For almost two decades, it was thought that HIV-1 selectively downregulated the highly expressed HLA-I molecules HLA-A and HLA-B from the cell surface in order to evade cytotoxic-T-cell recognition, while leaving HLA-C and HLA-E molecules unaltered. It was stipulated that HIV-1 infection thereby maintained inhibition of NK cells via inhibitory receptors that bind HLA-C and HLA-E. This concept was recently revised when a study showed that primary HIV-1 strains reduce HLA-C surface levels, whereas the cell line-adapted HIV-1 strain NL4-3 lacks this ability. Here, we demonstrate that infection with distinct primary HIV-1 strains results in significant downregulation of surface HLA-E levels. Given the increasing evidence for HLA-E as an important modulator of CD8+ T-cell and NKG2A+ NK cell functions, this finding has substantial implications for future immunomodulatory approaches aimed at harnessing cytotoxic cellular immunity against HIV.
KEYWORDS: HIV-1, HLA-E, Nef
ABSTRACT
Human immunodeficiency virus type 1 (HIV-1) has evolved elaborate ways to evade immune cell recognition, including downregulation of classical HLA class I (HLA-I) from the surfaces of infected cells. Recent evidence identified HLA-E, a nonclassical HLA-I, as an important part of the antiviral immune response to HIV-1. Changes in HLA-E surface levels and peptide presentation can prompt both CD8+ T-cell and natural killer (NK) cell responses to viral infections. Previous studies reported unchanged or increased HLA-E levels on HIV-1-infected cells. Here, we examined HLA-E surface levels following infection of CD4+ T cells with primary HIV-1 strains and observed that a subset downregulated HLA-E. Two primary strains of HIV-1 that induced the strongest reduction in surface HLA-E expression were chosen for further testing. Expression of single Nef or Vpu proteins in a T-cell line, as well as tail swap experiments exchanging the cytoplasmic tail of HLA-A2 with that of HLA-E, demonstrated that Nef modulated HLA-E surface levels and targeted the cytoplasmic tail of HLA-E. Furthermore, infection of primary CD4+ T cells with HIV-1 mutants showed that a lack of functional Nef (and Vpu to some extent) impaired HLA-E downmodulation. Taken together, the results of this study demonstrate for the first time that HIV-1 can downregulate HLA-E surface levels on infected primary CD4+ T cells, potentially rendering them less vulnerable to CD8+ T-cell recognition but at increased risk of NKG2A+ NK cell killing.
IMPORTANCE For almost two decades, it was thought that HIV-1 selectively downregulated the highly expressed HLA-I molecules HLA-A and HLA-B from the cell surface in order to evade cytotoxic-T-cell recognition, while leaving HLA-C and HLA-E molecules unaltered. It was stipulated that HIV-1 infection thereby maintained inhibition of NK cells via inhibitory receptors that bind HLA-C and HLA-E. This concept was recently revised when a study showed that primary HIV-1 strains reduce HLA-C surface levels, whereas the cell line-adapted HIV-1 strain NL4-3 lacks this ability. Here, we demonstrate that infection with distinct primary HIV-1 strains results in significant downregulation of surface HLA-E levels. Given the increasing evidence for HLA-E as an important modulator of CD8+ T-cell and NKG2A+ NK cell functions, this finding has substantial implications for future immunomodulatory approaches aimed at harnessing cytotoxic cellular immunity against HIV.
INTRODUCTION
In most individuals, untreated human immunodeficiency virus type 1 (HIV-1) infection progresses to severe acquired immunodeficiency syndrome (AIDS) within about 5 to 10 years. However, a small percentage of infected individuals are able to efficiently control HIV-1 replication and prevent disease progression for longer periods, even in the absence of antiretroviral therapy. The HLA class I (HLA-I) locus is a major determinant of host genetic HIV-1 control (1–3), and certain HLA-I alleles are consistently associated with slower progression to AIDS (4–6). The beneficial effects of some HLA-I alleles have been mostly attributed to effective recognition by cytotoxic CD8+ T lymphocytes (reviewed in reference 7). These can recognize HLA-I-presented HIV-1 epitopes and kill virus-infected cells, causing the initial drop in peak viremia following acute infection (8, 9). Natural killer (NK) cells are also sensitive to changes in HLA-I expression and HLA-I-presented viral peptides (10–13), and NK cell receptors have been associated with delayed HIV-1 progression (14–16).
Consequently, HIV-1 has evolved multiple immune evasion mechanisms targeting the surface expression of HLA-I on infected cells (17–19) to evade immune cell recognition. On one hand, HLA-A and -B downregulation is mediated by the HIV-1 accessory protein Nef. A tyrosine-based motif in the cytoplasmic tail of HLA-I molecules enables Nef-dependent recruitment of the clathrin-associated adaptor protein complex AP-1 (a cellular trafficking protein), resulting in rerouting of HLA-I molecules to lysosomal degradation (19). HLA-C, which lacks a tyrosine at a similar position, is resistant to Nef-mediated downregulation (18). In addition, Nef intervenes with a large variety of cellular pathways to modulate signal transduction (20) and cell survival (21). Nef also downmodulates cell surface expression of CD4 (22, 23) and CXCR4 (24), counteracts the restriction factor SERINC5 (25), and enhances viral replication and infectivity (26).
On the other hand, HLA-C expression at the surface of HIV-1-infected cells is disrupted by the viral protein U (Vpu), which does not affect HLA-A or -B levels (17). Vpu of NL4-3 lacks HLA-C downregulation properties (17), which explains why this phenomenon was not observed in earlier studies (18, 19). Downregulation of HLA-C can impair adaptive and innate antiviral responses (17, 27). Interestingly, there is novel evidence that the capacity to decrease HLA-C levels of different Vpu proteins varies due to adaptation to host genetic HLA-C allotypes (28). Other important functions of Vpu are counteraction of the host restriction factor tetherin, which prevents budding of virions from the cell surface (29), and suppression of cellular antiviral responses through inhibition of NF-κB during later stages of infection (20, 30). In summary, HIV-1 has developed intricate ways to disrupt classical HLA-I surface expression, but susceptibility to HLA-I downregulation varies among HLA-I molecules (31), as well as across different HIV-1 strains (32).
HLA-E is a ubiquitously expressed nonclassical HLA-I molecule that is well conserved among the human population. It mirrors the overall HLA-I content of a cell, as its surface expression is dependent on peptides derived from the signal sequences of other HLA-I molecules, which include a methionine at position −21 (33). This group includes HLA-A, HLA-C, and HLA-G, but only certain HLA-B allotypes (34, 35). Furthermore, presentation of viral peptides derived from cytomegalovirus (CMV), Epstein-Barr virus (EBV), and hepatitis C virus (HCV) by HLA-E molecules to HLA-E-restricted T cells has been described (36–38). In rhesus macaques, RhCMV-based simian immunodeficiency virus (SIV) gag vectors surprisingly elicited a broad and protective HLA-E-restricted antiviral CD8+ T-cell response (39). In humans, HIV-1-specific HLA-E-restricted T cells have not been reported yet, but an increasing number of HIV-1-derived peptides that stabilize HLA-E expression have been identified (40–42). HLA-E is also important for NK cell development and function (33), and it engages the inhibitory NKG2A receptor, as well as its activating counterpart, NKG2C, in a peptide-dependent manner (43). NKG2A+ NK cells display high activity against HIV-1-infected cells (40, 44). Recent data implicate HLA-E as a potential determinant of antiviral control. For example, Ramsuran et al. reported an association between increased HLA-A levels in HIV-1-infected subjects and poor viral immune control. All HLA-A molecules encode an HLA-E-stabilizing leader peptide. Elevated HLA-A levels are thus correlated with higher HLA-E expression, which inhibits the antiviral function of NKG2A+ NK cells (34). Thus, despite its low surface expression levels (45), HLA-E has important implications for both innate and adaptive anti-HIV-1 immune responses. So far, unchanged or upregulated levels of HLA-E expression on the surfaces of HIV-1-infected cells have been reported (39–41, 46–50). However, apart from a study using HIV-1 isolates from peripheral blood mononuclear cells (PBMCs) of HIV-1-infected donors (41), mostly cell line-adapted HIV-1 strains have been investigated (18, 46, 48, 49).
In this study, using infectious molecular clones of primary HIV-1 strains, as well as cell line-adapted viruses, we investigated whether HIV-1 modulates HLA-E surface levels on infected primary CD4+ T cells. We demonstrated that infection with a subset of primary HIV-1 strains indeed resulted in reduced HLA-E levels on the surfaces of infected CD4+ T cells. The results further showed that the HIV-1 accessory protein Nef plays a major role in modulating HLA-E surface expression, although it might cooperate with other viral proteins for full effect. Taken together, the results of this study provide novel insights into HIV-1-mediated modulation of HLA-E surface expression, which may be relevant to immune recognition of HIV-1-infected cells by both CD8+ T cells and NK cells.
RESULTS
Reduced HLA-E levels on the surfaces of primary HIV-1-infected CD4+ T cells.
HIV-1-mediated modulation of HLA-E surface expression was investigated using a panel of cell line-adapted strains (NL4-3, SF162, SF2, and LAI [BRU]), as well as seven infectious molecular clones (IMCs) representing primary HIV-1 strains (CH293, CH107, CH164, CH185, CH236, CH077, and CH198). An HLA-E-specific monoclonal antibody (clone 3D12) (51) was used to measure HLA-E surface levels on HIV-1-infected primary CD4+ T cells. In addition, the capacity of viral strains to downregulate HLA-I was assessed by using a pan-HLA-I antibody (clone W6/32). HIV-1-mediated reduction of total HLA-I surface content as measured by W6/32 has been previously shown to correlate well with the extent of virus-induced HLA-A2 downregulation (32). However, W6/32 also captures HLA-I molecules less susceptible to HIV-1-induced degradation. HLA-E surface levels were measured 72 h after infection, when HLA-I modulation by HIV-1 is observed in vitro, as previously determined (52). HIV-1-infected CD4+ T cells were defined as being HIV-1 p24+ CD4dim, and uninfected bystander cells within the same culture were defined as HIV-1 p24− CD4+ (gating strategy displayed in Fig. 1A).
FIG 1.
Infection with HIV-1 differentially modulates HLA-E surface levels on primary T cells. (A) Gating strategy for flow cytometric analyses of HLA-E staining on HIV-1-infected primary isolated CD4+ T cells. Lymphocytes were first defined by forward scatter area (FSC-A) and side scatter area (SSC-A) characteristics. After doublet exclusion using forward scatter width (FSC-W) and side scatter width (SSC-W), viable cells were identified as negative for Zombie NIR staining (viability dye) and positive for CD3. Subsequently, CD4 was plotted against HIV-1 p24 to gate on two subsets: HIV-1-infected cells, defined as p24+ CD4dim, and HIV-1-uninfected bystander cells, defined as p24− CD4+ subset of the same well. The gate for the p24− CD4+ subset was set on the no-virus control (not shown). The histograms display the fluorescence intensities of HLA-E surface staining (clone 3D12) on HIV-1-infected cells (red) and uninfected bystander cells (black). Histograms of the respective isotype control stainings (dotted red line, HIV-1-infected cells; shaded gray histogram, HIV-1-uninfected cells) are overlaid. Staining was performed with antibody panel A (Table 2). (B) (Top row) Representative dot plots of primary CD4+ T cells infected with several HIV-1 strains ranging from no (left) to the highest (right) extent of HLA-E downregulation. HLA-E surface staining is plotted against HIV-1 p24 intracellular staining. (Bottom row) Histograms displaying fluorescence intensities of HLA-E surface staining following gating on HIV-1-infected cells (red) or on HIV-1-uninfected cells (black) with the respective isotype controls as indicated on the right. The gating strategy was the same as for panel A; the HIV-1 strains correspond to those in the top row. (C) Each pair of connected dots displays the median fluorescence intensity (MFI) of HLA-E (red) or isotype control (gray) on HIV-1-uninfected bystander cells (filled dots) and HIV-1-infected CD4+ T cells (empty dots) from the same donor. HIV-1 strains are indicated below the x axis, as well as the no-virus control. Differences in HLA-E MFIs on HIV-1-infected and -uninfected CD4+ T cells were analyzed using Wilcoxon signed rank test for paired data; significant P values (<0.05) are marked with asterisks. FDR-adjusted P values are as follows: for SF162, LAI, SF2, and CH293, P > 0.1; for NL4-3, CH107, CH164, and CH185, P = 0.059; for CH236, CH077, and CH198, P = 0.037. (D) HIV-1 cell line-adapted strains and HIV-1 primary strains (x axis) are ordered from weakest (SF162 and CH293, respectively) to strongest (NL4-3 and CH198, respectively) median relative change (percent) in HLA-E MFI (y axis). The relative change (percent) in HLA-E MFI was calculated as follows: (MFI infected − MFI uninfected)/MFI uninfected × 100. The data are represented as box-and-whisker plots indicating IQRs and medians. In total, six to nine healthy donors were tested; samples with <150 infected CD4+ T cells were excluded, resulting in varying numbers of donors per viral strain (n = 6 to 9).
We observed that the cell line-adapted strains SF162 and SF2 did not affect HLA-E surface expression levels (Fig. 1B to D), in agreement with previous reports (40, 48). Similarly, infection with LAI, which has been reported to upregulate HLA-E on CEM×174 cells, the promonocytic cell line U-937 (46), and human CD4+ T cells (49), overall did not alter HLA-E levels on primary infected CD4+ T cells in our study, although slightly increased HLA-E levels were observed in two of the donors tested (Fig. 1C). The cell line-adapted strain NL4-3 induced only a marginal reduction in HLA-E surface expression levels (median relative change, infected versus uninfected, of −17% [interquartile range {IQR} = −24% to −12%]) (Fig. 1D).
The primary viruses tested in this study showed variable degrees of HLA-E downregulation (Fig. 1B to D). In particular, CH077 (subtype B) and CH198 (subtype C) mediated the strongest and significant reduction in HLA-E surface expression, with median relative changes of −35% (IQR = −43% to −31%) for CH077 and −40% (IQR = −51% to −30%) for CH198. Other HIV-1 primary strains were less effective in downregulating HLA-E (Fig. 1C and D), but some modulation of HLA-E was observed for almost all primary HIV-1 IMCs and was consistent across the donors tested. Only CH293 did not interfere with HLA-E surface expression (median relative change, −6% [IQR = −19% to 3%]). Altogether, a subset of primary HIV-1 strains (CH236, CH077, and CH198) downregulated HLA-E surface expression significantly, whereas this was not observed for cell line-adapted viruses.
HIV-1 CH077- and CH198-derived Nef proteins mediate HLA-E surface downregulation.
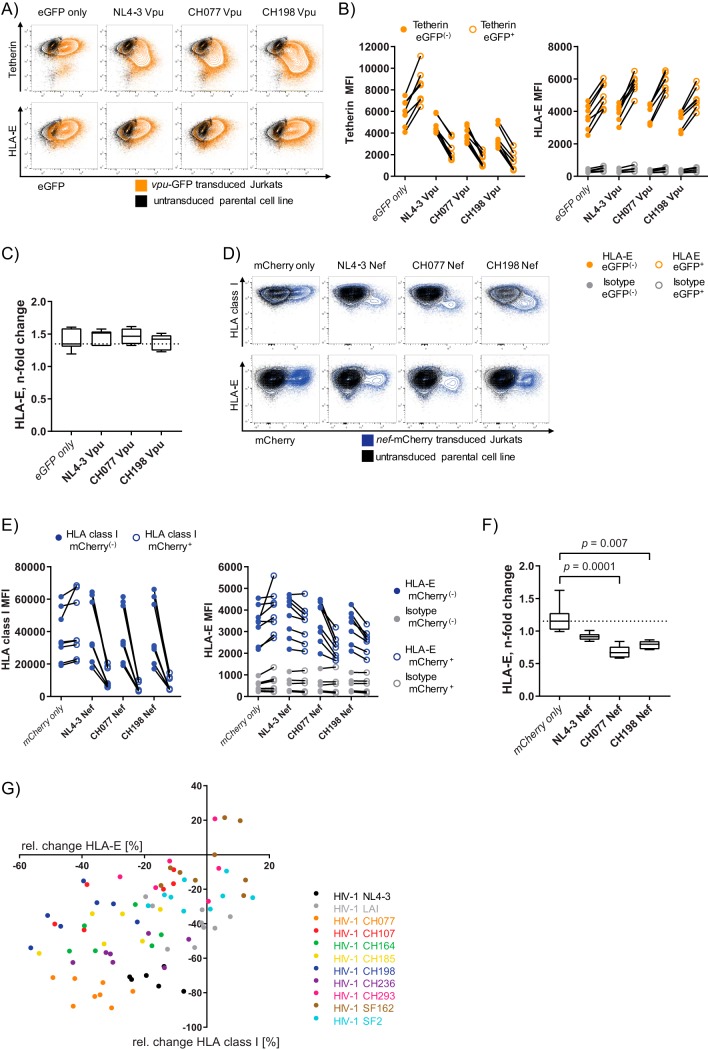
Downregulation of classical HLA-I (HLA-A/B and -C) is mediated by the HIV-1 accessory proteins Nef and Vpu in order to counteract CD8+ T-cell recognition (17, 53). Thus, for further investigation into the mechanism underlying the downregulation of HLA-E on primary CD4+ T cells infected with HIV-1 IMCs, we generated lentiviral vectors coexpressing either Vpu with enhanced green fluorescent protein (eGFP) (Fig. 2A to C) or Nef with mCherry (Fig. 2D to F). For subsequent testing, lentiviral vectors containing Nef and Vpu proteins of three viral strains—CH077 and CH198, which displayed the highest degree of HLA-E downregulation and represent different subtypes of HIV-1 group M, as well as the cell line-adapted strain NL4-3—were transduced into a Jurkat T-cell line. Unstimulated Jurkat cells express very low HLA-E surface levels, rendering evaluation of HLA-E surface modulation difficult. Therefore, Jurkat cells were prestimulated with interferon gamma (IFN-γ) prior to flow cytometric staining, as IFN-γ is well known to strongly induce HLA-I expression, in particular, HLA-E (54). The functionality of Vpu and Nef was confirmed by staining tetherin (Fig. 2A and B) and HLA-I (Fig. 2D and E), respectively. Transduction of Jurkat cells with Vpu led to a nonspecific increase in HLA-E median fluorescence intensity (MFI) that was also observed in cells transduced with vector control alone (Fig. 2B), potentially due to a cellular response to lentiviral transduction with the vector. Thus, no specific effect of Vpu on HLA-E surface expression was observed in this T-cell line (Fig. 2C).
FIG 2.
Nef proteins of HIV-1 CH077 and CH198 are sufficient to reduce HLA-E surface expression in Jurkat cells. (A) Flow plots displaying tetherin (top) and HLA-E (bottom) plotted against eGFP expression on vpu-eGFP-transduced Jurkat cells (orange). Untransduced Jurkat cells are overlaid in black to distinguish eGFP+ populations. (B) (Left) Graph displaying MFIs of tetherin staining on Jurkat cells. The dot-line graphs connect vpu-transduced (eGFP+, open orange dots) and eGFP− cells of the same sample (filled orange dots) for all three constructs and the vector control (eGFP alone) as indicated on the x axis. (Right) Graph displaying HLA-E MFIs on vpu-eGFP+ (empty orange dots) and vpu-eGFP− (filled orange dots) Jurkat cells from the same sample. The isotype control for HLA-E staining is depicted in gray. (C) n-fold change in HLA-E MFI following transduction with vpu-eGFP, calculated as the HLA-E MFI on vpu-eGFP+ divided by the HLA-E MFI on vpu-eGFP− cells. The dotted line indicates the median n-fold change of the eGFP vector control. Differences were analyzed using Friedman test, and only significant FDR-adjusted P values are depicted. The data are derived from two independent experiments (once with three technical replicates and once with four technical replicates [n = 7]). (D) Flow plots depicting HLA-I (top) and HLA-E (bottom) surface levels plotted against mCherry (x axis) on nef-mCherry-transduced Jurkat cells (blue) and the untransduced parental cell line (black). (E) (Left) Graph displaying MFIs of HLA-I staining on Jurkat cells. The lines connect nef-transduced (mCherry+, empty blue dots) with mCherry− cells from the same sample (filled blue dots) for all three constructs and the vector control. (Right) Graph displaying HLA-E MFIs derived from nef-mCherry− cells (filled blue dots) and nef-mCherry+ cells (empty blue dots) from the same well. Isotype control staining for HLA-E is shown in gray. (F) Box-and-whisker plots (medians and IQRs) representing n-fold change in HLA-E MFI calculated as HLA-E MFI nef-mCherry+ divided by HLA-E MFI nef-mCherry−. The dotted line indicates the median n-fold change of the vector control. The data are derived from five independent experiments (n = 8, once with two and once with three technical replicates). Differences were analyzed using Friedman test; all P values shown are FDR adjusted, and only significant P values are displayed (<0.05). Staining was performed using antibody panel C (Table 2). (G) Association between HLA-E surface expression and HLA-I surface levels on HIV-1-infected primary CD4+ T cells. Each dot represents the relative change (percent) in the HLA-E MFI (clone 3D12) on the x axis and the relative change (percent) in HLA-I surface expression (clone W6/32) on the y axis for one specific donor/virus-strain combination. The relative change (percent) in MFI was calculated as follows: (MFI infected − MFI uninfected)/MFI uninfected × 100. Color coding with the same color for the same HIV-1 strain used for infection of CD4+ T cells from different donors, as indicated on the right. The data are derived from the same experiments shown in Fig. 1 (n = 6 to 9).
A minor reduction of HLA-E surface expression was observed on Jurkat cells transduced with NL4-3 Nef (median n-fold change, 0.91 [IQR = 0.87 to 0.95]) compared to mCherry vector alone (median n-fold change, 1.15 [IQR = 1.02 to 1.28]) (Fig. 2F). In contrast, transduction of Jurkat cells with both Nef proteins derived from CH077 and CH198 resulted in a significant reduction in HLA-E MFI (Fig. 2F). A higher degree of HLA-E surface downmodulation was observed for CH077-derived Nef (median n-fold change, 0.67 [IQR = 0.59 to 0.76]). The median n-fold change in HLA-E surface levels induced by CH198-derived Nef was 0.8 (IQR = 0.72 to 0.85). In summary, the presence of HIV-1 Nef proteins derived from CH077 and CH198 was sufficient to reduce HLA-E surface expression in Jurkat cells. In contrast, all three Vpu proteins tested here did not influence HLA-E surface levels.
Reduced HLA-E surface levels on HIV-1-infected CD4+ T cells are associated with downregulation of HLA-I expression.
Given that—akin to downregulation of highly abundant HLA-A and -B—Nef caused downregulation of HLA-E in Jurkat cells, we assessed whether the ability of HIV-1 strains to modulate HLA-E surface expression parallels their ability to downregulate overall HLA-I expression from the cell surface. To this end, we reanalyzed the first data set (Fig. 1), where the pan-HLA-I antibody W6/32 was used to measure total HLA-I, which includes HLA-E. Nevertheless, HLA-E constitutes only a minor percentage of the total HLA-I content recognized, making pan-HLA-I staining levels more directly related to the highly expressed HLA-A and HLA-B molecules (32). Of note, in contrast to previous reports (48, 55), infection with HIV-1 SF162 did not reduce HLA-I surface levels in our experiments, a finding that may be ascribed to potential mutations accumulated during propagation of the viral stock (56). Employing a mixed-effect linear regression model, we observed that downregulation of HLA-I surface expression was significantly associated with reduced HLA-E surface levels on HIV-1-infected CD4+ T cells (for each +1% of HLA-E change, the mean surface HLA- I change was +0.72% [95% confidence interval, 0.44% to 0.99%; P = 0.0001]) (Fig. 2G). Color coding of HIV-1 strains indicated that this association was driven to a greater extent by differences between HIV-1 strains and less by interindividual donor differences upon infection with the same strain. CH077 (subtype B), which significantly downregulated HLA-E, displayed the greatest ability to reduce total HLA-I surface levels of all IMCs tested, in line with previous reports comparing subtype B and subtype C virus effects on HLA-I (57). Altogether, HIV-1 strains cluster according to their abilities to reduce HLA-E and total HLA-I levels from the surfaces of HIV-1-infected primary CD4+ T cells.
Nef targets the cytoplasmic tail of HLA-A*02:01 and HLA-E*01:03, but not that of HLA-C*04:01.
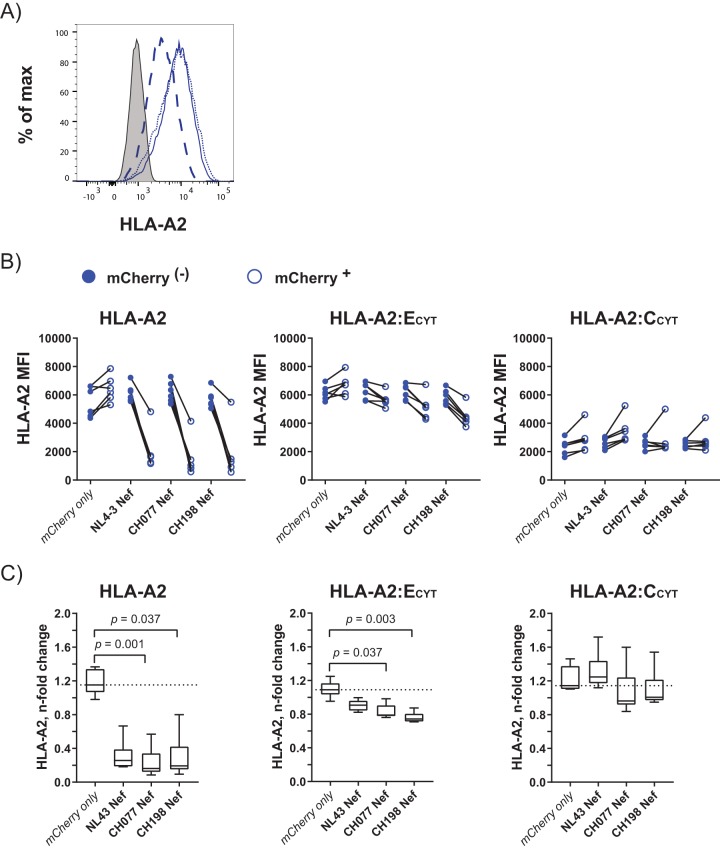
HIV-1 Nef targets the cytoplasmic tail of HLA-A and -B, resulting in degradation of the HLA-I molecule (19). Here, we aimed to investigate the dependency of Nef on the cytoplasmic tail of HLA-E to mediate HLA-E surface downmodulation. To test this, we generated three recombinant Jurkat cell lines: (i) Jurkat-HLA-A2 cells, expressing the full HLA-A*02:01 molecule as a positive control; (ii) Jurkat-HLA-A2:ECYT cells, expressing the extracellular and transmembrane domains of HLA-A2*02:01 fused to the cytoplasmic domain of HLA-E*01:03; and (iii) Jurkat-HLA-A2:CCYT cells, expressing the extracellular and transmembrane domains of HLA-A*02:01 fused to the cytoplasmic domain of HLA-C*04:01 as a negative control. Since HLA-E is constitutively displayed at low levels on the cell surface and is highly dependent on the availability of peptides derived from signal sequences of other HLA-Is, this approach allowed higher surface expression of the HLA-A2:ECYT construct compared to physiological HLA-E expression. Surface expression of transduced HLA molecules was determined by staining for HLA-A2 on Jurkat cells, which naturally are negative for HLA-A2 (Fig. 3A). Jurkat-HLA-A2 and Jurkat-HLA-A2:ECYT cells displayed similar levels of HLA-A2 surface expression, whereas surface HLA-A2 levels of Jurkat-HLA-A2:CCYT cells were lower, as expected (58). Next, these cell lines were transduced with nef-mCherry constructs of NL4-3, CH077, and CH198, respectively. Surface expression levels of HLA-A2 in nef-transduced cells were compared to those in untransduced (mCherry−) cells of the same culture. To control for nonspecific vector effects, a condition with mCherry alone was added. In agreement with previous studies, transduction of all three nef-mCherry constructs led to substantial downregulation of HLA-A2 surface levels in the Jurkat-HLA-A*02:01 cell line (Fig. 3B).
FIG 3.
CH077 Nef and CH198 Nef target the cytoplasmic tail of HLA-A*02:01 and HLA-E*01:03, but not that of HLA-C*04:01. (A) Histograms displaying fluorescence intensities of HLA-A2 surface staining on Jurkat cells transduced with the full HLA-A*02:01 molecule (HLA-A2; dotted blue line) or the extracellular and transmembrane domains from HLA-A*02:01 fused to the cytoplasmic domain of HLA-E*01:03 (HLA-A2:ECYT; blue line) or to the cytoplasmic domain of HLA-C*04:01 (HLA-A2:CCYT; dashed blue line). The HLA-A2 expression of nontransduced parental Jurkat cells is depicted as a gray-shaded histogram. (B) MFIs of HLA-A2 staining in mCherry− (filled blue dots) and mCherry+ (empty blue dots) Jurkat HLA-A2 (left), Jurkat HLA-A2:ECYT (middle), and Jurkat HLA-A2:CCYT (right) cells after transduction with nef-mCherry constructs or an mCherry vector control. Each dot represents one individual experiment, and each line connects MFIs from mCherry− and mCherry+ cells within the same culture. (C) n-fold changes in HLA-A2 MFIs on mCherry+ and mCherry− cells following transduction of Nef-derived constructs as indicated on the x axes, calculated as MFI HLA-A2 mCherry+ divided by MFI HLA-A2 mCherry−. The dotted line indicates the median n-fold change of the mCherry vector control for each cell line. Differences in HLA-A2 downregulation between nef constructs and the empty mCherry vector control were analyzed using Friedman test. Only significant FDR-adjusted P values are displayed (P < 0.05). Staining was performed with antibody panel D (Table 2). The data are derived from six independent experiments (n = 6).
Lentiviral transduction of Jurkat-HLA-A2:ECYT with mCherry control vector resulted in a minor increase in HLA-A2 (median n-fold change, 1.09 [IQR = 1.03 to 1.17]) (Fig. 3C, middle). In contrast, surface HLA-A2 levels of Jurkat-HLA-A2:ECYT cells were significantly reduced when exposed to Nef derived from CH077 (median n-fold change, 0.79 [IQR = 0.78 to 0.9]) and CH198 (median n-fold change, 0.74 [IQR = 0.71 to 0.8]). NL4-3-derived Nef affected HLA-A2 surface staining only marginally (median n-fold change, 0.91 [IQR = 0.84 to 0.96]) (Fig. 3C). Notably, the Nef-mediated HLA-A*2:ECYT downregulation did not reach the same extent as Nef-induced reduction of HLA-A*02:01 surface levels. In comparison, HLA-A2 surface levels of Jurkat-HLA-A2:CCYT following transduction with Nef proteins did not display a significant difference from the mCherry control vector (mCherry, median n-fold change, 1.14 [IQR = 1.11 to 1.38]; NL4-3, median n-fold change, 1.25 [IQR = 1.17 to 1.44]; CH077, median n-fold change, 0.96 [IQR = 0.92 to 1.24]; CH198, median n-fold change, 1.01 [IQR = 0.97 to 1.22]). This indicated that no change is induced by Nef on expression of the HLA-A*02:01:C*04:01CYT chimeric construct, in line with previous work (17). Altogether, the HLA tail swap experiments demonstrated the well-known ability of Nef to downregulate HLA-I via the cytoplasmic tail of HLA-A*02:01, but not HLA-C*04:01. The presence of the cytoplasmic tail of HLA-E was sufficient to allow HLA-E surface reduction, providing novel mechanistic insights into Nef-mediated HLA-E modulation.
Nef is necessary for downmodulation of HLA-E surface levels on HIV-1-infected primary CD4+ T cells.
To validate the finding that HLA-E surface downregulation is mediated by Nef in primary CD4+ T cells, IMCs harboring Δnef and Δvpu mutants were used to generate mutant virus of the HIV-1 strains CH077 and CH198, with NL4-3 as a control (Fig. 4A). As Nef targets CD4 for degradation (22, 23), our previous gating strategy to identify HIV-1-infected cells was modified for CD4+ T cells infected with Δnef mutant virus, where HIV-1-infected T cells were defined as HIV-1 p24+ tetherindim (Fig. 4B, top). The gating strategy for Δvpu mutants is depicted in Fig. 4B (bottom). Loss of Nef and Vpu functions in mutant viruses was validated by analyzing the surface levels of HLA-I (downregulated by Nef) and tetherin (downregulated by Vpu). All Δnef viruses exhibited a reduced ability to downregulate HLA-I surface expression compared to wild-type (WT) infection, demonstrating loss of Nef function (Fig. 4C). Furthermore, Δvpu HIV-1 NL4-3 and CH077 derivatives were unable to downmodulate tetherin surface expression compared to their respective WT viruses (Fig. 4D). Interestingly, a reduction of tetherin levels—although less prominent than in WT infection—was still observed following infection with the Δvpu mutant of CH198, indicating additional downregulation of tetherin independent of Vpu, potentially via Nef (59). In summary, we validated functional loss of Nef and Vpu in mutant viruses, enabling us to study the necessity of these HIV-1 accessory proteins for downregulation of HLA-E surface levels in primary T cells.
FIG 4.
Δnef mutants exhibit less reduction of HLA-E surface levels on HIV-1-infected primary T cells. (A) Dot plots of primary CD4+ T cells infected with the WT and Δnef and Δvpu mutants of HIV-1 NL4-3 (top), HIV-1 CH198 (middle), and HIV-1 CH077 (bottom). HLA-E surface staining (clone 3D12) is plotted against HIV-1 p24. The HLA-E MFI is displayed for HIV-1 p24-positive cells (upper right corner). (B) Gating strategy for flow cytometric analyses of isolated CD4+ T cells infected with Δnef (top) or Δvpu (bottom) mutants following doublet and dead cell exclusion. To identify the HIV-1-infected cell subset, CD4 or tetherin was plotted against HIV-1 p24 staining. HIV-1-infected T cells were defined as p24+ tetherindim for Δnef-infected cells and as p24+ CD4dim for Δvpu virus infection. Staining was performed with antibody panel B (Table 2). (C and D) Functional loss of Nef and Vpu in HIV-1 mutants was validated by analyzing surface expression of HLA-I (C) and tetherin (D). To allow comparison of mutant viruses with the WT, CD4+ T cells infected with WT virus were gated as the mutant virus of interest. The dot-line graphs display the respective MFIs for HIV-1-uninfected bystander cells and HIV-1-infected CD4+ T cells from the same donor. Non-virus-exposed CD4+ T cells (w/o) are depicted in gray as a control. (E and F) MFIs of HLA-E following infection with Δnef (E) or Δvpu (F) mutants compared to the WT for HIV-1 NL4-3 (left), CH198 (middle), and CH077 (right). The data are represented as dot-line graphs displaying MFIs of HIV-1-uninfected, bystander, and HIV-1-infected subsets as indicated with + and − on the x axes. Non-virus-exposed CD4+ T cells (w/o) are depicted in gray as a control. (G) Relative change in HLA-E MFIs on the surfaces of Δnef-infected (blue), Δvpu-infected (orange), or WT-infected (black) CD4+ T cells. The HLA-E MFI on HIV-1-infected CD4+ T cells was compared to the HLA-E MFI on HIV-1-uninfected bystander CD4+ T cells, and the relative change (percent) was calculated as follows: (MFI infected − MFI uninfected)/MFI uninfected × 100. The box-and-whisker plots display medians and IQRs (n = 7). Differences between WT and mutant viruses were analyzed using the Wilcoxon signed rank test for paired data (two tailed). Significant FDR-adjusted P values are marked with asterisks (*, P = 0.037); nonsignificant values (P > 0.05) are not shown in the graph. The data are derived from in vitro HIV-1 infection of primary CD4+ T cells from seven healthy donors (n = 7). MFIs are derived from measurements at two different analyzers (LSR Fortessa and FACSAria Fusion).
In assessing HLA-E levels following infection with viral mutants (Fig. 4E and F), we observed that HLA-E surface downregulation was completely lost following disruption of nef in CH198 (Fig. 4E), with a median relative change in HLA-E between Δnef HIV-1-infected and uninfected bystander cells of +11% (IQR = −8% to +33%) compared to a median relative change in HLA-E of −50% in WT infection (IQR = −55% to −26%) (Fig. 4G). For CH077, disruption of nef resulted in diminished capacity of the mutant virus to downmodulate HLA-E surface expression (median relative change, −25% [IQR = −33% to −8%]) compared to the WT (−52% [IQR = −57% to −42%]). However, unlike the CH198 Δnef mutant, loss of functional CH077-derived Nef did not fully restore HLA-E surface levels to the level of HIV-1-uninfected bystander CD4+ T cells (Fig. 4E). NL4-3 WT infection modulated HLA-E levels only marginally (Fig. 4A and E to G); therefore, no conclusions were drawn from changes in HLA-E surface levels of NL4-3 mutants compared to WT infection with regard to HLA-E downregulation.
Notably, for both primary HIV-1 strains, infection with the Δvpu mutants still moderately reduced HLA-E levels on infected CD4+ T cells, albeit to a lesser degree than WT infection (Fig. 4F). The effect of Vpu knockout in CH198 was less pronounced (median relative change, −28% [IQR = −36% to −24%]), and—unlike the Δnef mutant—did not fully restore HLA-E surface levels to HLA-E levels on uninfected bystander cells (Fig. 4G). However, the effect of CH077-derived Vpu knockout (median relative change, −29% [IQR = −35% to −23%]) was similar to the effect caused by loss of Nef protein in CH077 (−25% [IQR = −33% to −8%]) and significant compared to CH077 WT (−52% [IQR = −57% to −49%]) (Fig. 4G). Therefore, to investigate a potential additive effect of Nef and Vpu on HLA-E surface modulation, we tested the effect of a double mutant (Δvpu Δnef) of CH077 on HLA-E surface levels of infected primary T cells (Fig. 5A). As the CH077 Δvpu Δnef mutant was impaired in its ability to downregulate CD4, tetherin, and HLA-I (Fig. 5B), we stained with two different p24 antibodies (clone KC57 in fluorescein isothiocyanate [FITC] and clone 28B7 in allophycocyanin [APC]) and gated on the double-positive population, as described previously (60). Comparison of HIV-1-infected T cells (p24+ p24+) to uninfected bystander T cells (p24− p24−) showed that infection with the double mutant defective in Nef and Vpu resulted in elevated HLA-E levels (Fig. 5B). Loss of Nef and Vpu completely abrogated HLA-E downregulation compared to the WT. In conclusion, these data show that the accessory HIV-1 proteins Nef and, to some extent, Vpu contribute to HLA-E downregulation in primary CD4+ T cells following HIV-1 infection.
FIG 5.

Loss of Nef and Vpu in CH077 disrupts HLA-E surface downregulation in HIV-1-infected primary T cells. (A) Dot plots of primary CD4+ T cells infected with CH077 WT or a mutant defective in HIV-1 accessory genes nef and vpu (CH077 Δnef/Δvpu). HLA-E surface staining (clone 3D12) is plotted against HIV-1 p24 (clone KC57). The HLA-E MFI is displayed for the HIV-1 p24-positive cell subset (bottom right corner). (B) Relative changes (percent) in surface expression of the indicated proteins (x axis) upon infection of primary CD4+ T cells with CH077 WT (black dots) or the mutant virus CH077 Δnef Δvpu (gray dots). The MFI on HIV-1-infected CD4+ T cells (p24+ p24+) was compared to the MFI on HIV-1-uninfected bystander CD4+ T cells (p24− p24−), and the relative change (percent) was calculated as follows: (MFI infected − MFI uninfected)/MFI uninfected × 100. The data are presented as scatter plots with IQRs and medians and derived from three independent healthy donor infections (n = 3). Staining was performed with antibody panel E (Table 2).
DISCUSSION
HIV-1 has evolved an outstanding ability to escape immune cell recognition by controlling the display of surface ligands on HIV-1-infected cells (61–64). The concept of HLA-A and -B being selectively downregulated to avoid cytotoxic-T-cell recognition of HIV-1-infected cells while leaving HLA-C and -E on the surface to interact with inhibitory NK cell receptors was recently revised when primary virus strains were shown to downregulate HLA-C (17). Here, we investigated the impact of eleven HIV-1 strains on HLA-E surface levels upon infection of primary T cells. Unlike cell line-adapted viruses, a subset of primary strains—CH236, CH077, and CH198—showed significant downregulation of HLA-E. Transduction of CH077- and CH198-derived Nef into a T-cell line was sufficient to reduce HLA-E surface levels, also when only the cytoplasmic tail of HLA-E was present. Mutational analyses confirmed that lack of functional Nef reduced the efficiency of HLA-E downmodulation in primary T cells infected with mutants of CH077 and CH198. Notably, in primary CD4+ T cells, loss of Vpu in CH077 resulted in a lesser degree of HLA-E downmodulation, indicating that HLA-E downregulation can be multifactorial during HIV-1 infection.
Infection with HIV-1 primary strains reduced HLA-E surface staining on infected CD4+ T cells, albeit to varying degrees. In our study, HIV-1 strains clustered according to their abilities to modulate HLA-E and HLA-I expression. A previous study reported that this was not the case for HLA-C (32), in line with downregulation of HLA-A, -B, and -E being mediated by Nef and HLA-C downregulation being mediated by Vpu. Earlier work demonstrated that the property of Nef to downregulate HLA-I surface levels was decreased in viruses obtained from individuals who progressed to AIDS, indicating no selective advantage to maintain this Nef function at later stages of infection, when immune pressure is impaired (65). This may potentially explain why most cell line-adapted viral strains, which are adapted to conditions without immune pressure present and/or were isolated from AIDS patients (66), do not modulate HLA-E surface levels upon infection. Accordingly, the HIV-1 primary strain CH293, which was cloned from an HIV-1-infected individual at the chronic stage of infection (67), displayed only minor downregulation of HLA-I and no reduction in HLA-E surface expression. Increased HLA-E expression on CD4+ T cells from HIV-1-infected subjects has been shown to correlate with loss of CD4+ T cell counts (46). No difference in HLA-E (and also HLA-C) surface levels on infected CD4+ T cells was observed following HIV-1 reactivation from viremic patients (47). Interestingly, Bachtel et al. showed that the magnitude of HLA-C downregulation between HIV-1 strains varies to a higher degree than the overall well-preserved extent of HLA-A downregulation (28). Nattermann et al. reported elevated HLA-E levels upon HIV-1 infection when testing four HIV-1 isolates cultured from PBMCs of HIV-1-infected individuals (41). Together with the data presented here, this indicates that HLA-E downregulation may vary substantially between HIV-1 strains. Future studies will be needed to extend this investigation of HLA-E modulation to a larger panel of viruses, including IMCs sampled from different stages of HIV-1 infection.
In order to assess the mechanisms leading to reduced HLA-E expression by HIV-1 primary strains, we next investigated the role of the HIV-1 accessory proteins Nef and Vpu. Disruption of nef resulted in increased HLA-E surface levels compared to WT infection. Additionally, transduction of the Jurkat T-cell line with Nef derived from CH077 and CH198 was sufficient to reduce HLA-E surface levels. Early studies reported that Nef did not bind to the cytoplasmic tail of HLA-E (18, 61), but Nef in these initial studies was derived from NL4-3. Here, we exchanged the cytoplasmic tail of HLA-A*02:01 with that of HLA-E*01:03 and demonstrated that CH077- and CH198-derived Nef proteins are able to disrupt HLA-E surface expression via its cytoplasmic tail. Recruitment of the adaptor protein AP-1 by Nef to the cytoplasmic tail of HLA-I, which then reroutes trafficking of newly synthesized HLA-A and -B molecules (19), has been established as an important mechanism of HLA-I downregulation across a range of cell types, including primary T cells (reviewed in reference 68). Other mechanisms include increased turnover of HLA-I molecules from the cell surface (69) and coatomer complex (β-COP)-dependent lysosomal degradation (70). Three amino acids in the HLA-I cytoplasmic tail are critical for binding of AP-1 (Y320, A324, and D327) (71). The glutamic acid at position 324 alone (present in HLA-E) was shown to prevent coimmunoprecipitation of AP-1 (in the presence of NL4-3 Nef) with a mutated cytoplasmic tail of HLA-A2 (71). We did not observe a significant difference in Nef-mediated downregulation between a mutated cytoplasmic tail of HLA-E (HLA-A2:ECYT(Y320C, D327N)) and the HLA-A:ECYT construct in Jurkat cells (data not shown). Altogether, this indicates that involvement of AP-1 in Nef-dependent downregulation of the cytoplasmic tail of HLA-E is less likely, at least with regard to the known pathway targeting HLA-A and -B molecules. Further experimental data—including biochemical approaches, functional knockout of AP-1, and testing of a larger Nef panel—is needed to understand the mechanism of Nef-dependent HLA-E downregulation.
Structural studies have elucidated motifs in Nef that are critical for downregulation of HLA-A and -B. Residues that stabilize the transient interaction of AP-1 with the cytoplasmic tail of HLA-A or -B are (i) residues in the amino-terminal proximal α-helix, in particular, methionine at position 20 (M20) and tryptophan at position 13 (W13); (ii) a polyproline helix, P69/72/75/78 (PXXP motif); and (iii) an acidic cluster, E62–65 (72). In addition, an RXR17–19 motif in the proximal α-helix was reported to enhance HLA-I degradation via the β-COP-mediated pathway (70). M20, which is the consensus subtype B amino acid, was replaced with consensus subtype C isoleucine (I20) in the majority of subtype C HIV-1 strains tested here (namely, CH293, CH107, CH164, and CH198). Stronger downregulation of HLA-I by subtype B viruses (as observed for CH077) has been previously associated with this amino acid substitution (57, 73). W13, the PXXP motif, the RXR17–19 motif, and at least two acidic amino acids in the acidic cluster were present in all HIV-1 Nef sequences tested, indicating that sequence diversity in these particular motifs does not explain the variability in HLA-E and HLA-I downregulation observed between the investigated HIV-1 strains. However, previous studies highlighted the great difficulty of assigning functional Nef properties without considerable experimental data (74). Also, the ability to downregulate HLA-I was conserved in a variety of SIV- and HIV-derived Nef proteins, regardless of high sequence diversity in these regions (50). Here, we provide first evidence in a T-cell line that the HIV-1 protein Nef derived from two primary HIV-1 strains interferes with HLA-E surface expression by targeting its cytoplasmic tail via yet-unknown sequence motifs.
Despite Vpu not being sufficient to downregulate HLA-E in a Jurkat T-cell line, CH077-derived Vpu was partially necessary for HLA-E surface reduction in HIV-1-infected primary CD4+ T cells. There are several potential explanations. First, Jurkat T cells, as a tumor cell line, lack certain cellular factors (e.g., defects in T-cell receptor [TCR] signaling and O-linked glycosylation) (75), one of which may be necessary for Vpu to exert its effect on HLA-E. The rate of cellular HLA-I trafficking varies in general depending on the investigated cell type and may also be a contributing factor (68). Second, there may be an additive effect of HIV-1 (accessory) proteins in downregulating HLA-E surface levels, as was described for the downregulation of CD4 (76) and CD1d (77). In the case of the nonclassical HLA-I ligand CD1d, Vpu impairs recycling from endosomal compartments (77), whereas Nef potentially increases CD1d surface internalization (78), resulting in a cooperative effect of the two accessory proteins in reducing CD1d expression. Here, infection of primary CD4+ T cells with a double mutant of HIV-1 CH077 defective in nef and vpu fully disrupted tetherin, HLA-I, and HLA-E surface downregulation observed in WT infection, and HLA-E surface levels of infected primary T cells were slightly increased compared to HLA-E levels of HIV-1 uninfected bystander CD4+ T cells. In contrast, CD4 is modulated by three HIV-1 accessory proteins (Nef, Vpu, and Env) (76), and, in agreement with this, loss of Nef and Vpu did not fully restore CD4 surface levels. Finally, downregulation of HLA-E in the presence of CH077 Vpu in primary CD4+ T cells may be explained by a host cellular response to HIV-1 replication. A few studies have reported that endoplasmic reticulum (ER) stress results in posttranscriptional downregulation of surface HLA-E, but not of classical HLA-I levels. It was postulated that this selective loss of HLA-E may be due to HLA-E having a less stable tertiary structure than other classical HLA-I molecules and, thus, a shorter half-life at the plasma membrane (79). So far, few studies have investigated ER stress specifically in HIV-1, but it has been shown that an unfolded-protein response can be detected following in vitro HIV-1 infection of primary PBMCs (80). In conclusion, in addition to a direct effect of Nef on the cytoplasmic tail of HLA-E, Vpu may exert effects on HLA-E surface expression in a cell context-dependent manner.
Importantly, the effect of HLA-E downregulation by HIV-1 on the antiviral immune response remains to be investigated. In humans, no HLA-E-restricted T cells have been identified in HIV-1 infection to date. Human NKG2A+ NK cells show the highest functionality among NK cell subsets against HIV-1-infected cells (44), and high HLA-E levels result in impaired NKG2A+ NK cell activity (34). Perhaps a beneficial effect of HLA-E downregulation for HIV-1 is avoiding recognition via the activating NK cell receptor, NKG2C, which also recognizes HLA-E. Interestingly, SIV infection of rhesus macaques has been shown to induce an expansion of NK cells expressing NKG2C (81), and there is a high level of conservation of HLA-E and major histocompatibility complex class E (MHC-E) between humans and nonhuman primates (49). However, the role of NKG2C-mediated NK cell responses in HIV-1-infected cells is still unclear. Our finding that a subset of HIV-1 primary strains can downregulate HLA-E levels on HIV-1-infected primary CD4+ T cells may have implications for immune control of HIV-1. Further studies will be required to assess the functional impact of reduced HLA-E levels on HIV-1-infected CD4+ T cells for both innate immune recognition by NKG2A and/or NKG2C on NK cells and adaptive immune recognition by cytotoxic CD8+ T cells.
MATERIALS AND METHODS
Ethics statement.
PBMCs were obtained from healthy donors of the Healthy Cohort Hansestadt Hamburg (HCHH). The study was approved by the Ethical Committee of the Universitätsklinikum Hamburg-Eppendorf, and all donors gave written informed consent.
Cell culture.
Jurkat cells (clone E6-1; American Type Culture Collection [ATCC]) are a T lymphocytic cell line first derived from a child suffering from leukemia (82). MT-4 cells (a lymphocytic laboratory-stable cell line) were obtained through the NIH AIDS Research and Reference Reagent Program (83). Both nonadherent cell lines were cultured in RPMI 1640 l-glutamine medium (Sigma-Aldrich) supplemented with 10% heat-inactivated superior fetal bovine serum (FBS) (Biochrom AG). Adherent HEK 293T cells were obtained from ATCC and cultured in Dulbecco’s modified Eagle medium (DMEM) (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Biochrom AG). All cell lines were maintained at 37°C, 5% CO2, and 95% humidity.
Production of HIV-1 stock from infectious molecular clones.
Plasmids harboring the proviral genome of infectious molecular clones representing the HIV-1 primary strains CH077, CH293, CH198, CH107, CH164, CH185, and CH236 were kindly provided by the Hahn laboratory; IMCs of CH077, CH198, CH107, CH164, CH185, and CH236 were cloned in the Kappes laboratory (67, 84, 85) (Table 1 lists viral characteristics). pCH107.c was constructed by the Kappes laboratory following a strategy essentially as described previously (67). Briefly, the transmitted/founder (T/F) nucleotide sequence from subject 705010107 was inferred essentially as described. The entire proviral genome was then synthesized in 3 overlapping fragments; the two overlaps encompassed naturally occurring unique restriction sites (EcoRV and DraIII); MluI and BamHI sites were added directly upstream and downstream of the 5′ and 3′ ends, respectively, of the proviral coding region. The three fragments were then ligated consecutively into pUC57 via the unique restriction sites. The resulting plasmid DNA of pCH107.c was sequence confirmed to represent the inferred T/F nucleotide sequence. The following plasmids encoding infectious molecular clones were used to produce full-length replication-competent HIV-1 virions and the respective mutants via transfection: pBR_HIV-1 NL4-3 (WT and Δnef and Δvpu mutants), pCH077.t. (WT, Δnef, Δvpu, and Δnef Δvpu), pCH198.c (WT, Δnef, and Δvpu). The vpu and nef mutants were generated through insertion of premature stop codons using site-directed mutagenesis (86). For data included in Fig. 1, pNL4-3 (National Institutes of Health [NIH]; catalog no. 114, lot 140136) was used (87). Twelve micrograms of plasmid was diluted in Opti-MEM (Life Technologies) and used for Lipofectamine 2000-based transfection (Invitrogen) of HEK 293T cells in a T75 flask at 90% confluence according to the manufacturer’s protocol. Following 4 h of incubation, the medium was carefully removed and exchanged for fresh culture medium. The lentiviral supernatant was collected at 48 to 72 h posttransfection. For purity, the supernatant was centrifuged at 200 × g for 5 min and filtered with a Whatman 0.45-μl syringe filter (Sigma-Aldrich). Lentiviral aliquots were stored at −80°C until further use.
TABLE 1.
Characteristics and origins of HIV-1
| Group | Virus | Subtype | Source (reference) | Fiebig stagea | Coreceptor |
|---|---|---|---|---|---|
| Cell line-adapted virus | SF162 | B | Viral stock (isolated from cerebrospinal fluid of an individual diagnosed with AIDS) (66) | NA | CCR5 |
| LAI (BRU) | B | Viral stock (first laboratory-isolated strain from an AIDS patient; 1983) (92, 93) | NA | CXCR4 | |
| SF2 | B | Viral stock (patient isolate from 1983) (94, 95) | NA | CXCR4/CCR5 | |
| NL4-3 | B | Infectious molecular clone (chimera of HIV-1 NY5 isolate [5′] and the HIV-1 LAV isolate [3′]) (87) | NA | CXCR4 | |
| HIV-1 primary strains | CH293 | C | Infectious molecular clone (chronic) (67) | NA | CCR5 |
| CH107 | C | Infectious molecular clone (founder) | V | Unknown | |
| CH164 | C | Infectious molecular clone (founder) (67) | IV | CCR5 | |
| CH185 | C | Infectious molecular clone (founder) (67) | I/II | CCR5 | |
| CH236 | C | Infectious molecular clone (founder) (84) | II/III | CCR5 | |
| CH077 | B | Infectious molecular clone (founder) (85) | II/III | CXCR4/CCR5 | |
| CH198 | C | Infectious molecular clone (founder) (67) | I/II | CCR5 |
The Fiebig classification is used to describe six different stages of early HIV infection based on the results of standard diagnostic assays (99). NA, not applicable.
HIV-1 SF162 (catalog no. 276; lot 098300), HIV-1 SF2 (catalog no. 2525; lot 140081), and HIV-1 LAI (BRU) (catalog no. 2522; lot 110239) isolates were obtained through the NIH AIDS Reagent Program. Viral stocks were further propagated in MT-4 cells. MT-4 cells (1 × 106 cells/ml) were infected with HIV-1 (10 ng of p24) by spinfection (2 h at 37°C; 1,200 × g) and then cultured for 72 to 96 h at 37°C, 5% CO2 at 5 × 105 cells/ml before staining for HIV-1 p24 expression. The virus supernatant was harvested, centrifuged at 200 × g for 5 min, and filtered with a Whatman 0.45-μl syringe filter (Sigma-Aldrich).
Isolation and activation of CD4+ T cells.
PBMCs were isolated from EDTA-anticoagulated blood of healthy donors via density gradient centrifugation using Bicoll (Biochrom). Subsequently, CD4+ T cells were isolated from PBMCs through a negative-selection strategy using an EasySep human CD4+ T-cell enrichment kit (Stem Cell Technology) according to the manufacturer’s protocol. The cells were cultured at 3 × 106 cells/ml in RPMI containing 10% heat-inactivated FBS and 100 IU/ml human recombinant interleukin 2 (hrIL-2) (Peprotech). Isolated CD4+ T cells were activated with anti-CD3/CD28-coupled magnetic beads (Dynabeads; Human T-Activator CD3/CD28 [ThermoFisher]) at a bead-to-cell ratio of 1:1. The HIV-1 primary strains tested here largely depended on the CCR5 coreceptor for efficient entry (88), which has reduced expression after T-cell receptor triggering (89). In order to allow CCR5 reexpression, the beads were magnetically removed after 72 h, and the activated CD4+ T cells were cultured in fresh IL-2-containing medium for 72 h prior to HIV-1 infection.
HIV-1 infection of primary CD4+ T cells.
CD4+ T-cell blasts were resuspended in viral supernatant or cell culture medium (no-virus control) to perform spinfection for 2 h at 1,200 × g and 37°C with acceleration/deceleration at 3. After spinfection, the medium was replaced with fresh RPMI supplemented with 10% FBS and IL-2 (100 U/ml). The infected CD4+ T cells were cultured at 37°C, 5% CO2 at a concentration of 3 × 106 cells/ml. HLA-E surface expression was assessed at 72 h postinfection.
Generation of Jurkat-HLA-A2 cell lines.
Gene constructs for HLA-A*02:01ECD-TMD-CYT (HLA-A2), HLA-A*02:01ECD-TMDHLA-E*01:03CYT (HLA-A2:Ecyt), and HLA-A*02:01ECD-TMDHLA-C*04:01CYT (HLA-A2:Ecyt) were ordered from GeneArt (Life Technologies). All constructs included the mammalian Kozak sequence (GCCACC) and were subjected to mammalian gene optimization. The constructs were cloned into the lentiviral vector pSIP (containing a spleen focus-forming virus [SFFV] promoter driving the gene of interest and an internal ribosomal entry site [IRES]), followed by a puromycin resistance gene (vector kindly provided by Thomas Pertel), using NEBBuilder HiFi DNA assembly master mix (NEB). To produce lentiviral-like particles, HEK 293T cells (ATCC) were transfected using Lipofectamine 3000 (Life Technologies), a vesicular stomatitis virus G (VSV-G) envelope vector (pHEF-VSVg; NIH AIDS Reagent Program), an HIV-1 gag-pol packaging vector (psPAX2; NIH AIDS Reagent Program), and the lentiviral transfer vector encoding the specific HLA-A2 construct. After 72 h, the lentiviral supernatant was harvested and filtered. The filtered supernatant was used for transduction of Jurkat E6.1 cells, which were subsequently selected with puromycin (1 μg/ml; Sigma-Aldrich).
Generation of virus-like particles containing nef or vpu of CH077, CH198, and NL4-3.
Gene constructs of nef derived from NL4-3 and CH077 were ordered from GeneArt (Life Technologies). The constructs included the mammalian Kozak sequence, GCCACC, and EcoRI/NotI digestion sites. The nef gene derived from CH198 and vpu genes derived from NL4-3, CH077, and CH198 were amplified from the WT plasmids pBR-amp HIV-1 NL4-3, pCH077.t, and pCH198.c using primers with the Kozak sequence and EcoRI/NotI digestion sites. The nef constructs were cloned into the lentiviral vector pLVX-EF1α-IRES-mCherry (Clontech) containing an EF1α promoter and the IRES-driven fluorescent marker mCherry. The vpu constructs were cloned into the Lego-iG2 vector (90) (kindly provided by Boris Fehse) containing an SFFV promoter and the IRES-driven fluorescent marker eGFP.
Stimulation and lentiviral transduction of Jurkat cells with nef constructs.
The resulting nef- or vpu-containing transfer vectors were transfected into HEK 293T cells together with a VSV-G envelope vector and the HIV-1 gag-pol packaging vector using Lipofectamine 2000 (Invitrogen). As a vector control, transfection was performed using vectors without nef or vpu but containing the fluorescent marker. After 72 h, the supernatant containing lentiviral particles was collected and filtered as described above. The lentiviral particles were subsequently used to transduce Jurkat cells for stable expression of nef or vpu genes. Lentivirus-transduced Jurkat cells were stimulated overnight with 100 U/ml IFN-γ (PeproTech; catalog no. 300-02; lot no. 121527) prior to flow cytometric assessment of HLA-E surface levels. Lentivirus nef-transduced Jurkat-HLA-A2 cell lines were analyzed 72 h after transduction for downregulation of HLA-A2.
Flow cytometric assessment of surface markers and intracellular staining.
All the antibody information used for flow cytometry staining is listed in Table 2, and the respective panel used is indicated in each figure legend. The following protocol was used for surface staining: 1 × 106 cells were resuspended in the respective LIVE/DEAD marker for 20 min at 4°C to allow LIVE/DEAD discrimination. After a washing step, the cells were resuspended in 100 μl of surface antibody mixture for 30 min at 4°C. For intracellular detection of HIV-1 p24 Gag, cells were fixed and permeabilized using a Cytofix/Cytoperm solution kit (BD Biosciences) following the manufacturer’s instructions but adding an additional step of 20 min incubation in permeabilization buffer prior to intracellular staining (Fig. 1). For the experiments shown in Fig. 4, Fix & Perm cell permeabilization kit (ThermoFisher) was used following the manufacturer’s instructions for intracellular detection of HIV-1 p24. The cells were subsequently washed and resuspended in phosphate-buffered saline (PBS). In assays that did not include intracellular staining, cells were fixed with 4% paraformaldehyde in PBS for 20 min prior to flow cytometric analysis. Flow cytometric acquisition was performed using a BD LSR Fortessa cell analyzer and BD FACS Diva v.7.0. software or a BD FACS Aria fusion flow cytometer with BD FACS Diva v.7.0. software.
TABLE 2.
Antibodies used in this study
| Antibody | Clone | Fluorophorea | Manufacturer | Catalog no. | RRIDb |
|---|---|---|---|---|---|
| Panel A | |||||
| LIVE/DEAD | Zombie NIR | APC Cy7 | Biolegend | 423105 | |
| CD4 | RPA-T4 | BV711 | Biolegend | 300558 | AB_2564393 |
| CD3 | UCHT1 | BUV395 | BD Biosciences | 563546 | AB_2744387 |
| HLA class I | W6/32 | APC | ThermoFisher Scientific | 17-9983-42 | AB_10733389 |
| Tetherin | RS38E | PE | Biolegend | 348406 | AB_10564402 |
| HLA-E | 3D12 | BV421 | Biolegend | 342612 | AB_2721525 |
| IgG1(κ) isotype control | MOPC 21 | BV421 | Biolegend | 400158 | AB_11150232 |
| HIV-1 p24 | KC57 | FITC | Coulter | 6604665 | AB_1575987 |
| Panel B | |||||
| LIVE/DEAD | Zombie NIR | APC Cy7 | Biolegend | 423105 | |
| CD4 | OKT4 | BV711 | Biolegend | 317439 | AB_11219404 |
| HLA class I | W6/32 | Pacific Blue | Biolegend | 311418 | AB_493669 |
| Tetherin | RS38E | APC | Biolegend | 348410 | AB_2067121 |
| HLA-E | 3D12 | PE | Biolegend | 342603 | AB_1659250 |
| HIV-1 p24 | KC57 | FITC | Coulter | 6604665 | AB_1575987 |
| CD3 | UCTH1 | BUV395 | BD Biosciences | 563546 | AB_2744387 |
| CD8 | RPA-T8 | BV605 | Biolegend | 301040 | AB_2563185 |
| IgG1(κ) isotype control | MOPC 21 | PE | Biolegend | 400111 | AB_241956 |
| Panel C | |||||
| LIVE/DEAD | Zombie NIR | APC Cy7 | Biolegend | 423105 | |
| HLA-E | 3D12 | BV421 | Biolegend | 342612 | AB_2721525 |
| IgG1(κ) isotype control | MOPC-21 | BV421 | Biolegend | 400158 | AB_11150232 |
| CD3 | UCHT1 | BUV395 | BD Biosciences | 563546 | AB_2744387 |
| Tetherin | RS38E | PE | Biolegend | 348406 | AB_10564402 |
| HLA class I | W6/32 | APC | ThermoFisher Scientific | 17-9983-42 | AB_10733389 |
| Panel D | |||||
| LIVE/DEAD | Near-IR | APC-Cy7 | ThermoFisher Scientific | L34975 | |
| CD3 | UHCT1 | BUV737 | BD Biosciences | 564307 | AB_564307 |
| HLA-A2 | BB7.2 | FITC | Biolegend | 343304 | AB_1659245 |
| Panel E | |||||
| LIVE/DEAD | Near-IR | APC-Cy7 | ThermoFisher Scientific | L34975 | |
| CD4 | RPA-T4 | BV711 | Biolegend | 300558 | AB_2564393 |
| CD3 | UCHT1 | BUV395 | BD Biosciences | 563546 | AB_2744387 |
| HLA class I | W6/32 | PE-Cy7 | Biolegend | 311430 | AB_2561617 |
| Tetherin | RS38E | PE | Biolegend | 348406 | AB_10564402 |
| HLA-E | 3D12 | BV421 | Biolegend | 342612 | AB_2721525 |
| IgG1(κ) isotype control | MOPC 21 | BV421 | Biolegend | 400158 | AB_11150232 |
| HIV-1 p24 | KC57 | FITC | Coulter | 6604665 | AB_1575987 |
| HIV-1 p24 | 28B7 | APC | Medimabs | MM-0289-APC |
PE, phycoerythrin.
RRID, research resource identifier.
Data analysis, software, and statistics.
FlowJo software (v.10.1) was used to analyze all acquired flow cytometry data. Statistical analyses were performed using GraphPad Prism (8) and SAS (v.9.3). The relative change (percent) was calculated as follows: (MFI infected − MFI uninfected)/MFI uninfected × 100; n-fold change was calculated as follows: MFI transduced divided by MFI untransduced. To assess the association of relative change in HLA-E surface (percent) with the relative change in HLA-I content (percent), a mixed-effect linear regression model with a random intercept was employed, which takes into account the intradonor correlation (multiple measurements per donor). The data are reported as median and IQR (1st quartile to 3rd quartile) or in figures are displayed as median and IQR. For statistical testing, nonparametric paired tests were employed (the specific tests are indicated in the figure legends), and the test multiplicity for all tests was adjusted using the original false-discovery rate (FDR) method of Benjamini and Hochberg (91). All reported P values are FDR adjusted, and P values of <0.05 are significant.
Accession number(s).
GenBank accession numbers are as follows: NL4-3, AF324493.2; CH293, KC156216.1; CH107, MN202471; CH164, KC156127.1; CH185, KC156129.1; CH236, MN202472; CH077, JN944909.1; CH198, KC156130.1.
ACKNOWLEDGMENTS
The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 SF162 and HIV-1 SF2 MC from Jay Levy, HIV-1 LAI from Jean-Marie Bechet and Luc Montagnier, HIV-1 NL4-3 infectious molecular clone (pNL4-3) from Malcolm Martin, MT4-cells from Douglas Richman (83, 96, 97), psPAX2 from Didier Trono, and pHEF-VSVG from Lung-Ji Chang (98). We are also thankful to Maja Ziegler for her help in generating viral stocks of the infectious molecular clones tested here. LeGO-iG2 (90) was a kind gift from Boris Fehse.
J.I.A., T.V.S.T., A.N., and A.H. performed the experiments and analyzed the data (T.V.S.T., Fig. 1 and 2; A.N., Fig. 3; J.I.A., Fig. 4; A.H., Fig. 5), and A.H. and M.A. designed the experiments. C.T.F. generated viral stocks from infectious molecular clones. L.R. performed the mixed-effect linear regression analysis and provided important statistical input. D.S., C.M.S., F.K., H.L., J.C.K., C.O., and B.H.H. generated mutant HIV-1 constructs, constructed/provided infectious molecular clones, and provided important intellectual input. W.F.G.-B., G.M., A.H., and M.A. interpreted the data. J.I.A., T.V.ST., A.N., and A.H. wrote the manuscript.
C.T.F., D.S., and F.K. were supported by the DFG priority program Innate Sensing and Restriction of Retroviruses (SPP1923). F.K. is further supported by an ERC Advanced grant (Anti-Virome) and DFG SFB 1279. This work was supported by German Center for Infection Research (DZIF) grants to A.H., J.I.A., A.N., and M.A. (TI 07.002, TI 07.003, and TTU 04.810.) and by grants from the NIH to B.H.H. (R01 AI 114266 and UM1 AI 26620). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Work by J.C.K. and C.O. was supported by the NIH Center for HIV/AIDS Vaccine Immunology (CHAVI) (UO1-AI067854).
REFERENCES
- 1.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuniholm MH, Gao X, Xue X, Kovacs A, Anastos K, Marti D, Greenblatt RM, Cohen MH, Minkoff H, Gange SJ, Fazzari M, Young MA, Strickler HD, Carrington M. 2011. Human leukocyte antigen genotype and risk of HIV disease progression before and after initiation of antiretroviral therapy. J Virol 85:10826–10833. doi: 10.1128/JVI.00804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PIW, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O’Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DML, Vine S, Addo MM, Allen TM, Altfeld M, Henn MR, Le Gall S, Streeck H, Haas DW, Kuritzkes DR, Robbins GK, Shafer RW, Gulick RM, Shikuma CM, Haubrich R, Riddler S, Sax PE, Daar ES, Ribaudo HJ, Agan B, Agarwal S, Ahern RL, Allen BL, Altidor S, Altschuler EL, Ambardar S, Anastos K, Anderson B, Anderson V, Andrady U, Antoniskis D, Bangsberg D, Barbaro D, Barrie W, Bartczak J, Barton S, Basden P, Basgoz N, Bazner S, Bellos NC, Benson AM, Berger J, Bernard NF, Bernard AM, Birch C, Bodner SJ, Bolan RK, Boudreaux ET, Bradley M, Braun JF, Brndjar JE, Brown SJ, Brown K, Brown ST, Burack J, Bush LM, Cafaro V, Campbell O, Campbell J, Carlson RH, Carmichael JK, Casey KK, Cavacuiti C, Celestin G, Chambers ST, Chez N, Chirch LM, Cimoch PJ, Cohen D, Cohn LE, Conway B, Cooper DA, Cornelson B, Cox DT, Cristofano MV, Cuchural G, Czartoski JL, Dahman JM, Daly JS, Davis BT, Davis K, Davod SM, DeJesus E, Dietz CA, Dunham E, Dunn ME, Ellerin TB, Eron JJ, Fangman JJW, Farel CE, Ferlazzo H, Fidler S, Fleenor-Ford A, Frankel R, Freedberg KA, French NK, Fuchs JD, Fuller JD, Gaberman J, Gallant JE, Gandhi RT, Garcia E, Garmon D, Gathe JC, Gaultier CR, Gebre W, Gilman FD, Gilson I, Goepfert PA, Gottlieb MS, Goulston C, Groger RK, Gurley TD, Haber S, Hardwicke R, Hardy WD, Harrigan PR, Hawkins TN, Heath S, Hecht FM, Henry WK, Hladek M, Hoffman RP, Horton JM, Hsu RK, Huhn GD, Hunt P, Hupert MJ, Illeman ML, Jaeger H, Jellinger RM, John M, Johnson JA, Johnson KL, Johnson H, Johnson K, Joly J, Jordan WC, Kauffman CA, Khanlou H, Killian RK, Kim AY, Kim DD, Kinder CA, Kirchner JT, Kogelman L, Kojic EM, Korthuis PT, Kurisu W, Kwon DS, LaMar M, Lampiris H, Lanzafame M, Lederman MM, Lee DM, Lee JML, Lee MJ, Lee ETY, Lemoine J, Levy JA, Llibre JM, Liguori MA, Little SJ, Liu AY, Lopez AJ, Loutfy MR, Loy D, Mohammed DY, Man A, Mansour MK, Marconi VC, Markowitz M, Marques R, Martin JN, Martin HL, Mayer KH, McElrath MJ, McGhee TA, McGovern BH, McGowan K, McIntyre D, Mcleod GX, Menezes P, Mesa G, Metroka CE, Meyer-Olson D, Miller AO, Montgomery K, Mounzer KC, Nagami EH, Nagin I, Nahass RG, Nelson MO, Nielsen C, Norene DL, O’Connor DH, Ojikutu BO, Okulicz J, Oladehin OO, Oldfield EC, Olender SA, Ostrowski M, Owen WF, Pae E, Parsonnet J, Pavlatos AM, Perlmutter AM, Pierce MN, Pincus JM, Pisani L, Price LJ, Proia L, Prokesch RC, Pujet HC, Ramgopal M, Rathod A, Rausch M, Ravishankar J, Rhame FS, Richards CS, Richman DD, Rodes B, Rodriguez M, Rose RC, Rosenberg ES, Rosenthal D, Ross PE, Rubin DS, Rumbaugh E, Saenz L, Salvaggio MR, Sanchez WC, Sanjana VM, Santiago S, Schmidt W, Schuitemaker H, Sestak PM, Shalit P, Shay W, Shirvani VN, Silebi VI, Sizemore JM, Skolnik PR, Sokol-Anderson M, Sosman JM, Stabile P, Stapleton JT, Starrett S, Stein F, Stellbrink H-J, Sterman FL, Stone VE, Stone DR, Tambussi G, Taplitz RA, Tedaldi EM, Telenti A, Theisen W, Torres R, Tosiello L, Tremblay C, Tribble MA, Trinh PD, Tsao A, Ueda P, Vaccaro A, Valadas E, Vanig TJ, Vecino I, Vega VM, Veikley W, Wade BH, Walworth C, Wanidworanun C, Ward DJ, Warner DA, Weber RD, Webster D, Weis S, Wheeler DA, White DJ, Wilkins E, Winston A, Wlodaver CG, van’t Wout A, Wright DP, Yang OO, Yurdin DL, Zabukovic BW, Zachary KC, Zeeman B, Zhao M. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magierowska M, Theodorou I, Debré P, Sanson F, Autran B, Rivière Y, Charron D, Costagliola D. 1999. Combined genotypes of CCR5, CCR2, SDF1, and HLA genes can predict the long-term nonprogressor status in human immunodeficiency virus-1-infected individuals. Blood 93:936–941. [PubMed] [Google Scholar]
- 5.Flores-Villanueva PO, Yunis EJ, Delgado JC, Vittinghoff E, Buchbinder S, Leung JY, Uglialoro AM, Clavijo OP, Rosenberg ES, Kalams SA, Braun JD, Boswell SL, Walker BD, Goldfeld AE. 2001. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc Natl Acad Sci U S A 98:5140–5145. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder P. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 7.Kløverpris HN, Leslie A, Goulder P. 2015. Role of HLA adaptation in HIV evolution. Front Immunol 6:665. doi: 10.3389/fimmu.2015.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frater AJ, Brown H, Oxenius A, Günthard HF, Hirschel B, Robinson N, Leslie AJ, Payne R, Crawford H, Prendergast A, Brander C, Kiepiela P, Walker BD, Goulder PJR, McLean A, Phillips RE. 2007. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J Virol 81:6742–6751. doi: 10.1128/JVI.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapel A, Garcia-Beltran WF, Hölzemer A, Ziegler M, Lunemann S, Martrus G, Altfeld M. 2017. Peptide-specific engagement of the activating NK cell receptor KIR2DS1. Sci Rep 7:1–12. doi: 10.1038/s41598-017-02449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölzemer A, Thobakgale CF, Jimenez Cruz CA, Garcia-Beltran WF, Carlson JM, van Teijlingen NH, Mann JK, Jaggernath M, Kang SG, Körner C, Chung AW, Schafer JL, Evans DT, Alter G, Walker BD, Goulder PJ, Carrington M, Hartmann P, Pertel T, Zhou R, Ndung’u T, Altfeld M. 2015. Selection of an HLA-C*03:04-restricted HIV-1 p24 Gag sequence variant is associated with viral escape from KIR2DL3+ natural killer cells: data from an observational cohort in South Africa. PLoS Med 12:e1001900-27. doi: 10.1371/journal.pmed.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Teijlingen NH, Hölzemer A, Körner C, García-Beltrán WF, Schafer JL, Fadda L, Suscovich TJ, Brander C, Carrington M, Evans DT, Van Baarle D, Altfeld M. 2014. Sequence variations in HIV-1 p24 Gag-derived epitopes can alter binding of KIR2DL2 to HLA-C*03: 04 and modulate primary natural killer cell function. AIDS 28:1399–1408. doi: 10.1097/QAD.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunemann S, Martrus G, Hölzemer A, Chapel A, Ziegler M, Körner C, Garcia Beltran W, Carrington M, Wedemeyer H, Altfeld M. 2016. Sequence variations in HCV core-derived epitopes alter binding of KIR2DL3 to HLA-C*03:04 and modulate NK cell function. J Hepatol 65:252–258. doi: 10.1016/j.jhep.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelak K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, Ge D, De Luca A, Martinez-Picado J, Wolinsky SM, Martinson JJ, Jamieson BD, Bream JH, Martin MP, Borrow P, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Carrington M, Goldstein DB, Alter G, NIAID Center for HIV/AIDS Vaccine Immunology. 2011. Copy number variation of KIR genes influences HIV-1 control. PLoS Biol 9:e1001208. doi: 10.1371/journal.pbio.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MP, Gao X, Lee J-H, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 16.Martin MP, Naranbhai V, Shea PR, Qi Y, Ramsuran V, Vince N, Gao X, Thomas R, Brumme ZL, Carlson JM, Wolinsky SM, Goedert JJ, Walker BD, Segal FP, Deeks SG, Haas DW, Migueles SA, Connors M, Michael N, Fellay J, Gostick E, Llewellyn-Lacey S, Price DA, Lafont BA, Pymm P, Saunders PM, Widjaja J, Wong SC, Vivian JP, Rossjohn J, Brooks AG, Carrington M. 2018. Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B*57 protection against HIV-1. J Clin Invest 128:1903–1912. doi: 10.1172/JCI98463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apps R, Del Prete GQ, Chatterjee P, Lara A, Brumme ZL, Brockman MA, Neil S, Pickering S, Schneider DK, Piechocka-Trocha A, Walker BD, Thomas R, Shaw GM, Hahn BH, Keele BF, Lifson JD, Carrington M. 2016. HIV-1 Vpu mediates HLA-C downregulation. Cell Host Microbe 19:686–695. doi: 10.1016/j.chom.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 19.Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol 167:903–913. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotter D, Krabbe T, Reith E, Gawanbacht A, Rahm N, Ayouba A, Van Driessche B, Van Lint C, Peeters M, Kirchhoff F, Sauter D. 2017. Primate lentiviruses use at least three alternative strategies to suppress NF-κB-mediated immune activation. PLoS Pathog 13:e1006598-28. doi: 10.1371/journal.ppat.1006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenway AL, McPhee DA, Allen K, Johnstone R, Holloway G, Mills J, Azad A, Sankovich S, Lambert P. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J Virol 76:2692–2702. doi: 10.1128/jvi.76.6.2692-2702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross TM, Oran AE, Cullen BR. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol 9:613–621. doi: 10.1016/S0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 23.Manrique S, Sauter D, Horenkamp FA, Lülf S, Yu H, Hotter D, Anand K, Kirchhoff F, Geyer M. 2017. Endocytic sorting motif interactions involved in Nef-mediated downmodulation of CD4 and CD3. Nat Commun 8:442. doi: 10.1038/s41467-017-00481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venzke S, Michel N, Allespach I, Fackler OT, Keppler OT. 2006. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J Virol 80:11141–11152. doi: 10.1128/JVI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heigele A, Kmiec D, Regensburger K, Langer S, Peiffer L, Stürzel CM, Sauter D, Peeters M, Pizzato M, Learn GH, Hahn BH, Kirchhoff F. 2016. The Potency of Nef-Mediated SERINC5 antagonism correlates with the prevalence of primate lentiviruses in the wild. Cell Host Microbe 20:381–391. doi: 10.1016/j.chom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Münch J, Rajan D, Schindler M, Specht A, Rücker E, Novembre FJ, Nerrienet E, Müller-Trutwin MC, Peeters M, Hahn BH, Kirchhoff F. 2007. Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses. J Virol 81:13852–13864. doi: 10.1128/JVI.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Körner C, Simoneau CR, Schommers P, Granoff M, Ziegler M, Hölzemer A, Lunemann S, Chukwukelu J, Corleis B, Naranbhai V, Kwon DS, Scully EP, Jost S, Kirchhoff F, Carrington M, Altfeld M. 2017. HIV-1-mediated downmodulation of HLA-C impacts target cell recognition and antiviral activity of NK cells. Cell Host Microbe 22:111–119.e4. doi: 10.1016/j.chom.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachtel ND, Umviligihozo G, Pickering S, Mota TM, Liang H, Del Prete GQ, Chatterjee P, Lee GQ, Thomas R, Brockman MA, Neil S, Carrington M, Bwana B, Bangsberg DR, Martin JN, Kallas EG, Donini CS, Cerqueira B, Doherty UTO, Hahn BH, Jones RB, Brumme L, Nixon DF, Apps R. 2018. HLA-C downregulation by HIV-1 adapts to host HLA genotype. PLoS Pathog 14:e1007257. doi: 10.1371/journal.ppat.1007257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz P, Hahn BH, Hatziioannou T, Kirchhoff F. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langer S, Hammer C, Hopfensperger K, Klein L, Hotter D, De Jesus PD, Herbert KM, Pache L, Smith N, van der Merwe JA, Chanda SK, Fellay J, Kirchhoff F, Sauter D. 2019. HIV-1 Vpu is a potent transcriptional suppressor of NF-κB-elicited antiviral immune responses. Elife 8:1–25. doi: 10.7554/eLife.41930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahiti M, Toyoda M, Jia X, Kuang XT, Mwimanzi F, Mwimanzi P, Walker BD, Xiong Y, Brumme ZL, Brockman MA, Ueno T. 2016. Relative resistance of HLA-B to downregulation by naturally occurring HIV-1 Nef sequences. mBio 7:e01516. doi: 10.1128/mBio.01516-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ende Z, Deymier MJ, Claiborne DT, Prince JL, Mónaco DC, Kilembe W, Allen SA, Hunter E. 2018. HLA class I downregulation by HIV-1 variants from subtype C transmission pairs. J Virol 92:e01633-17. doi: 10.1128/JVI.01633-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz A, Djaoud Z, Nemat-Gorgani N, Blokhuis J, Hilton HG, Béziat V, Malmberg K-J, Norman PJ, Guethlein LA, Parham P. 2016. Class I HLA haplotypes form two schools that educate NK cells in different ways. Sci Immunol 1:eaag1672. doi: 10.1126/sciimmunol.aag1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsuran V, Naranbhai V, Horowitz A, Qi Y, Martin MP, Yuki Y, Gao X, Walker-Sperling V, Del Prete GQ, Schneider DK, Lifson JD, Fellay J, Deeks SG, Martin JN, Goedert JJ, Wolinsky SM, Michael NL, Kirk GD, Buchbinder S, Haas D, Ndung’u T, Goulder P, Parham P, Walker BD, Carlson JM, Carrington M. 2018. Elevated HLA-A expression impairs HIV control through inhibition of NKG2A-expressing cells. Science 359:86–90. doi: 10.1126/science.aam8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strong RK, Holmes MA, Li P, Braun L, Lee N, Geraghty DE. 2003. HLA-E allelic variants: correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J Biol Chem 278:5082–5090. doi: 10.1074/jbc.M208268200. [DOI] [PubMed] [Google Scholar]
- 36.Pietra G, Romagnani C, Mazzarino P, Falco M, Millo E, Moretta A, Moretta L, Mingari MC. 2003. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A 100:10896–10901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romagnani C, Pietra G, Falco M, Millo E, Mazzarino P, Biassoni R, Moretta A, Moretta L, Mingari MC. 2002. Identification of HLA-E-specific alloreactive T lymphocytes: a cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc Natl Acad Sci U S A 99:11328–11333. doi: 10.1073/pnas.172369799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte D, Vogel M, Langhans B, Krämer B, Körner C, Nischalke HD, Steinberg V, Michalk M, Berg T, Rockstroh JK, Sauerbruch T, Spengler U, Nattermann J. 2009. The HLA-E(R)/HLA-E(R) genotype affects the natural course of hepatitis C virus (HCV) infection and is associated with HLA-E-restricted recognition of an HCV-derived peptide by interferon-gamma-secreting human CD8(+) T cells. J Infect Dis 200:1397–1401. doi: 10.1086/605889. [DOI] [PubMed] [Google Scholar]
- 39.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, Ford JC, Selseth AN, Pathak R, Malouli D, Legasse AW, Axthelm MK, Nelson JA, Gillespie GM, Walters LC, Brackenridge S, Sharpe HR, López CA, Früh K, Korber BT, McMichael AJ, Gnanakaran S, Sacha JB, Picker LJ. 2016. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 351:714–720. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le Gall S, Planelles V, Campbell KS, Barker E. 2016. A conserved HIV-1-derived peptide presented by HLA-E renders infected T-cells highly susceptible to attack by NKG2A/CD94-bearing natural killer cells. PLoS Pathog 12:e1005421. doi: 10.1371/journal.ppat.1005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nattermann J, Nischalke HD, Hofmeister V, Kupfer B, Ahlenstiel G, Feldmann G, Rockstroh J, Weiss EH, Sauerbruch T, Spengler U. 2005. HIV-1 infection leads to increased HLA-E expression resulting in impaired function of natural killer cells. Antivir Ther 10:95–107. [DOI] [PubMed] [Google Scholar]
- 42.Hannoun Z, Lin Z, Brackenridge S, Kuse N, Akahoshi T, Borthwick N, McMichael A, Murakoshi H, Takiguchi M, Hanke T. 2018. Identification of novel HIV-1-derived HLA-E-binding peptides. Immunol Lett 202:65–72. doi: 10.1016/j.imlet.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llano M, Lee N, Navarro F, García P, Albar JP, Geraghty DE, López-Botet M. 1998. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol 28:2854–2863. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Lisovsky I, Isitman G, Song R, DaFonseca S, Tremblay-McLean A, Lebouché B, Routy J-P, Bruneau J, Bernard NF. 2015. A higher frequency of NKG2A+ than of NKG2A− NK cells responds to autologous HIV-infected CD4 cells irrespective of whether or not they coexpress KIR3DL1. J Virol 89:9909–9919. doi: 10.1128/JVI.01546-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apps R, Meng Z, Del Prete GQ, Lifson JD, Zhou M, Carrington M. 2015. Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. J Immunol 194:3594–3600. doi: 10.4049/jimmunol.1403234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martini F, Agrati C, D'Offizi G, Poccia F. 2005. HLA-E up-regulation induced by HIV infection may directly contribute to CD94-mediated impairment of NK cells. Int J Immunopathol Pharmacol 18:269–276. doi: 10.1177/039463200501800209. [DOI] [PubMed] [Google Scholar]
- 47.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, Barker E, Marcenaro E, Moretta A, Fauci AS. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog 4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonaparte MI, Barker E. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 49.Wu HL, Wiseman RW, Hughes CM, Webb GM, Abdulhaqq SA, Bimber BN, Hammond KB, Reed JS, Gao L, Burwitz BJ, Greene JM, Ferrer F, Legasse AW, Axthelm MK, Park BS, Brackenridge S, Maness NJ, McMichael AJ, Picker LJ, O’Connor DH, Hansen SG, Sacha JB. 2018. The role of MHC-E in T cell immunity is conserved among humans, rhesus macaques, and cynomolgus macaques. J Immunol 200:49–60. doi: 10.4049/jimmunol.1700841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Specht A, DeGottardi MQ, Schindler M, Hahn B, Evans DT, Kirchhoff F. 2008. Selective downmodulation of HLA-A and -B by Nef alleles from different groups of primate lentiviruses. Virology 373:229–237. doi: 10.1016/j.virol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. 1998. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol 160:4951–4960. [PubMed] [Google Scholar]
- 52.Martrus G, Niehrs A, Cornelis R, Rechtien A, García-Beltran W, Lütgehetmann M, Hoffmann C, Altfeld M. 2016. Kinetics of HIV-1 latency reversal quantified on the single-cell level using a novel flow-based technique. J Virol 90:9018–9028. doi: 10.1128/JVI.01448-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 54.Gustafson KS, Ginder GD. 1996. Interferon-gamma induction of the human leukocyte antigen-E gene is mediated through binding of a complex containing STAT1alpha to a distinct interferon-gamma-responsive element. J Biol Chem 271:20035–20046. doi: 10.1074/jbc.271.33.20035. [DOI] [PubMed] [Google Scholar]
- 55.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiselyeva Y, Nedellec R, Ramos A, Pastore C, Margolis LB, Mosier DE. 2007. Evolution of CXCR4-using human immunodeficiency virus type 1 SF162 is associated with two unique envelope mutations. J Virol 81:3657–3661. doi: 10.1128/JVI.02310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mann JK, Byakwaga H, Kuang XT, Le AQ, Brumme CJ, Mwimanzi P, Omarjee S, Martin E, Lee GQ, Baraki B, Danroth R, McCloskey R, Muzoora C, Bangsberg DR, Hunt PW, Goulder PJR, Walker BD, Harrigan PR, Martin JN, Ndung’u T, Brockman MA, Brumme ZL. 2013. Ability of HIV-1 Nef to downregulate CD4 and HLA class I differs among viral subtypes. Retrovirology 10:100–116. doi: 10.1186/1742-4690-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. 1988. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A 85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kluge SF, Mack K, Iyer SS, Pujol FM, Heigele A, Learn GH, Usmani SM, Sauter D, Joas S, Hotter D, Bibollet-Ruche F, Plenderleith LJ, Peeters M, Geyer M, Sharp PM, Fackler OT, Hahn BH, Kirchhoff F. 2014. Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe 16:639–650. doi: 10.1016/j.chom.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardons M, Baxter AE, Massanella M, Pagliuzza A, Fromentin R, Dufour C, Leyre L, Routy J-P, Kaufmann DE, Chomont N. 2019. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog 15:e1007619. doi: 10.1371/journal.ppat.1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams M, Roeth J, Kasper Fleis R, Przybycin C, Collins K. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. Society 76:12173–12184. doi: 10.1128/JVI.76.23.12173-12184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol 88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 63.Matusali G, Potestà M, Santoni A, Cerboni C, Doria M. 2012. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J Virol 86:4496–4504. doi: 10.1128/JVI.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sowrirajan B, Barker E. 2011. The natural killer cell cytotoxic function is modulated by HIV-1 accessory proteins. Viruses 3:1091–1111. doi: 10.3390/v3071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carl S, Greenough TC, Krumbiegel M, Greenberg M, Skowronski J, Sullivan JL, Kirchhoff F. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J Virol 75:3657–3665. doi: 10.1128/JVI.75.8.3657-3665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng-Mayer C, Levy JA. 1988. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol 23(Suppl):S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 67.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wonderlich ER, Leonard JA, Collins KL. 2011. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv Virus Res 80:103–127. doi: 10.1016/B978-0-12-385987-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 70.Schaefer MR, Wonderlich ER, Roeth JF, Leonard JA, Collins KL. 2008. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog 4:e1000131. doi: 10.1371/journal.ppat.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wonderlich ER, Williams M, Collins KL. 2008. The tyrosine binding pocket in the adaptor protein 1 (AP-1) μ1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J Biol Chem 283:3011–3022. doi: 10.1074/jbc.M707760200. [DOI] [PubMed] [Google Scholar]
- 72.Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y. 2012. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol 19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mann JK, Omarjee S, Khumalo P, Ndung’u T. 2015. Genetic determinants of Nef-mediated CD4 and HLA class I down-regulation differences between HIV-1 subtypes B and C. Virol J 12:1–8. doi: 10.1186/s12985-015-0429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neill E, Kuo LS, Krisko JF, Tomchick DR, Garcia JV, Foster JL. 2006. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J Virol 80:1311–1320. doi: 10.1128/JVI.80.3.1311-1320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gioia L, Siddique A, Head SR, Salomon DR, Su AI. 2018. A genome-wide survey of mutations in the Jurkat cell line. BMC Genomics 19:334. doi: 10.1186/s12864-018-4718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wildum S, Schindler M, Munch J, Kirchhoff F. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80:8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moll M, Andersson SK, Smed-Sörensen A, Sandberg JK. 2010. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 116:1876–1884. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu X-N. 2006. HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol 36:278–286. doi: 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- 79.Carlsten M, Namazi A, Reger R, Levy E, Berg M, St Hilaire C, Childs RW. 2019. Bortezomib sensitizes multiple myeloma to NK cells via ER-stress-induced suppression of HLA-E and upregulation of DR5. Oncoimmunology 8:e1534664. doi: 10.1080/2162402X.2018.1534664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borsa M, Ferreira PLC, Petry A, Ferreira LGE, Camargo MM, Bou-Habib DC, Pinto AR. 2015. HIV infection and antiretroviral therapy lead to unfolded protein response activation. Virol J 12:77. doi: 10.1186/s12985-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ram DR, Manickam C, Hueber B, Itell HL, Permar SR, Varner V, Reeves RK. 2018. Tracking KLRC2 (NKG2C)+ memory-like NK cells in SIV+ and rhCMV+ rhesus macaques. PLoS Pathog 14:e1007104. doi: 10.1371/journal.ppat.1007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schneider U, Schwenk HU, Bornkamm G. 1977. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer 19:621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 83.Pauwels R, De Clercq E, Desmyter J, Balzarini J, Goubau P, Herdewijn P, Vanderhaeghe H, Vandeputte M. 1987. Sensitive and rapid assay on MT-4 cells for detection of antiviral compounds against the AIDS virus. J Virol Methods 16:171–185. doi: 10.1016/0166-0934(87)90002-4. [DOI] [PubMed] [Google Scholar]
- 84.Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, Shaw GM, Hahn BH, Ochsenbauer C, Kappes JC, Borrow P. 2013. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 10:146–148. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kmiec D, Iyer SS, Stürzel CM, Sauter D, Hahn BH, Kirchhoff F. 2016. Vpu-mediated counteraction of tetherin is a major determinant of HIV-1 interferon resistance. mBio 7:1–10. doi: 10.1128/mBio.00934-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu Q, Huang X, Shattock RJ. 2010. C-C chemokine receptor type 5 (CCR5) utilization of transmitted and early founder human immunodeficiency virus type 1 envelopes and sensitivity to small-molecule CCR5 inhibitors. J Gen Virol 91:2965–2973. doi: 10.1099/vir.0.025270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Minang JT, Trivett MT, Barsov EV, Del Prete GQ, Trubey CM, Thomas JA, Gorelick RJ, Piatak M, Ott DE, Ohlen C. 2011. TCR triggering transcriptionally downregulates CCR5 expression on rhesus macaque CD4+ T-cells with no measurable effect on susceptibility to SIV infection. Virology 409:132–140. doi: 10.1016/j.virol.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber K, Bartsch U, Stocking C, Fehse B. 2008. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol Ther 16:698–706. doi: 10.1038/mt.2008.6. [DOI] [PubMed] [Google Scholar]
- 91.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 92.Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 93.Wain-Hobson S, Vartanian J, Henry M, Chenciner N, Cheynier R, Delassus S, Martins L, Sala M, Nugeyre M, Guetard D. 1991. LAV revisited: origins of the early HIV-1 isolates from Institut Pasteur. Science 252:961–965. doi: 10.1126/science.2035026. [DOI] [PubMed] [Google Scholar]
- 94.Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS. 1984. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science 225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez-Pescador R, Power M, Barr P, Steimer K, Stempien M, Brown-Shimer S, Gee W, Renard A, Randolph A, Levy J. 1985. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science 227:484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- 96.Harada S, Koyanagi Y, Yamamoto N. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 97.Larder BA, Darby G, Richman DD. 1989. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science 243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 98.Chang L-J, Urlacher V, Iwakuma T, Cui Y, Zucali J. 1999. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther 6:715–728. doi: 10.1038/sj.gt.3300895. [DOI] [PubMed] [Google Scholar]
- 99.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]