Immunodominant epitopes may suppress immune responses to more desirable determinants, such as those that elicit potentially protective neutralizing antibody responses. To overcome this problem, we attempted to mask immunodominant glycan holes by immunizing rabbits with ICs consisting of the BG505 SOSIP.664 gp140 trimer and MAbs that targeted the glycan holes. We found that IC vaccination likely diverted antibody responses, to some extent, away from the glycan holes and toward other regions of the trimer. IC vaccination resulted in slower decay of HIV-1-specific antibodies than did immunization with uncomplexed trimer. We did not observe a widening of the breadth or an increase in the potency of neutralizing antibody responses compared to uncomplexed trimers. Our results suggest that selective epitope dampening of BG505 trimers by ICs is rather ineffective. However, IC vaccination may represent a novel means of increasing the duration of vaccine-induced antibody responses.
KEYWORDS: HIV, SOSIP trimer, antibody, glycan hole, immune complexes
ABSTRACT
Immune complex (IC) vaccines have been successfully used to increase immune responses against various pathogens, including HIV-1. Additionally, IC vaccines can induce qualitatively different antibody responses, with distinct antigenic specificities compared to the same antigens used alone. Here we measured the HIV-1-specific antibody responses in female New Zealand White rabbits after immunization with ICs made from BG505 SOSIP.664 trimers (BG505 trimers) and three rabbit monoclonal antibodies (MAbs) with different neutralization profiles. Two of the MAbs were specific for a hole in the glycan shield of the BG505 trimer, while the third, which bound less avidly, was specific for determinants at the gp41-gp120 interface. We found that immunization with one of the glycan-hole-specific ICs resulted in lower levels of trimer-binding antibodies compared to vaccination with the uncomplexed trimer, and that ICs made using either of the glycan-hole-specific MAbs resulted in lower rates of anti-trimer antibody decay. We concluded that ICs based on MAbs that bound to the immunodominant glycan hole epitope likely diverted antibody responses, to some extent, away from this site and to other regions of the trimer. However, this outcome was not accompanied by a widening of the breadth or an increase in the potency of neutralizing antibody responses compared with uncomplexed trimers.
IMPORTANCE Immunodominant epitopes may suppress immune responses to more desirable determinants, such as those that elicit potentially protective neutralizing antibody responses. To overcome this problem, we attempted to mask immunodominant glycan holes by immunizing rabbits with ICs consisting of the BG505 SOSIP.664 gp140 trimer and MAbs that targeted the glycan holes. We found that IC vaccination likely diverted antibody responses, to some extent, away from the glycan holes and toward other regions of the trimer. IC vaccination resulted in slower decay of HIV-1-specific antibodies than did immunization with uncomplexed trimer. We did not observe a widening of the breadth or an increase in the potency of neutralizing antibody responses compared to uncomplexed trimers. Our results suggest that selective epitope dampening of BG505 trimers by ICs is rather ineffective. However, IC vaccination may represent a novel means of increasing the duration of vaccine-induced antibody responses.
INTRODUCTION
A major goal of HIV-1 vaccine design is to elicit neutralizing antibody (NAb) responses with activity against a broad array of virus strains. This task has proved to be difficult and will likely require immunogens that expose or mimic vulnerable sites on the native HIV-1 trimer (1). Toward that end, the BG505 SOSIP.664 envelope (Env) glycoprotein trimer has been used in multiple animal immunization studies (2–11). The BG505 Env glycoprotein lacks N-glycosylation sites at positions 241 and 289 (7, 8). The resulting holes in the glycan shield expose immunodominant targets that elicit NAbs specific to the sequence-matched (i.e., autologous) BG505.T332N virus, which lacks the same N-glycosylation sites (6–8). Antibodies against sites of vulnerability associated with neutralization breadth, such as the CD4 binding site (CD4bs), V1/V2 loop region, V3/Asn332 glycan patch, gp120-gp41 interface, or membrane proximal external region, have not yet been elicited consistently by immunization with the BG505 SOSIP trimer or other recombinant Env proteins (12, 13).
It has been proposed that suppressing immunodominant non-NAb or narrow-specificity NAb epitopes may help drive the emergence of neutralization breadth (14–17). One way to decrease the immunogenicity of immunodominant regions is via epitope masking (18–24). For example, adding N-glycosylation sites to the V3 region or the position 241/289 glycan hole epitope of the BG505 trimer suppresses the immunogenicity of its non-NAb epitopes and, in some cases, diverts the NAb responses to neoepitopes (20, 25, 26). Antibodies, by forming immune complexes (ICs) with antigens, can also be used to mask immunogenic epitopes (27–29). Antibody binding can also change antigen stability and thereby affect processing pathways (30, 31) and T cell epitope presentation (32). ICs have been shown to induce qualitatively different antibody responses with distinct antigenic specificities, compared with those elicited by antigens alone, and can enhance immune responses against various viral pathogens, including HIV-1 (27, 32–36). Guided by these observations, we immunized rabbits with ICs formed between the BG505 SOSIP.664 trimer and rabbit monoclonal antibodies (MAbs) that targeted either a glycan hole at positions 241 and 289 or an epitope located at the gp120-gp41 interface around residue 611 (37).
RESULTS
IC immunization modifies titers of anti-trimer antibodies.
Four groups of rabbits were immunized with uncomplexed BG505 SOSIP.664 trimers (group D) or ICs formed between the trimer and three different rabbit MAbs (Table 1). The ICs consisted of the trimer bound to MAb 11A (group A), MAb 11B (group B), or MAb 12A (group C) (8). MAbs 11A and 11B bind to similar glycan hole epitopes that are both in the vicinity of residue S241 and have very similar binding affinities (4.6 × 10−10 M and 4.5 × 10−10 M, respectively) (Table 1) (8). MAbs 11A and 11B neutralize the parental BG505.T332N virus with IC50 values of 0.17 and 0.11 μg/ml, respectively (8). MAb 12A binds to the gp41-gp120 interface close to the epitope for the PGT151 broadly neutralizing antibody (bNAb) and has a lower binding affinity for the BG505 trimer (7.9 × 10−8 M) than does MAb 11A or MAb 11B (Table 1) (37). MAb 12A very weakly neutralizes the parental virus (IC50 of 100 μg/ml) but very potently neutralizes the same virus from which the N611 glycan has been removed (IC50 of 1.06 μg/ml) (8).
TABLE 1.
Vaccination groups (n = 5 animals/group) and MAbs used to make SOSIP ICsa
| Group | Antibody | Specificity | Kd (M) | kon (M/s) | koff (1/s) |
|---|---|---|---|---|---|
| A | 11A | Glycan hole, C2; centered at S241 | 4.6 × 10−10 | 8.5 × 104 | 3.8 × 10−5 |
| B | 11B | Glycan hole, C2; centered at S241 | 4.5 × 10−10 | 4.6 × 104 | 2.1 × 10−5 |
| C | 12A | Overlap with MAb PGT151 determinants | 7.9 × 10−8 | 5.6 × 103 | 4.5 × 10−4 |
| D | None |
The BG505 trimer batch used for the immunization was confirmed to have an appropriate antigenic conformation by demonstration of its binding to MAbs 11A, 11B, and 12A (Fig. 1A), as well as to the trimer-specific MAb PGT145, but not to the gp120-monomer-specific MAb F105 (Fig. 1B). We found no allosteric changes induced upon IC formation, based on our antibody controls PGT145 and F105 (Fig. 2).
FIG 1.
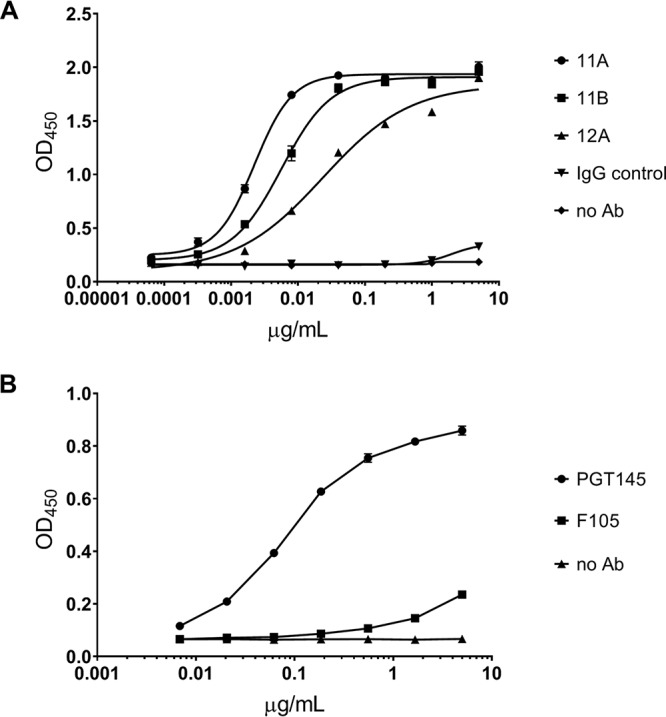
Binding of MAbs 11A, 11B, and 12A to BG505 SOSIP.664 trimers. (A) MAb binding to trimers immobilized with D7324 antibody was measured by ELISA. (B) BG505 SOSIP.664 trimer binds to the quaternary, configuration-dependent, human MAb PGT145 but not to the human MAb F105, which is directed against a non-NAb epitope associated with the CD4bs. MAbs were tested in duplicate. Curve fitting was performed with GraphPad Prism 8.0 software.
FIG 2.
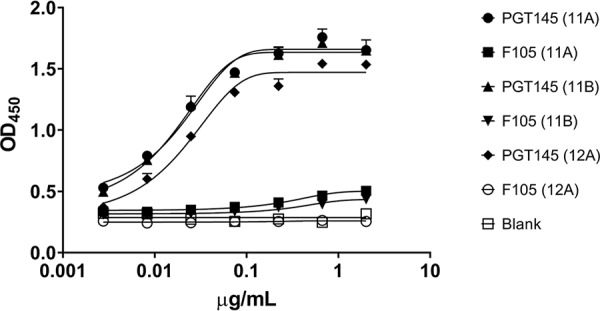
Trimer-MAb IC formation does not affect MAb PGT145 or MAb F105 binding. The antigenicity of ICs captured by polyclonal goat antibody directed against rabbit IgG was determined by binding with the conformation-dependent MAb PGT145 or the conformation-independent MAb F105. MAbs were tested in duplicate. Curve fitting was performed with GraphPad Prism 8.0 software.
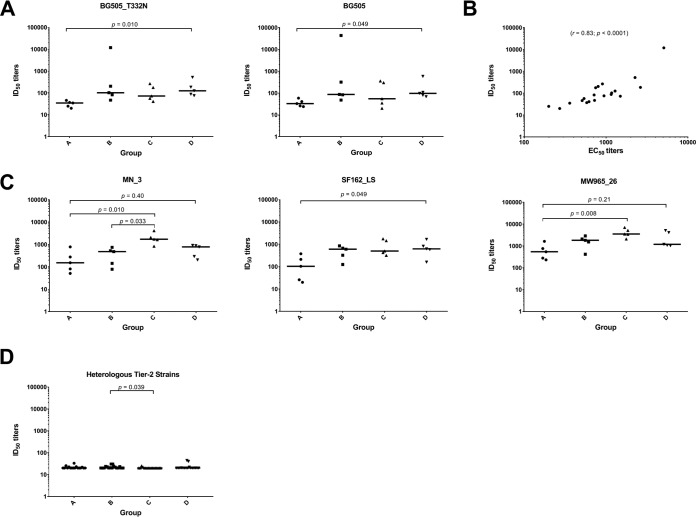
Serum samples from all immunization groups at weeks 0, 22, and 26 were assessed for anti-trimer binding, and the midpoint titers (i.e., 50% effective concentration [EC50] values) were calculated after applying a nonlinear regression fit to the antibody binding curves. Using serum samples obtained 2 weeks after the last immunization (i.e., week 22), we observed a significant difference in median anti-trimer binding antibody titers between the individual groups (P = 0.0056). In pairwise analyses, the difference was due to a lower median binding titer in group A (MAb 11A ICs), compared with the trimer-only immunogen group D (P = 0.002) (Fig. 3A). By week 26 (6 weeks after the last immunization), anti-trimer titers had declined in all four groups (Fig. 3B). Compared to group D, however, the rate of BG505-specific antibody midpoint titer decline was significantly lower for group A (P = 0.042, Kruskal-Wallis test with Dunn’s multiple-comparison test) and group B (P = 0.006) but not for group C (P = 1.0). There was a trend toward a lower rate of decline for group B, compared to group C (P = 0.090). Group A titers also declined more slowly than group C, but the difference was not statistically significant after adjustment for multiple comparisons (P = 0.39).
FIG 3.
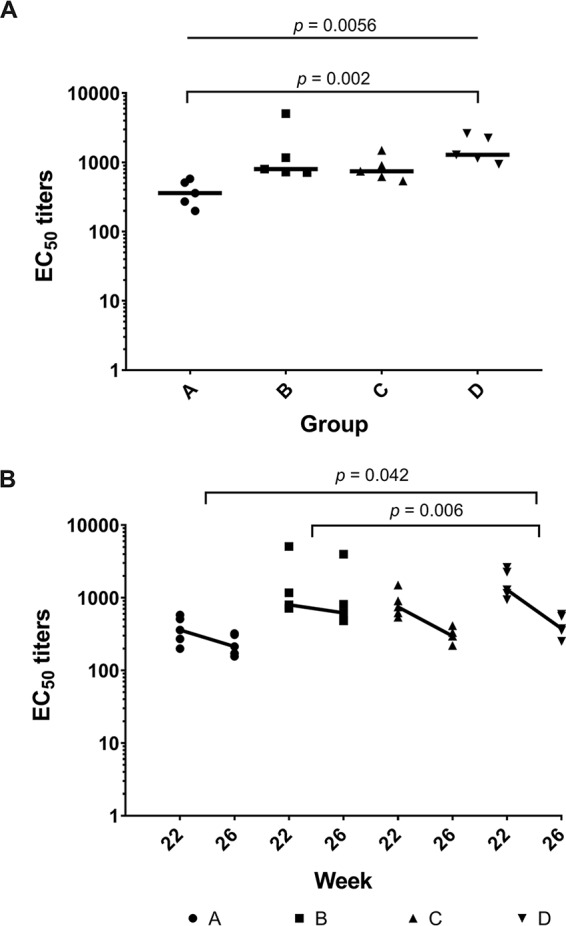
IC immunization may decrease anti-trimer antibody responses and the rate of antibody decay. (A) Antibody binding to the BG505 SOSIP.664 trimer in individual sera from animals in each group was measured by ELISA at week 22. (B) Binding responses at weeks 22 and 26 are plotted. Thick lines represent the median rate of decay. Median decay rates (log EC50/week) were −0.05 (group A), −0.04 (group B), −0.1 (group C), and −0.15 (group D). P values were calculated using the Kruskal-Wallis test with Dunn’s multiple-comparison test.
NAb responses are generally lower with IC immunization.
To determine whether IC vaccination had an impact on virus neutralization, sera were tested against a panel of tier 1 (n = 5) and tier 2 (n = 19) HIV-1 isolates at Duke University Medical Center (Fig. 4). Autologous NAb responses to the autologous BG505.T332N virus were significantly lower in group A animals than in group D animals (P = 0.01) (Fig. 5A). Correspondingly, NAb titers against the parental strain BG505 were also significantly lower in group A than in group D (P = 0.049) (Fig. 5A). As reported previously, there was a strong correlation (r = 0.83; P < 0.0001) between BG505.T332N NAb responses measured as the 50% inhibitory dilution (ID50) and BG505.T332N anti-trimer binding antibody titers measured as the EC50 (Fig. 5B) (38, 39).
FIG 4.
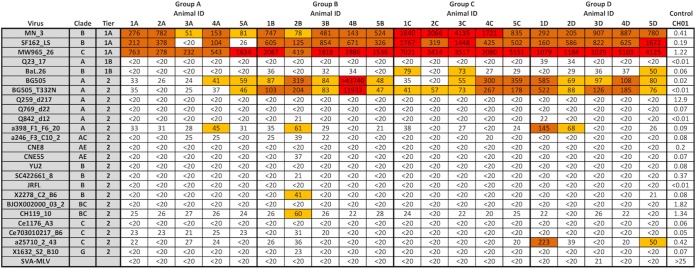
IC immunization does not alter the breadth of NAb responses. Data represent serum ID50 values measured from week 22 sera using Env-pseudotyped viruses and TZM-bl target cells. Boxes are color coded according to the magnitude of neutralization, i.e., ID50 of <40 (white), ID50 of 40 to 100 (yellow), ID50 of 100 to 1,000 (orange), or ID50 of >1,000 (red). Simian virus amphotropic murine leukemia virus (SVA-MLV) was used as a negative control virus, and MAb CH01 served as a positive control antibody. All serum samples were assayed in duplicate.
FIG 5.
IC immunization affects some NAb responses. (A) Autologous neutralization potency against BG505_T332N and BG505 is reduced in sera from group A animals. (B) NAb responses correlate with ELISA binding titers. Binding (EC50) and neutralization (ID50) results were analyzed by Spearman correlation. (C) For certain tier 1 HIV-1 strains, neutralizing activity is decreased by IC immunization. Results are shown for the three HIV-1 strains (indicated above each graph) that were neutralized at ID50 values of >20 by 4 of the 5 animals. (D) Neutralizing activity against tier 2 strains is limited and does not differ between vaccination groups except for group B versus group C (see also Fig. 4). For data analysis, ID50 values of <20 and >43,740 were considered to equal 20 and 43,740, respectively. P values were determined by the Kruskal-Wallis test followed by Dunn’s multiple-comparison test.
We also looked at NAb titers against tier 1 strains that were neutralized by sera from at least 4 of the 5 animals in each group (ID50 values of >20). Consistent with the antibody binding data, group A animals responded with lower NAb titers against each of these strains, compared to the control group D, although the differences were not always statistically significant P = 0.4 for MN_3, P = 0.049 for SF162_LS, and P = 0.21 for MW965_26 (Fig. 5C). In the case of MN_3, the NAb responses for both group A and group B were significantly lower than those for group C (P = 0.01 and P = 0.03, respectively). For MW965_26, the responses in group A were significantly lower than those in group C (P = 0.008) (Fig. 5C). Overall, neutralization breadth and potency against heterologous tier 2 strains (n = 17) in our virus panel were limited and did not differ between the groups except for NAb titers in group B, which were significantly higher than those in group C (P = 0.039) (Fig. 5D).
Epitope mapping reveals various NAb determinants.
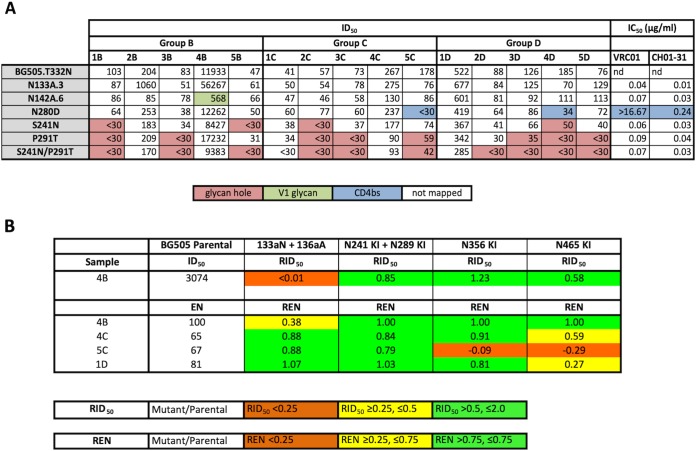
The most frequently targeted autologous NAb epitope in BG505-SOSIP-trimer-immunized rabbits is a hole in the glycan shield created by the absence of the N241 and N289 glycans (6, 8, 40). To assess whether the same or a different epitope (or epitopes) was targeted in the IC-immunized rabbits, we used the same method, based on BG505.T332N mutant viruses, to analyze all of the sera from groups B, C, and D (Fig. 6A). The group A sera were not tested, since the NAb titers against the wild-type BG505.T332N virus were too low to be mapped with any precision (6). According to the Duke University Medical Center mapping data, virus mutants with the N241 glycan and/or P291T substitution knocked in were predominantly resistant to neutralization by 3 of the 5 group B sera (sera 1B, 3B, and 5B) and group C sera (sera 2C, 3C, and 5C), as well as by 4 of the 5 group D sera (sera 2D, 3D, 4D, and 5D) (Fig. 6A). In contrast, the NAbs in sera 2B, 4B, 1C, 4C, and 1D did not target the N241/N289 glycan hole. This analysis suggests that, in most cases, immunization with ICs containing glycan-hole-specific MAb 11B or gp120-gp41-interface-specific MAb 12A did not divert the NAb response away from the glycan hole that is predominantly targeted in the trimer-only group D.
FIG 6.
Antibody responses to IC immunogens and uncomplexed trimers are predominantly directed against epitopes in the glycan hole. (A) BG505.T332N virus variants were used to map neutralizing determinants in all sera except those in group A, for which the NAb titers were too low. Determinants involved in neutralizing responses are color coded and were identified by ≥3-fold reductions in NAb titers against variants containing mutations in relevant epitopes. For values that were below the detection limit (ID50 of <30), one-half the cutoff value (ID50 of 15) was used for calculations. Mapping was conducted at Duke University Medical Center. (B) Further mapping was performed at WCMC using BG505 virus mutants with sequence changes affecting V1 (133aN plus 136aA), CD4bs (N356 KI), and a newly identified glycan epitope (N465 KI). RID50 refers to the ID50 against the mutant/ID50 against the parental BG505 strain. REN is the ratio of the extent of neutralization of the mutant versus the parental strain using IgG corresponding to a 1:50 dilution of serum.
We conducted further mapping studies at Weill Cornell Medical College (WCMC) to characterize the NAb responses of samples 4B, 4C, 5C, and 1D against a previously described immunodominant C3/465 epitope (6). Based on partial resistance of the N142A.6 mutant, the response in serum 4B targeted the V1 loop, a rare but not unprecedented response to BG505 SOSIP trimers (Fig. 6A) (6). The use of the 133aN plus 136aA virus mutants at WCMC confirmed that the neutralizing activity of serum 4B was directed against a V1 epitope (Fig. 6B) (6). For serum 5C, the NAbs were directed against the C3/465 epitope. It is of note that the neutralization potencies of serum from animal 5C and the CD4bs-specific MAb VRC01 were highly affected by the N280D mutation (Fig. 6A). NAbs in sera 4C and 1D recognized the C3/465 epitope, albeit to a lesser extent than did serum 5C (Fig. 6B).
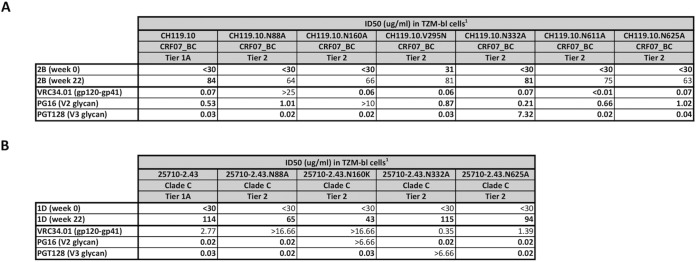
Serum from animal 2B, which had modest activity against the heterologous tier 2 virus HIV-1CH119.10 (Fig. 4) and did not target the N241/N289 glycan hole on the BG505.T332N virus (Fig. 6A), was further evaluated at Duke University Medical Center by using a panel of HIV-1CH119.10 variants that could be differentiated by binding to bNAbs VRC34.01 (gp120-gp41 interface), PG16 (V2 glycan), and PGT128 (V3 glycan) (Fig. 7A). Serum 1D, which neutralized the heterologous tier 2 virus HIV-1a25710_2_43 (Fig. 4), was studied in an analogous way with mutants of that virus (Fig. 7B). None of the HIV-1CH119.10 mutants was significantly resistant to serum 2B, implying that the NAbs present did not target the gp120-gp41 interface, V2 glycan, or V3 glycan epitopes; overall, the NAb activity present in this serum could not be mapped to a known epitope. In contrast, reduction of neutralization in serum 1D by the N160K mutation was consistent with targeting of the gp120-gp41 interface (VCR34.01-like). Targeting of V2 glycan (PG16-like activity) is suggested by the lack of a >3-fold reduction in activity with the N160K mutation; however, the <3 fold-reduction in the 1D serum associated with the N88A mutation makes V2 glycan targeting unclear.
FIG 7.
Mapping of neutralizing determinants in sera 2B and 1D. (A) Antibody mapping of serum from animal 2B at week 0 and week 22 was performed with variants derived from HIV-1CH119.10. (B) Serum from animal 1D was tested against a panel of variants derived from HIV-125710-2.43. The human MAbs VRC34.01, PG16, and PGT128 were used as controls. ID50 values are reported for serum samples and IC50 values for the control MAbs. Serum samples were tested in duplicate.
Vaccination with ICs made with glycan-hole-specific antibodies results in lower serum anti-V3 antibody responses.
HIV-1 tier 1 viruses are highly sensitive to anti-V3 Abs (41). To test whether V3-specific antibody to the V3 crown was made, we analyzed the immune sera of all groups against a set of HIV-1MN V3 peptides. Sera in groups A and B revealed lower binding signals (>2-fold) than did sera in groups A and B (Fig. 8A). The HIV-1MN V3-specific binding signals correlated with the HIV-1MN_3 neutralization titers (r = 0.45; P = 0.049) (Fig. 8B), suggesting the presence of NAbs against the V3 crown.
FIG 8.
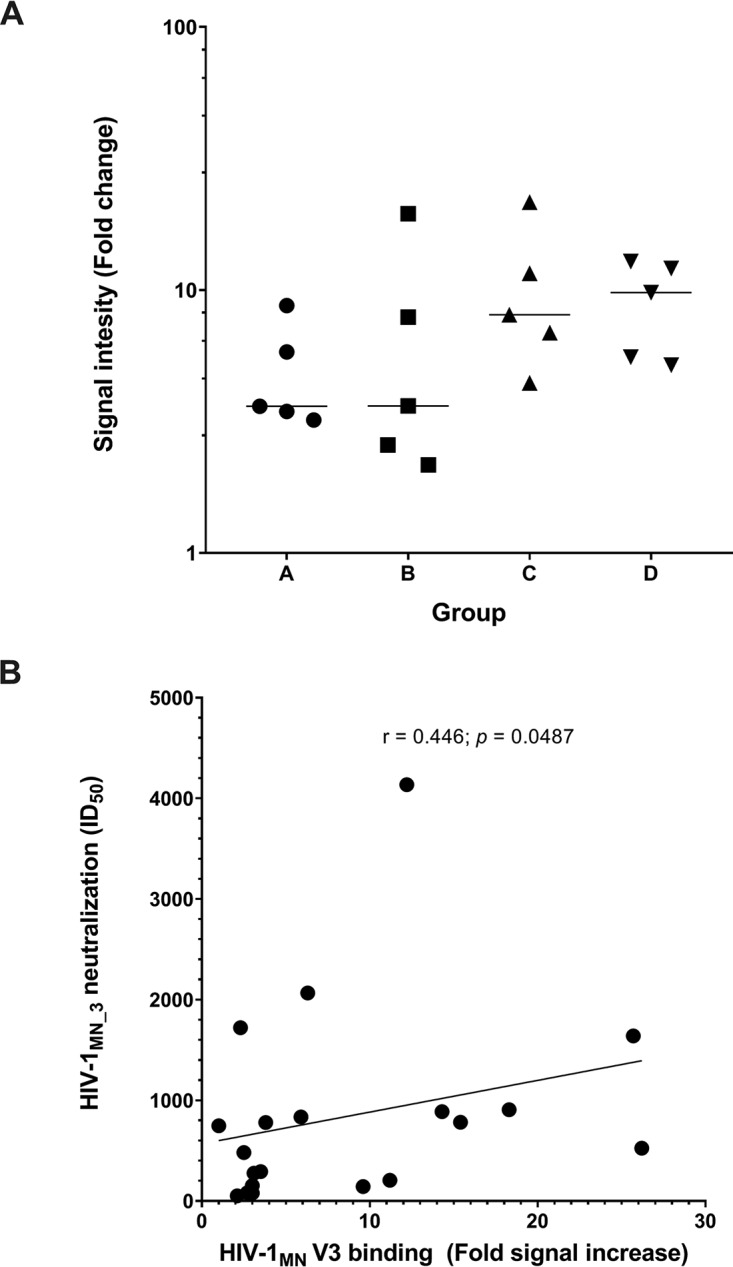
IC immunization resulted in variable binding to HIV-1 MN V3 peptides, which correlated with neutralizing activity against HIV-1MN_3. (A) Sera of all groups were tested by ELISA for binding against three overlapping V3 peptides. All samples were tested in duplicate. (B) HIV-1MN_3 neutralization correlates with HIV-1 MN V3 peptide binding, as analyzed by Spearman correlation.
DISCUSSION
In this study, we assessed the immunogenicity in rabbits of ICs composed of BG505 SOSIP.664 trimers and rabbit MAbs directed against N241/N289 glycan hole or gp120-gp41 interface epitopes. The ICs formed using MAb 11A (glycan hole epitope) induced lower titers of anti-trimer antibodies than did ICs made with the other MAbs (including MAb 11B, to a similar epitope) or with the uncomplexed BG505 SOSIP trimer. Furthermore, immunization with ICs based on either of the two glycan hole MAbs resulted in 2- to 3-fold lower rates of binding antibody decay, compared to the uncomplexed control group.
There was no increase in the potency or breadth of the NAb responses induced by ICs compared with the uncomplexed trimer. However, the formation of ICs using MAbs to the glycan hole epitope might have diverted the antibody responses, in some cases, away from that immunodominant autologous NAb epitope and to other regions of the BG505 virus.
Previous immunization studies with HIV-1 Env-based IC vaccines generally resulted in increased Env-specific antibody titers and tier 1 NAb titers, compared to uncomplexed vaccines (32, 34, 35, 41). However, the increase of Env-specific antibody responses was mostly attributed to allosteric effects between gp120- and CD4bs-specific antibodies, which stabilized the V3 loop for better recognition and also rendered the gp120 protein more resistant to proteolytic degradation (41). Here, we observed the opposite, with lower median BG505.T332N trimer-specific antibody titers and lower autologous NAb titers against HIV-1BG505.T332N in all three IC groups, compared to the uncomplexed trimer group, at week 22. We assume that direct masking or shielding of immunodominant determinants by antibody may account for the lower overall anti-trimer antibody responses we observed. This is in accordance with a recent study finding that immunization with ICs consisting of HIV-1JR-FL gp120 and the C2-specific MAb 1006-30D (which binds to epitopes that overlap MAb 11A and 11B epitopes) resulted in overall lower antibody titers against gp120, the V1/V2 loop, and the V3 loop, as well as lower NAb titers against the tier 1 isolate HIV-1SF162, compared to the uncomplexed gp120 control (41, 42).
Immunization with ICs containing MAb 11A resulted in notably less antibody binding and neutralization potency compared to the uncomplexed trimer. This outcome was not seen with ICs based on MAb 11B, despite binding to a similar glycan hole epitope (8). The difference in neutralizing activity may be related to the fact that the two MAbs vary slightly in their epitope-binding properties. MAb 11B binds closer to the Env apex than does MAb 11A, which binds closer to the viral membrane. In addition, MAb 11A, but not MAb 11B, binds to the BG505 SOSIP.664 trimer with a S241N mutation, suggesting more binding flexibility. Compared to MAb 11B, MAb 11A has a 1.8-fold greater kon rate (8.5 × 104 M/s versus 4.6 × 104 M/s) and a 1.8-fold greater koff rate (3.8 × 10−5 1/s versus 2.1 × 10−5 1/s) (Table 1). These different epitope-binding properties might affect the binding of other NAbs through steric hindrance. Indeed, competition assays with MAbs 11A and 11B against a panel of human gp120-gp41 interface MAbs revealed interference by MAb 11A, whereas MAb 11B had no effect (8). Finally, all of these differences could affect the antigenicity, stability, and half-life of ICs and thus the production of NAbs after rabbit immunization.
One of the primary goals of this study was to divert the immune response away from an immunodominant glycan hole at positions 241 and 289 by forming ICs with the glycan-hole-specific MAbs 11A and 11B. Although it was demonstrated previously, using a different strategy, that blocking undesired epitopes on BG505 trimers lowered tier 1 NAb responses (25), we found only 2 animals in group B, compared with 1 each in groups C and D, that had NAb responses against epitopes other than the glycan hole. Unfortunately, group A sera could not be evaluated thoroughly for glycan-hole-specific NAb responses because NAb titers against the autologous HIV-1 isolate BG505.T332N were too low. Thus, blocking of glycan hole reactivity by MAb 11B cannot be considered very effective. MAb 11B might be considered an inferior blocker of the glycan hole, compared with MAb 11A, based on the fact that MAb 11B cannot bind the BG505 trimer in the presence of a glycan at N241 (8). The fact that the gp120-gp41-interface-specific MAb 12A was ineffective in blocking immunodominant glycan-hole-specific epitopes is possibly due to its notably lower affinity for BG505 trimers and the minimal effect that the absence of an N241 glycan has on MAb 12A binding to BG505 trimers (8).
Most of the NAb responses were mapped to epitopes in the glycan hole. However, sera from some animals were of particular interest. In the case of animal 2B, we were not able to map the neutralizing activity to any known epitope. This serum had low-level cross-neutralizing activity, with ID50 values of >20 against 10 of 17 heterologous tier 2 HIV-1 strains. Animal 5C revealed potential activity directed against the CD4bs, since the BG505.T332N virus N280D mutant was markedly resistant to neutralization. However, the NAb activity in serum 5C was also likely directed against the C3/465 epitope cluster, given the 4-fold reduction in activity with the N241 knock-in (KI)/N289 KI mutant and an even stronger reduction with the N465 KI mutant. We think that, overall, the polyclonal neutralizing activity of serum 5C was mainly associated with the C3/465 epitope cluster, with possible indirect effects of the N282D mutation. We observed that deleting the N-glycosylation site from position N133 rendered the BG505.T332N virus 5-fold more sensitive to NAbs in the sera of animals 2B and 4B. It was shown recently that removal of specific N-glycosylation sites can have significant effects on viral infectivity and antibody-mediated neutralization (43). For example, a mutation in V1 at position N133 (N133Q) increased the sensitivity of the virus (HIV-1CRF07_BC Env, FE) to a V3-specific antibody (MAb 3869) 5-fold (43).
Finally, we observed 2- to 3-fold lower rates of antibody decay in rabbits immunized with ICs. Additional sampling at longer time intervals will be necessary to confirm this finding in future studies. To our knowledge, a decrease in antibody decay has not been previously ascribed to the use of IC immunogens. Although we have gone no further to investigate the mechanisms of this delay in antibody decay, it is plausibly associated with the recognition of ICs by Fcγ receptors expressed on the surface of antigen-presenting cells; the ICs may thus be processed differently or at a different rate (depot effect) than the uncomplexed antigen (44–48). For example, Fcγ receptor-mediated antigen processing could affect B cell activation and differentiation (49, 50) or germinal center memory B cells (51–53) and secondary antibody responses (54). In addition, the formation of ICs in the presence of complement factors could lead to more efficient deposition of antigen on follicular dendritic cells (55).
MATERIALS AND METHODS
Reagents.
BG505 SOSIP.664 trimers were expressed in CHO cells and purified as described previously (2). Rabbit MAbs 11A, 11B, and 12A were transiently expressed, affinity purified, and checked for purity and integrity as described previously (15). Human MAbs PGT145 and F105, as well as the HIV-1 MN Env (15-mer) V3 peptides CTRPNYNKRKRIHIG, RKRIHIGPGRAFYTT, and HIGPGRAFYTTKNII, were obtained from the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH.
Rabbit immunization.
Immunizations and blood sampling were carried out under subcontract by Pacific Immunology (Ramona, CA). Prior to immunization, the BG505 trimers (30 μg/rabbit) were incubated for 30 min at room temperature with or without one of the rabbit MAbs (32 μg/rabbit), at a molar ratio of 1:3, and then formulated in 75 units of Iscomatrix adjuvant. The immunization mixture was injected intramuscularly into female New Zealand White (NZW) rabbits (5 rabbits per group). The use of rabbit antibodies for IC formulation avoids anti-immunoglobulin responses after rabbit immunization. The animals were immunized at weeks 0, 4, and 20 and bled at weeks 0, 6, 8, 12, 16, 22, and 26, as described previously (40, 56).
Ethics statement.
The NZW rabbits were housed, immunized, and bled at Pacific Immunology, in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and in adherence to the National Research Council Guide for the Care and Use of Laboratory Animals.
ELISA for anti-trimer antibodies.
Ninety-six-well enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with the gp120-C5-epitope-specific antibody D7324 (500 ng/well). Wells were then washed and blocked in 5% blocking solution (5% nonfat dry milk in Dulbecco’s phosphate-buffered saline containing 0.05% Tween) for 1 h at 37°C (9). After washing and blocking, plates were further incubated with D7324-epitope-tagged BG505 trimers (50 ng/well) for 2 h at 37°C. Duplicates of serially diluted (1:5 in 1% blocking buffer) rabbit serum samples (starting at a dilution of 1:20) or of MAbs (starting at 5 μg/ml) were added and incubated for 1 h at 37°C. Unbound antibodies were washed away, and trimer-specific antibodies were detected with a goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP), diluted in 1% blocking solution. After 1 h, plates were washed, developed with 3,3′,5,5′-tetramethylbenzidine (TMB) solution, and subsequently read at 450 nm with a Synergy 2 plate reader (BioTek). The EC50 values were determined using GraphPad Prism 7.0 software after applying a nonlinear regression curve fit to the antibody binding curves.
IC conformation assay.
ELISA plates were coated with 2 μg/ml (100 ng/well) of a goat anti-rabbit Fc antibody and incubated overnight at 4°C. Plates were then washed and blocked with 5% blocking solution. In the meantime, MAbs 11A, 11B, and 12A (1 μg/ml) were mixed with 1 μg/ml of BG505 trimers and incubated for 45 min at 37°C. After blocking, ELISA plates were washed and incubated with the MAb/BG505 trimer mixture for 1 h at 37°C. IC formation was tested with a serial dilution (1:3) of human MAbs PGT145 and F105. Diluted antibodies were incubated for 1 h at 37°C. Bound antibodies were detected with a HRP-labeled goat anti-human Fab antibody. Plates were developed as described above.
Peptide ELISA.
HIV-1 MN V3 Env peptides (15-mers) were coated at 500 ng per well (10 μg/ml) and incubated overnight at 4°C. After washing, wells were blocked in 5% blocking solution for 1 h at 37°C. Replicates of diluted rabbit sera at week 0 (preimmune serum control) and week 22 (1:100 dilutions in 1% blocking buffer) were added and incubated for 1 h at 37°C. Unbound antibody was removed by washing and bound antibodies were detected by a goat anti-rabbit IgG-HRP conjugate. After 1 h at 37°C, plates were washed and analyzed as described above. Fold increases in signal intensity of week 22 serum versus week 0 serum from the same animal were calculated.
Neutralization assays.
Neutralization activity of the rabbit sera at week 22 was measured with Env-pseudotyped viruses in TZM-bl cells, as described previously (57). In brief, heat-inactivated test samples were serially diluted 1:3 in duplicate and incubated for 1 h at 37°C with a pretitered dose of the respective pseudotyped HIV-1 virions. Freshly trypsinized TZM-bl cells (10,000 cells per well) were then added to each well and incubated for 48 h at 37°C in the presence of DEAE-dextran (15 μg/ml). Luminescence was measured using the Britelite luminescence reporter gene assay system (PerkinElmer Life Sciences). A virus control (cells and virus) and a background control (cells only) were used to calculate neutralization titers (ID50), defined as the dilution at which relative luminescence units (RLU) were reduced by 50%, compared to the values for the virus control wells after subtraction of background control RLUs. All stocks of HIV-1 Env-pseudotyped viruses for neutralization assays were prepared by transfection in 293T cells and were titrated in TZM-bl cells, as described previously (57).
Epitope mapping assays.
Epitope mapping of selected rabbit serum samples was performed at Duke University Medical Center and WCMC. Mapping at Duke University Medical Center was conducted using the following BG505 virus mutants provided by WCMC: N133A, N142A, N280D, S241N, P291T, and S241N plus P291T mutants. Further mapping at Duke University Medical Center was carried out with the CH119.10 virus N88A, N160A, V295N, N332A, N611A, and N625A variants, as well as the 25710-2.43 virus N88A, N160K, N332A, and N625A mutants. Human MAbs VRC01, CH01-31, VRC34.01, PG16, and PGT128 were used as controls. All mutants were prepared and titrated as described previously (57). Differences in serum mapping were considered real when the calculated ID50 values for the parental strain and the respective mutant were at least 3-fold different. For values that were below our limit of detection (ID50 of <30), we used one-half the cutoff value (ID50 of 15) for calculations. Mapping at WCMC was performed using the BG505 virus 133aN plus 136aA, N241 KI plus N289 KI, N356 KI, and N465 KI mutants. Relative ID50 (RID50) and relative extent of neutralization (REN) were calculated at WCMC as described elsewhere (6). In brief, for RID50, the ID50 against the mutant was divided by the ID50 against the parental strain; for REN, the effect on neutralization was expressed as the extent of inhibition of mutant strains divided by that of the parental strain at a 1:50 dilution of serum.
Statistics.
Kruskal-Wallis tests with Dunn’s multiple-comparison tests were used to analyze differences in continuous variables between groups. Correlations were analyzed using Spearman’s rho. Statistical analyses were conducted using GraphPad Prism 8.0.
ACKNOWLEDGMENTS
We thank Thomas Ketas and Albert Cupo for their excellent technical support and Dennis Burton for providing MAbs 11A, 11B, and 12A, as well as for his support of L.E.M. through the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID).
This study was partially supported by the National Institute of Allergy and Infectious Diseases under awards R01 AI118581 (D.N.F.), R01 AI36082 (P.J.K. and J.P.M.), P01 AI110657 (M.J.V.G., P.J.K., R.W.S., and J.P.M.), HHSN272201800004C (C.C.L. and D.C.M.), and CHAVI-ID UM1AI100663 (L.E.M.).
REFERENCES
- 1.Sanders RW, Moore JP. 2017. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol Rev 275:161–182. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capucci S, Wee EG, Schiffner T, LaBranche CC, Borthwick N, Cupo A, Dodd J, Dean H, Sattentau Q, Montefiori D, Klasse PJ, Sanders RW, Moore JP, Hanke T. 2017. HIV-1-neutralizing antibody induced by simian adenovirus- and poxvirus MVA-vectored BG505 native-like envelope trimers. PLoS One 12:e0181886. doi: 10.1371/journal.pone.0181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Taeye SW, de la Pena AT, Vecchione A, Scutigliani E, Sliepen K, Burger JA, van der Woude P, Schorcht A, Schermer EE, van Gils MJ, LaBranche CC, Montefiori DC, Wilson IA, Moore JP, Ward AB, Sanders RW. 2018. Stabilization of the gp120 V3 loop through hydrophobic interactions reduces the immunodominant V3-directed non-neutralizing response to HIV-1 envelope trimers. J Biol Chem 293:1688–1701. doi: 10.1074/jbc.RA117.000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu JK, Crampton JC, Cupo A, Ketas T, van Gils MJ, Sliepen K, de Taeye SW, Sok D, Ozorowski G, Deresa I, Stanfield R, Ward AB, Burton DR, Klasse PJ, Sanders RW, Moore JP, Crotty S. 2015. Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J Virol 89:10383–10398. doi: 10.1128/JVI.01653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klasse PJ, Ketas TJ, Cottrell CA, Ozorowski G, Debnath G, Camara D, Francomano E, Pugach P, Ringe RP, LaBranche CC, van Gils MJ, Bricault CA, Barouch DH, Crotty S, Silvestri G, Kasturi S, Pulendran B, Wilson IA, Montefiori DC, Sanders RW, Ward AB, Moore JP. 2018. Epitopes for neutralizing antibodies induced by HIV-1 envelope glycoprotein BG505 SOSIP trimers in rabbits and macaques. PLoS Pathog 14:e1006913. doi: 10.1371/journal.ppat.1006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klasse PJ, LaBranche CC, Ketas TJ, Ozorowski G, Cupo A, Pugach P, Ringe RP, Golabek M, van Gils MJ, Guttman M, Lee KK, Wilson IA, Butera ST, Ward AB, Montefiori DC, Sanders RW, Moore JP. 2016. Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from clades A, B and C. PLoS Pathog 12:e1005864. doi: 10.1371/journal.ppat.1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy LE, van Gils MJ, Ozorowski G, Messmer T, Briney B, Voss JE, Kulp DW, Macauley MS, Sok D, Pauthner M, Menis S, Cottrell CA, Torres JL, Hsueh J, Schief WR, Wilson IA, Ward AB, Sanders RW, Burton DR. 2016. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep 16:2327–2338. doi: 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliepen K, Ozorowski G, Burger JA, van Montfort T, Stunnenberg M, LaBranche C, Montefiori DC, Moore JP, Ward AB, Sanders RW. 2015. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 12:82. doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrents de la Pena A, Julien JP, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens AJ, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW. 2017. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep 20:1805–1817. doi: 10.1016/j.celrep.2017.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward AB, Wilson IA. 2017. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev 275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AP Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. 2014. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angeletti D, Gibbs JS, Angel M, Kosik I, Hickman HD, Frank GM, Das SR, Wheatley AK, Prabhakaran M, Leggat DJ, McDermott AB, Yewdell JW. 2017. Defining B cell immunodominance to viruses. Nat Immunol 18:456–463. doi: 10.1038/ni.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng C, Pancera M, Bossert A, Schmidt SD, Chen RE, Chen X, Druz A, Narpala S, Doria-Rose NA, McDermott AB, Kwong PD, Mascola JR. 2016. Immunogenicity of a prefusion HIV-1 envelope trimer in complex with a quaternary-structure-specific antibody. J Virol 90:2740–2755. doi: 10.1128/JVI.02380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale GA, Shartouny JR, Jacob J. 2017. Quantifying the shifting landscape of B cell immunodominance. Nat Immunol 18:367–368. doi: 10.1038/ni.3695. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff MC, Kim EH, Luo W, Pulendran B. 2018. B cell competition for restricted T cell help suppresses rare-epitope responses. Cell Rep 25:321–327.e323. doi: 10.1016/j.celrep.2018.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed FK, Clark BE, Burton DR, Pantophlet R. 2012. An engineered mutant of HIV-1 gp120 formulated with adjuvant Quil A promotes elicitation of antibody responses overlapping the CD4-binding site. Vaccine 30:922–930. doi: 10.1016/j.vaccine.2011.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan H, Chen X, Boyington JC, Cheng C, Zhang Y, Jafari AJ, Stephens T, Tsybovsky Y, Kalyuzhniy O, Zhao P, Menis S, Nason MC, Normandin E, Mukhamedova M, DeKosky BJ, Wells L, Schief WR, Tian M, Alt FW, Kwong PD, Mascola JR. 2018. Glycan masking focuses immune responses to the HIV-1 CD4-binding site and enhances elicitation of VRC01-class precursor antibodies. Immunity 49:301–311.e305. doi: 10.1016/j.immuni.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, Goudsmit J, Nara PL. 1997. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol 159:279–289. [PubMed] [Google Scholar]
- 21.Pantophlet R, Wilson IA, Burton DR. 2003. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol 77:5889–5901. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. 2005. Comparing antigenicity and immunogenicity of engineered gp120. J Virol 79:12148–12163. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvarajah S, Puffer BA, Lee FH, Zhu P, Li Y, Wyatt R, Roux KH, Doms RW, Burton DR. 2008. Focused dampening of antibody response to the immunodominant variable loops by engineered soluble gp140. AIDS Res Hum Retroviruses 24:301–314. doi: 10.1089/aid.2007.0158. [DOI] [PubMed] [Google Scholar]
- 24.Sliepen K, van Montfort T, Melchers M, Isik G, Sanders RW. 2015. Immunosilencing a highly immunogenic protein trimerization domain. J Biol Chem 290:7436–7442. doi: 10.1074/jbc.M114.620534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringe RP, Ozorowski G, Rantalainen K, Struwe WB, Matthews K, Torres JL, Yasmeen A, Cottrell CA, Ketas TJ, LaBranche CC, Montefiori DC, Cupo A, Crispin M, Wilson IA, Ward AB, Sanders RW, Klasse PJ, Moore JP. 2017. Reducing V3 antigenicity and immunogenicity on soluble, native-like HIV-1 Env SOSIP trimers. J Virol 91:e00677-17. doi: 10.1128/JVI.00677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringe RP, Pugach P, Cottrell CA, LaBranche CC, Seabright GE, Ketas TJ, Ozorowski G, Kumar S, Schorcht A, van Gils MJ, Crispin M, Montefiori DC, Wilson IA, Ward AB, Sanders RW, Klasse PJ, Moore JP. 2019. Closing and opening holes in the glycan shield of HIV-1 envelope glycoprotein SOSIP trimers can redirect the neutralizing antibody response to the newly unmasked epitopes. J Virol 93:e01656-18. doi: 10.1128/JVI.01656-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsouchnikas G, Zlatkovic J, Jarmer J, Strauß J, Vratskikh O, Kundi M, Stiasny K, Heinz FX. 2015. Immunization with immune complexes modulates the fine specificity of antibody responses to a flavivirus antigen. J Virol 89:7970–7978. doi: 10.1128/JVI.00938-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JG, Tomer KB, Hioe CE, Zolla-Pazner S, Norris PJ. 2006. The antigenic determinants on HIV p24 for CD4+ T cell inhibiting antibodies as determined by limited proteolysis, chemical modification, and mass spectrometry. J Am Soc Mass Spectrom 17:1560–1569. doi: 10.1016/j.jasms.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Zarnitsyna VI, Ellebedy AH, Davis C, Jacob J, Ahmed R, Antia R. 2015. Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza. Philos Trans R Soc Lond B Biol Sci 370:20140248. doi: 10.1098/rstb.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien PC Jr, Cohen S, Tuen M, Arthos J, Chen PD, Patel S, Hioe CE. 2004. Human immunodeficiency virus type 1 evades T-helper responses by exploiting antibodies that suppress antigen processing. J Virol 78:7645–7652. doi: 10.1128/JVI.78.14.7645-7652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuen M, Visciano ML, Chien PC Jr, Cohen S, Chen PD, Robinson J, He Y, Pinter A, Gorny MK, Hioe CE. 2005. Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation. Eur J Immunol 35:2541–2551. doi: 10.1002/eji.200425859. [DOI] [PubMed] [Google Scholar]
- 32.Hioe CE, Visciano ML, Kumar R, Liu J, Mack EA, Simon RE, Levy DN, Tuen M. 2009. The use of immune complex vaccines to enhance antibody responses against neutralizing epitopes on HIV-1 envelope gp120. Vaccine 28:352–360. doi: 10.1016/j.vaccine.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Wilson R, O’Dell S, Guenaga J, Feng Y, Tran K, Chiang CI, Arendt HE, DeStefano J, Mascola JR, Wyatt RT, Li Y. 2016. An HIV-1 Env-antibody complex focuses antibody responses to conserved neutralizing epitopes. J Immunol 197:3982–3998. doi: 10.4049/jimmunol.1601134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar R, Tuen M, Li H, Tse DB, Hioe CE. 2011. Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation. Vaccine 29:9064–9074. doi: 10.1016/j.vaccine.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar R, Tuen M, Liu J, Nadas A, Pan R, Kong X, Hioe CE. 2013. Elicitation of broadly reactive antibodies against glycan-modulated neutralizing V3 epitopes of HIV-1 by immune complex vaccines. Vaccine 31:5413–5421. doi: 10.1016/j.vaccine.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R, Visciano ML, Li H, Hioe C. 2012. Targeting a neutralizing epitope of HIV envelope Gp120 by immune complex vaccine. J AIDS Clinic Res S8:5512. doi: 10.4172/2155-6113.S8-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi M, Turner HL, Nogal B, Cottrell CA, Oyen D, Pauthner M, Bastidas R, Nedellec R, McCoy LE, Wilson IA, Burton DR, Ward AB, Hangartner L. 2018. Electron-microscopy-based epitope mapping defines specificities of polyclonal antibodies elicited during HIV-1 BG505 envelope trimer immunization. Immunity 49:288–300.e288. doi: 10.1016/j.immuni.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gach JS, Gorlani A, Dotsey EY, Becerra JC, Anderson CT, Berzins B, Felgner PL, Forthal DN, Deeks SG, Wilkin TJ, Casazza JP, Koup RA, Katlama C, Autran B, Murphy RL, Achenbach CJ. 2016. HIV-1-specific antibody response and function after DNA prime and recombinant adenovirus 5 boost HIV vaccine in HIV-infected subjects. PLoS One 11:e0160341. doi: 10.1371/journal.pone.0160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR. 2017. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46:1073–1088.e1076. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torrents de la Pena A, de Taeye SW, Sliepen K, LaBranche CC, Burger JA, Schermer EE, Montefiori DC, Moore JP, Klasse PJ, Sanders RW. 2018. Immunogenicity in rabbits of HIV-1 SOSIP trimers from clades A, B, and C, given individually, sequentially, or in combination. J Virol 92:e01957-17. doi: 10.1128/JVI.01957-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hioe CE, Kumar R, Upadhyay C, Jan M, Fox A, Itri V, Peachman KK, Rao M, Liu L, Lo NC, Tuen M, Jiang X, Kong XP, Zolla-Pazner S. 2018. Modulation of antibody responses to the V1V2 and V3 regions of HIV-1 envelope by immune complex vaccines. Front Immunol 9:2441. doi: 10.3389/fimmu.2018.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nadas A, Zolla-Pazner S. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol 74:7096–7107. doi: 10.1128/JVI.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Nie J, Prochnow C, Truong C, Jia Z, Wang S, Chen XS, Wang Y. 2013. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 10:14. doi: 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddad EE. 2016. A Message from the immune system: a vaccine technology in needs of exploration! Int J Vaccines Vaccin 2:00039. doi: 10.15406/ijvv.2016.02.00039. [DOI] [Google Scholar]
- 45.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. 1992. Enhanced antigen presentation using human Fcγ receptor (monocyte/macrophage)-specific immunogens. J Immunol 149:3477–3481. [PubMed] [Google Scholar]
- 46.Leon B, Ballesteros-Tato A, Randall TD, Lund FE. 2014. Prolonged antigen presentation by immune complex-binding dendritic cells programs the proliferative capacity of memory CD8 T cells. J Exp Med 211:1637–1655. doi: 10.1084/jem.20131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platzer B, Stout M, Fiebiger E. 2014. Antigen cross-presentation of immune complexes. Front Immunol 5:140. doi: 10.3389/fimmu.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. 2002. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol 168:2240–2246. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 49.Fridman WH. 1993. Regulation of B-cell activation and antigen presentation by Fc receptors. Curr Opin Immunol 5:355–360. doi: 10.1016/0952-7915(93)90053-U. [DOI] [PubMed] [Google Scholar]
- 50.Soderberg LS. 1983. Antigen-antibody complex binding and cell interaction in stimulating normal rabbit lymphocytes. Proc Soc Exp Biol Med 174:229–238. doi: 10.3181/00379727-174-41730. [DOI] [PubMed] [Google Scholar]
- 51.Klaus GG. 1978. The generation of memory cells. II. Generation of B memory cells with preformed antigen-antibody complexes. Immunology 34:643–652. [PMC free article] [PubMed] [Google Scholar]
- 52.Nie X, Basu S, Cerny J. 1997. Immunization with immune complex alters the repertoire of antigen-reactive B cells in the germinal centers. Eur J Immunol 27:3517–3525. doi: 10.1002/eji.1830271253. [DOI] [PubMed] [Google Scholar]
- 53.Zheng B, Switzer K, Marinova E, Wansley D, Han S. 2007. Correction of age-associated deficiency in germinal center response by immunization with immune complexes. Clin Immunol 124:131–137. doi: 10.1016/j.clim.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Goins CL, Chappell CP, Shashidharamurthy R, Selvaraj P, Jacob J. 2010. Immune complex-mediated enhancement of secondary antibody responses. J Immunol 184:6293–6298. doi: 10.4049/jimmunol.0902530. [DOI] [PubMed] [Google Scholar]
- 55.Kranich J, Krautler NJ. 2016. How follicular dendritic cells shape the B-cell antigenome. Front Immunol 7:225. doi: 10.3389/fimmu.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]