoclonal antibody and bioactivity for capsid protein of porcine circovirus type II without nuclear localization signal. J Biotechnol 118:201–211. doi: 10.1016/j.jbiotec.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Strobel B, Miller FD, Rist W, Lamla T. 2015. Comparative analysis of cesium chloride- and iodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum Gene Ther Methods 26:147–157. doi: 10.1089/hgtb.2015.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booth DS, Avila-Sakar A, Cheng Y. 2011. Visualizing proteins and macromolecular complexes by negative stain EM: from grid preparation to image acquisition. J Vis Exp (58):e3227. doi: 10.3791/3227. [DOI] [PMC free article] [PubMed] [Google Scholar]


